Abstract
This work assessed some properties and effects of biocoal briquettes on environmental pollution compared with utilising coal alone. A mixture of coal fines and rice husks was prepared, with cassava starch gel as a binder, before compaction in a briquetting machine and drying with a solar dryer. Samples of the coal fines, rice husk, cassava flour and briquettes were analysed for their elemental and oxide concentrations via an energy-dispersive X-ray fluorescence analyser. The heat fuels were analysed for their thermal behaviour via thermogravimetric analyser (TGA), whereas the specific surface area, pore size and pore volume were determined using a Brunner–Emmett–Teller (BET) analyser. The conversion of the tested coals to biocoal briquettes resulted in a reduction in the level of arsenic in the briquettes. Additionally, the sulphur level decreased from 1.96 to 1.11% in the Okobo-Enjema briquettes and from 1.78 to 0.90% in the Onyeama briquettes. However, the levels of manganese increased from 0.04 to 0.22% in the Okobo-Enjema briquettes and from 0.04 to 0.21% in the Onyeama briquettes. Thermogravimetric analysis at different heating rates revealed that derivative weight loss increased with increasing heating rate, from 4.4% at 10 °C/min to 5.5% at 20 °C/min. BET analysis revealed that the briquettes had larger pore diameters, volumes and specific surface areas than did the coal samples. The coal samples have greater propensity to pollute the environment as the amount of potentially hazardous elements in the samples is greater than in their biocoal briquettes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Coal is a fossil fuel that is used as a primary energy fuel in several countries for electricity generation, heating and industrial processes [1]. Coal is commonly referred to as the dirtiest fuel [2, 3], because the exploitation and utilisation of coal release hazardous substances into the environment, which, may adversely affect human health and cause harm to other living resources and ecological systems. Some of the pollutants of environmental concern include carbon (IV) oxide (CO2), a greenhouse gas that exacerbates global warming, and climate change [3]; nitrogen oxides, which can cause acid rain, smog and respiratory diseases; and sulphur oxides, which contribute to acid rain and respiratory illnesses [4]. Additionally, some of the potentially hazardous elements (PHEs) released from coal contaminate the environmental compartments around coal mining sites [5]. These pollutants may enter humans through the food chain, resulting in adverse health consequences. Some of these PHEs are endocrine disruptors, carcinogenic, mutagenic and teratogenic, whereas others may cause neurological changes [6, 7]. Most. PHEs remain in the environment over a long period of time through the movement of water and atmospheric circulation, as they are nonbiodegradable and can accumulate in the biotic environment [8]. These elements, such as arsenic, cadmium, chromium, lead, nickel and zinc, are designated priority contaminants by the US Environmental Protection Agency. The occurrence of these elements in the environment should be controlled to mitigate their harmful effects [9].
Coal is an important natural resource in the energy mix of many countries. In addition to being used to generate electricity, coal is a raw material for briquette production. Briquettes are useful sources of fuel for domestic and industrial heating. The common types of briquettes are biomass and coal briquettes. Biomass briquettes are briquettes made from combustible materials of plant or animal origin. Briquettes made from coal are referred to as coal briquettes, whereas a blend of biomass and coal is used to produce biocoal briquettes. Many studies have been carried out on the compaction of Nigerian coals into briquettes. Some of these studies highlighted ways of enhancing the production of coal briquettes [10], assessed the quality of briquettes produced by blending coal with biomass [11] or studied the physical and combustion properties of some briquettes [12, 13].
To mitigate the level of CO2 emission during utilisation, some studies have investigated the conversion of coal to the liquid form [14] and the direct combustion of coal with biomass for power generation [15,16,17]. Ikelle et al. [18] noted that the CO2 released from coal is a greenhouse gas, whereas that from the combustion of biomass may not contribute much to the amount of CO2 in the environment, as CO2 is used by plants for photosynthesis. Therefore, biomass is regarded as carbon dioxide-neutral with respect to the greenhouse gas effect [19]. There are studies on biocoal briquettes produced from coal blended with different biomass materials. The biomass includes cassava stalks [12], sunflower husks and leaves [20], fermented cow dung [21], sawdust and maize husks [22] and groundnut shells [23]. Other types of biomass, such as rice husk, may be investigated for blending with coal. Rice husk is a byproduct of milling rice, which is abundantly available in rice-producing regions worldwide. Rice husk is essentially treated as a waste and burned in open air, thereby emitting toxic and particulate matter into the air, or used for landfilling, where it emits various gases, such as methane, in processes that add to air, soil and water pollution. Literature searches reveal scant information on its chemical composition, especially in relation to its impact on the environment during utilisation.
