Abstract
The Erdenet mine (Erdenet, Mongolia) is a copper–molybdenum open pit mine with a huge tailing pond and is located next to a residential area. As the sources of drinking water in this area rely on groundwater and can be categorized into public or private wells, we aimed to assess the groundwater quality of the different types of drinking water wells. To accomplish our goal, we analyzed 18 trace metals (As, Ba, Bi, Cd, Co, Cr, Cs, Cu, Ga, Mo, Ni, Pb, Se, Sr, Th, U, V, Zn) and 8 major metals (Al, Ca, Fe, K, Mg, Mn, Na, Si) samples using ICP-MS and ICP-OES, and the heavy metal pollution index, heavy metal evaluation index, and degree of contamination were calculated. The most concerning point is that the Mo concentration of waste water in the Erdenet mine tailing pond and at a natural spring located 300 m from the tailing pond was 1100 μg/L, which greatly exceeds the WHO permissible concentration for Mo of 70 μg/L. Furthermore, high Ca and Mg concentrations at all private wells indicated that consumers of these wells are more vulnerable to any type of water pollution, as these wells are not built with any disinfection or treatment system. A modified categorization of the water quality indices showed that the public and private wells have low–medium contamination and that the tailing pond seepage water, along with its nearest spring, have a high degree of contamination. Because many private wells are located along the tailing pond and used directly without any treatment, we suggest a continuous evaluation and monitoring of the groundwater quality in this area.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Groundwater pollution can be categorized by origin into four main types: industrial, domestic, agricultural, and environmental pollution [1]. Within these various sources of groundwater pollution, anthropogenic activities have various impacts and heavy metal pollution has become a concerning problem because of its harmful effects on organisms and its long-term adverse impacts [2]. Numerous research reports have shown that groundwater near industrial and mining areas is typically affected heavy metal pollution [3,4,5]. Groundwater in mining areas often has particularly high concentrations of certain heavy metals, which present a potential risk to human health [6,7,8,9]. Mongolia experiences problems with groundwater pollution similar to those in other countries in the world. Expanding mining and industrial operations, urbanization, and intensified land use for agriculture pose growing threats to Mongolia's limited water resources [10, 11]. Aside from being the main source of drinking water, groundwater is consumed in every sector [12]. Apartment households are supplied with groundwater from the central water supply, whereas the drinking water in the suburbs and ‘ger’ areas (a type of residential district in Mongolian settlements) is supplied from portable water or private wells [10]. As most people in ger areas are not connected to a central water supply, there is no sewer system, and ger district households usually have a pit toilet [13]. The quality and sanitation of the portable water supplies and private wells are highly questionable. The main feature of Erdenet, an industrial and mining town and the second largest city in Mongolia in terms of population, is the Erdenet copper–molybdenum mine located near the city. The mine has been operating since 1978 and has sufficient estimated reserves to continue operations for another 30 years [14]. In recent years, researchers have become very interested in the impact of the Erdenet mine on the environment. The source of drinking water for the population of the city of Erdenet is groundwater, and farmers and herdsmen distant from the city use surface water for livestock and irrigation. Over the years, a significant volume of waste has been generated by the mining operation, accumulating in the tailings pond close to river and caused negative impacts [15]. In 2013, a study of the groundwater, surface water, river sediment, and soil of Erdenet revealed high concentrations of Ca, Mg, Mo, and As. The study concluded that the mine tailings pond was directly affecting surface water and indirectly affecting groundwater, and it recommended that the impact of the mine on the environment be studied in greater detail and that the risks to human health and the environment be assessed to prevent potential adverse impacts [16]. Another study conducted research on surface water in Erdenet and concluded that the Mo concentration is notably higher than the world average concentration in surface water [17]. To investigate its behavior, these researchers continued their work with monthly observations of Mo in river water. As a result, groundwater used for cooling of the Erdenet power plant and mine tailing ponds were found to be the main sources of the Mo in river water [18]. Based on these research results and conclusions, it is apparent that the Erdenet mining activity has some adverse impacts on the environment, which need to be validated and clarified in greater detail.
