Abstract
This study assessed nutrients and microbial contamination in water and soil samples from Chileka Township, Blantyre City, Malawi. Elevated total and fecal coliforms (1300 cfu/100 mL and 290 cfu/100 mL) in groundwater (GW), and (34,000 cfu/100 mL and 8000 cfu/100 mL) in surface water (SW) were found, representing a risk of exposure to water-borne disease. While the criteria in the Malawi Standard for raw groundwater was mostly met, water from only 20% of the boreholes complied with the WHO requirements. Nitrate (NO3─) and Cl─ (47.8 mg/L and 263 mg/L) exceeded the WHO limits in GW. Cadmium (Cd) occurred in a few cases at concentrations up to 0.217 mg/L and 0.138 mg/L in GW and SW. Lead (Pb) and Cr were below detection limits, while Mn (0.319 mg/L and 0.640 mg/L) in GW and SW, and Fe (6.92 mg/L) in SW compromised taste. Though bacteriologically unfit for raw consumption by humans, both GW and SW chemically met FAO-acceptable limits for irrigation, and standards for livestock watering. The NO3─ and PO43─ maximum concentrations in soil were 58.9 mg/kg and 506 mg/kg, respectively. Lead (Pb) and Cd were not detected whereas Cr, Zn, Cu, Mn and Fe in soil were 27.7 mg/kg, 190 mg/kg, 60.4 mg/kg, 1307 mg/kg and 6552 mg/kg, respectively. Magnesium (Mg), Ca, Na and K were 20,523 mg/kg, 22,334 mg/kg, 544 mg/kg and 5758 mg/kg, respectively in soil. The human health risk assessment results, on the other hand, showed that at least 30% (6 out of 20) of the GW samples and 60% (3 out 8) of the SW samples had HI > 1 for adults, children and infants, indicating existence of non-carcinogenic risk. Similarly, at least 15% (3 out 20) of the GW samples and 18% (1 out of 8) of the SW samples had CR > 0.001 for adults, children and infants, suggesting a risk of developing cancer during a lifetime due to Cd exposure. Though both GW and SW are generally of good chemical quality, chronic exposure to nitrate and cadmium is a health risk in the area. The current trace metal levels are not worrisome, but soil nitrate and phosphate may need regular monitoring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Globally, environmental soil–water transfer of contaminants affects water quality thereby limiting community access to safe and clean water. However, both groundwater (GW) and surface water (SW) are key to human livelihood worldwide. While GW is generally the source of piped water supply in Europe, it is indispensable to support rural livelihood in Sub-Saharan Africa because of the added potential of possible use for agricultural purposes [1]. In the global quest, under the sustainable development goals of the United Nations (UN), for universal and equitable access to safe water by 2030, the intrusion into drinking water sources of contaminants of both geogenic and anthropogenic origin remains a major drawback [2]. Therefore, deliberate efforts are necessary to preserve the ecological integrity of water resources through a balance between exploitation for human benefit and protection against polluting influences. Although GW is relatively inexpensive for rural water supply in Sub-Saharan Africa, often having a satisfactory chemical profile and good microbial properties that require little or no treatment prior to human consumption, its quality is still susceptible to compromise by contaminants [3]. In the interest of public health, the World Health Organization (WHO) recommends limits for selected contaminants in drinking water [4]. Clean water, safe for drinking, is that which conforms to prescribed standards in terms of its chemical and microbial properties. In Malawi, common pollutants in both GW and SW include nutrients, trace metals and bacteria [5, 6]. These may originate in the soil and get transported downward into GW or laterally into SW bodies by surface runoff, and can be exchanged between GW and SW [7,8,9,10].
In both urban and rural environments, GW and SW might be contaminated with pathogens, nutrients, and trace metals, and the situation is worse in developing countries due to poor implementation of environmental management strategies. Although the natural subsoil profiles possess some potential of attenuating microbial and chemical contaminants originating from the surface [11], these contaminants are still capable of being mobilized under certain conditions. Thus, the contaminants eventually leach into GW, and shallow aquifers are frequently more exposed to contamination. Similarly, soil erosion and runoff processes on the land surface may constitute a vehicle transporting contaminants adsorbed to soil particles into SW. Microbial contamination of drinking water is responsible for water-borne diseases such as cholera, dysentery, typhoid and bilharzia [12]. Excess nutrients i.e., phosphate (PO43─) and nitrate (NO3─) cause eutrophication and fish kill in freshwaters [13], the latter has also been linked to blue baby syndrome (methemoglobinemia) in infants [14], and to livestock poisoning [15]. Excessive concentration of lead (Pb), cadmium (Cd), and chromium (Cr) in drinking water is toxic to humans whereas iron (Fe), copper (Cu), zinc (Zn), and manganese (Mn) compromise the aesthetic quality of drinking water in addition to affecting plants and animals, and the suitability of water for irrigation, and support of aquatic life [3, 16, 17]. In soil, the fate of contaminants is described by the natural attenuation processes of biodegradation, diffusion, dilution, sorption, volatilization and chemical and biochemical stabilization, including uptake by vegetation and animals [18]. Similarly, a contaminant that enters a body of water can be transported within the water body, volatilized or transported into the atmosphere, sorbed into the soil, dissolved into or precipitated out of the water, degraded by photolysis, or transformed by biodegradation. Contaminants that are not degraded by natural systems end up bio-accumulating in organisms. Generally, water pollution depletes aquatic ecosystems.
Groundwater is, like in other developing countries, still key to rural water supply in Malawi, with 62% of the national population relying on boreholes [19]. However, due to limited monitoring, water quality data is still fragmented and inadequate, with less than 20% of the 27,913 boreholes of the country tested before use in 2014 [6, 20]. Rural and peri-urban areas often offer cheaper livelihoods subsidized by agricultural activities and are home to the larger proportion of the population. Although mandated to provide potable water to residents of both the city and the outskirts, Blantyre Water Board (BWB) already faces capacity challenges to service city dwellers adequately. Therefore, expansion of service to the surrounding rural and peri-urban areas, including Chileka Township, is unlikely to happen soon. Currently, the people of Chileka mostly use raw GW for drinking purposes while the Likhubula River, which runs through the area and drains into the country’s mighty Shire River from which BWB draws water for treatment and subsequent distribution, largely provides water for non-potable uses. Cholera outbreaks have constituted a public health problem in Malawi, and Chileka in particular, in recent years [21, 22], implicating sources of drinking water among other possible routes of exposure. Faecal coliforms are predictors of the risk of waterborne diarrhoeal diseases that constitute a major cause of morbidity and mortality in developing countries [23]. In the semi-urban community of Chileka, drinking water of good quality is essential for not only human health but supporting livelihoods through agriculture. The purpose of this research, therefore, was to assess, in both GW and SW (Likhubula River), and in associated soils from Chileka Township, the extent of microbial (total and feacal coliforms), trace metal (Pb, Cd, Cr, Fe, Cu, Mn and Zn), nutrient (NO3─ and PO43─), chemical and organic matter (COD and BOD5) contamination, including mineral (Ca, Mg, Na, and K) content against prescribed standards.
