Abstract
Fish and fish tissue are effective bioindicators due to their sensitivity to pollution and are frequently used for assessing aquatic ecosystem health. Establishing baseline metal concentrations in freshwater fish tissues within aquatic ecosystems is important prior to establishing industrial activities to help determine potential future industrial impacts. Historically, North-Eastern European Russia has been an area with relatively low levels of industrial development and is still in pristine condition. In this region, the noise-to-background ratio for industrial contaminants may be disproportionately high. This study measured baseline metal concentrations in freshwater fish tissues collected from three study sites (the Bolshoi Patok, Maly Patok and Kara Rivers) in northeastern European Russia as bioindicators of overall aquatic ecosystem health. Seven fish species, namely, European Grayling, Arctic Char, Whitefish, Perch, Pike, Roach, and Peled, were studied over a three-year period between 2000 and 2003. The copper (Cu), lead (Pb), cadmium (Cd), and zinc (Zn) concentrations were analysed in fish tissue samples. Metal concentrations measured in fish tissues in this study were comparable to those measured at remote sites in Alaska, the United States and Slovenia. Despite the small variation between the sampling sites, the metal concentrations were relatively low and considered to be in pristine condition. Metal concentrations measured in fish tissues in this study represent baseline conditions, which will be important for comparison against monitoring programmes should the region experience future industrial development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Establishing baseline contaminant concentrations in different ecological matrices within aquatic ecosystems is important prior to establishing industrial activities to help determine potential future industry-related impacts [1]. Effective aquatic monitoring programs rely on acquiring reliable baseline data for use in comparing future conditions [2]. Once baseline conditions are established in various ecological media, future environmental effects monitoring (EEM) data can be used to compare positive or negative effects in aquatic environments, especially when threatened by industrial development [3, 4]. Aquatic monitoring programmes are designed to measure the effects of anthropogenic activities on aquatic receiving environments and thus are crucial components of industrial development [5]. Monitoring programmes vary by industry but are designed to identify and determine the magnitude of environmental effects through biological, chemical, and physical analyses while also evaluating the efficacy of regulations for protecting aquatic ecosystem health [3, 5, 6].
The Kara, Bolshoi Patok, and Maly Patok River basins located in northeastern European Russia are very sparsely populated and comprise mostly large swaths of uninhabited natural and untouched landscapes. The Komi Republic, the area that holds large parts of both the Kara, Bolshoi Patok, and Maly Patok River basins, has a population of approximately 900,000 people, with a population density of 2.2/km2 and is mostly (80%) urban; thus, most of the area is uninhabited and undeveloped despite immense potential for extractive activities within the region [7]. Although EEMs are common within extractive industries, many remote undeveloped areas do not have readily available baseline data. The Kara, Bolshoi Patok, and Maly Patok River basins are remote and only slightly anthropogenically disturbed [8, 9]. Therefore, measuring current metal concentrations is important because baseline aquatic monitoring can help establish predictions of environmental impact assessments (EIAs) [1, 2]. Baseline studies can also identify industry-related impacts and are a cornerstone for industrial compliance as well as environmental due diligence (e.g., metal mining or pulp and paper industries) [4, 6, 10].
Historically, northeastern European Russia has been an area with relatively low levels of industrial development and is still in pristine condition, where the noise-to-background ratio for industrial contaminants may be disproportionately high [8, 9, 11]. Thus, by establishing baseline concentrations of metal contaminants, future industrial development can mitigate the effects of wastewater effluent runoff through an understanding of pre-industrialization metal concentrations. In recent decades, the Kara, Bolshoi Patok, and Maly Patok River basins (rich in oil and gas) in northeastern European Russia have been actively used for the operation of oil and gas production facilities and construction and pipeline infrastructure [8, 9]. In the Komi Republic, the number of oil fields under development doubled (from 36 to 82) between 1995 and 2012. A total of 137 oil deposits have been discovered in the region. Between 2007 and 2012, accumulated oil production increased from 470 to 536 million tons. There are 138 known natural gas fields in the Komi Republic, of which only eight were developed by the end of 2012. Although most northern oil and gas reserves have not yet been developed, the region is regarded as one of the richest mineral resource bases in Russia and is poised for increased industrial development [9, 12].
Due to increased interest in resource extraction, environmental monitoring in the region is urgently needed to help establish baseline conditions and metal concentrations. Given that the European northeast includes tundra and forest-tundra zones, long-term monitoring in both zones is needed. Environmental monitoring of regions involved in intensive industrial development involves monitoring, evaluating, and predicting changes due to anthropogenic activity in natural ecosystems and their various components [1]. The aquatic monitoring system allows us to avoid some formalization inherent in the physical and chemical assessment of the degree of technogenic pollution, and in combination with the latter, it brings closer the solution of the problem of environmental quality management. Adequate monitoring of ecosystems is based on the use of functional (for example, bioproductivity, flows of substances) and structural (composition of communities, number of species, biomass) biological parameters [13].
