Abstract
The presence of heavy metals in agricultural soils has become a critical concern in the face of increased environmental scrutiny, highlighting the relationship between human and natural impacts on our land. This study focused on examining heavy metal contamination levels including Copper (Cu), Chromium (Cr), Cobalt (Co), Lead (Pb), Iron (Fe), Zinc (Zn), Nickel (Ni) and Manganese (Mn) conducting an ecological risk assessment in the Morigaon district's paddy fields, which are characterized by disturbed soils. Undisturbed playground soils of the Morigaon district were taken as control. Based on the averages of all locations and the corresponding contamination factor (Cf) for paddy field, it was found that the soil's Cr (0.56 to 0.84), Fe (0.11 to 0.13), Mn (0.38 to 0.78), and Zn (0.35 to 0.65) contamination is low, with Cf < 1 for all seasons. Observed levels of Cu, Ni, and Pb showed moderate contamination throughout seasons, with contamination factors (Cf) ranging from 1 to 3. Meanwhile, Co exhibited a greater amount of contamination in the disturbed soil, with Cf ranging from 3 to 6, indicating significant contamination. Higher degree of contamination (CD) of the sampling sites (10.71 to 14.72) might have been due to metal contamination, especially Co, Ni and Pb. Undisturbed soil showed a comparatively lesser degree of contamination because of an absence of physical or chemical disturbances. In particular, Ni contents of disturbed and undisturbed sites were excessively higher than the worldwide average. Significant variations from global averages were particularly noted for Co and Pb. Conversely, Cr, Mn, and Zn demonstrated minimal variations when compared to these averages. Additionally, metrics such as Enrichment Factors (EF), Potential Ecological Risk Index (PERI) and Ecological Risk Index (Er) were elevated in the disturbed soils relative to their undisturbed counterparts. The findings indicated that anthropogenic activities have significantly negatively influenced the Morigaon district paddy field's soil quality and agriculture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil contamination with heavy metals (HMs) is a pervasive environmental issue around the world, attributed to their enduring, toxic, and bioaccumulative characteristics [1, 2]. HMs accumulation not only diminishes the quality and yield of agricultural goods but also deteriorates soil quality and affects the soil's physical/chemical attributes directly [3, 4]. Over the last several decades, industrial expansion, urbanization, and agricultural techniques have resulted in HMs accumulation, or potentially harmful components in soil [5,6,7,8]. The eventual destination and consequences of such pollutants are determined by several geo-environmental factors, such as the parental rocks' composition and the kinds of operations humans engage in, such as agricultural chemicals [9].
Higher HMs levels in soil can increase the possibility of plant uptake of these metals [10]. As a result, extensive risk assessments of heavy metal accumulation in farmlands are required to ensure the safe use of inorganic fertilizers, organic waste, and pesticides during crop production [2]. While metals like Mn, Fe, Cu and Zn are required in trace levels for biological processes, others, like As, Cr, Cd and Pb, can be toxic even at low concentrations, posing significant dangers to plant, animal, and human health via the food chain [3, 8, 11, 12]. Manganese and iron are both more prevalent metals in the Earth's crust, and they are frequently found in oxides and hydroxides of Fe/Mn. These oxides and hydroxides are crucial for determining whether certain heavy metals will precipitate or dissolve in soil [13,14,15]. Conversely, metals like Zn and Cu are frequently added to animal feeds for their growth-promoting and antimicrobial benefits, with the majority typically being expelled through animal waste [16].
The presence of HMs in farm soils might harm public health via the consumption of contaminated crops and continual exposure to soil particulates [17,18,19]. HMs in soil can become concentrated in less soluble forms, penetrate soil solutions, and leach into groundwater, reducing water quality [20, 21]. These metals are xenobiotic (foreign to biological systems), have great mobility, and are persistent in the soil environment [22]. They are derived from soil, enter the food chain, and undergo biomagnification at higher trophic levels, causing metabolic abnormalities in the human body [19, 23]. As a result, they might jeopardize food safety and numerous biological aspects, as well as pose a risk to people's health [24]. The environmental concerns associated with HM concentrations in habitation land-use patterns, which are changed by various anthropogenic influences, should be assessed using conventional procedures [25]. Environmental risk evaluation is one of the prime methods for separating soil pollution from background levels of HMs [7, 8, 26]. Simultaneously, the use of several metrics, such as enrichment factor (EF), contamination factor (Cf), degree of contamination (CD), ecological risk index (Er), and potential ecological risk index (PERI), is imperative and advantageous in the determination of elemental contamination in soils [7, 8, 25].
The Morigaon district in Assam is predominantly an agricultural land area (out of the geographical location of 1,55,100 ha, 81% of the site is utilized as crop area), i.e., the soil-rice system, and villagers use a diverse range of agrochemicals for agricultural output. The Morigaon district is the backbone of the Assam state for agricultural production. Thus, the safety of soil used for agriculture has emerged as a critical concern. Despite India’s status as a preeminent producer and consumer of grains, there have been limited studies assessing HMs contamination in the soil-rice systems within the country [1, 27]. Additionally, most of the research focused only on specific metal pollution [3, 28, 29]. Further, not a single study comprehensively addressed the ecological risk assessment from the perspective of public health concerns. This work represents the first documentation of HMs pollution in the Morigaon district's soil-rice environment. Regional field evaluation is critical due to the diversity of soil-rice interactions in soil-rice ecosystems and its superior practical usefulness [3].
Hence, the study's primary objectives were to (i) evaluate HMs levels (Co, Cr, Cu, Fe, Mn, Ni, Pb, and Zn) within the agricultural soils used for rice cultivation (disturbed soil) of the Morigaon district in Assam, in comparison to undisturbed soils (soils from playgrounds); (ii) comparative evaluation of contamination status based on pollution measurement indices and (iii) comparative assessment of potential ecological risk. The output of the investigation can be utilized to formulate sustainable agricultural strategies for rice cultivation in acidic soils.
2 Materials and Methods
2.1 Study area
The Morigaon district, nestled in the state of Assam, India, is characterized by its predominantly rural landscape that stretches across the southern bank of the mighty Brahmaputra River, known for its significant role in agriculture and biodiversity in the region. The communities of Karbi-Anglong, Nagaon and Kamrup form the southern, eastern and western boundaries, respectively, and the Brahmaputra makes up the northern boundary. Alluvial plains cover a significant chunk of the landmass and are highly fertile, while wetlands, rivers, creeks, and marshy lands can be found throughout the district. Out of the geographical area of 1, 55,100 ha in the community, 81% can be described as the net crop area. The area under forest is 13% of the total geographical location [30]. Agriculture is a crucial component of the district’s economic framework, with approximately 80% of the rural population relying on it as their primary source of livelihood. The community has a subtropical climate, with a warm, humid and wet summer and a moderately cool and dry winter. The district experiences abundant yearly precipitation, with an average of 1319 mm. Most of this precipitation falls between June and September during the monsoon season, making up around 75% of the entire amount of precipitation. The average high temperature fluctuates between 32 and 38 ºC, while the average low temperature falls between 8 and 9 ºC. Based on physiography, soil quality, farming system, crop and cropping system and hydrological information, the district Morigaon could be classified into Alluvial flood zone, Alluvial flooded area and Char (Riverine sandy strip). Additionally, this district is more productive concerning agricultural output.