The characteristics (rank, type, and impurities) of each coal differ because coals are formed from different plant sources under different environmental and geological conditions [24]. These properties are related to the chemical composition, pore volume, size and specific surface area. They affect the potential for the release of toxic substances into the environment. The characteristics of specific coals and the biomass to blend can aid in understanding the fate of hazardous elements during conversion, whether they will be found in the final product and the best mechanism for mitigating the effects of these elements on the environment [25]. Vassilev et al. [26] noted the need to characterize biomass in terms of its chemical composition, mineral composition, and other properties.
In Nigeria, coal is an abundant, accessible and inexpensive fuel, as its deposits are found in many locations within the Anambra sedimentary basin. There are coal deposits in approximately thirteen states in Nigeria. No literature exists on the elemental and inorganic oxide concentrations of coal mined at Okobo-Enjema. No previous work has compared the level of PHEs in coal before and after conversion to biocoal briquette with rice husk. Additionally, no studies have considered pore volume, size and specific surface area as factors that may affect emissions from coal and biocoal briquettes. There is no literature on the compaction of mesoporous coals with Abakaliki rice husk variety.
Therefore, the present study investigated some properties and effects of briquettes prepared from mesoporous coal fines and Abakaliki rice husks with cassava starch as a binder on environmental pollution compared with the effects of utilizing coal alone. The objectives of this study are to determine the elemental and oxide compositions of the rice husk and cassava flour (binder); characterize Okobo-Enjema and Onyeama coals for their elemental and oxide compositions, surface area, pore size and pore volume; prepare biocoal briquette with coals mined at Okobo-Enjema and Onyeama coal mines blended with rice husk; characterize the biocoal briquette for its elemental and oxide compositions, surface area, pore size and pore volume; and analyse the thermal behavior of the fuels (coal, rice husk and briquettes) using TGA.
2 Materials and methods
2.1 Materials
Coal fines were obtained from mines at Okobo-Enjema (Kogi State) and Onyeama (Enugu State), Nigeria. Rice husks from Abakaliki rice were collected from a rice milling cluster at Abakaliki, Ebonyi State, Nigeria. Cassava roots were sourced from a farm at the National Centre for Energy Research and Development (NCERD), University of Nigeria, Nsukka. A solar dryer and a hydraulic press briquetting machine constructed at the NCERD were used. A Vecstar muffle furnace (model LF3), an MB 35 halogen moisture analyser, an EDXRF analyser (Thermo Fisher Scientific ARL Quant’x model), a BET analyser (Quantachrome Nova 4200e model) and a TGA (Perkin Elmer TGA 4000) were used for this study.
2.2 Preparation of biocoal briquettes
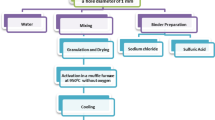
The as-received coal fines were pulverized via a manual wooden mortar and pestle, and then passed through a 250 µm sieve. Rice husk was weighed, pulverized and passed through a sieve. Equal amounts of coal and rice husk fines were mixed in a bowl. The mixture was gradually put into warm starch gel while stirring with a stick to form a paste. The quantity of cassava starch in relation to the feedstock was approximately 7%. This mixture was fed into the molding compartments in a briquetting machine for compaction and shaping into briquettes. The briquetting machine was fabricated at the Energy Research Centre, University of Nigeria, Nsukka. It has a hydraulic press and 12 cavities where the feed-stocks are loaded. The machine uses a hydraulic press of 160 N/m2 maximum pressure. Further details and schematic of the machine were reported by Anyanwu et al. [13].
The formed briquettes were kept in trays inside the drying chambers of a solar dryer for four days to remove excess moisture, prevent mold growth, improve combustion efficiency, and enhance the stability and handling of the briquettes. The dryer has two compartments: a solar collector and a drying chamber. The temperature of the drying chamber in the solar dryer during the briquette drying process ranged between 31 and 58 ℃. The solar dryer was fabricated at the Energy Research Centre, University of Nigeria, Nsukka. The briquettes were properly packaged in paper bags to protect them from moisture, contamination, and physical damage.
2.3 Proximate analyses and calorific values of the cassava flour, coal, biocoal briquettes and rice husks
This is an analysis of parameters such as ash, moisture, volatile matter and fixed carbon compositions in a material. The samples of the rice husks were analysed in accordance with the AOAC methods for biomass [27]. Additionally, the as-received coal samples were sieved with a Rupson sieve and analysed according to the standards of the American Society for Testing and Materials (ASTM). A moisture analyser (MB 35 halogen) was used to determine the moisture content of the pulverised coal and briquette samples at 110 °C, whereas the ash composition was determined with a Vecstar furnace in accordance with ASTM Standard D3174-11 [28]. The volatile matter content was determined according to the ASTM standard D3175-11 [29]. The fixed carbon value was calculated by reducing the total value of ash, moisture and volatile matter contents from 100.