2 Materials and methods
2.1 Description of the study area
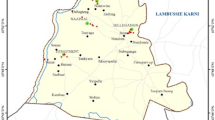
Malawi is a landlocked country located in South-Eastern Africa with a dense population. It is bordered by Tanzania to the North, Zambia to the North-West with Mozambique joining it on the East, South and West. The country has a tropical climate consisting of a dry season lasting from May to October and a wet (rainy) season extending from November to April. Chileka Township is a peri-urban area approximately 70 square miles (181.3 km2) situated about 16 km north-west of Blantyre city on a plain between the Shire Highlands and the Shire River, within latitudes 15.350–16.015˚ S and longitudes 34.725–35.125˚ E. The study area (Fig. 1) comprised eight villages, namely; Mpasuka, Magombo, Masitala, Kapitao, Gova, Gopani, Mapemba, and Singano where purposively selected 10 boreholes and four sites in Likhubula River were sampled (Fig. 1). Being a typical rural community, anthropogenic activities constituting sources of soil and water pollution within the area include indiscriminate waste disposal, cultivation of riverbanks, use of fertilizers, manures and pesticides, brick kilns, and sanitation facilities (pit latrines and septic tanks).
2.2 Collection of water and soil samples
A purposive sampling strategy was employed to select 10 boreholes (BH1 to BH10), and four sites (LK1 to LK4) in Likhubula River based on intensity of use by the community, surrounding land use activities, and accessibility for easy transportation of samples in both seasons. The water samples were collected in both the dry season (October, 2016) and the rainy season (February, 2017), once in each, following standard methods [24, 25]. At each sampling site, two sets of triplicate water samples were collected, filtered, and stored in new pre-cleaned polyethylene bottles. The first sample-set was acidified with HNO3 (to pH 2) for analysis of major cations while the second was stored unacidified for analysis of major anions. A separate sample for microbiological analysis was collected at each site. Before collection of this sample, each borehole was flame-sterilized with cotton wool lit after being soaked in 70% methanol. Consistently, at each borehole, water was pumped to waste for 30 s before sample collection to release any trapped water and thus ensuring collection of water freshly drawn from the aquifer. The general surroundings of the boreholes, including wellhead protection were assessed by visual examination and observation. All samples were, after on-site determination of non-conservable parameters, stored and transported on ice in a cooler box to the laboratory for subsequent analyses. In order to assess possible influence of soil contaminants on water quality, a soil sample of 500 g was collected at 0–20 cm and 20 cm–40 cm depths within a radius of 25 m of each borehole, and of each SW sampling point in crop fields near the river banks. The soil samples were taken using a hand-operated auger and collected in plastic bags [26].
2.3 Physicochemical analysis of water samples
All non-conservable parameters (temperature, pH, turbidity, EC, and TDS) were measured in-situ (Table 1). The water samples were analyzed by the methods in APHA (1999) [24] as follows: for chloride (Cl─) by the argentometric method (4500-Cl– B), phosphate (PO43─) by the vanadomolybdophosphoric acid colorimetric method (4500-P C), chemical oxygen demand (COD) by the open-reflux titrimetric method (5220B), and biochemical oxygen demand (BOD5) by iodometric methods (5210B and 4500-O D). Bicarbonate (HCO3) was determined by the titrimetric method (AOAC 920.194) [25], and nitrate (NO3─) by the sodium salicylate method [27]. Metals and mineral elements (Ca, Mg, Na, K, Cd, Cu, Cr, Fe, Mn, Pb and Zn) were determined by flame atomic absorption spectrometry (3111B). The trace metal detection limits of the GBC 732 model AAS used were 0.003 mg/L for cadmium, 0.025 mg/L for Cu, 0.001 mg/L for Pb, 0.005 mg/L for Zn, 0.002 mg/L for Mn, 0.003 mg/L for Cr, and 0.005 mg/L for Fe. Table 1 describes the equipment used in the laboratory analyses.
2.4 Microbiological analysis of water samples
The water samples were, immediately after collection, analyzed for total and feacal coliform bacteria using the membrane filtration technique (9222B) as in APHA [24]. A sample of water was passed through a presterilised 0.45 µm membrane filter. This filter was then placed in a petri dish containing membrane lauryl sulphate broth (MLSB), and subsequently incubated for 24 h at 37 °C. At the end of the incubation period, the filter was examined. Bacteria trapped on the membrane grew into colonies that were counted, and a bacterial density was calculated. All yellow-coloured colonies on the filter were identified as feacal coliforms. Total coliforms were pink to dark red in colour, and had a green metallic surface sheen. The colonies were enumerated and expressed in terms of colony forming units (CFU) per 100 mL. This was calculated using the formulae below.
Colonies/100 mL = (100 mL x colony count)/sample volume used.
2.5 Analytical methods used in the laboratory for soil samples
The soil samples were air-dried, crushed and passed through a 2-mm sieve. Next, a representative sample of about 200 g of the sieved soil was retained by coning and quartering, and stored in clean plastic bottles in readiness for analysis [26]. Soil pH was determined in a 1:1 (soil: water) suspension using a digital pH meter [28]. Soil NO3─ was determined by the sodium salicylate method in a 1:5 (soil: water) extract [27, 29]. To estimate total phosphorus, the soil samples were digested using the Perchloric Acid Digestion procedure described by O’Halloram & Cade-Menum [30]. Phosphorus was then determined in an aliquot of the clear supernatant by the Vanadomolybdophosphoric Acid Colorimetric Method (4500-P C) as in APHA [24]. Metals and mineral elements (Ca, Mg, Na, K, Cd, Cu, Cr, Fe, Mn, Pb and Zn) were extracted from soil samples following the Acid Digestion Procedure described by Bamgbose [28], and recovered by filtration through a Whatman 42 filter paper. The diluted extract was analysed for metals by Flame Atomic Absorption Spectrometry (3111B) as in APHA [24]. The precision, expressed as the relative standard deviation (RSD), of the analytical procedures ranged from 5 to 10%. Further, calculated recoveries of the metals from the spiked water samples were found to be within the range 90–110%. Throughout the analysis, blanks were run and, where necessary, corrections applied. All the observations were recorded in duplicate and average values are reported. The elemental concentration in soil of the measured species was expressed (on a dry weight basis) in milligrams of the element per kilogram of soil extracted.