Infiltration of leachate from solid waste disposal on land, liquid sewage, and sewage sludge can directly contaminate groundwater with metals. Other pathways include leachate from mine tailings and other mining wastes, liquid waste disposal in deep wells, seepage from industrial waste lagoons, and other spills or leaks associated with industrial metal processing facilities [19]. Humans may be exposed to heavy metals by either ingestion (drinking or eating) or inhalation (breathing), and living and working in or near industrial areas where heavy metals are used or where waste containing heavy metals is inappropriately discarded increases the risk. Moreover, heavy metals may synergistically increase the overall heavy metal toxicity [20,21,22]. Because heavy metals are harmful to the human body, it is essential to determine and evaluate their levels. This study aimed to assess the water quality and suitability for drinking and household use of different types of groundwater sources and highlight differences between these sources. There are two main sources of drinking water for the citizens of that city: the Selenge River basin and the area around Erdenet city, and it is interesting to note that there are differences between them: public and private wells. Due to a lack of studies that examined the differences in water quality between the different drinking sources in the study area, this study was conducted.
2 Materials and methods
2.1 Study area
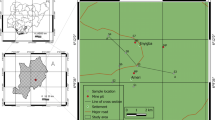
The study area was the city of Erdenet (49°00′–49°10′ N, 104°00′–104°15′ E), Mongolia, which is the residential area of the Erdenet copper–molybdenum mine (Fig. 1). The city is within a valley between the Selenge and Orkhon catchment areas, about 380 km northwest of Ulaanbaatar, the capital of Mongolia. The annual average air temperature is − 17.6 °C in January and 15.5 °C in July, and the annual average precipitation is approximately 314 mm. Most of the precipitation (60–70%) occurs during summer [23]. The population of the city of Erdenet was 108,713 in 2021 [24]. Approximately 35% of the total population lives in an apartment, and the remaining 65% live in the ger area. The Erdenet mining area is on the outskirts of the city of Erdenet, and three rivers flow through the city: the Erdenet, Govil, and Khangal. The Erdenet deposit is lies in the Orkhon-Selenge depression, which contains Permian volcanic rock intruded by the Selenge Intrusive Complex from the Permian and Early Triassic. Late Triassic and Early Jurassic volcanic rock overlay the depression [25]. The bed rocks consist of three distinct associations: gabbroids, granitoids, and subalkaline granites with sienogranites [26]. The deposit is categorized as porphyry molybdenum-copper type and contains productive levels of Re, Ag, and Se. Additionally, the ore holds notable amounts of metals including Pb, Zn, As, Sr, Bi, Co, Ni, Ge, Ta, Ga, In, and Cd [23]. Between 1971 and 1973, a groundwater study assessing the hydrogeological conditions and industrial water supply of the Erdenet copper–molybdenum ore deposit was conducted in cooperation with the Russian Federation (formerly the USSR). The study concluded that the groundwater resources in the valleys of small rivers around Erdenet are insufficient to provide a constant water resource for the Erdenet deposit. Therefore, water from the groundwater aquifer in the Selenge River Valley, 64 km from the deposit, was transferred by pipeline to meet the water needs of the city of Erdenet and the adjacent and mine [27]. This Selenge River Valley groundwater is used by the mine, industries and factories, and apartment residents in Erdenet. The Erdenet mine extracts 26 million tons of ore annually through open-pit mining, and it yields about 530,000 tons of copper concentrate as well as 4500 tons of molybdenum concentrates each year. The open-pit measures 2800 m in length and has a width of 1600 m with benches at intervals of 15 m [28]. The primary production facility is the mineral processing plant which involves multiple technological processes such as ore crushing and grinding (generating substantial dust), acidic flotation, filtration, drying, and transport of copper and molybdenum concentrates. Close to the Erdenet processing plant exists a smaller facility, set up in 1994, which extracts copper from discarded rock utilizing sulfuric acid [23]. Waste from the mineral processing plant near the open-pit mine is transferred by pipeline to a mine tailing pond located in the northeastern part of the city. The solids in the waste water settle to the bottom as sludge, and the remaining water flows through dams and eventually discharges into the Khangal River. The bottom of the tailings pond does not have any protective layer to prevent seepage [16].