Fish and fish tissue are effective bioindicators due to their sensitivity to pollution and are frequently used for assessing aquatic ecosystem health [14]. Fish are versatile bioindicators because they allow for the evaluation of toxic impacts, such as bioaccumulation of metals in different tissues; histological and hematological approaches; and the detection of morphological anomalies [15]. Fish are used as bioindicators because they occupy the upper trophic structure of aquatic ecosystems and serve as important food resources for humans [16,17,18]. The goal of this study was to measure baseline metal concentrations in freshwater fish species collected from three a priori ‘pristine’ rivers within the Pechora River basin in northeastern European Russia prior to extraction of ore deposits and industrial development as bioindicators of overall aquatic ecosystem health. The selection of fish species as bioindicators was based on their abundance and availability.
2 Materials and methods
2.1 Study area
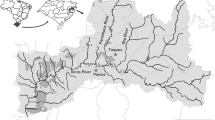
Northeastern European Russia has not been subject to widespread industrial development, but due to discovery of ore deposits, heavy industrialization in the area is poised for development (Fig. 1). The region has aquatic ecosystems that are sufficiently remote from sources of pollution such as the major coal mining city of Vorkuta and generally still preserve their pristine characteristics [9]. These pristine aquatic ecosystems include the Bolshoi and Maly Patok rivers located on the western slopes of the Subpolar and Polar Urals, as well as the Kara River located in the northeastern Bolshezemelskaya tundra (Fig. S1).
Coal deposits were discovered on the western slopes of the Polar Urals nearly two centuries ago, and in the 1920s, there was a boom in the coal industry, specifically in the Vorkuta River, which is located southwest of the Kara River Basin [19]. The largest town around the Kara River is Vorkuta (approximately 200 km to the South), an industrial town with a population of 52,500. The largest towns near the Bolshoi Patok and Maly Patok rivers are Pechora (population 43,500), located approximately 100 km northwest of Yugyd Va National Park, and Ukhta (population 99,500), located approximately 300 km west of Yugyd Va National Park. The Maly Patok and Bolshoy Patok River Basins are located > 100 km from the nearest pipelines that transport oil and gas from Siberia and the Yamal Peninsula. The basins of the Maly Patok and Bolshoy Patok rivers are located entirely within Yugyd Va National Park and are thus considered pristine [20, 21].
2.2 Characteristics of the Bolshoi, Maly Patok and Kara Rivers
The Bolshoi Patok and Maly Patok rivers (located in the North Taiga zone) are both second-order tributaries of the Pechora River, which is the largest European river draining into the Arctic Ocean. The Kara River is located to the north of the western slope of the northernmost part of the Polar Urals, eastern spurs of the Pai-Khoi range, and the outermost northeastern part of the Bolshezemelskava Tundra [22, 23]. The Kara River basin covers an area of 13,400 km2. The Kara River flows in the northernmost region of the Polar Urals and on the northeastern slopes of the Pai-Khoi ridge, located in the central part of the Ugra Peninsula. The Kara riverbed is located within the northernmost border between Europe and Asia. The general direction of the Kara River and most of its course are oriented from south to north. The Kara River (the Bolshaya and Malaya Kara Rivers) is located at 400–800 m a.s.l. Currently, the population living in the Kara River basin includes reindeer herders, fishermen and hunters who lead a nomadic seasonal lifestyle. In the last decade, the Yamal-Europe gas pipeline was constructed and now crosses the river. The Kara River Basin has a thin soil mantle that includes peat bogs and peat gleys as major soil types. Moss lichens, tundra shrubs, dwarf birches, and willows make up much of the foliage, accounting for less than 5% of the total boreal forest cover [23]. Snow is found in the river basin on average from 10th October to 5th June [24].
The Bolshoi Patok River is 121 km long with a catchment area of 2520 km2 and is the largest tributary of the Shchugor River; it is located on the western slopes of the subpolar Urals. This watercourse originated on the Research Ridge between the Orel and Kvartsitnaya peaks at an altitude of approximately 600 m a.s.l. (Fig. S1). The same area contains the sources of the Manya River (the basin of the Northern Sosva, Eastern slopes of the Ural Mountains). The basin of the Maly Patok River is located on the border of the southern part of the subpolar Urals. This watercourse is 73 km long and has a catchment area of 800 km2 [25]. Both the Bolshoi Patok and Maly Patok Rivers are located within Yugyd Va National Park. Both rivers flow in trough valleys with steep rocky riverbeds, which often form rapids and waterfalls [26]. These rivers are free from any form of human economic activity and have long been devoid of settlements and permanent populations. The variety of landscapes (mountain, plain, tundra) in this territory, the presence of many different types of mountain lakes, and the uniqueness of aquatic and terrestrial fauna help support rich aquatic and terrestrial biodiversity and pristine ecosystems [27].
2.3 Study area and fish tissue sampling
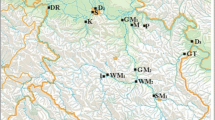
Three study sites (the Bolshoi Patok, 65°58′24″N 58°01′17″E, Maly Patok 64°18′54″N 59°04′40″E and Kara Rivers, 69°12′12.79″N, 62°36′28.03″E) were sampled for seven fish species, namely, Arctic Grayling (Thymallus arcticus), Arctic Char (Salvelinus alpinus), Whitefish (Coregonus lavaretus), Perch (Perca fluviatilis), Pike (Esox lucius), Roach (Rutilus rutilus), and Peled (Coregonus peled), over a three-year period (2000 to 2003) (Table 1).