The calorific values of the samples of rice husk, briquettes and coal were determined via a Hewlett 1242 adiabatic bomb calorimeter. This was done by combustion. The fuel is ignited in the calorimeter and the temperature starts increasing until the maximum temperature is reached. Then, the temperature starts falling slowly until it shows a steady rate and the readings are taken at regular intervals. The calorific value is calculated by measuring the mass of the samples, the temperature change it causes, and the amount of energy released per unit mass.
2.4 Determination of some elements and inorganic oxides in coal samples, rice husk, cassava flour and biocoal briquettes
This determination was done with an Energy Dispersive X-Ray fluorescence spectrometer (Thermo Fisher Scientific ARL Quant’x model). It consists of a radiation (X-ray) source, a sample chamber, a scanning electron microscope equipped with an energy-dispersive X-ray detector. and a computer for data processing. The principle involves a generation of X-ray radiation from a target sample. The characteristics of the radiation are used as fingerprint to compare with data from certified standards.
The sample was bombarded with X-rays in the EDXRF analyser, which caused it to emit fluorescent X-rays characteristic of its elemental composition. The emitted X-rays were then detected and analysed to determine their composition in the sample. It provides elemental and oxide composition information.
A weighed amount of the pulverized rice husk was placed in the sample holder and then covered with soft material. The moisture and oxygen were removed from the sample with a vacuum pump, before it was placed in the XRF spectrometer. The system is calibrated using certified samples of biological material. Calibrations for the elements and oxides were also carried out. The analysis of the as-received coal fines was performed separately, with the setting mode changed from biological to geological material.
2.5 Determination of specific surface areas, pore sizes and pore volumes of the coal samples and biocoal briquettes
These determinations by instrumental analysis require placing an amount of sample into a sample container of the instrument, then, outgassing to remove moisture and impurities, and taking measurements after increasing the pressure of the gas.
The parameters were investigated by using a BET analyser (Quantachrome Nova 4200e, USA). Briefly, the sample is properly weighed into the sample cell, and the filled sample cell bulb is inserted into the heating mantle. The clamp is placed around the mantle so that the sample cell is held firm. The sample cell stem is inserted into the sample preparation station, and the knurled ring is tightened clockwise to secure the sample cell at the preparation station. Then, outgassing is initiated by entering the control panel menu on the instrument. The outgassing temperature is set to 250 °C, as the system is instructed to start degassing for 3 h while the heater is switched on. Then, the heating mantle and the sample cell are allowed to cool, and the sample cell is reweighed to determine the post outgas sample weight. The dewar is filled to the internal upper mark with liquid nitrogen, and the sample cell containing an outgassed and weighed sample is placed into the analysis station to be used for analysis. After all the fields/selections on the start analysis menu are completed, ‘Start’’ is clicked, to begin the analysis.
2.6 Thermogravimetric analysis of rice husk, coal and biocoal briquette samples
TGA measures the mass loss of a material as a function of temperature or time during heating in an inert atmosphere. Thermogravimetric analysis of samples of rice husk, Okobo-Enjema coal samples and Okobo-Enjema biocoal briquettes was performed via TGA. Approximately 11.257 mg of rice husk was added to the analysis compartment and continually heated as nitrogen passed over the sample at a rate of 10 °C/min. As volatiles are removed, the losses in the mass of the sample are shown in a derivative thermogravimetric (DTG) profile. The analysis was repeated with the coal and the biocoal briquette samples. Further analyses of the biocoal briquette with TGA were carried out at 15 and 20 °C/min to study the effects at different heating rates.
2.7 Statistical analysis
The data reported are the means of duplicate measurements. Statistical analysis was carried out with a t-test (P < 0.05) via IBM SPSS statistics version 23 software. These analyses were performed to determine if there were significant differences between the coals (onyeama and Okobo-Enjema coals), coals and briquettes (Onyeama and Okobo-Enjema coals/Onyeama and Okobo-Enjema biocoal briquettes), and briquettes (Onyeama biocoal briquette and Okobo-Enjema biocoal briquette).
3 Results and discussion
All the data generated or analysed during this study are included in this article.
Several samples of biocoal briquettes were prepared from equal amounts of coal and rice husk. A sample is shown in Fig. 1.
The briquettes were strong, durable and without cracks or molds.
3.1 Proximate composition and calorific values
The results of proximate analyses of samples of rice husk, Onyeama and Okobo-Enjema coals are shown in Table 1.
The present study revealed that the Onyeama coal has a lower moisture content than the Okobo-Enjema coal. The moisture content of the United Kingdom (UK) (Daw mill) coal is 4.60% [30]. The moisture content of the rice husk is 7.40%, which is lower than the 8.10% obtained for a cereal co-product (CCP), a biomass in the United Kingdom [30].