2.2 Drinking water availability
Ger area residents use locally sourced public or private wells and carry their water from well to home. Public well users carry their drinking water 1–2 km away. In ger area, residents face increased water supply charges despite receiving a lower level of service compared to apartment residents [10]. Water resources for the ger area can be divided into three types: public well (centralized source), public well (self-source), and private well (Fig. 2). Water authority of Erdenet city monitors drinking water microbial, physical and some chemical components in public wells. For private wells, water quality test is conducted immediately after the well is constructed. However, it is at the discretion of the owner to determine when to assess water quality in further. Centralized-source public wells withdraw and transfer water from Burenbust groundwater wells which locates 64 km away from Erdenet area. Self-source public wells and private wells withdraw water directly from their own source. The difference between these two types of wells is that self-source public wells serve the public, whereas private wells are used only by the owner household. Public wells provide water to approximately 300–700 people, and private wells provide water for a family. Centralized-source public wells have chlorine disinfection, but self-source public wells and private wells do not have any disinfection [29]. Thus, their use of drinking water that is not sterilized or disinfected makes residents of the ger area vulnerable to water pollution.
2.3 Water sampling
After selecting a total of 40 sampling points from public and private wells, springs, and tailing pond seepages of the Erdenet mine, sampling was conducted in September 2021 and February 2022. In September 2021, we collected water samples from each sampling point (total of 40 samples). In February 2022, 29 samples were collected. The sampling points are illustrated in Fig. 2. The 40 sampling points include 9 centralized-source public wells, 9 self-source public wells, 17 private wells, 4 springs, and 1 tailing pond seepage point. We chose September and February as sampling periods because groundwater dilution is typically lowest at these times. September is the end of summer, when the amount of rain decreases, and February is the end of winter, the time just before the weather warms and snow begins to melt. We measured water temperature, pH, electrical conductivity (EC), dissolved oxygen (DO), and oxidation–reduction potential (ORP) on site. pH was measured using a pH meter calibrated with standard pH solutions (WM-32EP, TOA-DKK Corp., Tokyo, Japan), EC and ORP were measured with an EC/ORP meter (WM-32EP, TOA-DKK Corp.), and DO was measured with a DO meter (DO-31P, TOA-DKK Corp.). Water samples were filtered through a 0.45 μm mixed cellulose membrane filter and divided into three 50 mL plastic bottles. The plastic bottles were sterilized in 3% HNO3 for 24 h and washed with ion exchanged water. One 50 mL sample was stabilized with 60% HNO3 for measurement of cations and metals. A second 50 mL sample was used for measurement of anions, and a third was titrated with 0.1 N H2SO4 for measurement of alkalinity. Alkalinity was calculated by the Gran function method using the Web-based Alkalinity Calculator version 2.22 (https://or.water.usgs.gov/alk/). All water samples were transported to the laboratory and stored in a refrigerator at 4 °C until analysis. pH meter was calibrated by solutions with pH = 4.01, pH = 6.86 and pH = 9.18 everyday during the fieldwork.
2.4 Heavy metal analysis
Eighteen trace metal (As, Ba, Bi, Cd, Co, Cr, Cs, Cu, Ga, Mo, Ni, Pb, Se, Sr, Th, U, V, Zn) concentrations were determined using an inductively coupled plasma mass spectrometer (ICP-MS) (iCAP RQ, Thermo Fisher Scientific Corp., Waltham, MA, USA). The sample solutions for trace element measurements were diluted at least 10 times using ultrapure-grade 0.6% HNO3 solution. The concentrations of eight major metals (Al, Ca, Fe, K, Mg, Mn, Na, Si) were also determined by inductively coupled plasma optical emission spectroscopy (ICP-OES) (ES-710, Varian, Inc., Palo Alto, CA, USA). Both ICP-MS and ICP-OES were calibrated by calibration solution of each element before the measurement.
2.5 Pollution evaluation indices
After measuring the concentrations of heavy metals, water quality pollution indices were calculated. The heavy metal pollution index (HPI), heavy metal evaluation index (HEI), and degree of contamination (Cd) were employed to evaluate groundwater quality and examine its suitability for drinking.