Study sites were chosen based on previous interdisciplinary studies and biodiversity assessments of northeaestern European Russia (e.g., [28]). Due to the pristine and remote nature of the region, samples were collected opportunistically. Arctic Grayling and Arctic Char were collected from the Kara River in March 2003. Maly Patok River fish samples were collected in June 2002 and included five fish each, Whitefish, Perch, Arctic Grayling, Pike, and Roach. Bolshoi Patok River samples were collected in June 2002 and included five samples from Arctic Grayling, four samples from Perch and five samples from Peled. Fish sample collection was performed using standard ichthyofaunal methods [29, 30]. A standard set of Finnish gill nets, each 30 m long, 1.8 m high and varying in 10–60 mm mesh diameter, was used for fishing. Fish were also caught with rods and lines using spinners. During collection, the date, time, location of gear, size and composition of catches, as well as the indicators, were recorded. The following parameters were collected according to Ponomarev and Shubin [31]: fork length, standard fish length, total mass, sex and maturity. Age was determined in the laboratory by examining scales. Autopsies were performed on fish samples immediately in the field after capture according to Ponomarev and Shubin [31]. Extracted tissues were subsequently frozen at − 20 °C and transported to the laboratory until further analysis [20]. Although the limited number of samples per species is acknowledged as a limitation of this study, the relative metal concentrations in fish tissue samples will serve as a baseline for future studies should aquatic ecosystem health become impacted due to extraction of ore deposits and industrial development.
2.4 Analysis of metal concentrations in fish tissue
Samples were frozen until analysis for metal concentrations, at which point samples were processed at room temperature. Fish tissue samples were analysed for metal concentrations via atomic absorption spectroscopy in five different tissues, namely, the skeleton, liver, kidney, gills, and muscle. In total, fifteen metals were analysed, namely, copper (Cu), lead (Pb), cadmium (Cd), zinc (Zn), iron (Fe), calcium (Ca), manganese (Mn), nickel (Ni), chromium (Cr), cobalt (Co), magnesium (Mg), potassium (K), sodium (Na), aluminium (Al), and strontium (Sr), but only Cu, Pb, Cd, and Zn are presented because of their toxicity in aquatic environments [32]. The four metal concentrations presented in this study are also listed on the Agency of Toxic Substances and Disease Registry (ATSDR) substance priority list in the following rank order: Pb, 2nd; Cd, 7th; Zn, 74th; Cu, 120th [33].
Chemical analysis of the fish tissue samples was conducted in accordance with regulatory protocols of the Russian Federation. Fish tissue samples up to 0.5 g were digested by autoclaving in a microwave with a mixture of 10 mL of concentrated nitric acid and 1 mL of hydrogen peroxide. The organic matrix was digested using Minotaur-1 microwave mineralizers (Lumex group, Russia) in Teflon autoclaves at 200 °C and a pressure of 8 bars. Excess nitric acid was removed by evaporation without depressurizing the autoclave. Deionized water was added to the extract, which was cooled to room temperature and mixed. The resulting solution was transferred through a paper filter with a pore size of 8–12 microns to a plastic graduated tube. The walls of the extractor were washed with small portions of deionized water, which were subsequently transferred to the same graduated tube. The volume of the solution was adjusted to 14 mL, and the solution was thoroughly mixed. Blank samples were prepared in parallel with the analysed samples under identical conditions. At least one blank, one blind field duplicate and one laboratory duplicate was included for a maximum of every 12 samples. Metal concentrations in the digests were measured via atomic absorption spectroscopy in an air-acetylene flame and electrothermically using a Hitachi 180–60 (Japan) and a MGA-915 (Lumex group of companies, Russia), respectively.
2.5 Quality control
Certified mixtures prepared from single-element standard samples (Agilent Level III—ISO 17034 reference materials, Russia) were used for calibration. In accordance with the internal quality control methodology, the following calibration dependence characteristics were monitored: signal intensity of the idle solution, standard deviation of the blank solution intensity, coefficient of calibration dependence, and correlation coefficient (not less than 0.9998). The stability of the calibration dependences was evaluated with an acceptable deviation of no more than 10%. Verification of the accuracy of the calibration dependencies (trueness) was performed using multielement reference samples, with an acceptable deviation not exceeding 40% of the total error of the measurement method.
Reproducibility of measured metal concentrations was controlled using stock samples. Control and blank samples were analysed for every 10 samples. The accuracy of the measured metal concentrations was controlled using reference samples of fish tissues. If control standards were not met, measurements were repeated until satisfactory control results were obtained. Detection limits for Cu, Cd, Pb, and Zn were 0.1, 0.01, 0.01, and 1.0 µg/g, respectively.
2.6 Statistical analysis
Statistical analyses were performed in Minitab to assess significant differences in mean metal concentrations (µg/g dry weight, ± standard deviation, SD) between rivers for each tissue. Significant differences were determined by one-way analysis of variance (ANOVA) followed by Tukey’s test. Within each measured attribute, tissues or river sites with the same letters were not significantly different, and tissues or river sites with different letters were significantly different at the p value < 0.05 level.