2.2 Soil sampling
For three years, samples of soil were taken twice a year, from 0 to 15 cm depth, in the hot, humid summer months of June and July, and the cold and dry winter months of December and January, from every location. Sampling was conducted during the summer (S1, S2, S3) and winter (W1, W2, W3) across the three-year period. At each location, at least three soil samples were collected in polythene bags from within a 1 square meter area from different points and were taken to the laboratory. There, the air-dried soil was broken down, spread out on white paper, and combined to create a homogenous composite sample. Once dried, the soil was sifted through a 2 mm sieve to obtain the fraction needed for analysis and then sealed in polythene bags.
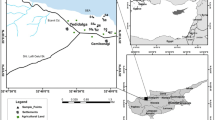
Every season, a total of thirty sites were routinely sampled: 25 samples from rice cultivation fields [tilled land or disturbed soil within the soil-rice system, designated as T1 to T25] and five samples (NT1 to NT5) from non-tilled areas or undisturbed soil, predominantly playgrounds. The sampling locations are depicted in Fig. 1, and specific details about each location are provided in Table 1.
2.3 Estimation of soil physicochemical characteristics
2.3.1 Soil texture
The Bouyoucos hydrometer method was employed to analyze soil texture. Initially, 50 g of soil dried in an oven were passed through a 2 mm sieve. The sieved soil then treated with 50 ml of a 6% hydrogen peroxide solution to break down organic particles. To decompose organic material, 50 ml of 6% hydrogen peroxide solution was added to the sieved soil. The surplus hydrogen peroxide was then dissipated by heating in a water bath. Once the mixture cooled to room temperature, 100 ml of 5% Sodium hexametaphosphate solution was introduced, and the solution was agitated using an electric stirrer for 10 min before being poured into a 1-L measuring cylinder. The soil suspension was then tested with a hydrometer, noting readings at the 4-min mark and again after 2 h. This procedure allowed for the calculation of samples' sand, clay and silt proportions, and the evaluation of the soil texture category based on the International Soil Science Society's (ISSS) texture triangle [31].
2.3.2 Soil pH and electrical conductivity
Using a digital pH tester (Elico101E), the pH of the soil was determined in 1:5 soil–water suspensions [14]. Similarly, soil electrical conductivity was measured with a conductivity bridge (Labtronics LT-16) also in 1:5 soil–water suspensions [14].
2.3.3 Soil organic carbon (SOC)
The SOC was analyzed following Walkey-Black Rapid Filtration method [31]. Briefly, 1.0 g soil is oxidized with K2Cr2O7 and concentrated H2SO4, followed by dilution with 200 ml distilled water. This was followed by adding 10 ml of 85% H3PO4 and diphenylamine indicator (1 ml), and the solution was titrated with 0.5N FeSO4(NH4)2SO4.6H2O aqueous solution. The SOC was calculated from the following relation:
where B = Vol. of 0.5 N FeSO4(NH4)2SO4.6H2O solution used for blank titration.
S = Vol. of 0.5 N FeSO4(NH4)2SO4.6H2O solution used for sample titration.
W = Wt. of the soil, and 1.3 is a correction factor.
1ml of 1N dichromate is equivalent to 3.0 mg of carbon = 0.003 g of org. C
2.4 Soil heavy metals (Co, Cr, Cu, Fe, Mn, Ni, Pb and Zn) estimation
To determine the total amounts of trace metals present in soil samples, extraction was done from 1 g of soil with a mixture of concentrated nitric, hydrochloric and sulphuric acids in 2:1:4 proportions and keeping the variety overnight following the methodology described by Adhikari and Bhattacharyya [14]. Following that, the mixture underwent filtering using Whatman 45 and was diluted to a level of 100 millilitres using Milli-Q water. Finally, metals quantity was analyzed in an Atomic Absorption Spectrophotometer (PerkinElmer Analyst 200) using air acetylene flame described by Adhikari and Bhattacharyya [14]. The AAS was individually calibrated employing a minimum of 3 standard solutions for every metal prior to measurement. The determined concentrations were converted to mg kg−1 using the formula:
2.5 Quality assurance and quality control
Calibration curves were generated to ascertain the concentration HMs levels present in the sample solutions. Standard solutions of intermediate concentration (100 mg/L) for each metal were made by diluting stock solutions with a concentration of 1000 mg/L for each metal. The process of dilution using distilled water produced the metal solutions for suitable working standards. To improve sensitivity and set appropriate working standards, the samples were measured according to the instructions in the instrument operation handbook. The absorbance values of each sample were then recorded. Calibration curves were constructed by plotting absorbance against concentrations (mg/L) of several metal standards. Following the calibration process utilizing the standard solutions, the sample solutions were introduced into the device, and an immediate measurement of the metal concentrations was obtained.
During the investigation, analytical grade (AR) substances were utilized without additional purification. All of the reference materials and chemicals were purchased from Merck India. De-ionized water was utilized for preparing all solutions and calibrating standards. Each evaluation was performed three times. Using NIST reference material in triplicate, the analytical procedures' accuracy and precision were examined (Department of Commerce, USA). It was found that the procedure's recovery and precision were 88% to 110% and less than 4%, respectively.
2.6 Data analysis and statistics
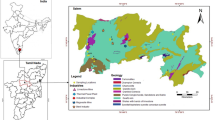
Different data were spatially plotted and visualized. IBM SPSS Statistic 26.0 were used for analyzing the data. By applying Pearson Correlation Analysis, the relationship between different variables was ascertained. The main components (PCs) across sampling sites were ascertained using principle component analysis (PCA), which was also used to evaluate regional differences in soil and potential metallic origins. Further, the various patterns of selected heavy metals were identified by mapping soil parameters using the Inverse Distance Weighted (IDW) interpolation protocol. The IDW method operates under the assumption that objects or phenomena nearby exhibit greater similarity compared to those located at greater distances. To estimate a value at a location where no measurements have been taken, the IDW method uses the measured values in the vicinity of the prediction site. The predicted value is more heavily influenced by the measured values that are in close proximity to the prediction location, as opposed to those that are located further away. Higher weight is assigned by the interpolation to those points closest to the predicted location; the weightings decrease with increasing distance. The interpolation was carried out in ArcGIS software and spatially visualized. This interpolation method was chosen for geographical visualization since it is a widely used approach in GIS and gives a smooth depiction of the data distribution. It enables the identification of locations with more significant or lower concentrations of heavy metals, which is critical for determining the degree of pollution and possible exposure concerns in various places.
2.6.1 Assessment of pollution indices
2.6.1.1 Contamination factor (Cf)
The Cf is determined by following formula,
where Ci is mean HM level and Cref concentration of the selected reference back ground [32]. Categories of contamination levels as [33]: low degree (Cf < 1), moderate degree (1 < Cf < 3), considerable degree (3 < Cf < 6) and very high degree (Cf > 6).
2.6.1.2 Degree of contamination (CD)
The following equation is utilized to evaluate CD of the soil suggested by Hakanson [33]. CD (total Cf of all the metals) is calculated by following formula,
Based on Hakanson [33] equation categorization of CD are as follows: lower contamination degree (CD < 6), moderate contamination degree (6 < CD < 12), considerable contamination degree (12 < CD < 24) and high degree of contamination (CD > 24).
2.6.1.3 Enrichment factor (EF)
The EF is employed to determine anthropogenic origin of HMs into soil [35]. EF is calculated as,
Fe is used as a normalization element because of its uniform abundance in soil and Fe being mostly non-anthropogenic in origin [36]. Sakan et al. [37] have shown that the EF could be interpreted as enrichment, zero (EF < 1), minimal (1 < EF < 3), moderate (3 < EF < 5), moderately high (5 < EF < 10), high (10 < EF < 25), very high (25 < EF < 50) extremely high (EF > 50).