2.6 Heavy metal pollution index (HPI)
Based on the weighted arithmetic quality mean method, the HPI represents water quality as it relates to heavy metals. When the HPI is less than 100, the heavy metal is at a low level and does not pose a threat to human health. When the HPI value is equal to 100, threshold risk is present, along with adverse health effects. If the HPI value exceeds 100, the water is not suitable for drinking and must not be consumed. The HPI is expressed by Eqs. (1), (2), and (3) [6, 30,31,32,33,34]:
where Wi is the unit weight of i-th parameter, Qi is the sub-index of the parameter, n is the number of parameters considered, Mi is the monitored value of the heavy metal, and Ii and Si are the ideal and standard values of the i-th parameter, respectively (Table 1).
2.7 Heavy metal evaluation index (HEI)
The HEI, another water quality indexing method, is calculated using Eq. (4) [6, 30,31,32,33,34]:
where Hc is the monitored value and Hmac is the maximum admissible concentration (MAC) of the i-th parameter. Hmac is taken to be the same as the Si of the HPI (Table 1). The threshold value for the HEI is 1 (HEI > 1), and a higher HEI value indicates that the water is unsuitable for domestic use [34].
2.8 Degree of contamination (Cd)
The Cd value is calculated separately for each sample of water analyzed based on the sum of the contamination factors for each constituent exceeding the upper permissible value for that component. Thus, Cd is a measure that summarizes the combined effects of a number of quality parameters considered relevant to household water supplies. It is calculated by Eqs. (5) and (6) [6, 30,31,32,33,34]:
where Cfi is the contamination factor of the i-th parameter, CAi is the analytical value of the i-th parameter, and CNi is the upper permissible concentration of the i-th component (N denotes the “normative value”). CNi is taken to be the same as the Hmac of the HEI. When analytical values are below the upper permissible concentration value, they are not considered in the calculation. As long as heavy metal concentrations are below the upper permissible concentration, the groundwater quality is not adversely affected. The Cd value is categorized into low (Cd < 1), medium (1 < Cd < 3), and high (Cd > 3) values.
3 Results and discussion
3.1 Physicochemical parameters
In general, the sample pH ranged within the optimal pH values (6.5–8.5) established by WHO [35], with the exception of a few unusually low (6.21) and high (9.54) points among the private wells and springs. The water pH was higher in February, which is the cold and dry season in Mongolia. The pH values of private wells, natural springs, and tailing pond seepage water were lower than those of public wells. The calculated alkalinity values in September 2021 of the centralized-source public well, self-source public well, private well, and natural spring samples were 2.47–3.9 meq/l, 3.65–6.53 meq/l, 2.81–5.82 meq/l, and 1.44–4.6 meq/l, respectively,. In February 2022, these values were 2.6–4.83 meq/l, 3.54–7.27 meq/l, 2.94–5.47 meq/l, and 4.17 meq/l, respectively. DO values in September 2021 and February 2022 for centralized-source public well, self-sourced public well, private well, and natural spring samples were 2.77–4.57 mg/l, 2.26–4.6 mg/l, 2.45–4.91 mg/l, and 3.28–4.54 mg/l, respectively. The DO value was generally stable between the sampling periods. The EC values during the sampling periods of the centralized-source public well, self-sourced public well, private well, and natural spring samples were 29.6–78.70 mS/m, 45.7–74.2 mS/m, 34.5–114 mS/m, and 16.6–182 mS/m, respectively. The ideal EC level by WHO is 150 mS/m [35], so the EC of groundwater in the Erdenet area is within this level, with the exception of some private well and tailing pond seepage points. According to the groundwater EC classification of fresh water (< 150 mS/m), brackish water (150–300 mS/m), and saline water (> 300 mS/m) [36], sampling points B12 (spring) and B9 (tailing pond seepage) fell into the brackish water category, with values of 182 mS/m and 195.2 mS/m, respectively. The other sampling points were in the freshwater category. The EC of water depends on the salinity and the amount of dissolved minerals in the water [37, 38]. These two points had the highest heavy metal values, which is the reason for the higher EC. ORP values of the centralized-source public well, self-sourced public well, private well, and natural spring samples were 121–208, 11–149, 69–159, and 53–164 mV, respectively. The lowest ORP value was observed in a self-sourced public well in September 2021.
