Abstract
Cyclodextrins are cyclic oligosaccharides characterized by a hydrophobic interior and a hydrophilic outside linked by α-1,4 glycosidic bonds. Cyclodextrins are biodegradable and generally considered environmentally friendly. These are utilized in diverse applications, encompassing environmental cleanup which is enhanced through the processes of cross-linking or surface modification. These molecules have a unique structure that enables them to form inclusion complexes with various guest molecules, including organic pollutants, pesticides, dyes, pharmaceuticals, and hydrocarbons. When used in water treatment, cyclodextrins can act as molecular sponges, trapping pollutants within their cavities through non-covalent interactions such as hydrogen bonding, van der Waals forces, and hydrophobic interactions. When cyclodextrins are introduced into water containing pollutants, the pollutants can enter the cavities of cyclodextrin molecules, forming inclusion complexes. This process effectively sequesters the pollutants from the surrounding water, reducing their concentration. It can often be regenerated and reused multiple times, making them cost-effective for water treatment applications. This review presents the primary applications of cyclodextrins for the adsorption of contaminants from various pollutants from diverse sources, based on recent publications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Water pollution has adverse effects on both ecosystems and human beings as a result of organic cationic contaminants, such as dyes and pesticides [1]. The development of environmental concerns is an outcome of the modern era. Dye effluent, a byproduct of industrial dye production, poses a substantial environmental risk in a variety of industries, including but not limited to paper manufacturing, food processing, leather, and paint [2]. Specific synthetic cationic and anionic dyes have been recognized for their detrimental effects on the environment, whereas the inadequate management and misuse of antibiotics have led to their emergence as an emergent organic pollutant [3]. Sustainable development, which is fundamental to both economic and social advancement, encompasses enhanced availability of sanitation facilities and pure water, thereby reducing pollution levels and subsequently improving water quality. By 2030, the Global Sustainable Development Goals (SDGs) of the United Nations stipulate that everyone should have affordable, equitable, and universal access to secure, clean water [4]. Adsorption has emerged as an ecologically sound approach to remediate contaminated water, prompting the creation of numerous innovative adsorbents constructed from sustainable and cost-effective substances. The increased interest in this process in recent years can be attributed to its numerous advantages, which include its simplicity, minimal energy requirement, reusability, ease of operation, and suitability for technology transfer [5]. The fundamental aspect of adsorption, which is currently utilized to remove pollutants from aquatic environments, revolves around the meticulous choice of adsorbents. The fundamental material of copper sulphide nanocomposites modified with nitrogenated graphene oxide is polysulfide membranes. Requirements such as regeneration (solvent consumption or energy consumption), adsorbent disposal at the end of its useful life, and the expense of reactants are some of the method's disadvantages. A multitude of adsorbents have been devised to facilitate the process of adsorption. These include cyclodextrin nano sponges, cellulose nanofiber, biobased materials, cyclodextrin polymers coated on textiles, and kaolin [6].
Adsorbents comprised of innovative cyclodextrin absorb and eliminate pollutants from a variety of media. By incorporating cyclodextrins into solid supports such as nanoparticles or polymers, these adsorbents become more stable and effective. Purification of air and water is one of their numerous environmental remediation applications. By encapsulating contaminants selectively in the hydrophobic cavities of cyclodextrin, they can be effectively extracted from aqueous or gaseous environments. Additionally, cyclodextrin chemistry can be modified to optimize adsorption capabilities or target particular pollutants. Reusability, selectivity, and high adsorption capacity are characteristics that set apart cyclodextrin-based adsorbents from conventional ones. Cyclodextrins (CDs) molecules possess distinctive attributes that enable them to establish an inclusion complex with organic compounds of an appropriate size. This is the rationale behind the limited utilization of cyclodextrin-based adsorbents in environmental applications [7]. In order to enhance the capacity for adsorbing organic components, a three-dimensional cross-linked polymer was produced by reticulating CD with citric acid (CTR), an environmentally safe trifunctional cross-linking agent [8].
Cyclodextrin’s efficacy in adsorbing water-soluble pollutants can be attributed to the presence of numerous hydroxyl groups within its structure. Its recycling process was simpler than that of particle adsorbents, and it could be incorporated into solutions to facilitate adsorption and the elimination of pollutants. It possesses the capability to be converted into a compact filtration apparatus capable of efficiently absorbing minute organic contaminants. This apparatus would be inexpensive, user-friendly, biodegradable, and highly effective for adsorption [9]. The primary advantage of incorporating lipophilic compounds into cyclodextrins is that it enhances their stability, bioavailability, and water solubility [10].
Chemical modifications can be applied to cyclodextrins to enhance their efficacy and precipitation, thereby rendering them suitable for the removal of pollutants. Despite this, there are numerous gaps that can be addressed. Its efficiency and functionalization can be optimized for a variety of contaminants found in various domains. Additionally, its size, shape, feasibility, and other impacts can be evaluated in order to expand its applications. This research paper elucidates the prospective factors and sustainable utilization of the subject matter. By combining it with nanoparticles or polymers, these hybrid CDs can be transformed into a highly efficient adsorbent that facilitates the removal of contaminants. Furthermore, the reusability of these adsorbents demonstrates that they are both cost-effective and environmentally sustainable.
1.1 History
Cyclodextrins α- and β- were discovered in 1891 as residues of potato starch digestion utilizing Bacillus amylobacter bacteria, according to a French scientist and chemist. CDs, or "crystalline dextrin," were initially isolated from diverse starch sources by Franz Schardinger, an Austrian microbiologist who later gained recognition as the "founding father" of CD chemistry, following digestion by Bacillus macerans [11].
The cyclodextrin patent was granted in 1953 after the demonstration of the viability of cyclodextrin-inclusion complex formation. The American biochemist and scientist proposed two larger CDs, which were composed of nine and ten glucose monomers, respectively [12]. However, it was observed that the larger CDs possessed fewer physicochemical properties that were conducive to complex formation, in contrast to the smaller CDs. During the latter half of the twentieth century, research was primarily focused on cyclodextrins' ability to form inclusion complexes with other molecules, as more knowledge regarding their structure and properties became accessible. Cyclodextrins have numerous intriguing applications due to their capacity to protect delicate organic guest molecules from oxidation and volatilization and their solubilizing effect on polar guests.
1.2 General uses
There has been a meteoric rise in the use of cyclodextrins in the pharmaceutical, cosmetic, and food chemistry industries since their toxicological safety was determined, and their reach has only broadened to include (re-)emerging fields like nutrients and natural products. Cyclodextrins were mostly seen as inactive ingredients or excipients in all these goods [13].