3 Results
Metal concentrations measured in fish skeletal, liver, kidney, gill, and muscle tissues, for each study site are shown in Table 2. Figure 2 shows differences in Cu, Cd, Zn, and Pb concentrations in the Arctic Grayling (Thymallus arcticus) across all three study sites (except for Zn in the Bolshoi Patok River). Different letters denote significant differences for which the p value was < 0.05.
In addition, the Arctic Grayling from the Bolshoi Patok River had higher Cu concentrations in four tissues (excluding the skeletal tissue) than did the other rivers (p ≤ 0.013) (Fig. 3). Similarly, the Zn concentrations in all five tissues from the Bolshoi Patok River were significantly greater than those in the other rivers (18.88 ± 4.6 to 90.58 ± 21.6 µg/g, p = < 0.0002). Zinc concentrations in the Arctic Grayling River from the Kara and Maly Patok Rivers ranged from 4.68 ± 1.64 to 24.4 ± 5.8 µg/g and 3.06 ± 1.08 to 28 ± 7 µg/g, respectively. Although Cd concentrations varied markedly between different tissues Arctic Grayling, there were no significant differences between rivers. Lead concentrations were only measured in the Arctic Grayling in the Kara and Maly Patok Rivers and were similar for all tissues, except for the kidneys, where the Kara River had significantly greater concentrations (p = 0.01). Mean Pb concentration in Kara River kidney tissue samples was 0.40 ± 0.15 µg/g but was an order of magnitude lower in the Maly Patok River (0.03 ± 0.02 µg/g) (Table 2).
Copper concentrations in Perch (Perca fluviatilis) liver, gill, and muscle tissues from the Bolshoi Patok River (ranging from 0.83 ± 0.43 to 4.93 ± 2.27 µg/g) were significantly greater (p = 0.004, 0.005 and 0.017, respectively) than those from the Maly Patok River (ranging from 0.31 ± 0.18 to 1.9 ± 0.86 µg/g) (Fig. 3). Similarly, Cd concentrations in skeletal, gill and muscle tissues from the Bolshoi Patok River were significantly greater than those in tissues from the Maly Patok River (p < 0.02 for all three tissues) but not significantly different in liver tissues (p = 0.07). Cadmium concentrations in the Bolshoi Patok River ranged from 0.06 ± 0.03 to 0.14 ± 0.06 µg/g but were much lower in the Maly Patok River which ranged from 0.01 ± 0.003 to 0.54 ± 0.12 µg/g. Zinc concentrations in all Perch tissues from the Bolshoi Patok River were significantly greater than those from the Maly Patok River (p < 0.009 for all four tissues). Zinc concentrations in the Bolshoi Patok River ranged from 20.25 ± 4.75 to 91.95 ± 21.5 µg/g but were much lower in the Maly Patok River which ranged from 5.32 ± 1.86 to 41.6 ± 10 µg/g. Lead concentrations were only measured in the Maly Patok River Perch, so no statistical comparisons were made (Table 2).
Metal concentrations in Arctic Char (Salvelinus alpinus) tissues are shown in Table 2 and Fig. S2. Arctic Char was only sampled in the Kara River, so no statistical comparisons were made. Copper concentrations in all tissues in Arctic Char ranged from 0.32 ± 0.19 to 11 ± 3.16 µg/g, which were higher than ranges measured in Arctic Grayling and Perch in the same river. Cadmium concentrations in Arctic Char tissues were significantly lower (p < 0.005) than those in other species measured in the Kara River and ranged from 0.02 ± 0.01 to 0.22 ± 0.09 µg/g. Zinc concentrations in Arctic Char tissues ranged from 5.42 ± 1.92 to 32.2 ± 7.8 µg/g and were comparable to Zn concentrations in Arctic Grayling in the Kara and Maly Patok Rivers but were lower than the Zn concentration in the Bolshoi Patok River. Lead concentrations in Arctic Char tissues ranged from 0.04 ± 0.03 to 1.30 ± 0.41 µg/g, which are comparable to Pb concentrations in gills and muscle measured in other species from other rivers.
Mean Cu, Cd, Zn and Pb concentrations were measured in Whitefish (Coregonus lavaretus) tissues from the Maly Patok River (Table 2; Fig. S3). Mean Cu concentrations for all tissues, except kidney tissues, were comparable to those of the Grayling samples collected from the same river and ranged from 0.41 ± 0.24 to 3.08 ± 1.44 µg/g and were not significantly different. Mean Cd concentrations ranged from 0.03 ± 0.01 to 0.54 ± 0.18 µg/g and were lower than those of species from the same river but comparable to Cd concentrations measured in the Kara River, with no significant differences. Mean Zn concentrations ranged from 6.12 ± 2.14 to 82.8 ± 20 µg/g and were greater than those of species from the same river and the Kara River, but not significantly different. Mean Pb concentrations ranged from 0.02 ± 0.01 to 0.40 ± 0.17 µg/g.
Mean Cu, Cd and Zn concentrations were measured in Peled (Coregonus peled) tissues from the Bolshoi Patok River (Table 2; Fig. S4). Mean Cu concentrations ranged from 0.37 ± 0.21 to 6.83 ± 3.1 µg/g and were comparable to Cu concentrations measured in Whitefish from the Maly Patok River. In contrast, mean Cd concentrations ranged from 0.005 ± n/a to 0.75 ± 0.20 µg/g and were lower than Cd concentrations measured in Whitefish from the Maly Patok River. Mean Zn concentrations measured in all five tissues ranged from 23.05 ± 5.4 to 212.5 ± 50 µg/g and were much greater than Zn concentrations measured in Whitefish from the Maly Patok River.