3.2 Metal concentrations
As mentioned previously, we measured 8 major metals and 18 trace metals. Among these, Al, Fe, Mn, Ga, Bi, Th (< 0.00001 µg/l), and Se (< 0.0005 µg/l) concentrations were below the detection limit of the measuring equipment for all samples. The WHO Guidelines for Drinking Water Quality were used as the standard permissible levels of the metals. To reveal any cases exceeding the standard, the ratios between the measured concentrations and standard levels of the heavy metals are listed in Table 2. The concentration exceeding a value of 1 indicates that it is above the WHO standards for safe drinking water. The ratio of the measured value of water samples in Erdenet to the WHO guideline shows that centralized public wells (groundwater source 64 km away from Erdenet) have significant differences from groundwater sources in the Erdenet area, especially in concentrations of Ca and Mg. Water with high calcium and magnesium concentrations is considered hard water. While continuous use of hard water is not directly harmful to human health, it should be treated and softened as part of the water supply process. A non-centralized source of drinking water, which does not have a treatment system, increases the risk for the users.
The results of the comparison showed that Ca and Mg concentrations exceeded the permissible levels at the majority of sampling points and that Co, Mo, Sr, and Zn were in exceedance at a few sampling points. Other measured metal concentrations were below the permissible levels for all samples. The highest Ca concentrations of 277 and 261 mg/l occurred at sampling points B12 (natural spring) and B9 (tailing pond seepage), respectively, in September 2021. These two sampling points were located approximately 300 m apart. Overall, the Ca concentration exceeded the guideline value (75 mg/l) in 77.5% of all samples in September 2021 and 79% of all samples in February 2022. As a group, in September 2021, high Ca concentrations were observed in 56% of centralized-source public well, 56% of self-sourced public well, 100% of private well and 75% of natural spring sample February 2022, high Ca concentrations were observed in 45% of centralized-source public well, 50% of self-sourced public well, and 100% of private well samples. The tailing pond seepage point had a high Ca concentration in both sampling periods. It is noteworthy that the private wells, which were located mostly near the mine tailing pond, had high Ca concentrations (Fig. 3). Mg concentrations showed a trend similar to the trend of Ca. Mg concentrations exceeded the permissible level established by WHO in all samples of private wells in both sampling periods. Usage of lime (CaO) to neutralize acidic wastewater from the mining area could be a major source of Ca [16]. Because of its solubility in water, the Ca may have infiltrated into the groundwater. High lime concentration deposits known as “white dust” are another issue associated with the mining activity in Erdenet [16]. The high concentrations of Ca and Mg in this study are in agreement with the results of the study by [16]). They concluded that the tailing pond is the likely source of groundwater contamination, but other sources also provide a continuous supply of metals. Sampling point B174, which is a centralized-source public well, is located between the mining open pit and the tailing pond and is surrounded by private wells with high Ca and Mg concentrations. Even so, point B174 contained noticeably lower concentrations of heavy metals because its water source is the “Buren Bust” central source. The difference implies a common source of Ca and Mg in private wells, which is probably related to tailing pond seepage and the bedrock geological formation.
According to the geological map of Erdenet [25], the western and northern parts of the tailing pond and the surrounding of open pit, where the private wells are located, have Permian Selenge Complex granodiorite–granite suite bedrock, whereas the residential area of Erdenet, where the centralized-source public well are located, lies in Quaternary cover. The mean, minimum, and maximum values of Ca and Mg concentration in the private wells all exceed the WHO drinking water guideline (Fig. 4). The private wells do not have any treatment system, which makes the private-well water vulnerable to any pollution and risky for consumption. Mo concentration was high at four sampling points in September 2021 (Fig. 5). Sampling point B9 (tailing pond seepage water) had a level of 1100 µg/l in September 2021, which is 2 times higher than Mongolian National Standard for Industrial Waste Water, and 650 µg/l in February 2022. Furthermore, sampling point B12 (natural spring), which was close to the tailing pond, had a similar concentration of 1056 µg/l in September 2021, which is 15 times higher than the permissible level.