2.6 Data analysis
2.6.1 Statistical analysis
The data obtained was entered and processed in IBM SPSS v20 software and the statistical comparison of mean values in water quality and soil parameters were done at 5% level of significance. It should be noted that before performing any statistical test, the data was first subjected to a homogeneity test to determine whether it was normally distributed or not. If the data was not normally distributed, then the common technique of taking the log of the data embedded in SPSS statistics was employed to transform non-normal distributed data into normal distributed in order to apply two-sample t-tests. The variations were further illustrated by box-and-whisker plots. The parameter values obtained in this study were compared to thresholds prescribed by both the World Health Organization (WHO), and the Malawi Bureau of Standards (MBS) for drinking water, and to literature and available regional standards for soil parameters. Canonical correspondence analysis (CCA) biplot, and hierarchical agglomerative cluster analysis (HCA) were invoked on the water data to examine correlation between the parameters. The correlation between species in GW, SW and topsoil was examined using Pearson’s correlation.
2.6.2 Human health risk (HHR) assessment of drinking water
The HHR assessment, established by the United States Environmental Protection Agency (USEPA), is a widely used tool for evaluating human health risk. Nitrate and cadmium concentrations were selected for HHR assessment in this study because the other toxics (Cr and Pb) were below detection in water. Both nitrate and cadmium primarily enter the human body through ingestion. Therefore, the rate of pollutant ingestion into the human body through drinking water was evaluated by calculating the chronic daily intake (CDI), hazard quotient (HQ), hazard index (HI), and carcinogenic risk (CR).
2.6.3 Hazard quotient
The adverse effects of exposure to nitrate as a non-carcinogenic pollutant was evaluated using HQ) calculated as follows:
where RfD is the reference dosage (1.6 mg/kg/day for nitrate, and 0.0005 mg/kg/day for cadmium) [31, 32]. The chronic daily intake (CDI) was calculated using the following equation:
where “C” is the measured concentration of the contaminant (NO3─ or Cd) in the water (mg/L), “IR” is the rate at which a person drinks water (2 L/day for adults, 1.5 L/day for children, and 0.8 L/day for infants, “ED” is the duration of exposure in years (40 years for adults, 10 years for children, and 1.0 year for infants, “EF” is the exposure frequency in days (d) (365 days for adults, children, and infants, “BW” is the average body weight in kg (70 kg for adults, 20 kg for children, and 10 kg for infants, and “AT” is the average time (14,600 days for adults, 3650 days for children, and 365 days for infants), according to [33].
2.6.4 Hazard index
The following equation was used to determine the HI of nitrate and cadmium associated with the HQ values, defined as the sum of the HQ of these measured parameters.
If HQ > 1, carcinogenic health impacts are said to potentially exist whereas HQ < 1 indicates none. Similarly, HI < 1 indicates a minimal or non-existent risk of adverse non-cancer health, whereas HI > 1 denotes a high risk. Chronic risk (HQ or HI) is categorised as negligible (where HQ or HI < 0.1), low (where 0.1 ≤ HQ or HI < 1), medium (where 1 ≤ HQ or HI < 4), and high (where HQ or HI ≥ 4) [34].
2.6.5 Carcinogenic risk
In the case of exposure to carcinogens, CR estimates a person’s lifetime risk of developing any sort of cancer [35] calculated by the following formula:
where, “SF” (038 mg/kg/day for Cd) is the cancer slope factor [36]. The “CR” is described as ranging from very low (where CR < 1 × 10−6) to very high (where CR > 1 × 10−3) [34].
3 Results and discussion
3.1 Microbial characteristics of the water samples
Coliform bacteria constitute a useful indicator of microbial quality and can signal presence of pathogenic organisms in drinking water. The results of analysis of GW samples (BH01 to BH10) and SW samples (LK01 to LK04) for total coliforms and feacal coliforms are presented in Table 2. The WHO recommends a 0 cfu/100 mL maximum limit for total and faecal coliform count in drinking water [4]. Site BH03 was a protected shallow well (Table 2) and the higher bacterial densities were probably due to seepage and compromised well-head protection observed at this site. Out of all samples analyzed (n = 20) in the two seasons, 60% did not meet the WHO standard, having tested positive for coliform colonies, with faecal coliform colonies observed in 25% of the samples. Only two sites (BH02 and BH08) consistently produced samples that met the WHO limit in both seasons while 50% complied with the threshold of 50 cfu/100 mL prescribed in the Malawi Standard for raw GW [37]. More boreholes were contaminated in the rainy season than in the dry season probably due to transfer of microbes through recharge [38]. However, the seasonal variation of both total coliform and faecal coliform counts was not statistically significant (Table 3) due to dilution. Thus, this research found that GW was mostly bacteriologically contaminated and, therefore, a potential source of risk of exposure to water-borne diseases.
In Likhubula River, all samples (n = 8) complied with neither the WHO standard nor the national standard MS 733:2005 [37] for GW. The total coliform and faecal coliform densities showed that SW was grossly contaminated bacteriologically, and that it could not be consumed without prior treatment for risk of water-borne diseases. The variation in microbial pollution between seasons was not statistically significant (Table 3) for total coliforms probably due to dilution during the rainy season. These results are consistent with the findings of another study conducted in Blantyre’s Lunzu Township [39]. Sources of microbial contamination in the river include animal dung and human faeces washed from the banks into the river. A comparison of GW and SW (Tables 4 and 3) shows that in both seasons both total coliforms and feacal coliforms were significantly higher (p < 0.05) in density in SW.
3.2 Physical characteristics of water samples
The physical parameters of GW and SW samples from Chileka are shown in Table 5, and illustrated in Fig. 2. The values are compared to limits prescribed by the WHO. Though not particularly regulated in water intended for drinking, temperature may, especially if excessively high or low, compromise palatability. The temperatures recorded in both seasons in the present study were below the 30 °C threshold proposed by WHO and, therefore, not worrisome though slightly higher than those reported in a similar study in Blantyre [40]. Except at BH02 where an acidic pH falling outside the WHO range (6.5–8.5) was recorded in the dry season, the rest of the samples complied with the criteria. All samples complied with the WHO threshold of 1500 μS/cm for electrical conductivity (EC). On the other hand, except at BH02 in the dry season, all samples (n = 20) in both seasons met the WHO threshold of 600 mg/L for TDS. Therefore, based on Table 6, GW in the area may be classified as freshwater [41, 42]. Total hardness (TH) values were above the acceptability limit of 500 mg/L [4] only at few sites (BH02, BH05, and BH08). Hardness of water due to magnesium and calcium content is characterized by precipitation of soap scum, and by the need for excessive amounts of soap to achieve cleaning during washing [4]. Total hardness (TH) (as CaCO3) being in the range 119–859 mg/L (Table 5) shows that the GW may be categorized as ranging from moderately hard to very hard (Table 7) [41]. All samples registered turbidity values within the WHO limit of 5 NTU. Generally, GW physical parameters mostly conformed to standards, rendering the waters mostly acceptable in both seasons.