The ash content of a sample is a measure of the amount of inorganic noncombustible material it contains. It is an indicator of the propensity of a material to contribute to environmental pollution. The ash content in Onyeama coal is 8.69%, which is lower than the 9.35% obtained for Okobo-Enjema coal and the 4.20% obtained for UK coal [30].
Materials with higher volatile matter contents have a greater propensity to pollute the environment with volatiles. The present study revealed that rice husk has a higher volatile matter content than coal does. The tested UK biomass and coal have 70.80 and 31.30% volatile matter, respectively [30].
Fixed carbon has a direct relationship with heat content, which is the energy stored in a material. The relative percentage of carbon increases with rank, with anthracite being the coal with the highest rank, and lignite, the lowest [31]. The present study indicates that both the Onyeama and Okobo-Enjema coals are subbituminous coals on the basis of their volatile matter contents being above 31% and calorific values [32]. Carbon in coal is of environmental concern because, upon complete combustion, the carbon combines with oxygen to release CO2. The CO2 from fossil fuels is a greenhouse gas that causes global warming and climate change. The present study indicates that the combustion of Onyeama coal has a greater propensity to pollute the environment with CO2 as it has a greater amount of carbon, which can be oxidized to CO and then CO2. CO is classified as a criteria pollutant because of its potential to cause harmful effects. It is generated from the incomplete combustion of fuels such as coal, natural gas, and agricultural residue [33]. The combustion of rice husk results in the production of less CO2 than coal does. Additionally, CO2 emissions from the combustion of biomass combustion are considered neutral with respect to the effects of greenhouse gases [34]. Rice husks can supplement a portion of coal required for combustion. Therefore, blending coal with biomass will reduce the quantity of coal to use and consequently the amount of CO2 that will be released into the environment.
The calorific value, also known as the heating value, is a measure of the total energy content of a material when it is burned [35]. The calorific values indicate that rice husk has a lower value than coal.
3.2 Elemental composition
Table 2 shows the average elemental compositions of the cassava flour (binder), rice husk, coals and biocoal briquettes.
The ash content of a fuel is the inorganic matter obtained after volatilization of water and organic components. The inorganic elements are found in ashes where they occur in compounds such as oxides, phosphates, chlorides and silicates. The chemical composition of the ash impacts on the combustion characteristics of the fuel and the combustion equipment. Briquette with high ash content will generate low amount of heat when compared with the same quantity of briquette with low ash content. High ash content reduces combustion efficiency and increases burning time of a fuel [37]. Inorganic elements are found in the ash.
Elements such as Cu, Fe, Mo, Mn, Ni and Zn are required for growth and physiological activities. However, they may cause toxic symptoms when their levels are above tolerable limits. Some heavy metals are highly toxic and have no biological benefits. Examples include Cr, Cd, Pb and Sr, and metalloids such as Sb and As [36]. Ca and Mg contribute to hardness in water and may be released into the environment when the fuels are utilized.
Amendments to the United States Clean Air Act (1990) designated 23 trace elements as being of greatest environmental concern [38]. This was based on the potential hazards of these elements to living organisms. They are antimony, arsenic, boron, beryllium, bromine, cadmium, chlorine, chromium, cobalt, copper, fluorine, iodine, lead, manganese, mercury, molybdenum, nickel, selenium, sulphur, thorium, uranium, vanadium and zinc.
In the present study, out of the list of potentially hazardous elements, As, Br, Cl, Cu, Cr, Ni, Mn, Pb, S, Sb, Zn and V are present in the tested coals and biocoal briquettes produced from the tested coals (Table 3).
Table 3 compares the mean compositions of potentially hazardous elements in this study to those of coals obtained from other countries.
The mean values show that the deviations in the total PHE levels of Okobo-Enjema coal and Okobo-Enjema briquettes; Okobo-Enjema coal and Onyeama coal; Onyeama coal and Onyeama briquettes; and Okobo-Enjema briquette and Onyeama briquettes are not statistically significant at p ≤ 0.05.
The biocoal briquettes contained lower levels of Sb, As, Br, Cr, S and V than their levels in the coal samples. Therefore on utilisation of these fuels, lower amount of these elements may be released from biocoal briquettes into the environment. Compared with that in the coal, the level of Cl in the briquette formed from the Okobo-Enjema coal was lower. However, the conversion resulted in increased amounts of Mn and Zn in the biocoal briquettes. The levels of Cu, Ni and Pb increased in the biocoal briquettes after the conversion of the Okobo-Enjema coal, whereas they decreased in the briquettes after the conversion of the Onyeama coal. Table 3 shows that the total amount of potentially hazardous elements in the Okobo-Enjema coal samples decreased by 36.83% after conversion to biocoal briquettes.
The elements found in the coal samples are derived from plants, water and geological conditions. They differ across samples, as different plants and depositional environment were involved during coalification.