The WHO guideline mentions that high Mo concentrations of more than 200 µg/l may in some cases occur in mining areas [35]. Several other studies have reported high Mo concentrations, likely originated from the earth’s crust, around mining areas [39]. Sampling point B10 (self-source public well) also showed a high Mo concentration of 122 µg/l in September 2021, but the value was lower (55.0 µg/l) in February 2022. Sampling point B150, a private well located near the open pit of the Erdenet mine, had a Mo concentration of 118 µg/l in September 2021. The highest Sr concentrations observed in September 2021 were 3540 µg/l at B150 (private well), 3170 µg/l at B12 (spring), and 2640 µg/l at B9 (tailing pond seepage). We used the health reference level of Sr from the WHO guideline (1500 µg/l) as the standard value. However, if we consider the minimum reporting level, which is 0.3 µg/l, all samples exceed this limit. [40] considered 0.3 µg/l as a threshold value, and all of their samples (n = 60) in northwest China exceeded it. However, their health risk assessment indicated a low level of potential carcinogenic risk from Sr, and they concluded that the Sr concentration in the drinking water in the study area would have no adverse effects on human health [40].
However, the origin of Sr in sourced groundwater can be aquifer bedrock, pipeline, or water container, and proper health risk assessment is crucial for public health [41]. Sampling point B9, an output of tailing pond point, and point B12, located 300 m distant, showed very similar trends of heavy metal concentrations, which suggests that the tailing pond seepage directly affects the proximal groundwater. In Table 3, we compared our results with those of other countries' mining areas. As compared with the elements measured in our study, measurements of As, Ba, Mo, Sr, and U were not performed in most of the other countries that we compared, with the exception of Iran. In Bangladesh and Iran, all measured element concentrations were higher than the results of our study. These researchers stated that the high heavy metal concentrations were related to anthropogenic and geogenic sources and that consumers of the groundwater were under health risk [6, 33]. An old silver mine site in Greece, where mining operations had been stopped a long time prior, had the lowest concentrations. A coal mine site in China showed results similar to the results of our study, and the authors concluded that the carcinogenic risk in this area, especially the carcinogenic risk from Cr, is higher than the acceptable limits established by various organizations [40]. The elevated concentrations of Ca and Mg in the groundwater samples are likely a result of mining activities in Erdenet, specifically from the tailing pond and other sources such as the bedrock geological formation. The study’s findings suggest that the tailing pond and other sources, including the bedrock geological formation, are contributing to the contamination of groundwater in Erdenet with heavy metals such as Ca, Mg, and Mo. Private wells located near the tailing pond and surrounded by bedrock geological formations showed higher concentrations of heavy metals such as Ca, Mg, and Mo in their groundwater samples. These findings highlight the potential risks associated with consuming groundwater from private wells in areas affected by mining activities and emphasize the need for proper water treatment systems to ensure safe drinking water for the residents. The study revealed that the presence of the tailing pond and the bedrock geological formation in Erdenet are contributing factors to the contamination of groundwater with heavy metals such as Ca, Mg, and Mo. This poses a significant risk to the residents relying on private wells for their drinking water, as these sources are highly susceptible to pollution from mining activities.
Table 4 shows the results of Kolmogorov–Smirnov and Shapiro–Wilk tests of normality (tests statistic, degrees of freedom, p-value). Since we have less than 50 observations (September 2021 N = 40, February 2022 N = 29), we will interpret the Shapiro–Wilk test results. In September 2021, p-value of EC p = 0.335, other measures are less than 0.05. In February 2022, p-value of alkalinity, pH and Mg are greater than 0.05, other measures are less than 0.05. The strongest correlations were between Mo and Cd (r = 0.987 in Sep., r = 0.947 in Feb.), Mg and Ca (r = 0.871 in Sep., r = 0.893 in Feb.), Zn and Pb (r = 0.869 in Sep., r = 0.686 in Feb.), Sr and Mg (r = 0.842 in Sep., r = 0.886 in Feb.), and Mg and EC (r = 0.816 in both Sep. and Feb.) (Table 5). Heavy metal concentrations had correlations moderately positive to moderately negative with alkalinity, weakly positive to moderately negative with pH, and moderately negative to strongly positive with EC.