SW registered temperatures that were regular and reasonably comparable to those reported in a similar study in Mudi River in Blantyre [9]. All pH values (n = 8) recorded in two seasons fell within the WHO range (6.5–8.5). The WHO threshold of 750 μS/cm for electrical conductivity (EC) was exceeded by all dry season samples probably due to concentration of salts resulting from reduced flow volume in the river. On the other hand, all samples (n = 8) met the WHO limit for TDS. Based on Table 6, the water from Likhubula River may be classified as freshwater. All total hardness (TH) values were below the WHO threshold of 500 mg/L and may be classified as ranging from moderately hard to very hard (Table 7) [41]. Table 3 shows significant seasonal variation (p < 0.05) of EC, TDS and TH, all being lower during the rainy season due to dilution as recorded in similar studies [45]. All turbidity values exceeded the 5 NTU limit recommended by WHO. This high turbidity was mostly due to disturbance of the water caused by brick-making activities along the banks during the dry season, and by cultivation during the rainy season. Turbidity was significantly higher (p < 0.05) during the rainy season due to runoff. A comparison of GW and SW (Tables 4 and 3) shows that TH was significantly higher (p < 0.05) in GW during the rainy season, and that while temperature, EC and TDS were significantly higher (p < 0.05) in SW during the dry season, pH and turbidity were higher (p < 0.05) in SW in both seasons.
3.3 Chemical parameters of water quality
The results of chemical analysis of GW samples are presented in Table 8 (BH01 to BH10) and illustrated in Fig. 3. Nutrients (NO3─ and PO43─) were detected in all GW samples (n = 20). All samples complied with the WHO requirement for \({\text{NO}}_{3}^{-}\) NO3─ in drinking water (Table 8) implying no health risk. The values recorded in this study also compare reasonably with those reported previously in the neighbouring Machinjiri rural area of Blantyre [40]. In natural waters, NO3─ usually falls below 10 mg/L [46]. Nitrate concentrations above 13 mg/L in GW are rather excessive and a probable result of anthropogenic interference [47]. In 70% of the samples (n = 20), NO3─ was above 13 mg/L suggesting anthropogenic contribution of nitrate into the aquifer. This may be due to agriculture which is a dominant activity in the area. On the other hand, PO43─ was of concern to ecological integrity only [13], mostly exceeding the USEPA limit of 0.1 mg/L set for environmental protection of SW bodies [48]. The present results are similar to those reported in other local GW studies [49,50,51]. Groundwater can contribute phosphate to SW through base flow, with potential of enhancing eutrophication [10]. Sources of nitrogen and phosphorus in the area include human and animal wastes, manure and fertilizers.
Except for once at BH02 (where Cl─ exceeded the WHO limit), concentrations in GW of all major constituents (Na, K, Mg, Ca, Cl─ and HCO3) either fell below the WHO criteria (Tables 8) or were considered common and normal. While K, Mn and Cu were significantly higher (p < 0.05) in concentration during the rainy season (Table 3), Cd was significantly higher during the dry season, being below detection during the rainy season.. Except for Mn during the rainy season, the trace metals (Pb, Cr, Cu, Fe, and Zn) invariably occurred below concentrations of health significance (Tables 8). However, virtually all samples in the dry season manifested Mn levels that could affect taste, laundry and plumbing fixtures. Similarly, while Cd was consistently below detection limit in the rainy season samples, a few dry season samples (BH01, BH02, BH03 and BH4) representing 20% (n = 20) registered concentrations exceeding the WHO threshold limits (Table 8). Continuous and prolonged (or lifetime) exposure to Cd concentration in the range 0.010–0.020 mg/L is a health risk, and above 0.020 mg/L, kidney damage may result [14]. Otherwise, GW was largely of satisfactory chemical quality. The lower Cd and Mn concentrations in the rainy season are attributable to dilution. Sources of manganese in the area include solid wastes while cadmium might have originated from historical use of phosphatic fertilizers. A comparison of GW and SW (Tables 4 and 3) shows that during the rainy season, Ca, Mg and HCO3─ were significantly higher (p < 0.05) in GW while Mn was significantly higher (p < 0.05) in SW. Similarly, NO3─ and PO43─ were in both seasons significantly higher (p < 0.05) in GW and SW, respectively.
In similarity with GW, all SW samples (n = 8) in the two seasons met the WHO limit for NO3─ in drinking water (Table 8), and ranged from excellent to acceptable (Table 9). The PO43─ concentrations exceeded the USEPA limit of 0.03 mg/L set for environmental protection of SW bodies [48]. Therefore, PO43─ is a threat to the ecological integrity of the river. The PO43─ levels found in this study are within the ranges reported in SW in some studies [45, 52] and were slightly higher in the rainy season probably due to input from agriculture. All major constituents (Na, K, Mg, Ca, Cl─ and HCO3) were either within the WHO thresholds (Tables 8) or considered common and normal. The trace metals (Pb, Cr, Cu and Zn) consistently occurred below concentrations of health significance (Tables 8). Concentrations of Fe and Mn were such as would be of both health and aesthetic concern particularly in the rainy season [4]. Table 4 shows that HCO3─, Cl─, BOD5 and COD were significantly higher (p < 0.05) during the dry season while Fe, Mn and Cu were lower. The higher Cu, Fe and Mn concentrations in the rainy season (Table 3) were likely consequent to erosion into the river of solid wastes containing copper and manganese, and of the relatively iron-rich soils (Table 10). Cadmium (Cd) exceeded the WHO limit at 3 out of 4 sites but later underwent dilution by rainfall recharge to below the detection limit. Sources of Cd contamination in SW include pesticides and solid wastes. However, the water was mostly of fair chemical quality.