Rice husk, the primary biomass feedstock, contains trace amounts of metals absorbed from the soil during plant growth. Compared with coal, cassava flour has greater amounts of Na, K, and P, while cassava flour, which is a binder, may introduce small quantities of metals into the briquette composition. More Na and Pb are present in cassava flour than in coal.
The elements found in the biocoal briquettes originate from the coal and biomass feedstocks, the binder, water and any other additives and from the briquetting process.
In the present study, the water used in the briquette production process is deionized; therefore, its contribution to the metal content in the briquettes is expected to be minimal.
Some of the elements that were not detected in Nigerian coals, such as Bo and Be, are present in UK, Australian, Indonesian and Chinese coals [40]. The amounts of Cr, Cu, I, Mn, Ni and Pb in Nigerian coals are greater than those in Australian, Indonesian and Chinese coals [40]. This information reinforces the need for studies on specific coals, as their compositions differ because of differences in their deposition.
The values for sulphur show that after the conversion of the coals to briquettes, the values decreased to within the allowable concentration limit (Table 3). The reduction may be ascribed to the biomass composition, as its alkali and alkaline earth contents react with oxides of sulphur to form alkali sulphate. Yuan et al. [24] noted that when the concentrations of PHEs exceed the ACL and MACL, these elements impose adverse effects on the environment and human health.
3.3 Inorganic oxide composition
Table 4 shows the inorganic oxides in samples of rice husk, Onyeama, Okobo-Enjema coals and biocoal briquettes.
Table 4 shows that compaction of the coals with rice husk reduced the amounts of some minerals, including Fe2O3, HfO2, MgO, SO3, TiO2, V2O5 and, As2O3, in the biocoal briquettes. However, compaction increased SiO2, CaO, CuO, PbO, and Ta2O5, and Nb2O5 contents in the coals. Some minerals decreased in the conversion of the Okobo-Enjema coal but increased in the Onyeama coal, and vice versa.
3.4 Surface area, pore volume and pore diameter data for the Okobo-Enjema coal and its biocoal briquette
In the International Union of Pure and Applied Chemistry (IUPAC) conventions, porous materials can be categorized into three types: macroporous (pore diameter > 50 nm), mesoporous (2–50 nm), and microporous (< 2 nm) [41].
Table 5 shows the results of the BET analysis of the coal sample and the biocoal briquette.
The average pore diameters for the coal indicate that tey are mesoporous. The biocoal briquette is also mesoporous, although the briquette has a larger pore size. In a study conducted by Mangalassery et al. [42], increased CH4 emissions were recorded as the aggregate size decreased. A report revealed that carbonized materials with high surface areas, pore volumes, and small pore sizes have the highest adsorption capacity [43]. Since the biocoal briquette has a larger pore size, larger surface area and larger pore volume than the coal samples, it is expected to emit its constituents more effectively, as larger volumes cause greater exposure of coal to gases. The rice husks contributed to the pore characteristics of the biocoal briquette. This results indicate that rice husks will emit its constituents faster since it has larger pore sizes than the coals and biocoal briquette.
3.5 TG/DTG analysis of rice husk, Okobo-Enjema coal and Okobo-Enjema biocoal briquette
The results of the thermogravimetric analysis are presented in Figs. 2–3. Figures 2 and 4 show the patterns of weight loss at various temperatures at a heating rate of 10 °C/min for rice husk and Okobo-Enjema coal, respectively, whereas Figs. 5 and 6 show the DTG profiles on the basis of the rates of weight loss of rice husk and Okobo-Enjema coal, respectively, at different temperatures at a heating rate of 10 °C/min.
3.6 TG/DTG analysis of the Okobo-Enjema biocoal briquette at different heating rates
Figures 7, 8 and 9 show the patterns of weight loss at various temperatures during the thermal treatment of the Okobo-Enjema biocoal briquette at heating rates of 10, 15 and 20 °C/min, respectively. Figures 10, 11 and 3 show the DTG profiles on the basis of the rate of weight loss of the Okobo-Enjema biocoal briquette at different temperatures at heating rates of 10, 15 and 20 °C/min, respectively.
Table 6 shows that the thermal decomposition process of the studied fuels occurs in three stages [44], namely, dehydration, devolatisation and carbonization. The first stage is related to the release of moisture and low molecular weight compounds [45]. This occurs from room temperatures. The second stage involves the release of the remaining volatiles [46]. The highest rate of weight loss occurs at this stage. This is followed by the third stage involving the slow decomposition and conversion of lignin into char [47].
Thermal analysis provides information on the temperatures at which moisture and volatiles (pollutants) are released from fuels. The results showed that emissions will occur at lower temperatures in biocoal briquettes than in coals. The derivative weight loss increased with increasing heating rate, from 4.4% at 10 °C/min, 4.7% at 15 °C/min to 5.5% at 20 °C/min.