1.2.1 Food additive
Japan views native CDs as natural goods, which allows for their widespread usage in food and medicine. In the West, the Joint WHO/FAO Expert Committee on Food Additives regulates the consumption of native cyclodextrins [14]. There are no limits on the use of α-CD and γ-CD, 5 mg per kg of weight per day is the maximum recommended oral consumption of β-CD. Native cyclodextrins are subject to somewhat stricter limitations when it comes to parenteral administration. The European Medicines Agency advises against injecting α-CD and β-CD directly into the circulation because of the risk of kidney damage. It has been observed that native cyclodextrins may induce hemolysis in vitro, with α-, β-, and γ-cyclodextrins causing this at doses of 6, 3, and 16 mM, because of the phospholipid and cholesterol extraction from the erythrocyte membrane. Functionalization of native cyclodextrins allows to produce a vast array of derivatives, with over 1500 distinct compounds being accessible [15].
1.2.2 Pharma agent
Limited to topical products and a maximum concentration of 1.5%, 2-hydroxypropyl-β-cyclodextrin (HPβCD) and 2-hydroxypropyl-γ-CD (HPγ-CD) are both recognized as inert substances that can be used as excipients by the U.S. Food and Drug Administration.
1.2.3 Pollutant removal
Because of their special molecular structure, which allows them to create inclusion complexes with a broad range of contaminants, cyclodextrins are being used in the process of removing pollutants more and more. Cyclodextrins are effective adsorbents used in environmental remediation for pollutants found in both the water and the air. Organic contaminants can be encapsulated in the water treatment process by cyclodextrins. Among these contaminants include medications, pesticides, and pigments. Because they create inclusion complexes, cyclodextrins greatly increase the effectiveness with which these pollutants are eliminated from water supplies. The immobilization of cyclodextrin-based products onto solid supports facilitates the simple filtration or adsorption processes for the easy separation of contaminants from water. In a same vein, cyclodextrins are used in air purification to capture various pollutants and volatile organic compounds (VOCs) that are transported by air. Cyclodextrin-based adsorbents are efficient in capturing volatile organic compounds (VOCs) in their hydrophobic cavities, therefore improving the air quality both inside and outside of the structure. Selection of certain pollutants by cyclodextrins suggests that they can remove specific impurities. Reducing this selectivity lowers the possibility of unfavorable results by reducing interference with harmless chemicals already present in the environment [16, 17].
1.2.4 Wastewater treatment and reuse
Municipal wastewater is produced worldwide annually at 330 km3. The European Investment Bank projects that by 2025, 800 million people would live in places without access to clean water [18]. A major threat to both people and the environment are xenobiotics, or pollutants of emerging concern. These materials can accumulate in living things and are frequently not broken down naturally in both surface and groundwater. Sometimes these microscopic pollutants are detected in public water supplies, which could be dangerous for people as well [19]. The bacteria used in wastewater secondary treatment—that is, after sedimentation, the first treatment—are xenobiotics; conventional techniques cannot efficiently remove these compounds from the wastewater. Microcontaminants must be avoided from the receiving environment, which includes the sea, river, lake, wetlands, ground, etc. Therefore, effective, and reasonably priced treatment methods must be developed.
Reusing water, which is crucial for human survival, is a major problem across the globe. The scientific community is always looking for new ways to purify water, as expected, to guarantee its quality and safety [20]. The aquatic environment is constantly being polluted by Emerging Contaminants (EC) and their metabolites, including pesticides, herbicides, insecticides, fungicides, medicinal chemicals, personal care items, and many more. Antibiotics are among the most widely utilized ECs in both animal and human medicine, and this is especially true when considering pharmaceutical substances [21]. Antibiotic resistance and other serious human health issues can result from this class of compounds being put into water [22]. In line with the principles of "Green Chemistry" and "Green Economy," this work proposes a safer and highly effective adsorbent for possible industrial uses by utilizing a polymer based on cyclodextrins and crosslinked with 1–4 butanediol diglyceryls ether. The challenge was to find efficient, low-cost, and sustainable processes that can recycle both the adsorbent and the pollutant. The adsorption capacities of different adsorbents for pollutants are as shown in Table 1.
The cross-linking of Cyclodextrins with epichlorohydrin has, unsurprisingly, been extensively studied before, although epichlorohydrin is supposedly extremely harmful to both people and animals. In fact, adsorbents were not disposed of as secondary hazardous waste after extensive research on adsorption/desorption cycles. The morphological, FTIR, ATR, and XRD properties of the adsorbent were maintained during adsorption and desorption [23].
1.3 Types of cyclodextrins
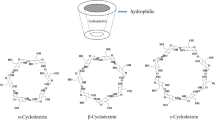
Cyclodextrins are cyclic oligosaccharides composed of glucose units, typically classified based on the number of glucose units they contain. The most common types of cyclodextrins are as shown in Fig. 1 includes:
-
α-Cyclodextrin: Comprising six glucose units, α-cyclodextrin has a cone-shaped structure with a hydrophobic cavity, making it suitable for encapsulating small hydrophobic molecules.
-
β-Cyclodextrin: This type consists of seven glucose units and is widely used due to its versatility in forming inclusion complexes with a variety of guest molecules, including drugs, fragrances, and pollutants.
-
γ-Cyclodextrin: With eight glucose units, γ-cyclodextrin has a larger cavity compared to α- and β-cyclodextrins, enabling it to encapsulate bulkier molecules. It finds applications in pharmaceuticals, food, and environmental remediation [24].
Besides these primary types, modified cyclodextrins have been developed to enhance specific properties or functionalities. Examples include:
-
Hydroxypropyl β-Cyclodextrin: Modified with hydroxypropyl groups, this derivative exhibit improved water solubility, making it suitable for applications requiring aqueous environments.
-
Methylated Cyclodextrins: Methylation of cyclodextrins alters their physicochemical properties, such as solubility and stability, expanding their applicability in various industries, including pharmaceuticals and food.
-
Sulfobutylether β-Cyclodextrin: Sulfobutylether modification enhances the water solubility of β-cyclodextrin, facilitating its use in pharmaceutical formulations, particularly for poorly water-soluble drugs.
These various types of cyclodextrins offer a range of functionalities and applications across industries, from pharmaceuticals and food to environmental remediation and beyond. Properties of Cyclodextrin are as shown in Fig. 2.