Mean Cu, Cd, Zn and Pb concentrations measured in Pike (Esox lucius) tissues sampled from the Maly Patok River ranged from 0.31 ± 0.18 to 3.28 ± 1.52 µg/g, 0.004 ± 0.002) to 0.11 ± 0.05 µg/g, 11.1 ± 3.04 to 109.2 ± 25.4 µg/g, and 0.01 ± 0.005 to 0.01 ± 0.01 µg/g, respectively (Table 2; Fig. S5). Mean Cu, Cd, Zn and Pb concentrations measured in Roach (Rutilus rutilus) tissues sampled from the Maly Patok River ranged from 0.34 ± 0.19 to 20.18 ± 5.2 µg/g, 0.02 ± 0.01 to 0.30 ± 0.13 µg/g, 39.24 ± 9.08 to 180.8 ± 44.8 µg/g, and 0.01 ± 0.01 to 0.33 ± 0.17 µg/g, respectively (Table 2; Fig. S6).
4 Discussion
Until recently, monitoring of pollution loads in aquatic ecosystems in the Russian Arctic has received little attention outside of Russia. This study measured and compared baseline metal concentrations in fish species in the relatively untouched and pristine Bolshoi Patok, Maly Patok and Kara River basins. Despite small variation between sampling sites, metal concentrations were relatively low and considered to be in pristine condition. For example, metal concentrations measured in fish tissues in this study were more favorable than those measured in remote sites in Alaska in the United States and Slovenia [34, 35] (Table 3). Metal concentrations measured in fish tissues in this study represent baseline conditions, which will be important for comparison against monitoring programmes should the region experience future industrial development [1]. Background metal concentrations measured in fish tissue in this study differ markedly from those measured at sites located near metal mining and metal smelting facilities in the Russian Federation and Canada [36,37,38] (Table 3).
According to Moiseenko and Kudryavtseva [38], the Cu and Zn concentrations in whitefish and Arctic char tissues were well above the background levels measured in other studies (Table 3). The authors measured metal concentrations in Whitefish and Arctic char tissues from contaminated lakes on the Kola Peninsula, an area widely known as the most industrially developed Arctic region [39]. Pollution around the metallurgical complexes on the Kola Peninsula has been classified as severe, and the area has been described as an ‘industrial desert’ [39, 40]. Copper-nickel, iron, rare earth metals, and other ores have been mined in the region since the 1930s and peaked in the 1980s with corresponding pollution impacts to aquatic ecosystems [39, 41]. However, since the 1990s, there has been a decline in metal contaminant loads in nearby aquatic ecosystems [42]. For example, Gashkina et al. [42] reported a 2- to tenfold reduction in metal contaminant loads in the kidneys of Whitefish collected from Lake Imandra.
High concentrations of metals such as Zn, Cu, Pb, and Cd in aquatic environments can result in toxicity in aquatic biota (e.g., fish) and humans who consume contaminated fish tissues [32]. This study was designed to establish baseline metal concentrations in fish tissues to be used as a reference for comparison for future development in the region. However, there were significant differences in the concentrations of some metals among the different river metal concentrations. For example, Zn concentrations were greater in all five tissues in both perch and graylings in the Bolshoi Patok River. Aside from the varying levels of Zn, there were also significant differences found between Cu concentrations in Graylings and Bolshoi Patok River samples with elevated concentrations. This could be due to naturally elevated levels of Zn-Cu sulfide deposits in the Polar Urals where the Pechora River originates [43]. The western slopes of the northern and subpolar Urals are composed of mountain tributaries of the Pechora River, such as the Bolshoi Patok River and Maly Patok River [20]. This mountainous area comprises natural deposits that affect the Bolshoi River more than the other two regions involved in the study due to the geographic position of the river. The Bolshoi River is the southernmost tributary of the Pechora River, much like the Maly Patok River, which is located slightly further north. These rivers flow southeast to northwest, with the Bolshoi Patok River flowing into the mouth of the Pechora before the Maly Patok River. The southeastern region of the Bolshoi Patok River subjects this river to runoff from the polar mountains before the other two rivers are involved in the study, especially the Kara River, which is not a tributary of the Pechora River Basin.
Certain metals that occur naturally in geological deposits within the mountains are primarily concentrated in the Bolshoi Patok River. Deposits of Zn-Cu in the Urals are found due to bimodal rhyolite-basalt assemblages [37]. These deposits located in the Ural Mountains explain the significantly higher concentrations of both Zn and Cu in the Bolshoi Patok River (Fig. 2). Both Zn and Cu are essential elements, and elevated levels of these elements could also be attributed to varying physiological requirements between species involved in the study [37]. However, since both perch and graylings captured from the Bolshoi had higher levels of both Zn and Cu than did those from the other study sites, we can safely assume that these differences are due to the Cu–Zn sulfide deposits of the Polar Urals as well as the southeastern position of the Bolshoi Patok River.