The values obtained for the parameters in the briquette were due to the input of the various feedstocks and the briquette-making processes. Therefore, the amount of the greenhouse gas CO2 will decrease if the coal component is supplemented with biomass.
Coal is vilified for its release of environmental pollutants such as toxic elements, which can cause human health problems; carbon (IV) oxide, a greenhouse gas that contributes heavily to global warming and climate change; particulate matter (PM), which has been linked to respiratory illnesses; sulphur oxides and nitrogen oxides that cause acid rain, which can negatively affect soil productivity, destroy vegetation and aquatic life, and attack man-made structures. The compaction of coals with biomass produces briquettes in which some elements in the coal samples may be reduced in the briquettes, whereas some may increase. This occurs because of the compositions of the coals, biomass, binder, water, other additives and the briquette-making process. The conversion of coal with biomass into biocoal briquette occurs with a reduction in the amount of coal used. This will reduce the level of emissions from coal. Additionally, rice husk and coal fines, which may pollute the environment, are densified, which helps in waste management. The characteristics of coal, biomass and processing methods should be considered in the conversion and utilisation of biocoal briquettes for pollution mitigation.
4 Conclusions
This research investigated some coal properties and the properties of briquettes prepared from compacting the coals with rice husks. The levels of some potentially hazardous elements in the coals diminished after the conversion of the coals to biocoal briquettes. However, some increased in the biocoal briquettes after conversion. Additionally, some minerals decreased, whereas some increased after conversion. The studies on specific coals and other feedstocks revealed that each has peculiar properties. Analysis with a BET analyser revealed that the pore volume, pore size and pore surface area of the biocoal briquette were larger than those of the coals, which indicates that emission increases with the biocoal briquette. Thermogravimetric analysis revealed the temperatures at which volatiles are released from the rice husks, coals and biocoal briquettes. This occurred at lower temperatures than those in the coals. A study using different heating rates revealed that derivative weight loss increased with increasing heating rate. As observed from this study, environmental pollution from biocoal briquettes are as a result of contributions the feedstock and the briquetting-making process. It is necessary to be aware of the sources of toxic elements in briquettes and control their levels to minimize adverse environmental and health impacts. Additionally, it is important to determine the potentially hazardous elements in briquettes before their utilisation.
Data availability
All data generated or analysed during this study are included in this article.
Code availability
Not applicable.
References
Yıldız I. Fossil Fuel. In: Dincer I, editor. Comprehensive energy systems. Elsevier: Amsterdam, Netherlands; 2018.
Edwards GAS. Coal and climate change. Wiley Interdiscipl Rev. 2019;10(5): e607.
EIA. Coal explained, U.S. Energy Information Administration, 2022.https://www.eia.gov/energyexplained/coal/). Accessed 13 April 2024
UCEM. The issue of coal consumption. 2024.https://www.ucem.ac.uk/whats-happening/articles/coal-consumption-in-the-built-environment/). Accessed 15 April 2024
Ugwu KE, Ukoha PO. Polycyclic aromatic hydrocarbons in surface sediments near a mining site in Okobo-Enjema, Nigeria: concentrations, source apportionment and risk assessment. Environ Geochem Health. 2018;40:359–73. https://doi.org/10.1007/s10653-017-9916-7.
Li Y-J, Wang Z-K, Qin F-X, Fang Z-G, Li X-L, Li G. Potentially toxic elements and health risk assessment in farmland systems around high-concentrated arsenic coal mining in Xingren China. J Chem. 2018. https://doi.org/10.1155/2018/2198176.
Farooqi ZUR, Hussain MM, Ayub MA, Qadir AA, Ilic P. Potentially toxic elements and phytoremediation Opportunities and challenges. In: Bhat RA, Tonelli FMP, Dar GH, Hakeem K, editors. phytoremediation. London; 2022. https://doi.org/10.1016/B978-0-323-89874-4.00020-0.
Nieder R, Benbi DK. Potentially toxic elements in the environment - a review of sources, sinks, pathways and mitigation measures. Rev Environ Health. 2023. https://doi.org/10.1515/reveh-2022-0161.
Okoro HK, Orosun MM, Oriade FA, Momoh-Salami TM, Ogunkunle CO, Adeniyi AG, Zvinowanda C, Ngila JC. Potentially toxic elements in pharmaceutical industrial effluents: a review on risk assessment, treatment, and management for human health. Sustainability. 2023;15(8):6974. https://doi.org/10.3390/su15086974.
Okafor IF, Anyanwu CN. Production of smokeless briquette fuel from sub-bituminous coal for domestic and industrial uses. Sci J Energy Eng. 2015;3(4):33–9. https://doi.org/10.11648/j.sjee.20150304.11.