Cyclodextrin supramolecular structure is akin to those of cryptands, calixarenes, cyclophanes, spheres, and crown ethers. Most of these exchanges go under the "host–guest" category. The first of the supramolecular hosts mentioned above are cyclodextrins. They can fundamentally change the properties of the materials they interact with by their ability to form inclusion complexes. Cyclodextrin is widely used in many technical applications, analytical instruments, and industrial products because of the molecular complexation phenomena. A key feature of cyclodextrins is their very low cytotoxic effects. Numerous uses include rug carrier, food and flavor, cosmetics, packaging, textiles, separation procedures, environment protection, fermentation, and catalysis areas [25].
The starch or starch derivatives used to make cyclodextrins are a class of oligosaccharides. In them, you may find glucopyranose monomers connected by α-1,4-glycosidic linkages in quantities ranging from six (αCD) to eight (γ-CD). This unique structure allows cyclodextrins to dissolve in water and concurrently host molecules that are hydrophobic and lipophilic. The main benefit of adding these lipophilic compounds to cyclodextrins is that they become more soluble in water. Research has shown that natural compounds like propolis and bioactive found in plants (such as tocotrienol, pentacyclic triterpenoids, and curcumin) can be enhanced in stability, bioavailability, and bioactivity when combined with γ-CD. The human intestinal flora ferments α- and β-CD, which are not digestible in the intestines, when taken orally, but α-amylase nearly entirely breaks down γ-CD to glucose cells. According to recent research, there may be additional potential for metabolic activity when empty γ-CD supplementation is taken alone. Another class of cyclic oligosaccharides called cyclodextrins (CDs) is synthesized by the bacterial enzyme cyclodextrin glycosyltransferase (CGTase) from starch or starch derivatives. α-1,4-glycosidic linkages connect at least six glucopyranose monomers in a CD. Out of all the members of the cyclodextrin family, α-, β-, and γ-CD stand out with their respective numbers of six, seven, and eight glucopyranose units [26].
Along with CS, β-cyclodextrin (β-CD) is widely used since it is affordable and can form several inclusion complexes. The cyclic oligosaccharide β-CD possesses distinctive affinity and inclusion properties due to its nontoxicity, ease of modification, water solubility, and unique cavity shape [27]. It is not convenient to adsorb β-CD in aqueous solutions since it is water-soluble [28]. There have been many endeavors to create water-insoluble polymers for pollution removal by cross-linking β-CD with CS. Metal ions and dyes were removed using the resulting composite adsorbent i.e. the β-cyclodextrin polymer (β-CDP) by combining Ų-CD with citric acid (CA) [29].
By combining CDs, composite materials have been created, allowing each material's unique properties to shine. Researchers have so far only employed composites for pollutant removal in water, CS/CD/MIL-68(Al) composites, in which CS is cross-linked with cyclodextrin and loaded with MIL-68(Al) simultaneously, were reported to adsorb hydrophobic and hydrophilic chemicals [30]. By using a β-CD/CTR/PVOH-functionalized cotton cord to adsorb PQ, MB, and CV, three soluble cationic pollutants, or coated β-CD with CTR in the presence of PVOH on cellulose material was also reported. Detailed chemical and physical properties of cyclodextrin are shown in Table 2. The initial investigation in this work focused on the coating of a cotton rope with anionic cyclodextrin polymer, which was produced by the reticulation of CTR and β-CD in the presence of poly (vinyl alcohol). The pharmaceutical, cosmetic, and home chemical industries mostly utilize cyclic oligosaccharides (CDs) that comprise 6–8 glucose units (α-, β-, and γ-CD) [31].
1.4 Synthesis and mechanism
Three types of oligosaccharide CD, α-CD, β-CD, and γ-CD are produced by actinic hydroxylation of starch and joined by α-1, 4-glycosidic linkages. Experiments carried out following the synthesis of enzymatic hydrolysis have demonstrated that there are many distinct CD derivatives with comparable properties to the three most common forms of CDs [32]. Three classes can be broadly distinguished among the processes of modification and synthesis; these will be briefly discussed below. Figure 3 shows the synthesis scheme for CD based materials.
These compounds can combine to create non-covalent inclusion complexes with a wide range of organic pollutants (e.g., synthetic herbicides, active components of pharmaceuticals and cosmetics, etc.) and microscopic contaminants (dissolved in water) present in freshwater. Chemical oxidation of pollutants in soil and in situ/ex situ microbial degradation are two soil remediation techniques that can benefit from the enhanced desorption of pathogens from soil by CD derivatives such hydroxypropyl and methylated CDs [33]. Beads and the microfibers of nano sponges are only two examples of the sorbents based on CDs that can be made by immobilizing CDs onto the surfaces of natural or artificial polymers. Research indicates that these absorbing materials can function as both samplers and removers of small-scale adulterants from wastewater.
Mechanism: The results amply illustrated the adsorption mechanism by indicating that the good adsorption capacity of the β-CDCS-EDTA adsorbent would be mostly determined by the presence of multiple functional groups. The main process of binding the metal ions to the adsorbent was EDTA chelation. Use of elemental mapping, FTIR spectra, and EDX confirmed the successful heavy metal ion adsorption process. The colorful elemental spots, which show that ciprofloxacin and nickel are similarly distributed throughout the whole adsorbent surface, validate the fact that the organic contaminants and metal ions were efficiently adsorbed onto the equally distributed adsorption sites on β-CD-CS-EDTA. The elemental composition and EDX spectrum are corresponding to the elemental mapping [34]. Figure 4 displays the cyclodextrin based adsorbent schematic diagram
The results of the isotherm adsorption experiment, which demonstrated that the synthetic adsorbent was more effective at removing metal ions from solution, are strongly corroborated by this [35]. When the O–H (or N–H) peak widened during adsorption, it was established that there was electrostatic interaction and accessible functional groups. Based on the fact that the distinctive peak at 1037 cm−1 in β-CD-CS-EDTA, which was attributed to glucose, shifted to 1023 cm−1 in β-CD-CS-EDTA-CIP, the prior research on other organic pollutants validated the formation of a host–guest inclusion complex with the β-CD, the FTIR spectrum for the adsorption of Ni(II) confirms that the two species are adsorbed simultaneously through chelation, electrostatic interaction, and the creation of a host–guest inclusion complex [36, 37].