Biochemical oxygen demand (BOD5) and Chemical oxygen demand (COD) are among the key indicators of pollution in a water body. While BOD5 is a surrogate of organic matter content in water, COD measures both organic matter and chemical contents. Based on the BOD5 and COD concentrations measured in this study (Table 11), the river in the study area may be said to range from slightly polluted to polluted (Table 9) [53], with the pollution slightly increasing in the dry season. Both BOD5 and COD were significantly higher (Table 3) in the dry season than in the rainy season due to dilution due to rainfall recharge [6, 50]. The area being dominated by agricultural activities including rearing of cattle, the increased pollution in the dry season may be attributed to concentration of animal and plant wastes in the river water that was much lower in volume and slower in flow-speed at the time.
3.4 Suitability of water for human and agricultural use
3.4.1 Human health risk assessment for nitrate and cadmium exposure
According to USEPA, no convincing data exists to suggest nitrate in drinking water is associated with any adverse effect other than methemoglobinemia [54]. The HHR assessment was performed by calculating the hazard index (HI) (Eq. 3) for NO3─ and Cd, and the carcinogenic risk (CR) (Eq. 4) for Cd. The risk assessment data is summarised in Table 12. The results showed that HI > 1 for adults, children and infants, for at least 30% (6 out of 20) of the GW samples and for 60% (3 out 8) SW samples. Habineza et al. [55] reported HI values of 6.13E + 01 mg/kg/day and 1.71E + 02 mg/ kg-day for adults and children, respectively, which are significantly greater than 1 for both age groups. Similar findings are also reported in this study, raising a concern for non-carcinogenic adverse health effects associated with water usage. Similarly, CR > 0.001 for adults, children, and infants, for at least 15% (3 out 20) of the GW samples and for 18% (1 out of 8) of the SW samples. Habineza et al. [55] reported the CR of 0.013951 for Cd which is higher than the value reported in this study. However, USEPA recommended range for CR is 10–4 and 10–6 [55]. This suggests that the values reported in this study fall outside the range (10–4–10–6). Thus, there is a risk of developing cancer during a lifetime due to Cd exposure. Though both GW and SW are generally of good chemical quality, some chronic exposure to nitrate and cadmium is a health risk in the area.
3.4.2 Suitability for irrigation and livestock watering
The pH values of both GW and SW generally measured within the range of 6.5–8.4 prescribed by FAO (1985) [43] for irrigation water. Further, TDS values recorded in this study (Table 5) suggest that while both GW and SW exclusively exceeded the 100 mg/L threshold set for livestock watering [44], both GW and SW were according to FAO guidelines [43] suitable for use in irrigation either with no restriction (< 450 mg/L), or with slight to moderate restrictions (450–2000 mg/L). On the other hand, the chemical data and the standards in Table 8 show that Cd, Fe and Mn concentrations exceeded the prescribed limits for water intended for irrigation, and for livestock watering in a few samples. Otherwise, Pb, Cr, Cu, and Zn concentrations fell within the stipulated quality criteria. Similarly, NO3─, Cl─, Na, Mg, and Ca concentrations complied with both standards (Table 8). Therefore, both GW and SW in the area is mostly suitable for both irrigation and livestock watering [43, 44] at least with respect to the parameters investigated in this study.
3.5 Canonical correspondence analysis (CCA) biplot, and hierarchical agglomerative cluster analysis (HCA) of water samples
The canonical correspondence analysis (CCA) biplot between trace elements and ions, and between trace elements and physicochemical parameters is given in Fig. 2. The arrow length indicates the significance of the variable and shows positive or negative correlations with the axis. Manganese (Mn), Cu, Cd, Pb, and Cr indicated strong association and are positively correlated with Na. Iron (Fe) showed a strong positive correlation with HCO3, Mg, Ca, K, and Cl, and a negative correlation with Cr. In Fig. 3b, Cr and Cd positively correlated with temperature, NO3─, COD and BOD5 but not with pH that instead showed negative correlation. Similarly, while Zn negatively correlated with EC, TDS and TH, it is positively correlated with TC and CF. Manganese negatively correlated with temperature, COD and NO3─, and positively correlated with pH.
Sampling sites in the same cluster in Fig. 3 have similar characteristics and natural background. This suggests that they could be affected by similar factors. This also applies to the parameters in the same cluster. According to Edet [56], correlation between chemical species in GW may indicate derivation from a common source. The dissolution of minerals such as halite, calcite, dolomite and gypsum could explain at least part of the contained Na, Ca, Mg and Cl─.
3.6 Pearson correlation analysis for selected GW, SW and topsoil physicochemical parameters
To establish comparative relationships between samples from the topsoil, GW and SW matrices across the different sites, we invoked the Pearson correlation analysis. The results, demonstrating the strength and direction of linear relationships between different variables, are presented in Tables 13 and 14. The correlation tables indicate the extent of association between species within and across matrices at a significance level of either p ≥ 0.05 or p ≥ 0.01. The values of the Pearson coefficient (r) show both positive and negative correlations between the parameters of soil, GW and SW, where r <|0.600| and r >|0.600| denote weak and strong associations, respectively. In GW (Table 13), weak negative correlations show for the pairs pH-NO3─, K-Cd, Cd-Mn and Cd-Cu, a weak positive association for Ca-Mg, and strong positive associations for Na-PO43─, K-Mn, K-Cu and Cd-Zn. Similarly, there exists in SW (Table 14) strong negative associations between the pairs Ca-Cu and Mg-Cu, and strong positive correlations between the pairs Ca-Mg, K-Fe, K-PO43─, Fe–Mn, Fe-Cu and Mn-Cu. Between the parameters of each GW sample and those of its corresponding topsoil sample (Table 13), weak negative correlations manifest for Mg-Ca, Mg-Mg, Cu- PO43─ and Mn–Mn, and weak positive correlations for Ca-Na, Na-Zn, K-pH, Cd-Na and Cu-pH. According to Table 14, while between the SW and topsoil parameters strong negative associations exist for the pairs NO3─-PO43−, Mn-PO43─, Zn- PO43─, Mn-K, Mn-Fe, and Cu-Fe, strong positive correlations show for the pairs Ca-Fe, Mg-Fe, Mg-Cu, Fe-pH, Fe-Ca, Fe-K, Cd-Na, Cd- PO43─, Cu-pH, Cu-K, Cu-NO3─. Within the topsoil (Table 14), Ca correlates positively with Mg, K, PO43─ and Zn, and negatively with Cu. Further, Mg correlates positively with K, PO43─, Cr, and Zn, and negatively with Cu. Similarly, K correlates positively with Cr and Zn, and negatively with Cu. Other positive correlations exist between the pairs Cu-NO3─, Cr-, PO43─ Zn- PO43─, Cr-Mn and Cr-Zn.