Adekunle Joshua O, Olorunfemi Bayode J, Adejuyigbe Samuel B. Performance of some biocoal briquettes produced from mixture of sawdust and varying Nigeria coals as composite domestic fuel. J Eng Tech. 2021. https://doi.org/10.4679/fuoyejet.v6i1.569.
Ikelle II, Nworie FS, Ogah AO, Ilochi NO. Study on the combustion properties of bio-coal briquette blends of cassava stalk. Chem Search J. 2017;8(2):29–34.
Anyanwu CN, Animoke CJ, Agu BU, Okafor IF, Ogbuagu NJ, Bentson S, Ojike O. Physical and emission properties of blended bio-coal briquettes derived from agro-wastes in Nigeria. ASTESJ. 2022;7(3):116–22.
Ugwu KE, Ofomatah AC, Eze SI. Influence of solid concentration on the flow characteristics and settling rate of coal-water slurries. J Energy Natural Res. 2013;2(3):21–4. https://doi.org/10.11648/j.jenr.20130203.11.
Li P, Ren Q, Han S, et al. Coal-biomass preheating combustion characteristics in cement precalciner part 1 preheating of coal/biomass. J Therm Sci. 2023. https://doi.org/10.1007/s11630-023-1893-9.
Akhtar J, Yaseen A, Munir S. Effect of rice husk co-combustion with coal on gaseous emissions and combustion efficiency. Energy Sources A. 2018;40(8):1010–8. https://doi.org/10.1080/15567036.2018.1468513.
Ahu SG, Chakraborty N, Sarkar P. Coal-biomass co-combustion: An overviewRenew. Sustain Energy Rev. 2014;39:575–86. https://doi.org/10.1016/j.rser.2014.07.106.
Ikelle II, Nworu JS, Nworie FS, Jedidiah J, Ogbuagu JO, Nwabueze IE. Thermal analyses of briquette fuels produced from coal dust and groundnut husk. ACMY. 2020;4(1):24–7. https://doi.org/10.2478/acmy-2020-0004.
Kanwal F, Ahmed A, Jamil F, Rafiq S, Ayub HMU, Ghauri M, et al. Co-combustion of blends of coal and underutilised biomass residues for environmental friendly electrical energy production. Sustainability. 2021;13:4881. https://doi.org/10.3390/su13094881.
Nikiforov A, Akmaral K, Evgeniy P, Amangeldy K, Sholpan N. Analysis of the characteristics of bio-coal briquettes from agricultural and coal industry waste. Energies. 2023;16(8):3527. https://doi.org/10.3390/en16083527.
Mursito AT, Widodo Arifin DN. Characterization of bio-coal briquettes blended from low quality coal and biomass waste treated by Garant® bio-activator and its application for fuel combustion. Int J Coal Sci Technol. 2020;7:796–806. https://doi.org/10.1007/s40789-020-00309-0.
Nwabue FI, Unah U, Itumoh EJ. Production and characterization of smokeless bio-coal briquettes incorporating plastic waste materials. Environ Technol Innov. 2017;8:233–45. https://doi.org/10.1016/j.eti.2017.02.008.
Akuma O, Charles M. Characteristic analysis of bio-coal briquette (coal and groundnut shell admixtures). IJSRST. 2017;3(3):30–8.
Yuan Y, Tang S, Zhang S. Concentrations and modes of occurrence of some potentially valuable and toxic elements in the No 5 coal from the Yanzishan Mine, Datong Coalfield, Shanxi Province China. Energy Explor Exploit. 2019;37(6):1694–720. https://doi.org/10.1177/0144598719861272.
Kentucky Geological Survey (KGS). Major, Minor, and Trace Elements. 2024. https://www.uky.edu/KGS/coal/coal-major-minor-trace-elements.php. Accessed 26 April 2024
Vassilev SV, Baxter D, Andersen LK, Vassileva CG. An overview of the composition and application of biomass ash. Part 1. Phase-mineral and chemical composition and classification. Fuel. 2013;105:40–76. https://doi.org/10.1016/j.fuel.2012.09.041.
Borah R, Gogoi N, Konwar LJ. Fabrication of biomass briquettes using mango seed shell and effect of binder on combustion characteristics. J Therm Anal Calorim. 2018;132(1):287–99.
Standard ASTM. Test method for ash in the analysis sample of coal and coke. ASTM International: West Conshohocken; 2011.
Standard ASTM. Test method for volatile matter in the analysis sample of coal and coke. ASTM International: West Conshohocken, PA; 2011.
Hussain T, Khodier AHN, Simms NJ. Co-combustion of cereal co-product (CCP) with a UK coal (Daw Mill): combustion gas composition and deposition. Fuel. 2013;112:572–83. https://doi.org/10.1016/j.fuel.2013.01.001.