Although cadmium concentrations were highest in the Bolshoi Patok River for Grayling muscle and in Perch skeleton, gill, and muscle tissues, these concentrations were comparable to those in pristine areas measured elsewhere (Table 3). Lead concentrations measured in all fish tissues in Kara and Maly Patok were relatively low and below the Canadian Food Inspection Agency (CFIA) guidelines of 0.5 µg/g, except for kidney tissue Pb concentrations (1.30 µg/g) measured in Arctic Char in the Kara River. CFIA tissue data are based on dry weight. Allen-Gil and Martynov [37] stated that neither Pb nor Cd has a recognized physiological function in fish but can vary naturally due to external factors such as diet. For example, Cd can be elevated in certain tissues if a species’ diet comprises primarily crustaceans [37]. Elevated Pb concentrations in kidney could also be due to the presence of metallothionein, which is a lead-binding protein present in the liver and kidney [37, 44]. While implementing data from five different tissues is a powerful tool for comparative analysis, it may not be as effective as just focusing on one tissue. Toxins and contaminants vary greatly across tissues due to each tissue’s physiological function. Kidney and liver tissues often have higher concentrations of contaminants than muscle, or skeletal tissue. Authman et al. [45] explained that metals naturally accumulate at much higher concentrations in fish kidneys and livers and may not always be adequate bioindicators for environmental monitoring studies. Thus, studies geared toward the human consumption of fish, rather than entire ecosystem health, have studied only the edible parts of fish, which are primarily muscle tissue [46].
The practice of capturing and analysing metal concentrations in freshwater fish tissue as a means of environmental monitoring is common. To quantify and assess metal concentrations in fish tissues present in these three rivers, five other similar studies were used for comparison. These studies were conducted in Sudbury, Canada, and Arctic Alaska, US. Compared to the data from other regions, the metal contaminants in the study sites measured in this study were considerably lower than those in regions that have undergone some type of industrial activity. For example, the Perch muscle in Sudbury, Ontario, Canada, had a Cu concentration of 30.64 ± 1.23 µg/g [36]; however, compared to those in the Maly and Bolshoi Patok Rivers (0.31 ± 0.18 and 0.83 ± 0.43 µg/g dry weight, respectively), the Cu concentrations in Perch muscle tissue were approximately 26 × greater in Sudbury, where Cu smelters have been operating for decades [36]. Allen-Gil et al. [35], who sampled Grayling muscle tissue in Arctic Alaska, USA, reported a Cu concentration of 1.68 µg/g dry weight, which is higher than but comparable to that of the Bolshoi Patok River (1.30 ± 0.62 µg/g dry weight). However, this amount is eight times greater than that of the Grayling muscle tissue in the Kara River and more than six times greater than that in the Maly Patok River. Evidently, areas with industrial activity will have higher concentrations of metals in surrounding ecosystems than the Maly Patok, Kara and Bolshoi Patok Rivers, as they are considered untouched and undeveloped.
Disparities between regions highlight the importance of baseline monitoring in the extractive mining industrial sector. Fish species selected for this study are extremely widespread in northeastern European Russia. Therefore, metal concentrations present in fish tissues obtained from the rivers of these protected areas of the Maly and Bolshoi Patok Rivers in Yugyd Va National Park and from remote areas of the Kara River in the Bolshezemelskaya tundra offer useful monitoring tools for comparing regions already involved in intensive economic development or those that will be commercially exploited in the foreseeable future. By establishing baseline metal concentrations using fish bioindicators via aquatic monitoring, future developers could use background levels as a point of reference in EEMs and EIAs. Environmental monitoring plays an integral role in risk management for large industrial operations where there is potential for environmental contamination; therefore, this study provides a solid foundation for sustainable management of future economic development in the Pechora and Kara River basins.
5 Conclusions
This study measured baseline Cu, Cd, Pb and Zn concentrations in Arctic Grayling, Arctic Char, Whitefish, Perch, Pike, Roach, and Peled Rivers over a three-year period between 2000 and 2003 in the Bolshoi Patok, Maly Patok and Kara Rivers in northeastern European Russia. Despite the small variation between sampling sites, metal concentrations were relatively low and considered to be pristine compared to those at remote sites in Alaska in the U.S. and Slovenia. Metal concentrations measured in the fish tissues in this study represent baseline conditions that can be used for comparison to help determine potential future industrial impacts should the region experience future industrial development. The limited number of tissue samples per fish species for each given tissue type (i.e., five) was too low to conduct correlation analyses, thus potential relationship may appear to be present even though they may not exist. Although limited metal pollution loads were detected in fish tissues sampled between 2000 and 2003 (over 20 years ago at the time of publication), much of the region remains semi-pristine allowing for any future changes in environmental pollution in aquatic ecosystems, specifically fish, to be easily recognized.
Data availability
All the data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Roach B, Walker TR. Aquatic monitoring programs conducted during environmental impact assessments in Canada: preliminary assessment before and after weakened environmental regulation. Environ Monit Assess. 2017;189(3):109.
MacKinnon, A.J., Duinker, P.N., Walker, T.R. (2018). The application of science in environmental impact assessment. Routledge. 150 p.
Kilgour BW, Dubé MG, Hedley K, Portt CB, Munkittrick KR. Aquatic environmental effects monitoring guidance for environmental assessment practitioners. Environ Monit Assess. 2007;130:423–36.