2 Applications of cyclodextrins
The percentage of βCD in worldwide CD manufacturing was over 70% in 2017, with αCD and γ-CD each accounting for about 15% and 5%. Although it was previously manufactured in lower quantities, γ-CD is now gaining popularity as a pharmaceutical excipient because of its excellent toxicological profile. Their status as natural goods, native cyclodextrins in Japan are widely used in both food and medicine. The formation of inclusion complexes with hazardous medications to increase their solubility has been the primary use of cyclodextrins in food and pharmaceutical items for a long time [38]. Cyclodextrins have been shown in earlier studies to be a useful material for antibacterial food packaging when mixed with natural antibacterial agents like thymol and carvacrol. Including an antibacterial agent inside food packaging is one method to stop bacteria from growing there. Electro spun nanofibers were prepared by using the THY-γ-CD complex containing zein. Zein-THY-γ-CD-NF was more effective against Escherichia coli and Staphylococcus aureus than was zein-THY-NF without γ-CD. As materials for food packaging, the webs have promise. Especially γ-CD has drawn a lot of interest in oral bioavailability studies and is seen as a possible nutrition delivery technique [39].
The bioavailability and bioactivity of tocotrienols, pentacyclic triterpenoids, propolis, and curcumin inclusion complexes with γ-CD can stabilize a wide range of food components, including tastes, colorants that are sensitive to fat, vitamins that are soluble in fat, acids that are polyunsaturated, and emulsions of lipids and oils. Hydrophilic cyclodextrins can only serve as a means of transport for lipophilic guest molecules when taken orally; this helps them reach the gastrointestinal tract's absorption surface through an aqueous medium, which increases their stability until they pass through biological membranes like the intestinal epithelium [40]. It is not unexpected that CDs do not typically enhance the permeability of hydrophilic guest molecules through lipophilic biological membranes. This is because cyclodextrins have a relatively high molecular weight and many hydrogen donors and acceptors, which make them poorly absorbed through biological membranes [41]. Applications of cyclodextrins to remove pollutants from wastewater is shown in Table 3.
Cyclodextrins do not make class I drugs more bioavailable. Because of their high solubility in water and permeability of the membrane, oral administration of them can efficiently absorb them into the circulation. To improve their bioavailability, cyclodextrins are not usually employed to encapsulate these substances; instead, a thorough examination of medication absorption after different application techniques, such as oral administration, is provided. Applications of cyclodextrin are illustrated in Fig. 5. Cyclodextrins have a short half-life in the systemic circulation and are likewise quickly eliminated in urine. During the little time the complex is in systemic circulation, it may not get to its target organ. The drug might not get to the organ or tissue it is meant to reach if the complexes separate.
2.1 Removal of dyes
Adsorbents based on cyclodextrin are highly effective in removing dyes. Research on dye adsorption has been extensive. The interactions between the analyte molecules and the adsorbent determine the adsorption efficiency. The dye-uptake capabilities of various adsorbents are matrix-dependent. As of the interactions between the cyclodextrin and hydrophobic dye, a surface area of 0.1 mg/g may adsorb despite the abundance of oxygen on the β-CD surface, CR and MB have a maximal capacity of 1.80–10–2 mmol/g [42]. The hydrophilic surface of β-cyclodextrin experiences mild contact forces with MB. In recent years, host–guest complexation has emerged as a promising and cost-effective adsorption method. A recent study investigated the efficacy of γ-cyclodextrin, and oil orange SS (OOSS) azo dye complexes made using the coprecipitation process for water purification [43]. The validation of the OOSS dye encapsulation with the β-CD hydrophobic cavity was supported by thermal analysis and FTIR data. The creation of host–guest complexes enhanced stability in the matrix during complexation. The results of an additional research that used the electrospinning method to create β-CD and ε-polycaprolactone (PCL) composite fibers using the host–guest complexation process. To characterize the adsorbents, SEM, XRD, EDXS, and FT-IR were used. When applied to 4-aminoazobenzene, the dye elusion capacity reached 24.1 mg/g. Dye adsorption on a large scale might be facilitated by the electropunk fibers’ sensitivity, selectivity, and adsorption stability [44]. They include a variety of active functional groups, natural polysaccharides can modify the surface of GO.
2.2 Removal of functionalized polymer
A recent study examined the effectiveness of a cross-linked GO/β-CD composite in MB adsorption. To characterize the novel material, SEM, XRD, FT-IR, Raman, and TGA analyses were ran. The greatest adsorption capacity of GO/ β-CD was 76.4 mg/g, as shown in six successive processes of adsorption followed by desorption. The polymeric chain contains several active groups, which are highly sensitive, readily available, and efficiently separated, making functionalized polymer composites a hot topic. Excellent adsorption capabilities are induced in materials by combining functionalized polymers with nanoparticles. A magnetic polymer nanocomposite was described, which was created by combining β-cyclodextrin, activated charcoal (AC), sodium alginate (Alg), and Fe3O4 nanoparticles (NPs). Elements and scanning electron micrographs of the synthetic adsorbent. The adsorption investigations with the produced polymer composite, according to the Langmuir isotherm, showed an MB 10.63 mg g−1 of adsorption capacity and a rate of elution of 99.53% [7, 17, 45].
2.3 Elimination of metals
In most cases, ion exchange processes, complexation, or contact can be used to remove metals. Optimal pH and preparation method are two of several criteria that determine the adsorbent's effectiveness. Under basic circumstances, the deprotonated hydroxyl groups of the cyclodextrin polymer form a strong bond with the metal. By combining polyacids with the Poly-cyclodextrin/metal ion complex, the stability is improved in relation to environmental conditions [46]. To absorb Cu (II) ions, the electrospinning of several Poly cyclodextrin and PVA solutions into Poly-cyclodextrin/PVA fibers was done. Using scanning electron microscopy (SEM), researcher studied the fiber shape, and FTIR and TGA analyzed the physical–chemical characteristics [47]. Analyzing the adsorption of Cu2+ and Cd2+ by ICP-OES allowed them to investigate the capacity of the insoluble cyclodextrin-based fibers for removal heavy metals from wastewater. An impressive maximum adsorption capacity of 48.15 mg/g was demonstrated by poly-CD/PVA fibers, particularly with respect to Cu (II).
2.4 Elimination by using complexes
In a separate investigation, nano-sponges were created by cross-linking linecaps and cyclodextrin with citric acid, a process known as complexation. To evaluate the results, repeated the process with pyromellitic dianhydride to create another nano-sponge of pyromellitate. At a metal concentration of 500 pm, the response was recorded, and the citrate-based nano-sponges had a lower retention capability than pyromellitate. Pyromellitate adsorbent was able to remove 272 mg/g of lead and 81 mg/g of copper from the sample, according to previous research [48]. One recent development in adsorption technology is the use of dual adsorbents. Reports of single-class pollutants are common, and it is difficult to separate inorganic and organic pollutants using the same adsorbents. A study by Verma et al. revealed the use of β-CD-CS-EDTA adsorbent to remove ciprofloxacin (CIP) and heavy metals (Ni (II), Cu(II), and Pb(II)). Using elemental mapping, EDX, and FTIR, the adsorption mechanism was investigated. In comparison to the CIP, the adsorption capacity of heavy metals was much higher; specifically, 118.90 mg/g for Ni (II), 161 mg/g for Cu(II), and 330.90 mg/g for Pb(II) compared to 25.40 mg/g for the CIP. It points the way for future studies that aim to find ways to use a single adsorbent to remove both organic and inorganic contaminants from water simultaneously [49].