3.7 Soil physicochemical parameters
The physico-chemical data for soil in Chileka are presented in Table 10. The soil pH fell in the acidic ranges 4.42–5.62, and 4.47–6.59 in the dry and rainy seasons, respectively. These results compare well with those reported by Lakudzala and Khonje [57] in another study conducted in Blantyre. Soil pH affects the sorption and mobility of trace elements within the soil matrix. According to Rieuwerts [58], lower pH tends to decrease sorption thereby enhancing mobility and bioavailability of most forms of trace metals. Therefore, acidic soils would support movement of trace metals between the soil and water matrices. However, the current trace metal concentrations in GW suggest little or no interference from the surface. Soil quality standards have not been developed for Malawi, but for all the samples (n = 14 in each season), the concentrations of Pb, Cd, Cr, Mn, Cu, Fe and Zn reported in the present study were generally low and comparable to values considered ambient elsewhere (Table 15). Further, the current trace metal profile of the soils at Chileka fall within the limits prescribed in some standards in the region (Table 15), and may essentially be of no environmental concern. Sources of trace metals in Chileka include fertilizers, animal manure, pesticides, welding, burning of tyres during baking of bricks, and solid waste disposal. These sources might require close monitoring and control to prevent long-term cumulative effects. The minerals K, Na, Mg, and Ca occurred in abundance in the soils, and are known to naturally constitute 99% of the elemental composition of the earth’s crust [59]. Soil mineral abundance may vary with geographical region due to lithological differences. GW mineral content is influenced by dissolution from practically all solids and rocks, but especially from limestone, dolomite, and gypsum.
Nitrate (NO3─) occurred in the ranges 0.681–39.9 mg/kg and 4.85–58.9 mg/kg, and phosphate (PO43─) in the ranges 61.4–395 mg/kg and 65.0–506 mg/kg in the dry and rainy seasons, respectively. These nutrient levels in soil agree with those reported in a study done by Chidya [45] in Zomba, Malawi. Sources of nitrogen and phosphorus in the study area include fertilizers and animal manure. Although phosphorous manifests reduced mobility due to adsorption to soil particles, it may be more readily transported by erosion and runoff into SW bodies, where it is a limiting nutrient for eutrophication, than into GW by percolation [13]. In contrast, NO3─ is highly mobile in soil and can be transported into both environmental compartments, with its excessive presence in GW intended for drinking being of public health significance [4, 14]. The results of this study suggest that NO3─ and PO43─ from the soil might constitute a threat to the quality of GW and SW, respectively, in the study area.
3.8 Seasonality of soil chemistry and impact on water quality
The seasonal variation of physicochemical parameters in soil is shown by box-and-whisker plots in Fig. 4a and b, and assessed by the Two-Sample Independent t-Test.
In both the topsoil and the subsoil, pH (Fig. 4a and Table 16) was higher (p < 0.05) in the rainy season than in the dry season probably due to the prevalent use of animal manures for agricultural purposes in the area. However, the abundance of NO3─ in subsoil was significantly higher (p < 0.05) during the dry season. Similarly, a comparison of topsoil and subsoil shows that pH and PO43─ were significantly higher (p < 0.05) in topsoil than in subsoil during the dry season. Similarly, NO3─ was significantly higher (p < 0.05) in subsoil than in topsoil during the rainy season. Otherwise, the variation of Ca, Mg, Na, K, Cr, Cu, Fe, and Mn both between the seasons and between the topsoil and subsoil was not significant. This suggests limited mobility of the minerals in the soil matrix due to pH changes. But due to high mobility, nitrate leached from the surface resulting in the higher concentrations in the dry season than in the rainy season in subsoil. In contrast, the seasonal variation of topsoil NO3─ was not significant probably due to compensation on the surface, through contribution from agriculture, of leached nitrate. Similarly, seasonal variation of PO43─ concentration in the topsoil was not significant likely consequent to low mobility due to sorption. Again, the higher PO4-P concentration in topsoil during the dry season is attributable to solid waste disposal and use of manures. Phosphorus is one of the major chemical constituents that constitute over 99% of the total elemental composition of the earth’s crust [59]. Thus, phosphate in soil derives from the rock parent material, and from possible anthropogenic addition especially to agricultural soils through application of fertilizers and manures. Therefore, while contamination of GW by nitrate and SW by phosphate may occur in Chileka during the rainy season through transfer from the soil, the phenomenon is limited and unlikely for trace metals.
4 Conclusions and recommendations
The purpose of this research was to assess the extent of contamination with trace metals, nutrients, and microbes (total coliforms and faecal coliforms) of water and associated soils in Chileka Township, Blantyre. The results show that both raw GW and SW in Likhubula River are not safe for drinking mainly due to bacterial contamination. The river is also rated as ranging from slightly polluted to polluted. On the other hand, GW is largely of satisfactory chemical quality but with a possibility of Cd exposure to the communities in excess of WHO thresholds especially in the dry season. Further, the communities are exposed to Mn beyond WHO limits. Nitrate, being mobile in soil, threatens GW quality whereas phosphate may, due to adsorption, potentially affect SW quality only. The trace metal levels in soil do not yet constitute an environmental problem. It is recommended that responsible authorities should continue to promote good sanitation, and include NO3─ and Cd under surveillance studies in the area. The transfer of nitrate and phosphate from soil into SW may be minimized through proper solid waste disposal, and securing riverbanks against cultivation. Although the study has reached its aims, other equally important contaminants such as fluoride and arsenic were not included in this study due to financial and time constraints. The purposive sampling strategy employed and the limited number of samples taken probably did not adequately capture spatial and temporal variation in the characteristics of GW and SW. Therefore, a study including more boreholes and shallow wells, and more sampling sites along the Likhubula river is recommended. There is also a need to investigate whether or not on-site sanitation systems (pit-latrines and septic tanks) in the area might be contributing to nitrate, phosphate and chloride in groundwater.
Data availability
All the data for the study has been provided in this manuscript. The authors are also available to provide any data that may be needed by the journal.
References
CobbingHiller JB. Waking a sleeping giant: realizing the potential of groundwater in Sub-Saharan Africa. World Dev. 2019;122:597–613.
Li P, Karunanidhi D, Subramani SMK. Sources and consequences of groundwater contamination. Arch Environ Contam Toxicol. 2021;80:1–10. https://doi.org/10.1007/s00244-020-00805-z.
MacDonald AM, Calow RC. Developing groundwater for secure rural water supplies in Africa. Desalination. 2009;248(1–3):546–56. https://doi.org/10.1016/j.desal.2008.05.100.