2.6.1.4 Potential ecological risk index
HMs ecological risk was measured in soils [38] using the potential ecological risk index (PERI) proposed by Hakanson [33]. A more thorough assessment of the possible risk associated with soil concentrations of heavy metals is achieved by utilizing this technique to ascertain the integrated ecological and environmental impact in conjunction with toxicological [39]. This index is calculated from the following equations.
where, Tr is each metal’s toxic response coefficient standard values of Cr = 2, Mn = 1, Ni = 5, Co = 5, Zn = 1, Cu = 5, Pb = 5 [33, 34, 40, 41] and Cf = contamination factor.
Based on Hakanson [33] the ecological risk factor (Eri) is classified as: lower risk (Eri < 40), moderate risk (40 < Eri < 80), considerable risk (80 < Eri < 160), higher risk (160 < Eri < 320), very serious risk (Eri > 320). Similarly, according to Hakanson [33] the PERI rated as, low risk (PERI < 95), moderate risk (95 < PERI < 190), considerable risk (190 < PERI < 380) and very higher risk (PERI > 380).
3 Results and discussion
3.1 Physicochemical characteristics and correlation with metal
The physicochemical properties of the soil samples of disturbed (rice paddy field) and undisturbed soil are presented in Table 2. Additionally, Tables 3 and 4 summarize the correlation studies between the examined trace metals and particular soil parameters of disturbed and undisturbed soils, respectively.
The retention and release of trace metals are predominantly regulated by soil texture [14]. The textural analysis results showed the sand predominance in all sites, with a marked increase in the undisturbed soil. Disturbance in terms of agricultural practices has served to change the texture by decreasing the sand content. Correspondingly, there is an increase in the clay content (i.e., finer-grained soils) of the agricultural soil samples. Finer-grained soils have a larger surface area and a net negative surface charge than their coarser-grained equivalents, which helps with the trace metals' electrostatic sorption [42]. Increased soil aggregation and soil organic carbon retention are traits of heavy soils. Aggregates in the soil impede the motion of solutes. Soil aggregates absorb both water and solutes due to capillary action at work. These forces increase with soil texture fineness, soil organic carbon content, and soil dry phase [14]. Sand dominance of the soil is likely to induce water deficit during the growing season and may affect the earth's productivity. Textural composition for cultivated alluvial soil at 0–15 cm depth, reported earlier from Tezpur, Assam (Sand; 60.19%, Silt; 21.10% and Clay; 18.80%) agrees with those found in this work [43]. Usually, sand-dominated soils typically exhibit spatial variation at different scales in terms of morphology, chemistry, and ecology [44]. Therefore, it is expected that there will be appreciable spatial variation in the soil quality from one site to another.
The soil is acidic at all the sites in conformity with the normally paddy-growing Assam soil. The acidifying effect of inorganic fertilizer, lower buffering capacity and minimal soil organic content impact on acidic nature of soil [45]. Further, lower soil pH might render heavy metal(loids) more mobile and soluble, which increases their uptake by plants because of their comparatively higher accessibility [46]. As a whole, the research location's acidic soil (acidic pH) might have a considerable influence on the accessibility of HMs in soil and its uptake by plants [3]. It has also been reported that the soil acidity in North-Eastern region of India is mainly due to the more rainfall, with other management factors (e.g.: chemical fertilization, being comparatively minor influence) [47]. Additionally, the combination of anaerobic and aerobic environments created by the sporadic flooding and non-flushing situations contribute to the complexity of the soil-rice system, which in turn influences the metals’ accessibility and intake in a direct/indirect manner [3, 14]. The surface soil of Tripura, India, has also been found to be acidic in nature (pH: 4.5–5.4) favouring rice-based cropping systems [48]. For the disturbed soil, the data for the three years from 25 rice cultivated soil sites yield a mean SOC varying from 0.68 to 1.67%, a comparatively wide range. For the undisturbed soil, the mean SOC values went from 0.87 to 1.17%. Soil organic carbon in soil provides the metals with appropriate chelating agents such that the trace metals can be held in complex forms and made available to the plants [49].
The EC for the disturbed soil ranges from 0.05 to 0.33 mS cm−1, and that for the undisturbed soil from 0.12 to 0.22 mS cm−1, i.e., non-saline soil containing dissolved ions [3]. Identical outputs reported by Kettler et al. [50] and Obade and Lal [51] have measured electrical conductivity in a range of 0.1 to 1 mS cm−1 relying on the cropping practices and soil particle size. Clay-rich soils have high electrical conductivity compared to sandy soil. In the present work, the soil had much lower values of EC, as the soils are not clay-rich but sand-dominated.
This study observed a negative association between chromium (Cr) and pH, with correlation coefficients of − 0.52 and − 0.74 for disturbed and undisturbed soil, respectively. These findings support the idea that factors beyond pH, such as other parameters and anthropogenic influence, have a role in determining the availability of chromium in soil. Nevertheless, a noteworthy negative association was identified between pH and the levels of cobalt (Co), iron (Fe), and nickel (Ni) in undisturbed soil. Several researchers have discovered a negative association between soil pH and metal’s mobility and availability [3, 52, 53]. A noteworthy inverse correlation between Fe and pH suggested that sediments with lower pH levels had more Fe availability. The results align with what Rahman et al. [53] found for Bangladeshi paddy soils. Numerous investigations have demonstrated a comparatively larger accessibility to soluble Fe at lower soil pH [52, 54]. The increase in soil acidity is associated with an elevated HMs mobility and bioavailability [55]. Consequently, this facilitates the uptake of heavy metals by plants, posing a possible health risk to humans. Furthermore, a report has suggested that soil pH is the primary factor influencing the solubility and mobility of chromium (Cr), lead (Pb), and zinc (Zn) in sandy soils with a light texture [56].
The best correlation is found between SOC and Ni. SOC is positively correlated with Ni for both the disturbed soil (r = + 0.87) and the undisturbed soil (r = + 0.97). It suggests that SOC has a substantial impact on the retention of Ni by the soil, independent of whether the soil is tilled or non-tilled and whether it is summer or winter. SOC is significantly correlated with other metals for the tilled soil, but it shows a positive correlation for the undisturbed soil with Co (r = + 0.78), Cr (r = + 0.65), Fe (r = + 0.77) and Zn (r = + 0.67). A notably significant positive connection (p < 0.05) was discovered in agricultural soil between the elemental pairings Cu–Zn (0.73) and Fe–Mn (0.73), suggesting that these metals had comparable origins preferably by human activities [3]. On the other hand, elemental pairing Cu–Mn (− 0.93) and Zn–Mn (− 0.71) had a negative correlation in tilled or agricultural soil. To completely understand the physicochemical mechanisms behind metal attachments, mobilization, and destiny of chemicals in the studied region, more investigation is necessary (Tables 3 and 4).