The study utilized the weighted-pair group method for statistical analysis to demonstrate the similarity in nature and origin of various elements within the research area. This approach is commonly employed in interpreting data on heavy metal pollution in environmental studies. Classification in the same cluster shows that the elements have similar sources and behavior in the groundwater system, indicating potential common pollution sources or geological processes influencing their distribution[43]. Figure 6 shows 2 main clusters A and B. Cluster B is including points with the highest Mo, Sr concentration among all sampling points which are tailing pond discharge point, a spring close to tailing pond discharge and a private well next to open pit. Among cluster B, centralized sourced public wells are tend to be together and mixed with private wells. Measured heavy metals distribution behavior similarity is shown in Fig. 7. A dendrogram exhibits distinct divisions into clusters A and B. Elements like As, Cr, Co, Cd and Pb serve as indicators for anthropogenic activities [44, 45]. The fact that these elements are grouped together in the same clusters implies that the metals in cluster A originate from human activities, particularly mining operations in our research area. Meanwhile, in cluster B, Strontium levels linked to natural geological processes. The findings align with research on the Erdenet region, which indicates that the soil in this area is significantly impacted by the extensive presence of volcanic rocks enriched with Sr [46].
3.3 Pollution evaluation indices
Water quality indices facilitate comparability and understandability of results. The HPI, HEI, and Cd indices are commonly used for water quality analyses of heavy metals. The HPI threshold value is 100. If a calculated value exceeds this value, the water is defined as unsuitable for drinking and other households purpose [32]. A similar definition is used for HEI, but the threshold value is 1. The Cd indicates the degree of pollution, with categories of low (Cd < 1), medium (1 < Cd < 3), and high (Cd > 3). The water chemical pollutants of As, Ba, Cd, Cr, Cu, Mo, Ni, Pb, and U concentrations and the ideal and maximum admissible levels of each metal established by WHO were used for calculation of water quality indices (Table 6). The most concerning points are B9 (tailing pond seepage) and B12 (spring). The water at B9 flows directly to the river. According to an interview with local residents, people take water from point B12 because it is a natural spring. However, the number of people who use water from this point, the usage purposes, and the profile remain uncertain. Using the water without consideration of these water characteristics or any treatment increases the vulnerability to heavy metal pollution. In September 2021, the HPI values of the centralized-source public well, self-source public well, private well, and spring samples were 43.9–50.8, 41.5–54.8, 46.9–51.4, and 47.8–74.5, respectively. The HEI values were 0.2–0.9, 0.1–2.8, 0.07–2.3, and 0.15–16.9, respectively, and the Cd values were 0, 0–0.75, 0–0.7, and 0–14.7, respectively. The water quality indices were slightly lower in February 2022. The HPI values were 46.9–49.6, 47.7–56.8, 40.5–49.9, and 50.4, respectively. The HEI values were 0.14–0.7, 0.11–1.5, 0.2–0.8, and 0.8, respectively, and Cd was 0 at all groundwater wells. For better understanding and more accurate classification, we used the mean value method to obtain a modified classification (Table 7) [31]. Degree of pollution is classified as ‘low’—mean value of a group sample, ‘medium’—between mean and 2 times of mean value a group sample, ‘high’—excess value than 2 times of mean value of a group sample. According to the modified classification of water quality indices, “high” degree of pollution is not observed among centralized sourced public well. Whereas, self-sourced public wells, private wells and spring water show “high” degree of pollution with 9–25% of samples. As centralized sourced public wells water source is not in Erdenet area, which is 64 km away, showing significant water quality difference compared to water source in Erdenet area. Nevertheless, the contents of Ca, Mg, Mo, and Sr exceed the maximum permissible values for drinking water established by the WHO Guidelines. In particular, high Ca and Mg contents occur in private wells, for the following reasons. First, the private wells are located near the mine tailing pond, which is likely to be a source of contamination due to seepage of waste water from the mine through soil pores [47].