Walker TR. Environmental effects monitoring in Sydney Harbor during remediation of one of Canada’s most polluted sites: a review and lessons learned. Remediat J. 2014;24(3):103–17.
Walker SL, Ribey S, Trudel L, Porter E. Canadian environmental effects monitoring: experiences with pulp and paper and metal mining regulatory programs. Environ Monit Assess. 2003;88:311–26.
Munkittrick KR, McGeachy SA, McMaster ME, Courtenay SC. Overview of freshwater fish studies from the pulp and paper environmental effects monitoring program. Water Qual Res J Can. 2002;37:49–77.
Russian Federal State Statistics Service. (2011). 2010 Russian Census. http://www.gks.ru/free_doc/new_site/perepis2010/croc/perepis_itogi1612.htm
Walker TR, Crittenden PD, Young SD, Prystina T. An assessment of pollution impacts due to the oil and gas industries in the Pechora basin, northeastern European Russia. Ecol Ind. 2006;6(2):369–87.
Walker TR, Crittenden PD, Dauvalter VA, Jones V, Kuhry P, Loskutova O, Mikkola K, Nikula A, Patova E, Ponomarev VI, Pystina T. Multiple indicators of human impacts on the environment in the Pechora Basin, northeastern European Russia. Ecol Ind. 2009;9(4):765–79.
Hoffman E, Bernier M, Blotnicky B, Golden PG, Janes J, Kader A, Kovacs-Da Costa R, Pettipas S, Vermeulen S, Walker TR. Assessment of public perception and environmental compliance at a pulp and paper facility: a Canadian case study. Environ Monit Assess. 2015;187:1–13.
Walker TR, Young SD, Crittenden PD, Zhang H. Anthropogenic metal enrichment of snow and soil in northeastern European Russia. Environ Pollut. 2003;121(1):11–21.
Gulas S, Downton M, D’Souza K, Hayden K, Walker TR. Declining Arctic Ocean oil and gas developments: opportunities to improve governance and environmental pollution control. Mar Policy. 2017;75:53–61.
Gilyarov, M.S. Biological Encyclopedic Dictionary Edited by M.S. Gilyarov. Moscow, Soviet Encyclopedia, 1986. p. 832.
Naigaga I, Kaiser H, Muller WJ, Ojok L, Mbabazi D, Magezi G, Muhumuza E. Fish as bioindicators in aquatic environmental pollution assessment: a case study in Lake Victoria wetlands, Uganda. Phys Chem Earth A/B/C. 2011;36(14–15):918–28.
Chovanec A, Hofer R, Schiemer F. Fish as bioindicators Trace metals and other contaminants in the environment. Amsterdam: Elsevier; 2003.
Nikolsky GV. Ecology of fish. Moscow: Higher School; 1974. p. 368.
Abakumov VA. Productive aspects of biomonitoring of freshwater ecosystems. In: Productive-hydrobiological studies of aquatic ecosystems. Leningrad: Nauka; 1987. p. 51–61.
Krivolutsky DA, Shalanki Ya, Gusev AA. International cooperation in the field of bioindication of anthropogenic environmental changes. In: Bioindication and biomonitoring. Moscow: Nauka; 1991. p. 5–9.
Chernov GA. From the history of the discovery of the Pechora coal basin. Syktyvkar: Komi Publishing House; 1989.
Ponomarev VI. Aquatic fauna of water bodies in the Maly Patok River Basin (the Subpolar Urals). Fishes Inland Water Biol. 2019;12(4):384–92.
Ponomarev VI, Loskutova OA. Effect of elevation gradient on the structure of aquatic communities in the Vangyr River Basin, the subpolar urals. Russ J Ecol. 2020;51(1):72–81.
Brittain JE, Gilason GM, Ponomarev VI, Bogen J, Brors S, Jensen AJ, Khokhlova LG, Kochanov SK, Kokovkin AV, Melvold K, Olafsson JS, Pettersson L-E, Stenina AS. Arctic rivers Rivers of Europe. Amsterdam: Elsevier; 2009.
Brittain JE, Gilason GM, Olafsson JS, Ponomarev VI, Aamodt KO, Bogen J, Brors S, Jensen AJ, Kochanov SK, Kokovkin AV, L’Abee-Lund JH, Melvold K, Stenina AS. Arctic rivers Rivers of Europe. 2nd ed. Amsterdam: Elsevier; 2021.
Atlas of the Komi Republic on Climate and Hydrology (1997). Moscow, Drofa; DiK, p. 115.
Zhila, I.M., Alyushinskaya, N.M. (1972). Resources of surface water of the USSR. Volume 3. The Northern Region. Edited by I.M. Zhila and N.M. Alyushinskaya. Leningrad, Hydrometeoizdat, p. 664.
Kemmerich AO. Academy of sciences hydrography of the northern circumpolar and polar. Urals Moscow: Publishing House of the USSR; 1961.
Ponomarev, V.I. Maly Patok River Basin: Wildlife. Edited by V.I. Ponomarev. 2007. Syktyvkar, p. 216
International scientific cooperation. Tundra degradation in the Russian Arctic (TUNDRA). 2024. https://ib.komisc.ru/en/international-scientific-cooperation/1088-tundra
Pravdin IF. Guide to the study of fish (mainly freshwater). Moscow: Food Industry; 1966. p. 376.