Plotting of the data on the positive and negative sides of PC1 and PC2 is presented in Fig. 2. Plotting elements in close proximity to one another indicates a strong connection, while plotting them at a greater distance indicates a weaker relationship, with the exception of the parameters which are placed diametrically opposite to one another, which denotes a low correlation [57]. Twenty-five samples with fourteen parameters showed five significant eigenvalues, PC1 and PC2, which accounted for twenty-eight and twenty-four percent, respectively for disturbed soil. PC3 showed 13% variability, however PC4 and PC5 showed ten percent and eight percent variability. The Eigenvalues larger than 0.5 were found in first five components. A significant connection is believed to exist when the factor loading value is close to + / − 1. It is considered significant if value is higher than 0.5. PC1, which is displayed in Table 5, shows 35% variability regarding significant loadings for EC, SOC, clay, sand, Cu, Ni, Mn and Zn. Significant loadings of Fe, Mn, Pb, Cu and pH were linked to 24% variability in PC2. EC and silt are loaded into PC3, whereas PC4 is packed with silt, Cr and Pb. PC5 loaded with Co. PC1 showed notable cations from both natural and anthropogenic sources, as shown by systematic data analysis. On the other hand, five samples with fourteen parameters showed three significant eigenvalues, PC1 and PC2, which accounted for sixty-three and fifteen percent, respectively for undisturbed soil. PC3 showed 15% variability. PC1, which is displayed in Table 5, shows 63% variability regarding significant loadings for EC, SOC, clay, Co, Zn, Cr, Fe, Cu, Ni, sand, pH and Mn. Significant loadings of silt and Pb were linked to 15% variability in PC2, while pH and Cu are loaded into PC3.
3.2 Comparison of the metal concentrations with the world averages
The level of HMs found in this work is compared to the global average data provided by Kabata-Pandias [58]. The concentration of Ni is excessively higher than the worldwide average. The investigation's measured mean concentrations of Ni and Fe were more significant compared to those found in the rice soil of Haryana [27] and Murshidabad district, West Bengal [59]. The average value of all HMs in soil samples was somewhat less than the comparable baseline values in reference soil.
The pH is a vital soil parameter influencing the availability of Ni [60]. Low pH levels in soil may rise Ni toxicity in soil-grown plants as more Ni would go into the soil solution [61]. Lower deviations from the world averages are observed for both all-season averages and the all-site averages of Cr, Mn and Zn. The lowest concentration of Zn in agricultural sandy soil was found by Baize [62]. A lower concentration of Mn is very rare for agricultural soil. Flooding, however, can potentially raise soil pH and reduce Mn uptake [63]. Since the overall Mn content of acid soils is often low, plants are typically not exposed to Mn toxicity [64]. The levels of Cu, Ni, and Pb may be greater than the global norms due to the overuse of various pesticides and fertilizers, particularly phosphate fertilizers [65].
3.3 Ecological risk assessment through pollution indices Contamination factor (Cf)
3.3.1 Contamination factor
The contamination factors of the disturbed soil and the undisturbed soil are presented location-wise in Table S1 and Fig. 3 and season-wise in Table S2 and Fig. 4. The CD and Cf are employed to investigate contamination level and the impact of human activity on soil quality by individual elements [66]. The mean values taken from the data for all the seasons indicate that Cf < 1.0 for Cr, Fe, Mn and Zn for both types of soil and therefore, the soil has deficient contamination levels of these four metals. The disturbed soil and the undisturbed soil are moderately contaminated concerning Cu and Pb (1 < Cf < 3) for all seasons and sites. In the case of Co and Ni, all the areas (disturbed and undisturbed) have considerable pollution levels (3 < Cf < 6) (Fig. 5). Cr, Fe, Mn and Zn contamination of the soil is low with C f < 1 for all the seasons (Table S2). Moderate contamination is observed with Cu, Ni and Pb in most seasons (1 < Cf < 3). Soluble Fe–Mn oxide proportion from deposition of home, industrial, and agricultural sewages and increased sedimental persistence of soluble oxide account for the frequently elevated Pb levels detected in this study compared to Ni and Cu [67]. The soil has considerable contamination (3 < Cf < 6) from Co, particularly for the disturbed soil.
The higher Cf observed in the summer (rainy) season is also compatible with the data of Naveedullah et al. [68], who determined agricultural soil contamination due to HMs from Yuhang County, Zhejiang, China. Higher Cf value in the summer season may be due to input of the metals from stormwater discharge and severe erosions by the runoff [69]. Morrison et al. [70] have reported that the poor vegetation cover of the soil can be a contributing factor to Ni-accumulation in the soil. Cobalt is quickly taken up by organic matter in the soil, increasing its content in the soil [71].
It was reported that Zn availability concerning Cf are lower than reference values in all sites and seasons. The paddy fields are generally known for deficiency of Zn throughout Asia [72]. It is suggested that under constantly flooded conditions, the formation of the relatively insoluble zinc sulfide is promoted under strongly reducing conditions, decreasing the available Zn contents. Opper is usually related to applying commercial fertilizers for the soil [73] and longer-duration use of Cu-based pesticides and fungicides, leading to their accumulation in the soil [74]. On the other hand, fallout and emissions from industries and vehicular exhausts could be the primary sources of Pb contamination of agricultural soil [75]. Mineral fertilizer application is also behind the increase in Pb levels in soil [76], and P-fertilizers have often been considered the primary source of Pb input to the cultivated soil [77].
3.3.2 Degree of contamination
The location-wise (from all-season means) and season-wise (from all-location mean) distribution of CD for the disturbed soil and the undisturbed soil are presented in Tables S3 and S4, respectively. The nature of variation of CD in the two cases is presented in Fig. 6. The CD of the disturbed soil is from T15 (10.71) to T22 (14.72). The CD at the sites T22, T11, T4, T6, T21, T1, T2, T7, T25, T17 and T23 is considerable and belong to the category 12 < CD < 24 (considerable degree of contamination) while the sites T20, T5, T13, T19, T18, T8, T24, T12, T16, T9, T10, T3 and T15 indicate moderate level of contamination with 6 < CD < 12 (Tables S3 & S4). In the case of undisturbed soil, the CD is from NT5 (9.95) to NT3 (12.53), and only NT3 falls in the level of considerable degree of contamination. In contrast, the other undisturbed sites depicted moderate contamination.
CD for the seasons S1, W1, and S3 showed considerable contamination with 12 < CD < 24), while the seasons W3, S2 and W2 showed moderate levels of contamination with 6 < CD < 12. For the undisturbed soil, CD-based contamination levels are intermediate CD in all seasons. The high degree of contamination (CD) of the sampling sites might have been due to metal contamination, especially Co, Ni and Pb. The undisturbed soil showed a comparatively lesser degree of contamination because these sites were free from physical or chemical disturbances. On the other hand, applying chemical pesticides and fertilizers might have been responsible for the increased contamination level of the disturbed soil [78].
The quantity of clay in soil and the organic carbon content are crucial in determining the metals’ distribution and behaviour. According to Ma et al. [79], soil worm uptake of lead can be significantly impacted by pH and organic matter concentration. Compared to soils with greater levels of these characteristics, this may be especially powerful in soils with lower pH and organic matter. High Co levels (122 mg kg−1 in Australia and 116 mg kg−1 in Japan) have been reported in soils; these findings have been linked to pollution or particular enrichment in roadside soils and street dust, which are known to be enriched in Co [64]. A critical source of Ni may be the usage of phosphate-based fertilizers and sludges. In addition, synthetic sources of Ni, particularly those derived from industrial production, have led to a notable rise in soil Ni concentration [68].