World Health Organization. Guideline for drinking water quality: fourth edition incorporating the first and second addenda. Geneva: Switzerland; 2022.
Kaonga CC. Master’s degree thesis. Zomba: University of Malawi, Chancellor College; 2007.
Mapoma HWT, Xie X. Basement and alluvial aquifers of Malawi: an overview of groundwater quality and policies. Afr J Environ Sci Technol. 2014;8(3):190–202. https://doi.org/10.5897/AJEST2013.1639.
Ritter L, Solomon K, Sibley P, Hall K, Keen P, Mattu G, Linton B. Sources, pathways and relative risks of contaminants in surface water and groundwater: a perspective prepared for the Walkerton inquiry. J Toxicol Environ Health A. 2002;65(1):1–142. https://doi.org/10.1080/152873902753338572.
Murray K, Rogers S, Kaufman DT. Dissolved heavy metals in shallow ground water in a Southeastern Michigan urban watershed. J Am Water Resour Assoc. 2006;42(3):777–92. https://doi.org/10.1111/j.1752-1688.2006.tb04492.x.
Kumwenda S, Tsakama M, Kalulu K, Kambala C. Determination of biological, physical and chemical pollutants in Mudi River, Blantyre, Malawi. J Basic Appl Sci Res. 2012;2(7):6833–9.
Holman IP, Whelan MJ, Howden NJK, Bellamy PH, Willby NJ, Rivas-Casado M, McConvey P. Phosphorus in groundwater-an overlooked contributor to eutrophication? Hydrol Process. 2008;22(26):5121–7. https://doi.org/10.1002/hyp.7198.
Foster SSD, Chilton PJ. Groundwater: the processes and global significance of aquifer degradation. Philosophical Trans Royal Soc B Biol Sci. 2003;358(1440):1957–72. https://doi.org/10.1098/rstb.2003.1380.
Ashbolt NJ. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology. 2004;198(1–3):229–38. https://doi.org/10.1016/j.tox.2004.01.030.
Mueller DK, Helsel DR, Kidd MA. Nutrients in the nation’s waters: Too much of a good thing? Washington, DC: US Government Printing Office; 1996.
Kempster PL, Vliet HR, Kuhn A. The need for guidelines to bridge the gap between ideal drinking-water quality and that quality which is practically achievable and acceptable. Water SA. 1997;23(2):163–7.
Tredoux G, Talma AS. Nitrate pollution of groundwater in Southern Africa. In: Yongxin X, Usher B, editors. Groundwater Pollution in Africa. London: Taylor & Francis Group; 2006.
Govind P, Madhuri S. Heavy metals causing toxicity in animals and fishes. Res J Animal Vet Fishery Sci. 2014;2(2):17–23.
Nagajyoti PC, Lee KD, Sreekanth TVM. Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett. 2010;8(3):199–216. https://doi.org/10.1007/s10311-010-0297-8.
Peijnenburg W. Fate of contaminants in soil. Dev Soil Sci. 2004;29:245–80.
Government of Malawi. Malawi population and housing census report. Zomba: National Statistical Office; 2018.
Chimphamba JB, Phiri OL. Borehole water pollution and its implication on health on the rural communities of Malawi. Malawi J Sci Technol. 2014;10(1):32–9.
Khonjeb A, Metcalf CA, Diggle E, Mlozowa D, Jere C. Cholera outbreak in districts around Lake Chilwa, Malawi: lessons learned. Malawi Med J. 2012;24(2):29–33.
Mello-Guyett LD, Cumming O, Rogers E, D’hondt R, Mengitsu E, Mashako M, Checchi F,. Identifying transferable lessons from cholera epidemic responses by Médecins Sans Frontières in Mozambique, Malawi and the Democratic Republic of Congo, 2015–2018: a scoping review. Confl Heal. 2022;16(1):12.
Hodge J, Chang HH, Boisson S, Collin SM, Peletz R, Clasen T. Assessing the association between thermotolerant coliforms in drinking water and Diarrhea: an analysis of individual-level data from multiple studies. Environ Health Perspect. 2016;124(10):1560–7.
American Public Health Association. Standard methods for the examination of water and wastewater. 20th ed. Washington DC, USA: American Public Health Association; 1999.
Association of Official Analytical Chemists. Official methods of analysis (15th ed., Vol. 1). Virginia: Association of Official Analytical Chemists (AOAC), Inc; 1990.
Alexander I, Anderson JM, Baker KF, Barrios E, Bohren L, Bowen GD, Young A. Tropical soil biology and fertility: a handbook of methods. 2nd ed. Wallingford: CAB International; 1993.
Yang JE, Kim JJ, Skogley EO, Schaff BE. A simple spectrophotometric determination of nitrate in water, resin, and soil extracts. Soil Sci Soc Am J. 1998;62(4):1108–15. https://doi.org/10.2136/sssaj1998.03615995006200040036x.
Bamgbose O, Odukoya O, Arowolo TOA. Earthworms as bioindicators of metal pollution in dump sites of Abeokuta City. Nigeria Revista de Biologia Tropical. 2000;48(1):229–34.
Buurman P, van Lagen B, Velthorst EJ. Manual for soil and water analysis. Leiden: Backhuys Publ; 1996.
Halloram IPO, Cade-Menum BJ. Total and organic phosphorus. 2nd ed. London: Taylor & Francis Group; 2006.
Yuting Z, Jianhua W, Bin X. Human health risk assessment of groundwater nitrogen pollution in Jinghui canal irrigation area of the loess region, northwest China. Environ Earth Sci. 2018;77:273. https://doi.org/10.1007/s12665-018-7456-9.
IRIS (2012) Integrated Risk Information System. Washington, DC: U. S. Environmental Protection Agency; Cadmium. http://www.epa.gov/iris/subst/index.html,Accessed 22 Feb 2012.
Alharbi T, El-Sorogy AS. Health risk assessment of nitrate and fluoride in the groundwater of central Saudi Arabia. Water. 2023;15:2220. https://doi.org/10.3390/w15122220.
United States Environmental Protection Agency. A risk assessment—multi way exposure spread sheet calculation Tool. Washington DC: United States Environmental Protection Agency; 1999.
United States Environmental Protection Agency. Exposure factors handbook 2011 edition (Final Report). Washington DC: United States Environmental Protection Agency; 2011.
United States Department of Energy’s Oak Ridge Operations Office (ORO). The Risk Assessment Information System (RAI). 2011.
Malawi Standard (MS) 733:2005. Borehole and shallow well water quality—Specification. 1st ed. Blantyre: Malawi Bureau of Standards Board; 2005.