Mina MV, Klevezal GA. Animal growth. Moscow: Nauka; 1976. p. 292.
Ponomarev, V.I., Shubin, Y.P. Holistic estimation of the Timan Uhta river (tributary of the Pechora) ichthyofauna state. Proceedings of the Komi Science Centre, Ural Division, 142, Russian Academy of Sciences, Syktyvkar. 1995. P. 20–29.
Carolin CF, Kumar PS, Saravanan A, Joshiba GJ, Naushad M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: a review. J Environ Chem Eng. 2017;5(3):2782–99.
ATSDR (Agency for toxic substances and disease registry). ATSDR's substance priority list. 2019. https://www.atsdr.cdc.gov/spl/index.html.
Bajc Z, Gacnik K, Jencic V, Doganoc D. The contents of Cu, Zn, Fe, and Mn in slovenian freshwater fish. Slov Vet Res. 2005;42(1/2):15–21.
Allen-Gil SM, Gubala CP, Landers DH, Lasorsa BK, Crecelius EA, Curtis LR. Heavy metal accumulation in sediment and freshwater fish in US Arctic lakes. Environ Toxicol Chem Int J. 1997;16(4):733–41.
Bradley RW, Morris JR. Heavy metals in fish from a series of metal-contaminated lakes near Sudbury, Ontario. Water Air Soil Pollut. 1985;27(3):341–54.
Allen-Gil SM, Martynov VG. Heavy metal burdens in nine species of freshwater and anadromous fish from the Pechora River, northern Russia. Sci Total Environ. 1995;160:653–9.
Moiseenko TI, Kudryavtseva LP. Trace metal accumulation and fish pathologies in areas affected by mining and metallurgical enterprises in the Kola Region. Russia Environ Poll. 2001;114(2):285–97.
Moiseenko, T.I., Dauvalter, V.A., Lukin, A.A., Kudryavtseva, L., Iliashuk, B., Iliashuk, E., Vandysh, O., Sharov, A., Sharova, Y., Koroleva, I. (2002). Anthropogenic modifications of the Imandra Lake ecosystem. Science: Moscow, Russia, p. 1–403.
Reimann C, Banks D, Caritat PD. Impacts of airborne contamination on regional soil and water quality: the Kola Peninsula. Russia Environ Sci Technol. 2000;34(13):2727–32.
Pavlova AS, Kashulin NA, Denisov DB, Terentev PM, Kashulina TG, Dauval’ter VA. Distribution of chemical elements between the components of the ecosystem of arctic lake Bolshoy Vudyavr (Khibiny, Murmansk oblast). Contemp Probl Ecol. 2019;12:280–97.
Gashkina NA, Moiseenko TI, Kudryavtseva LP. Fish response of metal bioaccumulation to reduced toxic load on long-term contaminated Lake Imandra. Ecotoxicol Environ Saf. 2020;191: 110205.
Prokin VA, Buslaev FP, Nasedkin AP. Types of massive sulfide deposits in the Urals. Miner Deposita. 1998;34(1):121–6.
Klaassen, C.D. (1986). Distribution, excretion, and absorption of toxicants. Casarett and Doull's Toxicology. The Basic Science of Poisons.
Authman MM, Zaki MS, Khallaf EA, Abbas HH. Use of fish as bioindicator of the effects of heavy metals pollution. J Aquacul Res Develop. 2015;6(4):1–13.
Storelli MM, Marcotrigiano GO. Fish for human consumption: risk of contamination by mercury. Food Addit Contam. 2000;17(12):1007–11.
Canadian Food Inspection Agency (CFIA) (2011). Canadian Guidelines for chemical contaminants and toxins in fish and fish products. http://www.inspection.gc.ca/english/fssa/fispoi/man/samnem/app3e.shtml (March, 2021).
Acknowledgements
The authors thank Lyudmila Adamova and Natalia Badulina of the eco-analytical laboratory for analysing fish tissue metal data.
Funding
Funding was provided by the NSERC Discovery Grant RGPIN-2018-04119 to T.R.W.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Claire Hughson, Vasily I. Ponomarev, Boris M. Kondratenok, and Tony R. Walker. The first draft of the manuscript was written by Claire Hughson and Tony R. Walker, and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Fish samples were collected and processed according to standard methodology developed in the Laboratory of Ecology of Aquatic Organisms, Institute of Biology, Komi Science Centre (KSC), Ural Division, Russian Academy of Sciences (RAS), Syktyvkar, Komi Republic, Russian Federation. The protocol for fish sampling and fish analysis was approved by the Institute of Biology, KSC, animal ethics review committee in accordance with the Institute of Biology, KSC, animal ethics guidelines and regulations which ensures the procedures are within established guidelines and are humane.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare that they are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hughson, C., Ponomarev, V.I., Kondratenok, B.M. et al. Metal concentrations in fish tissues from Kara, Bolshoi Patok, and Maly Patok River basins, Northeastern European Russia. Discov Environ 2, 27 (2024). https://doi.org/10.1007/s44274-024-00058-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44274-024-00058-w