3.3.3 Enrichment factor (EF)
The EF generally utilized to determine the degree of human influence and distinguish factors originating from both human and natural origins [67]. The location-wise and the season-wise distribution of EF of the disturbed and the undisturbed soil are presented in Tables S5 and S6, respectively. The variations are also shown in Figs. 7 and 8. The location-wise enrichment levels of Co can be interpreted as very severe for both the disturbed soil and the undisturbed soil (25 < EF < 50) with the exception of the undisturbed site, NT5 which can be categorized as severe enrichment (10 < EF < 25) (Fig. 9). The seasonal Co enrichment is also very severe for the disturbed and the undisturbed soil (25 < EF < 50), except for the undisturbed soil in W2 season where severe enrichment (10 < EF < 25) is seen (Fig. 9). Cr has moderately high enrichment factor (5 < EF < 10) for the disturbed soil sites of T22, T16, T20, T18, T23, T11, T13, T21, T12, T5, T15, T10, T2, T4, T17, T14, T8, T3, T9, T25, T24 and for the undisturbed soil sites of NT3, NT2, NT1, NT4 in that order (Fig. 9). Moderate enrichment (3 < EF < 5) is observed for the disturbed soil at T19, T6, T1 and T7, as well as the undisturbed soil at NT5. Considering season-wise Cr enrichment, moderately sever enrichment (5 < EF < 10) is observed in the seasons, S1, S2, W2, S3 and W3 for the disturbed soil and S1, S2, W2 and S3 for the undisturbed soil. Only moderate Cr enrichment (3 < EF < 5) is observed for the season, W1 for the disturbed soil and the seasons, W1, W2 for the undisturbed soil. The location-wise Cu enrichment is severe (10 < EF < 25) for the disturbed soil sites of T8, T14, T5, T11, T2, T16, T7, T15, T4, T6, T29, T9, T10, T17, T22, T13 and the undisturbed soil sites of NT5 and NT3 (Fig. 9). Moderately severe enrichment (5 < EF < 10) of Cu is observed for the disturbed soil at T3, T21, T12, T1, T18, T25, T24, T19, T23 and for the undisturbed soil at NT2, NT1 and NT4 in that order (Fig. 9).
The seasonal Cu enrichment is severe (10 < EF < 25) for the disturbed soil in the seasons S1, W1, S2, S3, W3 and for the undisturbed soil in the season, S3 (Fig. 8). The disturbed soil in W2 season and the undisturbed soil in the seasons S1, W1, S2, W2, S3 show moderately high enrichment (5 < EF < 10). Mn shows moderately high enrichment (5 < EF < 10) for the disturbed soil sites, T12, T24, T19, T23, T18 and T25 followed by moderate enrichment (3 < EF < 5) for T21, T3, T13, T15, T9, T1, T10, T6, T16, T4, T20, T17, T22, T2, T7, T5, T11, T8 and T14 (Fig. 9). For the undisturbed soil, moderate Mn enrichment (3 < EF < 5) is observed for NT2, NT4, NT5 and NT1 and minor enrichment for NT3. With respect to seasonal distribution, no enrichment of Mn (EF < 1) is observed for all the seasons of the disturbed soil and the seasons, W1, S2 and W2, for the undisturbed soil. Only moderate Mn enrichment (3 < EF < 5) is observed for the seasons, S1, S3 and W3 for the undisturbed soil (Fig. 9).
For the disturbed soil, Ni shows very severe enrichment (25 < EF < 50) for T22, T11, T4 and T5 in that order and severe enrichment (10 < EF < 25) for T21, T25, T6, T2, T13, T1, T23, T7, T14, T8, T20, T12, T10, T9, T18, T16, T15, T19, T3 and T4. In case of the undisturbed soil, all the sites could be categorized as having severe Ni enrichment. With respect to seasonal enrichment, severe Ni enrichment (25 < EF < 50) is observed in the season S1 for the disturbed soil and S1 and W2 for the undisturbed soil (Figs. 8 and 9). Severe Ni enrichment (10 < EF < 25) is seen for the seasons, W1, S2, W2, S3 and W3 for the disturbed soil and W1, S2, S3 and W3 for the undisturbed soil. All the disturbed soil and the undisturbed soil have severe Pb enrichment (10 < EF < 25). The seasonal enrichment of Pb is also severe (10 < EF < 25). For the disturbed soil, Zn has moderately severe enrichment (5 < EF < 10) for T14, T8, T11 for the disturbed soil and NT4 for the undisturbed soil. Moderate enrichment (3 < EF < 5) is observed for the disturbed soil at T16, T7, T22, T17, T4, T2, T20, T1, T13, T15, T9, T6, T3, T12, T10, T21, T5, T25, T8, T24 and T23, and for the undisturbed soil at NT5, NT4, NT2 and NT1. Only minor enrichment is observed for the disturbed soil site of T19. Moderate seasonal enrichment (3 < EF < 5) of Zn is observed in all the seasons of the disturbed and the undisturbed soil, except that Zn enrichment is minor for S3 season of the undisturbed soil.
EF values close to unity usually indicate that the soil is of crustal origin and is not contaminated. If the EF > 10, the soil is considered to be of non-crustal source having large anthropogenic influence and if EF < 1.0, it suggests a possible mobilization or depletion of the metals [80]. Application of animal manure may have introduced high levels of Cu and Zn. These metals may have come from the animal nutrition/drugs used to boost animal growth and to prevention of disease [81], and pesticides or fungicides that contain high levels of Cu [41]. It has been reported that accumulation of Cd and Zn in soil could strongly stimulate by the dissolved phosphorous [82]. It may also be account of the extensive application of phosphate fertilizers [83].
3.3.4 Potential ecological risk index
Generally, the PERI was applied to evaluate the effect of heavy metal in the sediment with their ecological and toxicological impact. This method is utilized to estimate the contamination levels in the sediments as well as combined effects of ecology and environment with toxicology for a significant determination of the possible risk of heavy metal accumulation in the soil [39]. The location-wise and season-wise ecological risk index (Eri) values are presented in Tables S7 and S8, respectively, and their variations are shown in Figs. 10 and 11, respectively. In this work, all the sampling sites and seasons offer low ecological risk (Eri < 40) (Fig. 12). Potential environmental risk index data are summarized in Tables S9 and S10 for location-wise and season-wise distribution and their variation patterns are presented in Fig. 13. Again, it is observed that location-wise and season-wise PERI is < 95, signifying the low level of ecological risk. Still, more specifically, higher accumulation of accumulation of Co, Pb and Ni may pose a threat to the ecosystem shortly. According to PERI and Eri’s general findings, soils may be at risk of pollution soon. This could be the result of applying agricultural inputs progressively, such as fungicides, pesticides, contaminated fertilizers, and irrigation water, which also can increase the load of heavy metals and cause heavy metal pollution in agricultural soil [84].
4 Conclusion
In the case of other metals, the quantity of Ni in both the disturbed and the undisturbed sites is excessively higher than the worldwide average. Higher deviations from the world averages are observed for both the Co and Pb concerning the all-season and all-sample are averages. Lower deviations from the world averages are observed for both all-season averages and the all-sample averages of Cr, Mn and Zn. Frequent use of phosphate fertilisers may be responsible for higher levels of Cu, Ni and Pb, giving them much higher values than the world averages. The risk assessment results indicated that the undisturbed soil showed a comparatively lesser degree of contamination because these sites were free from physical or chemical disturbances. On the other hand, applying chemical pesticides and fertilisers might have been responsible for the increased contamination level of the disturbed soil. The findings will be beneficial in assessing the quality of agricultural soil and determining appropriate farming methods to ensure the production of safe crops while safeguarding the natural world and human well-being.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Qi H, Zhao B, Li L, Chen X, An J, Liu X. Heavy metal contamination and ecological risk assessment of the agricultural soil in Shanxi Province, China. Royal Soc Open Sci. 2020;7(10):200538. https://doi.org/10.1098/rsos.200538.