U.S. Geological Survey. What are the types of coal? 2024, https://www.usgs.gov/faqs/what-are-types-coal. Accessed 23 June 2024
Kentucky Geological Survey. Bituminous coal. https://www.uky.edu/KGS/coal/coal- bituminous.php. Accessed 23 June 2024
EPA. Carbon Monoxide basics. 2024. https://www.epa.gov/co-pollution Accessed 13 May 2024.
Ibeto CN, Anisha MC, Anyanwu CN. Evaluation of the fuel properties and pollution potentials of lignite coal and pellets of its blends with different biowastes. Am Chem Sci J. 2016;14(1):1–12.
Himbane PB, Ndiaye LG, Napoli A, Kobor D. Physicochemical and mechanical properties of biomass coal briquettes produced by artisanal method. AJEST. 2018;12(12):480–6. https://doi.org/10.5897/AJEST2018.2568.
Thalassinos G, Petropoulos SA, Grammenou A, Antoniadis V. Potentially toxic elements: A review on their soil behavior and plant attenuation mechanisms against their toxicity. Agriculture. 2023;13(9):1684. https://doi.org/10.3390/agriculture13091684.
Sun R, Ismail TM, Ren X, Abd E-S. Effect of ash content on the combustion process of simulated MSW in the fixed bed. Waste Manag. 2016;48:236–49. https://doi.org/10.1016/j.wasman.2015.10.007.
Sia SG, Abdullah WH. Geochemistry of trace elements as one of the important coal quality parameter an example from Balingian coal Malaysia. Sains Malays. 2017;46(3):387–92. https://doi.org/10.1757/jsm-2017-4603-05.
Singh AL, Singh PK, Kumar A, Yadav A, Singh MP. Experimental study on demineralization of coal with Pseudomonas mendocina strain B6–1 bacteria to obtain clean fuel. Energy explor Exploit. 2014;32(5):831–46.
Dale L. Trace elements in coal, Australian coal association research programme. 2024. https://www.acarp.com.au/Media/ACARP-WP-3-TraceElementsinCoal.pdf, Accessed 12 March 2024.
Funabashi H, Takeuchi S, Tsujimura S. Hierarchical meso/macro-porous carbon fabricated from dual MgO templates for direct electron transfer enzymatic electrodes. Sci Rep. 2017;7:45147. https://doi.org/10.1038/srep45147.
Mangalassery S, Sjögersten S, Sparkes DL, Sturrock CJ, Mooney SJ. The effect of soil aggregate size on pore structure and its consequence on emission of greenhouse gases. Soil Tillage Res. 2013;132:39–46. https://doi.org/10.1016/j.still.2013.05.003.
NurAisyah S, Khairunnisa MP, NorRuwaida J, Md Ali AH, Dewika M, Rashid M. Soil with high porosity as an excellent carbon dioxide adsorbent carbon-rich soil as an effective adsorbent for carbon dioxide environ. Earth Sci. 2020. https://doi.org/10.1088/1755-1315/476/1/012124.
Waheed MA, Akogun OA, Enweremadu CC. Influence of feedstock mixtures on the fuel characteristics of blended cornhusk, cassava peels, and sawdust briquettes. Biomass Convers Bior. 2023;13:6211–16226. https://doi.org/10.1007/s13399-023-04039-6.
Ravindran B, Sravani R, Mandal AB, et al. Instrumental evidence for biodegradation of tannery waste during vermicomposting process using Eudrilus eugeniae. J Therm Anal Calorim. 2013;111:1675–84.
Li G, Hu R, Hao Y, Yang T, Li L, Luo Z, Xie L, et al. CO2 and air pollutant emissions from bio-coal briquettes. Environ Technol Innov. 2023;29: 102975. https://doi.org/10.1016/j.eti.2022.102975.
Jones K, Ramakrishnan G, Uchimiya M, Orlov A, Castaldi MJ, LeBlanc J, Hiradate S. Fate of higher-mass elements and surface functional groups during the pyrolysis of waste pecan shell. Energ Fuel. 2015;29(12):8095–101. https://doi.org/10.1021/acs.energyfuels.5b02428.
Acknowledgements
NCERD is hereby acknowledged for provided facilities for this research.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by K.E.U, C.G.E, C.N.I and I.F.O. The first draft of the manuscript was written by K.E.U and all authors commented on all versions of the manuscript. All authors reviewed and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ugwu, K.E., Ezema, C.G., Ibeto, C.N. et al. Properties of biocoal briquettes from mesoporous coals and rice husk and their effects on environmental pollution. Discov Environ 2, 106 (2024). https://doi.org/10.1007/s44274-024-00141-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44274-024-00141-2