Elisante E, Muzuka ANN. Sources and seasonal variation of coliform bacteria in groundwater around the slopes of Mount Meru, Arusha. Tanzania Environ Monitoring Assess. 2016;188(395):394–5.
Palamuleni LG, Dolozi MB, Masamba WRL, Claudio-Jeke A. Bacterial contamination of water in urban poor areas: a case study of south Lunzu Township, Blantyre, Malawi. Malawi J Sci Technol. 2004;7:26–33.
Mapoma HWT, Xie X, Zhang L, Nyirenda MT, Maliro A, Chimutu D. Hydrochemical characteristics of rural community groundwater supply in Blantyre, southern Malawi. J Afr Earth Sc. 2016;114:192–202. https://doi.org/10.1016/j.jafrearsci.2015.11.023.
Heath RC. Basic Ground-Water Hydrology: U.S. Geological Survey Water-Supply Paper 2220. 1983;86.
Robinove CJ, Langford RH, Brookhart JW (1958) Saline-water resources of North Dakota. Water Supply Paper 1428, U.S. Government Printing Office, Washington, D.C. https://doi.org/10.3133/wsp1428
Food and Agricultural Organisatio. Water quality for agriculture. FAO Irrigation and drainage paper 29 Rev. 1. Rome: Food and Agricultural Organization; 1985.
DWAF. South African water quality guidelines. Agricultural use: livestock watering. Vol. 5. Department of Water Affairs and Forestry of the Republic of South Africa. 1996.
Chidya RCG, Sajidu SMI, Mwatseteza JF, Masamba WRL. Evaluation and assessment of water quality in Likangala river and its catchment area. Phys Chem Earth Parts A/B/C. 2011;36(14–15):865–71. https://doi.org/10.1016/j.pce.2011.07.070.
Elisante E, Mazuka ANN. Occurrence of nitrate in Tanzanian groundwater aquifers: a review. Appl Water Sci. 2017;7(1):71–87. https://doi.org/10.1007/s13201-015-0269-z.
Burkart MR, Kolpin DW. Hydrologic and land-use factors associated with herbicides and nitrate in near-surface aquifers. J Environ Qual. 1993;22(4):646–56. https://doi.org/10.2134/jeq1993.00472425002200040002x.
United States Environmental Protection Agency. Quality criteria for water. Washington, D.C.: United States Environmental Protection Agency; 1986.
Kamanula JF, Zambasa OJ, Masamba WRL. Quality of drinking water and cholera prevalence in Ndirande Township, City of Blantyre, Malawi. Phys Chem Earth Parts A/B/C. 2014. https://doi.org/10.1016/j.pce.2014.09.001.
Sajidu SMI, Masamba WRL, Henry EMT, Kuyeli SM. Water quality assessment in streams and wastewater treatment plants of Blantyre, Malawi. Phys Chem Earth Parts A/B/C. 2007;32(15–18):1391–8. https://doi.org/10.1016/j.pce.2007.07.045.
Kanyerere T, Levy J, Xu Y, Saka J. Assessment of microbial contamination of groundwater in upper Limphasa river catchment, located in a rural area of northern Malawi. Water SA. 2012. https://doi.org/10.4314/wsa.v38i4.14.
Kuyeli SM, Masamba WR, Fabiano E, Sajidu SM, Henry EM. Temporal and spatial physicochemical water quality in Blantyre urban streams. Malawi J Sci Technol. 2009;9(1):5–10.
Prati L, Pavanello R, Pesarin F. Assessment of surface water quality by a single index of pollution. Water Res. 1971;5(9):741–51.
National Research Council (US) Subcommittee on Nitrate and Nitrite in Drinking Water (1995) Nitrate and Nitrite in Drinking Water. Washington (DC): National Academies Press (US); 5, Risk Characterization. https://www.ncbi.nlm.nih.gov/books/NBK231050/
Habineza E, Makwinja R, Inagaki Y. Contamination and health risks of trace metals in water and sediments of May Sieley stream, Ethiopia. Phys Chem Earth. 2023;129:103315. https://doi.org/10.1016/j.pce.2022.103315.
Edet A, Nganje TN, Ukpong AJ, Ekwere AS. Groundwater chemistry and quality of Nigeria: a status review. African J Environ Sci Technol. 2011;5(13):1152–69. https://doi.org/10.5897/AJESTX11.011.
Lakudzala DD, Khonje JJ. Nutritive potential of some “edible” soils in Blantyre city Malawi. Malawi Med J. 2011;23(2):38–42.
Rieuwerts JS. The mobility and bioavailability of trace metals in tropical soils: a review. Chem Speciat Bioavailab. 2007;19(2):75–85. https://doi.org/10.3184/095422907X211918.
Alloway BJ. Heavy metals in Soils, vol. 22. Dordrecht: Springer, Netherlands; 2013. https://doi.org/10.1007/978-94-007-4470-7.
Government of Tanzania (2007) Environmental management (soil quality standards) regulations.
Kaonga CC, Kosamu IBM, Lakudzala D, Mbewe R, Thole B, Monjerezi M, Sajidu SMI. A review of heavy metals in soil and aquatic systems of urban and semi-urban areas in Malawi with comparisons to other selected countries. Afr J Environ Sci Technol. 2017;11(9):448–60. https://doi.org/10.5897/AJEST2017.2367.
Towett EK, Shepherd KD, Tondoh JE, Winowiecki LA, Lulseged T, Nyambura M, Cadisch G. Total elemental composition of soils in Sub-Saharan Africa and relationship with soil forming factors. Geoderma Reg. 2015;5:157–68. https://doi.org/10.1016/j.geodrs.2015.06.002.
Lindsay WL. Chemical equilibria in soils. New York: John Wiley & Sons Inc; 1979.
Funding
This study did not receive any form of funding but the authors are grateful to Malawi Bureau of Standards for supporting the first author in sample collection and analysis.
Author information
Authors and Affiliations
Contributions
G. Dzinjalamala, F. Thulu, U. Mawenda collected and analyzed samples. C. Kaonga, S. Kumwenda and H. Sakugawa supervised the work and checked the manuscript. R. Makwinja, I. Kosamu and T. Kanyerere drafted the manuscript and analyzed data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dzinjalamala, G.D., Kaonga, C.C., Kumwenda, S. et al. Human health risk assessment of microbial contamination and trace metals in water and soils of Chileka Township, Blantyre, Malawi. Discov Environ 2, 62 (2024). https://doi.org/10.1007/s44274-024-00096-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44274-024-00096-4