Rashid A, Schutte BJ, Ulery A, Deyholos MK, Sanogo S, Lehnhoff EA, Beck L. Heavy metal contamination in agricultural soil: environmental pollutants affecting crop health. Agron. 2023;13(6):1521. https://doi.org/10.3390/agronomy13061521.
Baruah SG, Ahmed I, Das B, Ingtipi B, Boruah H, Gupta SK, Chabukdhara M. Heavy metal (loid)s contamination and health risk assessment of soil-rice system in rural and peri-urban areas of lower Brahmaputra valley, Northeast India. Chemosphere. 2021;266:129150. https://doi.org/10.1016/j.chemosphere.2020.129150.
Singh BP, Choudhury M, Samanta P, Gaur M, Kumar M. Ecological risk assessment of heavy metals in adjoining sediment of river ecosystem. Sustainability. 2021;13(18):10330. https://doi.org/10.3390/su131810330.
Paul S, Choudhury M, Deb U, Pegu R, Das S, Bhattacharya SS. Assessing the ecological impacts of ageing on hazard potential of solid waste landfills: a green approach through vermitechnology. J Clean Prod. 2019;236: 117643. https://doi.org/10.1016/j.jclepro.2019.117643.
Choudhury M, Jyethi DS, Dutta J, Purkayastha SP, Deb D, Das R, Bhattacharyya KG. Investigation of groundwater and soil quality near to a municipal waste disposal site in Silchar, Assam. India Int J Energy Water Res. 2021. https://doi.org/10.1007/s42108-021-00117-5.
Xiao P, Zhou Y, Li X, Xu J, Zhao C. Assessment of heavy metals in agricultural land: A literature review based on bibliometric analysis. Sustainability. 2021;13(8):4559. https://doi.org/10.3390/su13084559.
Sánchez-Castro I, Molina L, Prieto-Fernández MÁ, Segura A. Past, present and future trends in the remediation of heavy-metal contaminated soil-Remediation techniques applied in real soil-contamination events. Heliyon. 2023;9: e16692. https://doi.org/10.1016/j.heliyon.2023.e16692.
Rai PK, Lee SS, Zhang M, Tsang YF, Kim KH. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ Int. 2019;125:365–85. https://doi.org/10.1016/j.envint.2019.01.067.
Ghori NH, Ghori T, Hayat MQ, Imadi SR, Gul A, Altay V, Ozturk M. Heavy metal stress and responses in plants. Int J Environ Sci Technol. 2019;16:1807–28. https://doi.org/10.1007/s13762-019-02215-8.
Purkayastha SP, Choudhury M, Deb D, Paul C. Arsenic contamination in ground water is a serious threat in the North Karimganj block of Karimganj district, Southern part of Assam, India. J Chem Pharm Res. 2015;7(8):371–8.
Zoroddu MA, Aaseth J, Crisponi G, Medici S, Peana M, Nurchi VM. The essential metals for humans: a brief overview. J Inorg Biochem. 2019;195:120–9. https://doi.org/10.1016/j.jinorgbio.2019.03.013.
Jones C, Jacobsen J. Micronutrients: cycling, testing and fertilizer recommendations. Nutrient management modules 7. Bozeman: Montana State University Extension Service; 2009. p. 1–16.
Adhikari G, Bhattacharyya KG. Ecotoxicological risk assessment of trace metals in humid subtropical soil. Ecotoxicol. 2015. https://doi.org/10.1007/s10646-015-1522-9.
Ali W, Mao K, Zhang H, Junaid M, Xu N, Rasool A, Yang Z. Comprehensive review of the basic chemical behaviours, sources, processes, and endpoints of trace element contamination in paddy soil-rice systems in rice-growing countries. J Hazard Mater. 2020;397:122720. https://doi.org/10.1016/j.jhazmat.2020.122720.
Chen TB, Wong JW, Zhou HY, Wong MH. Assessment of trace metal distribution and contamination in surface soils of Hong Kong. Environ Pollut. 1997;96:61–8. https://doi.org/10.1016/S0269-7491(97)00003-1.
Liu B, Ai S, Zhang W, Huang D, Zhang Y. Assessment of the bioavailability, bio accessibility and transfer of heavy metals in the soil-grain-human systems near a mining and smelting area in NW China. Sci Total Environ. 2017;609:822–9. https://doi.org/10.1016/j.scitotenv.2017.07.215.
Gan Y, Huang X, Li S, Liu N, Li YC, Freidenreich A, Dai J. Source quantification and potential risk of mercury, cadmium, arsenic, lead, and chromium in farmland soils of Yellow River Delta. J Clean Prod. 2019;221:98–107.
Khanam R, Kumar A, Nayak AK, Shahid M, Tripathi R, Vijayakumar S, Pathak H. Metal (loid) s (As, Hg, Se, Pb and Cd) in paddy soil: Bioavailability and potential risk to human health. Sci Total Environ. 2020;699:134330. https://doi.org/10.1016/j.scitotenv.2019.134330.
Kelepertzis E. Accumulation of heavy metals in agricultural soils of Mediterranean: Insights from Argolida basin Peloponnese, Greece. Geoderma. 2014;221–222:82–90. https://doi.org/10.1016/j.geoderma.2014.01.007.
Zhuang Z, Mu HY, Fu PN, Wan YN, Yu Y, Wang Q, Li HF. Accumulation of potentially toxic elements in agricultural soil and scenario analysis of cadmium inputs by fertilization: a case study in Quzhou county. J Environ Manage. 2020;269:110797. https://doi.org/10.1016/j.jenvman.2020.110797.
Huang Y, Chen Q, Deng M, Japenga J, Li T, Yang X, He Z. Heavy metal pollution and health risk assessment of agricultural soils in a typical peri-urban area in southeast China. J Environ Manag. 2018;207:159–68. https://doi.org/10.1016/j.jenvman.2017.10.072.
Aschale M, Sileshi Y, Kelly-Quinn M, et al. Pollution assessment of toxic and potentially toxic elements in agricultural soils of the city Addis Ababa, Ethiopia. Bull Environ Contam Toxicol. 2017;98(2):234–43. https://doi.org/10.1007/s00128-016-1975-4.
Singh BP, Choudhury M, Gupta P, Chadha U, Zewude D. Physicochemical and biological characteristics of River Hindon at Galheta station from 2009 to 2020. Environ Qual Manag. 2023. https://doi.org/10.1002/tqem.22115.
Ngole-Jeme VM. Heavy metals in soils along unpaved roads in south west Cameroon: contamination levels and health risks. Ambio. 2016;45(3):374–86. https://doi.org/10.1007/2Fs13280-015-0726-9.
da Silva FBV, do Nascimento CWA, Araújo PRM, et al. Soil contamination by metals with high ecological risk in urban and rural areas. Int J Environ Sci Technol. 2017;14(3):553–62. https://doi.org/10.1007/2Fs13762-016-1170-5.
Yadav P, Singh B, Garg VK, Mor S, Pulhani V. Bioaccumulation and health risks of heavy metals associated with consumption of rice grains from croplands in Northern India. Hum Ecol Risk Assess. 2017;23(1):14–27.