2.5 Removal of organic compounds
As of increasing needs of the people for resources, such as food or medicine, industrial processes are likewise progressing alongside the expansion of civilization. The manufacture of pharmaceuticals inevitably results in the creation of massive volumes of wastewater. Sewage residues will be discharged into the environment and eventually humans because of insufficient treatment, leading to long-term detrimental effects. The situation is analogous in the food and packaging sector, which manufactures food packaging using dangerous endocrine-active chemicals (EDCs) [50]. They become ingested with food when the temperature rises, as happens, for example, when food is heated. Improper waste management frequently results in the entry of harmful EDCs into water sources in the form of cans or plastic packaging. When these harmful EDCs dissolve in water, they transform into micro-and eventually nano plastic, which is harmful to a wide variety of marine and terrestrial organisms. As a result, endocrine-active substances and medications wind up in contaminated water, where they build up and eventually catch people's notice. Most of the organic contaminants included here have the characteristics of bioaccumulation, long-term water persistence, and extreme toxicity to both humans and other animals [51].
Cyclodextrins are a great material with a lot of potential for the removal of organic pollutants because they can rearrange to self-adapt their structure to that of the adsorbed pollution. Materials based on cyclodextrin were tested to find out how well they adsorb to remove organic pollutants. Prior studies have shown that cyclodextrins are helpful in eliminating medications, endocrine-active chemicals, and chemical compounds. The kinds of contaminants mentioned are removed using model molecules like ciprofloxacin, ibuprofen, carbamazepine, estrogen, nonylphenol, naproxen, and bisphenol A. Determinants of Endocrinopathy Bisphenol A, or BPA, is a commercially accessible phenolic chemical that has found widespread use since the 1960s. The chemical industry has long employed polycarbonate resins and polymers that include BPA. Among the several disorders to which exposure to bisphenol A has been associated include diabetes, obesity, metabolic syndrome, cancer, and infertility problems [52].
Mesoporous magnetic clusters coupled with β-cyclodextrin, known as CD-MG, were created, and investigated to eliminate substances that interfere with the endocrine system's operation. It was determined that 52.7 mg/g of BPA was absorbed using adsorption isotherms. Reusability test investigations conducted after four cycles also revealed an excellent grade of 84.5% for the adsorbent. Their ability to rapidly remove active endocrine chemicals from the environment is greatly enhanced by their high adsorption ability, CD-MG adsorption mechanism, and rapid process kinetics [53]. The production of porous poly (chloromethyl styrene) resin changed with β-cyclodextrin and a spongy cross-linked material, specifically PS@CM-CDP, was demonstrated in another study as an easy and environmentally friendly way to remove organic contaminants from water solutions quickly and effectively. The PS@CM-CDP that was produced had an impressive maximum adsorption capability for BPA, which exceeded 8.25 mg/g. Natural β-cyclodextrin polymers might be reused up to six times without any noticeable decrease in performance due to the resin's circular nature. The research also included examples of real-world uses of the collected data, which shed light on the water treatment column from several angles. According to the study, a cyclodextrin substance was developed, manufactured, and used in 2021 to eradicate Pb (II) and BPA, two contaminants that might coexist in water, according to the study. Fe3O4 loading and effective β-cyclodextrin grafting were confirmed by the characterization results. The material that was created, β-CD@MRHC, had remarkable magnetic characteristics that allowed effective regeneration with water, without being impacted with the adsorption of pollutants [54]. The synthesized material achieved adsorption equilibrium in just 7.5 min and exhibited remarkable adsorption efficiency, with a maximum bisphenol A uptake of 412.8 mg/g. The material also achieved Pb (II) and BPA synergistic removal by reversing their competing tendencies, which was made possible by various holding mechanisms. Results showed that the adsorbent worked well with high-efficiency magnetic recovery to remove organic matter and heavy metals from water resources at the same time. Bisphenol A from wastewater by creating silica particles using β-cyclodextrin.
2.6 Removal of pharmaceutical contaminants
Pharmaceutical contaminants from pharmaceuticals have been discovered in water sources, which harm the environment. To remove pesticides, pharmaceutical residues, and other chemicals that disturb the endocrine system from treated wastewater, many sorbents having cyclodextrin have developed. An example would be carbamazepine, a dibenzoazepine derivative that serves like mood stabilizer, anticonvulsant, or psychiatric medication, primarily for the treatment of bipolar disorder and epilepsy. The substance has been found in water samples from all throughout North America and Europe. Hydroids, stinging beetles, crabs, and green algae have all been shown to have it. It is difficult to break down this harmful substance. Studies have shown that maximum adsorption ability (Qmax) of carbamazepine by cyclodextrin material is 136.4 mg/g [55]. This is because discovery of drugs in water is not an unusual incident, as previously mentioned. Medicines like ibuprofen, ketoprofen, and diclofenac are examples of non-steroidal anti-inflammatory medicines (NSAIDs).
Cyclodextrins are utilized in pharmaceutical applications to assemble complexes with lipophilic drugs. In this sense, parental administration is another crucial factor to consider. Especially, intravenous administration of βCD molecules is preferred, just like HPβCD. Use of αCD and βCD is not recommended because of their known nephrotoxicity. One further disadvantage of giving βCD intravenously is its low water solubility, claims. Furthermore inappropriate for parenteral formulations is γ-CD, which in water-based solutions forms obvious aggregates. One of the publications explained how to use nano-filters and cyclodextrins in concert to remove these pollutants from water. Examined were many nano-filters with different thicknesses and chemical compositions. By measuring the removal of pharmaceutical residue from municipal wastewater, it was possible to calculate the maximum adsorption capacity using ibuprofen as an example. Chemical Qmax is increased by ethanol regeneration of nanofilters after use [56].