Golui D, Mazumder DG, Sanyal SK, Datta SP, Ray P, Patra PK, Bhattacharya K. Safe limit of arsenic in soil in relation to dietary exposure of arsenicosis patients from Malda district, West Bengal-a case study. Ecotoxicol Environ Saf. 2017;144:227–35. https://doi.org/10.1016/j.ecoenv.2017.06.027.
Sharma S, Kaur I, Nagpal AK. Assessment of arsenic content in soil, rice grains and groundwater and associated health risks in human population from Ropar wetland, India, and its vicinity. Environ Sci Pollut Res. 2017;24(18836):18848. https://doi.org/10.1007/s11356-017-9401-y.
Directorate of Economics and Statistics, Assam, India. 2020. https://des.assam.gov.in/. Accessed 04 Dec 2022.
Baruah TC, Barthakur HP. A textbook of soil analysis. New Delhi: Vikash Publishing House Pvt. Ltd.; 1997. p. 1–334.
Jiang X, Lu WX, Zhao HQ, Yang QC, Yang ZP. Potential ecological risk assessment and prediction of soil heavy-metal pollution around coal gangue dump. Nat Hazards Earth Syst Sci. 2014;14:1599–610. https://doi.org/10.5194/nhess-14-1599-2014.
Hakanson L. An ecological risk index for aquatic pollution control, a sedimentological approach. Water Res. 1980;14:975–1001. https://doi.org/10.1016/0043-1354(80)90143-8.
Rehman IU, Ishaqa M, Ali L, Khan S, Ahmad I, Din IU, Ullah H. Enrichment, spatial distribution of potential ecological and human health risk assessment via toxic metals in soil and surface water ingestion in the vicinity of Sewakht mines, district Chitral, Northern Pakistan. Ecotoxicol Environ Saf. 2018;154:127–36.
Ho HH, Swennen R, Van Damme A. Distribution and contamination status of heavy metals in estuarine sediments near Cua Ong harbor, Ha Long Bay, Vietnam. Geol Belg. 2002;13(1–2):37–47.
Varol M. Assessment of heavy metal contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. J Hazard Mater. 2011;195:55–364. https://doi.org/10.1016/j.jhazmat.2011.08.051.
Sakan SM, Đorđević DS, Manojlović DD, Predrag PS. Assessment of heavy metal pollutants accumulation in the Tisza River sediments. J Environ Econ Manage. 2009;2009(90):3382–90.
Chai L, Li H, Yang Z, Min X, Liao Q, Liu Y, Men S, Yan Y, Xu J. Heavy metals and metalloids in the surface sediments of the Xiangjiang River, Hunan, China: distribution, contamination, and ecological risk assessment. Environ Sci Pollut Res. 2017;24:874–85.
Ke X, Gui S, Huang H, Zhang H, Wang C, Guo W. Ecological risk assessment and source identification for heavy metals in surface sediment from the Liaohe River protected area, China. Chemosphere. 2017;175:473–81.
Xu SX, Shi XZ, Zhao YC, Yu DS, Li CS, Wang SH, Tan MZ, Sun WX. Carbon sequestration potential of recommended management practices for paddy soils of China, 1980–2050. Geoderma. 2011;166:206–13.
Lu A, Wang J, Qin X, Wang K, Han P, Zhang S. Multivariate and geostatistical analyses of the spatial distribution and origin of heavy metals in the agricultural soils in Shunyi, Beijing. China Sci Total Environ. 2012;425:66–74.
Tack FM. Trace elements: general soil chemistry, principles and processes. In: Hooda PS, editor. Trace elements in soils. Hoboken: Wiley; 2010. p. 9–37.
Saikia P, Bhattacharya SS, Baruah KK. Organic substitution in fertilizer schedule: Impacts on soil health, photosynthetic efficiency, yield and assimilation in wheat grown in alluvial soil. Agric Ecosyst Environ. 2015;203:102–9. https://doi.org/10.1016/j.agee.2015.02.003.
Pedrera-Parrilla A, Brevik EC, Giráldez JV, Vanderlinden K. Temporal stability of electrical conductivity in a sandy soil. Int Agrophys. 2016;30:349–57. https://doi.org/10.1515/intag-2016-0005.
Ratnayake RR, Perera BMACA, Rajapaksha RPSK, Ekanayake EMHGS, Kumara RKGK, Gunaratne HMAC. Soil carbon sequestration and nutrient status of tropical rice based cropping systems: Rice-Rice, Rice-Soya, Rice-Onion and Rice-Tobacco in Sri Lanka. CATENA. 2017;150:17–23. https://doi.org/10.1016/j.catena.2016.11.006.
Feng W, Guo Z, Xiao X, Peng C, Shi L, Ran H, Xu W. Atmospheric deposition as a source of cadmium and lead to soil-rice system and associated risk assessment. Ecotoxicol Environ Saf. 2019;180:160–7.
Kumar M, Khan MH, Singh P, Ngachan SVN, Rajkhowa DJ, Kumar A, Devi MH. Variable lime requirement based on differences in organic matter content of iso-acidic soils. Indian J Hill Farming. 2012;25(1):26–30.
Singh AK, Chakraborti M, Datta M. Improving rice-based cropping pattern through soil moisture and integrated nutrient management in mid-tropical plain zone of Tripura, India. Rice Sci. 2014;21(5):299–304. https://doi.org/10.1016/S1672-6308(13)60190-0.
Vega FA, Covelo EF, Andrade ML, Marcet P. Relationships between heavy metals content and soil properties in minesoils. Anal Chim Acta. 2004;524:141–50. https://doi.org/10.1016/j.aca.2004.06.073.
Kettler TA, Lyon DJ, Doran JW, Powers WL, Stroup WW. Soil quality assessment after weed-control tillage in a No-Till Wheat-Fallow cropping system. Soil Sci Soc Am J. 2000;64:339.
de Pal OV, Lal R. Soil quality evaluation under different land management practices. Environ Earth Sci. 2014;72:4531–49.
Colombo C, Palumbo G, He JZ, Pinton R, Cesco S. Review on iron availability in soil: interaction of Fe minerals, plants, and microbes. J Soils Sediments. 2014;14:538–48.
Rahman MS, Biswas PK, Al Hasan SM, Rahman MM, Lee SH, Kim KH, Islam MR. The occurrences of heavy metals in farmland soils and their propagation into paddy plants. Environ Monit Assess. 2018;2018(190):1–18.
Robin A, Vansuyt G, Hinsinger P, Meyer JM, Briat JF, Lemanceau P. Iron dynamics in the rhizosphere: consequences for plant health and nutrition. Adv Agron. 2008;99:183–225.
Du Laing G, Vanthuyne DRJ, Vandecasteele B, Tack FMG, Verloo MG. Influence of hydrological regime on pore water metal concentrations in a contaminated sediment-derived soil. Environ Pollut. 2007;147:615–25.
Speir TW, Schaik APV, Percival HJ, Close ME, Pang LP. Heavy metals in soil, plants and groundwater following high-rate sewage sludge application to land. Water Air Soil Pollut. 2003;150:319–58.
Mughal A, Sultan K, Ashraf K, Hassan A, Zaman QU, Haider FU, Shahzad B. Risk Analysis of heavy metals and groundwater quality indices in residential areas: a case study in the Rajanpur district. Pakistan Water. 2022;14:3551. https://doi.org/10.3390/w14213551.
Kabata-Pendias A. Trace elements in soils and plants. 4th ed. Boca Raton: CRC Press; 2011.