2.7 Transportation of micro pollutants
Aside from cyclodextrin-intensified biodegradation of contaminants oxidation of micropollutants adsorbed through cyclodextrin polymer (CDP) with the help of KMnO4, there are more approaches that utilize cyclodextrins for wastewater purification. Mineralizing organic pollutants into comparatively innocuous chemicals is possible by oxidation processes that rely on the in-situ production of very reactive species. Recent studies have concentrated on photocatalysis as it relates to various methods. One such method is photodegradation, which uses light energy to break down pollutants. The location of the light-sensitive bonds in the molecule determines whether cyclodextrin can facilitate or impede photodecomposition. Take, for example, how β-cyclodextrin in water-based solutions improved bisphenol A photodegradation of it while β-cyclodextrin either prevented or accelerated the photodecomposition of related pesticides (parathion and paraoxon), the medication can be protected from light, or its breakdown can be accelerated by the complex formation.
Several photocatalysts that have been altered using cyclodextrins have been detailed. As an example, graphene oxide/ŗ-cyclodextrin/titanium dioxide improved the removal of phenol and Cr (VI) [57]. Graphene thin sheets stabilized by ϗ-CD and self-assembled with a TiO2 nanolayer improved the photo blemish of methylene blue. cyclodextrin-functionalized Fe3O4/TiO2 was an effective catalyst in the photodecomposition of endocrine disrupting materials like bisphenol A and dibutyl phthalate. Photodecomposition of several organic contaminants is facilitated by titanium dioxide, which generates powerful oxidizing radicals (hydroxyl and superoxide radical ions). The catalyst's surface is where the photocatalytic reactions occur under the influence of natural or artificial ultraviolet light. The process involves immobilizing TiO2 on a surface (such as glasswool mats or ceramic plates) then treating a layer of wastewater with solar radiation to decompose organic contaminants.
Nanoparticle application (nano-TiO2) is another method for surface enhancement. Particles of this size can easily come together to create bigger clumps, which have less surface area and less catalytic activity. Aggregation occurs slowly in purified water but rapidly in environments with salts, such as municipal water systems, surface waterways, and wastewater treatment plants. γ-CD, which is adsorbed on the surface of the nanoparticles, can be utilized to stabilize colloidal TiO2 systems—at least in distilled water. The photo simulant activity of nano-TiO2 particles is improved through the adsorption of β-CD, which also improves their colloidal stability. When connected to nano-TiO2 colloids, the latter limits charge-hole recombination by contributing electrons and collecting holes. Using inclusion complexation to retain the ligands at the surface of nanoTiO2 further improves efficiency. CDs slowed down the photocatalytic breakdown of toluene. Most of the published studies have only used distilled water, even though several operational factors have already been studied (pollutant concentration, pH, irradiation period, etc.) [58].
As stated in a recent review on cyclodextrins for environmental biotechnologies, it is known that many of the chemical compounds that pollute the environment may create inclusion complexes with cyclodextrins, which increases their solubility. Organic contaminants in water may be effectively removed using micelle-clay composites in environmental rehabilitation projects [59]. In this study, the researchers investigated the possibility of combining cyclodextrins—which have been found to form inclusion complexes and layers onto negatively charged surfaces when mixed with cationic surfactants—with clays in a composite form. They follow a protocol like that described for the preparation of micelle-clay composites for the purpose of removing bacteria from water. Important matter(s) or issues(s) handled It is not possible to absorb many developing toxins directly in clays without modifying them, as they are electrically neutral.
3 Conclusion and key issues
By adsorption, cyclodextrins lower the amount of pollutants; the amount of cyclodextrin utilized is a measure of their effectiveness. Furthermore, displaying the binding capacity are the isotherms. Investigated the speed at which the adsorption process ends. Important contaminants like bisphenol A are known to form inclusion complexes with CDs. Researchers have developed and designed easily recyclable, reasonably priced filters to guarantee sustainability. Cyclodextrins and cationic surfactants, by use of cationic exchange, turn it into a new substance. Several techniques (such as UV, FTIR, and SAXS) are being used by researchers to get further understanding of the modified structure of cyclodextrins and their removal effectiveness. Complementary studies are assessing how well the different chemicals under research eliminate. There is still reason for worry about cyclodextrin stability in different settings. The elements oxygen, temperature, and the presence of other compounds can break down cyclodextrin complexes, therefore progressively reducing their effectiveness. After pollutants are removed, cyclodextrin complexes must be considered. If proper control is not taken, leftover cyclodextrins could stay in the environment and cause unexpected ecological effects. Another challenge is that cyclodextrins are reusable and regenerable. Recovering contaminants and recycling cyclodextrins with the least amount of costs and environmental impact requires effective recovery methods. The potential of unexpected side effects or interactions with non-target chemicals eventually raises safety concerns. Thorough investigation is essential to properly assess the environmental risks associated with cyclodextrin-based remediation techniques. To effectively use cyclodextrins' sustainable pollutant removal capabilities, these issues must be resolved by putting into practice technological breakthroughs, cost-cutting strategies, and thorough risk assessments.
Data availability
No datasets were generated or analysed during the current study.
References
Pandey S, Singh A, Kumar A, Tyagi I, Karri RR, Gaur R, Javadian H, Verma M. Photocatalytic degradation of noxious p-nitrophenol using hydrothermally synthesized stannous and zinc oxide catalysts. Phys Chem Earth, Parts A/B/C. 2024;133: 103512.
Lavanya G, Anandaraj K, Selvam K, Gopu M, Selvankumar T, Govarthanan M, Kumar P. Green synthesis of gold nanoparticles using macroalgae Halimeda macroloba extract and their photocatalytic degradation of methylene blue and methyl orange. Polym Adv Technol. 2024;35(4): e6383.
Ahmad W, Ahmed S, Kumar S. Facile one step microwave assisted biofabrication of Fe2O3 NPs: potential application as solar light-driven photocatalyst in the photodegradation of acridine orange. Int J Environ Anal Chem. 2024. https://doi.org/10.1080/03067319.2024.2344707.
Pandey S, Twala B, Singh R, Gehlot A, Singh A, Montero EC, Priyadarshi N. Wastewater treatment with technical intervention inclination towards smart cities. Sustainability. 2022;14(18):11563.
Vakili R, Xu S, Al-Janabi N, Gorgojo P, Holmes SM, Fan X. Microwave-assisted synthesis of zirconium-based metal organic frameworks (MOFs): optimization and gas adsorption. Microporous Mesoporous Mater. 2018;260:45–53.
Sulaiman NS, Zaini MAA, Arsad A. Evaluation of dyes removal by beta-cyclodextrin adsorbent. Mater Today Proc. 2021;39:907–10.
Ozelcaglayan ED, Parker WJ. β-Cyclodextrin functionalized adsorbents for removal of organic micropollutants from water. Chemosphere. 2023;320: 137964.