Roychowdhury T, Uchino T, Tokunaga H, Ando M. Arsenic and other heavy metals in soils from an arsenic-affected area of West Bengal, India. Chemosphere. 2002;49(6):605–18. https://doi.org/10.1016/s0045-6535(02)00309-0.
Peijnenburg V, Posthuma WJGM, Zweers M, Baerselman PGPC, de Groot R, Van Veen AC, Jager T. Prediction of metal bio-availability in Dutch field soils for the oligochaete Enchytraeus crypticus. Ecotoxicol Environ Saf. 1999;43:170–86.
Dan T, Hale B, Johnson D, Conard B, Stiebel B, Veska E. Toxicity thresholds for oat (Avena sativa L.) grown in Ni-impacted agricultural soils near Port Colborne, Ontario, Canada. Can J Soil Sci. 2008;88:389–98. https://doi.org/10.4141/CJSS07070.
Baize D. Total content of metallic trace elements in soils (France). References and strategies of interpretation. Paris: INRA Editions; 1997. p. 1–410.
Kashem MA, Singh BR. Metal availability in contaminated soils: I. Effects of flooding and organic matter on changes in Eh, pH and solubility of Cd, Ni and Zn. Nutr Cycl Agroecosyst. 2001;61(3):247–55. https://doi.org/10.1023/A:1013762204510.
Kabata-Pendias A, Pendias H. Trace elements in soils and plants. 3rd ed. Boca Raton: CRC Press; 2001.
Marrugo-Negrete J, Pinedo-Hernández J, Díez S. Assessment of heavy metal pollution, spatial distribution and origin in agricultural soils along the Sinú River Basin, Colombia. Environ Res. 2017;154:380–8. https://doi.org/10.1016/j.envres.2017.01.021.
Radomirović M, Ćirović Ž, Maksin D, Bakić T, Lukić J, Stanković S, Onjia A. Ecological risk assessment of heavy metals in the soil at a former painting industry facility. Fron EnvironSci. 2020;8: 560415. https://doi.org/10.3389/fenvs.2020.560415.
Soleimani H, Mansouri B, Kiani A, Omer AK, Tazik M, Ebrahimzadeh G, Sharafi K. Ecological risk assessment and heavy metals accumulation in agriculture soils irrigated with treated wastewater effluent, river water, and well water combined with chemical fertilizers. Heliyon. 2023;9(3):e14580. https://doi.org/10.1016/j.heliyon.2023.e14580.
Hashmi MZ, Yu C, Shen H, Duan D, Shen C, Lou L, Chen Y. Risk assessment of heavy metals pollution in agricultural soils of Siling reservoir watershed in Zhejiang Province, China. Biomed Res Int. 2013;2013:590306. https://doi.org/10.1155/2013/590306.
Chakravarty M, Patgiri AD. Metal pollution assessment in sediments of the Dikrong River, N.E. India. J Hum Ecol. 2009;27(1):63–7. https://doi.org/10.1080/09709274.2009.11906193.
Morrison JM, Goldhaber MB, Lee L, Holloway JM, Wanty RB, Wolf RE, Ranville JF. A regional-scale study of chromium and nickel in soils of northern California, USA. Appl Geochem. 2009;24(8):1500–11. https://doi.org/10.1016/j.apgeochem.2009.04.027.
Taylor RM. The association of manganese and cobalt in soils-further observations. J Soil Sci. 1968;19:77–80. https://doi.org/10.1111/j.1365-2389.1968.tb01522.x.
Adriano DC. Trace elements in the terrestrial environment. Berlin: Springer; 1986.
Acosta JA, Faz A, Martínez-Martínez S, Arocena JM. Enrichment of metals in soils subjected to different land uses in a typical Mediterranean environment (Murcia city, southeast Spain). Appl Geochem. 2011;26:405–14. https://doi.org/10.1016/j.apgeochem.2011.01.023.
Epstein L, Bassein S. Pesticide application of copper on perennial crops in California, 1993 to 1998. J Environ Qual. 2001;22:335–48. https://doi.org/10.2134/jeq2001.3051844x.
Facchinelli A, Sacchi E, Mallen L. Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environ Pollut. 2001;114:313–24. https://doi.org/10.1016/S0269-7491(00)00243-8.
Atafar Z, Mesdaghinia A, Nouri J, Homaee M, Yunesian M, Ahmadimoghaddam M, Mahvi AH. Effect of fertilizer application on soil heavy metal concentration. Environ Monit Assess. 2010;160:83–9. https://doi.org/10.1007/s10661-008-0659-x.
Nziguheba G, Smolders E. Inputs of trace elements in agricultural soils via phosphate fertilizers in European countries. Sci Total Environ. 2008;390:53–7. https://doi.org/10.1016/j.scitotenv.2007.09.031.
Liu M, Yang Y, Yun X, Zhang M, Wang J. Concentrations, distribution, sources, and ecological risk assessment of heavy metals in agricultural topsoil of the three Gorges Dam region, China. Environ Monit Assess. 2015;187:187. https://doi.org/10.1007/s10661-015-4360-6.
Ma W, Edelman T, van Beersum I, Jans T. Uptake of cadmium, zinc, lead and copperby earthworms near a zinc-smelting complex: influence of soil pH and organic matter. Bull Environ Contam Toxicol. 1983;30:424–7. https://doi.org/10.1007/BF01610155.
Pan Y, Chen M, Wang X, Chen Y, Dong K. Ecological risk assessment and source analysis of heavy metals in the soils of a lead-zinc mining watershed area. Water. 2022;15(1):113. https://doi.org/10.3390/w15010113.
McBride MB, Spiers G. Trace element content of selected fertilizers and dairy manures as determined by ICP-MS. Commun Soil Sci Plant Anal. 2001;32:139–56. https://doi.org/10.1081/CSS-100102999.
Lin Q, Liu E, Zhang E, Li K, Shen J. Spatial distribution, contamination and ecological risk assessment of heavy metals in surface sediments of Erhai Lake, a large eutrophic plateau lake in southwest China. CATENA. 2016;145:193–203.
Zahra A, Hashmi MZ, Malik RN, Ahmed Z. Enrichment and geo-accumulation of heavy metals and risk assessment of sediments of the Kurang Nallah-feeding tributary of the Rawal Lake Reservoir. Pakistan Sci Total Environ. 2014;470:925–33.
Mahmud U, Salam MTB, Khan AS, Rahman MM. Ecological risk of heavy metal in agricultural soil and transfer to rice grains. Discov Mater. 2021;1:1–13.
Acknowledgements
The authors acknowledged the Faculty of Science, Sadiya College and the Department of Chemistry, Morigaon College, Assam, India. Authors were grateful for the resources and assistance provided for this study. The authors are also thankful to Voice of Environment (A Scientific and Environmental Research Organization), Guwahati, Assam, for their kind guidance during the research paper writing. Authors are thankful to the respective reviewers for their valuable suggestions for improving the manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
NG, AS, MC, MF and SS: conceptualization, writing original draft and figures; NG, AS, MC, PS: final draft; NG, AS, MC, PS: reviewed the manuscript. All authors approved the submission.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gogoi, N., Sarma, A., Choudhury, M. et al. Soil heavy metal pollution and ecological risk assessment in disturbed and undisturbed soil of Morigaon, Assam. Discov Environ 2, 13 (2024). https://doi.org/10.1007/s44274-024-00039-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44274-024-00039-z