Zhou Q, Fellows A, Flerchinger GN, Flores AN. Examining interactions between and among predictors of net ecosystem exchange: a machine learning approach in a semi-arid landscape. Sci Rep. 2019;9(1):2222.
Huang X, Hadi P, Joshi R, Alhamzani AG, Hsiao BS. A comparative study of mechanism and performance of anionic and cationic dialdehyde nanocelluloses for dye adsorption and separation. ACS Omega. 2023;8(9):8634–49.
Yu Y, Yu L, Koh KY, Wang C, Chen JP. Rare-earth metal based adsorbents for effective removal of arsenic from water: a critical review. Crit Rev Environ Sci Technol. 2018;48(22–24):1127–64.
Chandra L, Jagadish K, Karthikeyarajan V, Jalalah M, Alsaiari M, Harraz FA, Balakrishna RG. Nitrogenated graphene oxide-decorated metal sulfides for better antifouling and dye removal. ACS Omega. 2022;7(11):9674–83.
Fenyvesi É, Sohajda T. Cyclodextrin-enabled green environmental biotechnologies. Environ Sci Pollut Res. 2022;29(14):20085–97.
Rakmai J, Cheirsilp B, Mejuto JC, Torrado-Agrasar A, Simal-Gándara J. Physico-chemical characterization and evaluation of bio-efficacies of black pepper essential oil encapsulated in hydroxypropyl-beta-cyclodextrin. Food Hydrocolloids. 2017;65:157–64.
Kroes R, Verger P, Larsen JC. Safety evaluation of certain food additives (α-cyclodextrin—Addendum). WHO Food Addit Ser. 2006;54:3–15.
Nitalikar MM, Sakarkar DM, Jain PV. The cyclodextrins: a review. J Curr Pharm Res. 2012;10(1):1–6.
Okasha AT, Abdel-Khalek AA, Alenazi NA, AlHammadi AA, Al Zoubi W, Alhammadi S, Ko YG, Abukhadra MR. Progress of synthetic cyclodextrins-based materials as effective adsorbents of the common water pollutants: comprehensive review. J Environ Chem Eng. 2023;11(3): 109824.
Chen J, Bao C, Chen M, Wang Y, Xu X, Yang T, Shang C, Zhang Q. β-cyclodextrin-scaffolded crosslinked poly (ionic liquid) s for ultrafast removal of multiple pollutants: insight into adsorption performance and mechanism. Chem Eng J. 2023;464: 142526.
Rizzi V, Gubitosa J, Fini P, Petrella A, Romita R, Agostiano A, Cosma P. A “classic” material for capture and detoxification of emergent contaminants for water purification: the case of tetracycline. Environ Technol Innov. 2020;19: 100812.
Mehrabi F, Dil EA. Investigate the ultrasound energy assisted adsorption mechanism of nickel (II) ions onto modified magnetic cobalt ferrite nanoparticles: multivariate optimization. Ultrason Sonochem. 2017;37:37–46.
Yadav R, Chundawat TS, Verma M, Vaya D. β-cyclodextrinbased magnetic ternary nanocomposite for efficient adsorption and photocatalytic degradation of Rhodamine Blue, Gunner 500, Cefixime, and p-Nitrophenol. J Phys Chem Solids. 2024;188: 111930.
Lu D, Xu S, Qiu W, Sun Y, Liu X, Yang J, Ma J. Adsorption and desorption behaviors of antibiotic ciprofloxacin on functionalized spherical MCM-41 for water treatment. J Clean Prod. 2020;264: 121644.
Fan H, Ma Y, Wan J, Wang Y, Li Z, Chen Y. Adsorption properties and mechanisms of novel biomaterials from banyan aerial roots via simple modification for ciprofloxacin removal. Sci Total Environ. 2020;708: 134630.
Zhou Y, Cheng G, Chen K, Lu J, Lei J, Pu S. Adsorptive removal of bisphenol A, chloroxylenol, and carbamazepine from water using a novel β-cyclodextrin polymer. Ecotoxicol Environ Saf. 2019;170:278–85.
Poulson BG, Alsulami QA, Sharfalddin A, El Agammy EF, Mouffouk F, Emwas AH, Jaremko L, Jaremko M. Cyclodextrins: structural, chemical, and physical properties, and applications. Polysaccharides. 2021;3(1):1–31.
Kataky R, Morgan E. Potential of enzyme mimics in biomimetic sensors: a modified-cyclodextrin as a dehydrogenase enzyme mimic. Biosens Bioelectron. 2003;18(11):1407–17.
Łagiewka J, Girek T, Ciesielski W. Cyclodextrins-peptides/proteins conjugates: synthesis, properties and applications. Polymers. 2021;13(11):1759.
Jiang LW, Zeng FT, Zhang Y, Xu MY, Xie ZW, Wang HY, Wu YX, He FH, Jiang HL. Preparation of a novel Fe3O4/graphite oxide nanosheet/citric acid-crosslinked β-cyclodextrin polymer composite to remove methylene blue from water. Adv Powder Technol. 2021;32(2):492–503.
Alam G, Ihsanullah I, Naushad M, Sillanpää M. Applications of artificial intelligence in water treatment for optimization and automation of adsorption processes: recent advances and prospects. Chem Eng J. 2022;427: 130011.
Liu J, Wang S, Fu J, Ding X, Zhao J. Zn2+ adsorption from wastewater using a chitosan/β-cyclodextrin-based composite membrane. J Food Biochem. 2020;44(12): e13483.
Ma J, Zhong L, Peng X, Xu Y, Sun R. Functional chitosan-based materials for biological applications. Curr Med Chem. 2020;27(28):4660–72.
Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem Rev. 1998;98(5):1743–54.
Wei JZ, Gong FX, Sun XJ, Li Y, Zhang T, Zhao XJ, Zhang FM. Rapid and low-cost electrochemical synthesis of UiO-66-NH2 with enhanced fluorescence detection performance. Inorg Chem. 2019;58(10):6742–7.
Gupta VK, Agarwal S, Sadegh H, Ali GA, Bharti AK, Makhlouf ASH. Facile route synthesis of novel graphene oxide-β-cyclodextrin nanocomposite and its application as adsorbent for removal of toxic bisphenol A from the aqueous phase. J Mol Liq. 2017;237:466–72.
Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3(12):1023–35.
Chen M, Xu P, Zeng G, Yang C, Huang D, Zhang J. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: applications, microbes and future research needs. Biotechnol Adv. 2015;33(6):745–55.
Usman M, Ahmed A, Yu B, Wang S, Shen Y, Cong H. Simultaneous adsorption of heavy metals and organic dyes by β-Cyclodextrin-Chitosan based cross-linked adsorbent. Carbohyd Polym. 2021;255: 117486.
Cai N, Larese-Casanova P. Sorption of carbamazepine by commercial graphene oxides: a comparative study with granular activated carbon and multiwalled carbon nanotubes. J Colloid Interface Sci. 2014;426:152–61.
Hamedi A, Anceschi A, Patrucco A, Hasanzadeh M. A γ-cyclodextrin-based metal–organic framework (γ-CD-MOF): a review of recent advances for drug delivery application. J Drug Target. 2022;30(4):381–93.
Jansook P, Loftsson T. Self-assembled γ-cyclodextrin as nanocarriers for enhanced ocular drug bioavailability. Int J Pharm. 2022;618: 121654.
Patil NV, Netravali AN. Cyclodextrin-based “green” wrinkle-free finishing of cotton fabrics. Ind Eng Chem Res. 2019;58(45):20496–504.
Anceschi A, Caldera F, Bertasa M, Cecone C, Trotta F, Bracco P, Zanetti M, Malandrino M, Mallon PE, Scalarone D. New poly (β-cyclodextrin)/poly (vinyl alcohol) electrospun sub-micrometric fibers and their potential application for wastewater treatments. Nanomaterials. 2020;10(3):482.
Jia S, Tang D, Peng J, Sun Z, Yang X. β-Cyclodextrin modified electrospinning fibers with good regeneration for efficient temperature-enhanced adsorption of crystal violet. Carbohyd Polym. 2019;208:486–94.
Torres-Alvarez C, Castillo S, Sánchez-García E, Aguilera Gonzalez C, Galindo-Rodríguez SA, Gabaldón-Hernández JA, Báez-González JG. Inclusion complexes of concentrated orange oils and β-cyclodextrin: physicochemical and biological characterizations. Molecules. 2020;25(21):5109.
Guo R, Wang R, Yin J, Jiao T, Huang H, Zhao X, Zhang L, Li Q, Zhou J, Peng Q. Fabrication and highly efficient dye removal characterization of beta-cyclodextrin-based composite polymer fibres by electrospinning. Nanomaterials. 2019;9(1):127.
Verma M, Hong Y, Kumar V, Bisht B, Vlaskin MS, Chae SH, Shah KJ, Kim H. Enhanced removal and stepwise recovery of inorganic and organic micropollutants from water using novel graphene oxide doped multifunctional β− cyclodextrin chitosan polymer. J Phys Chem Solids. 2023;182: 111615.
Shi S, Ocampo-Pérez R, Lv J, Liu Q, Nan F, Liu X, Xie S, Feng J. Diatomite cross-linked β-Cyclodextrin polymers: a novel vision of diatomite adsorbent for the removal of bisphenol A. Environ Technol Innov. 2021;23: 101602.
Sanaei-Rad S, Ghasemzadeh MA, Razavian SMH. Synthesis of a novel ternary ZIF-8/GO/MgFe2O4 nanocomposite and its application in drug delivery. Sci Rep. 2021;11(1):18734.
Rubin Pedrazzo A, Smarra A, Caldera F, Musso G, Dhakar NK, Cecone C, Hamedi A, Corsi I, Trotta F. Eco-friendly β-cyclodextrin and linecaps polymers for the removal of heavy metals. Polymers. 2019;11(10):1658.
Verma M, Lee I, Sharma S, Kumar R, Kumar V, Kim H. Simultaneous removal of heavy metals and ciprofloxacin micropollutants from wastewater using ethylenediaminetetraacetic acid-functionalized β-cyclodextrin-chitosan adsorbent. ACS Omega. 2021;6(50):34624–34.
Saleh IA, Zouari N, Al-Ghouti MA. Removal of pesticides from water and wastewater: chemical, physical and biological treatment approaches. Environ Technol Innov. 2020;19: 101026.
Saifi A, Joseph JP, Singh AP, Pal A, Kumar K. Complexation of an azo dye by cyclodextrins: a potential strategy for water purification. ACS Omega. 2021;6(7):4776–82.
Li X, Zhou M, Jia J, Ma J, Jia Q. Design of a hyper-crosslinked β-cyclodextrin porous polymer for highly efficient removal toward bisphenol a from water. Sep Purif Technol. 2018;195:130–7.
Gontard N, Sonesson U, Birkved M, Majone M, Bolzonella D, Celli A, Angellier-Coussy H, Jang GW, Verniquet A, Broeze J, Schaer B, Batista AP, Sebok A. A research challenge vision regarding management of agricultural waste in a circular bio-based economy. Crit Rev Environ Sci Technol. 2018;48(6):614–54.
Quesada HB, Baptista ATA, Cusioli LF, Seibert D, de Oliveira Bezerra C, Bergamasco R. Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: a review. Chemosphere. 2019;222:766–80.
Hamann E, Gruber-Vodicka H, Kleiner M, Tegetmeyer HE, Riedel D, Littmann S, Chen J, Milucka J, Viehweger B, Becker KW, Dong X, Stairs CW, Hinrichs KU, Brown MW, Roger AJ, Strous M. Environmental Breviatea harbour mutualistic Arcobacter epibionts. Nature. 2016;534(7606):254–8.
Jurecska L, Dobosy P, Barkács K, Fenyvesi É, Záray G. Characterization of cyclodextrin containing nanofilters for removal of pharmaceutical residues. J Pharm Biomed Anal. 2014;98:90–3.
Wang Z, Zhang B, Fang C, Liu Z, Fang J, Zhu L. Macroporous membranes doped with micro-mesoporous β-cyclodextrin polymers for ultrafast removal of organic micropollutants from water. Carbohyd Polym. 2019;222: 114970.
Lannoy A, Kania N, Bleta R, Fourmentin S, Machut-Binkowski C, Monflier E, Ponchel A. Photocatalysis of volatile organic compounds in water: towards a deeper understanding of the role of cyclodextrins in the photodegradation of toluene over titanium dioxide. J Colloid Interface Sci. 2016;461:317–25.
Prochowicz D, Kornowicz A, Justyniak I, Lewiński J. Metal complexes based on native cyclodextrins: synthesis and structural diversity. Coord Chem Rev. 2016;306:331–45.
Author information
Authors and Affiliations
Contributions
T. U. and M. M. wrote the entire manuscript, S. P. modifed and made changes to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Urooj, T., Mishra, M. & Pandey, S. Unlocking environmental solutions: a review of cyclodextrins in pollutant removal. Discov Environ 2, 65 (2024). https://doi.org/10.1007/s44274-024-00090-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44274-024-00090-w