Second, the depth of private wells ranges from 8 to 80 m, whereas the depth of self-source public wells ranges from 70 to 90 m. Moreover, previous research indicated that the tailing pond does not have a protection layer at its bottom [16]. An additional reason is dissolution of the mineral contents in the bedrock of the area. A previous study of the surface water in the Erdenet area by [17] concluded that surface water in this area is CaSO4-rich type water. Due to rivers in this area are sourced from springs, the Ca and Mg concentrations may occur naturally. However, more detailed research will be required to establish the sources of the mineral contents in the groundwater of Erdenet. The Mo content is high at a few points, and those close to the mine tailings ponds and mine sites are the most concerning. The HPI, HEI, and Cd had not been used previously for groundwater quality assessment in this study area. Therefore, the results of this study are particularly useful for further studies on the water quality. Different water quality indices show slightly different results due to how each index is calculated. As shown on Fig. 8, we assume that HEI is showing more “high” category. Sampling points with high Mo concentration are categorized with “high” degree of heavy metal pollution and these wells are not suitable for drinking water supply.
4 Conclusion
This study revealed some points of concern. Samples from private wells showed high concentrations of Ca and Mg. These private wells are located along the western side of the tailing pond and the northern side of the open pit of the Erdenet mine. Because the wells are located near the tailing pond, they are at risk for contamination, and seepage water from the tailing pond through soil pores is a very likely source of contamination. As a result of cluster analysis, all measured heavy metal concentration (except Sr) is related to anthropogenic activity.
Consumers of water from private wells should consider their drinking water quality more carefully because treatment or disinfection methods are not utilized at these wells. The locations of these wells also make consumers more vulnerable to risk than consumers of water from the centralized-source public wells.
An additional concern is that wastewater with very high concentrations of Mo is seeping into the environment. Proof of this fact is provided by a natural spring located 300 m from the tailing pond that shows a result similar to that of the tailing pond seepage water. The presence of Mo is a well-known environmental concern in mining areas [48]. Future potential risk from Mo and Sr must be clarified in this area due to waste water from the tailing pond.
A modified categorization of the water quality indices for the groundwater wells in the study area revealed that the wells generally have “low–medium” level, and at few points “high” level of heavy metals. However, because some elements such as Mo and Sr are high at the tailing pond and in wells near the tailing pond, additional detailed analyses and human health risk assessments are essential for complete assessment of water quality and public health risk.
It is highly important to consider the water source and characteristics of wells in detail in the future when conducting groundwater research in mining areas. Specifically, consumers in our study area obtain their drinking water from a variety of sources and wells, and their daily behavior may affect their risk level.
Data availability
Data and materials will be available on request.
References
JJ. Fried, Groundwater Pollution. in ISSN. Elsevier Science, 1975. https://books.google.co.jp/books?id=8cKxHnsOKCkC
Zamani AA, Yaftian MR, Parizanganeh A. Multivariate statistical assessment of heavy metal pollution sources of groundwater around a lead and zinc plant. Iran J Environ Health Sci Eng. 2012;9(1):29. https://doi.org/10.1186/1735-2746-9-29.
He B, Yun Z, Shi J, Jiang G. Research progress of heavy metal pollution in China: sources, analytical methods, status, and toxicity. Chin Sci Bull. 2013;58(2):134–40. https://doi.org/10.1007/s11434-012-5541-0.
Gong X, Chen Z, Luo Z. Spatial distribution, temporal variation, and sources of heavy metal pollution in groundwater of a century-old nonferrous metal mining and smelting area in China. Environ Monit Assess. 2014;186(12):9101–16. https://doi.org/10.1007/s10661-014-4069-y.
Wang L, Tao Y, Su B, Wang L, Liu P. Environmental and health risks posed by heavy metal contamination of groundwater in the sunan coal mine, China. Toxics. 2022. https://doi.org/10.3390/toxics10070390.
Jahanshahi R, Zare M. Assessment of heavy metals pollution in groundwater of Golgohar iron ore mine area, Iran. Environ Earth Sci. 2015;74(1):505–20. https://doi.org/10.1007/s12665-015-4057-8.
Mehrabi B, Mehrabani S, Rafiei B, Yaghoubi B. Assessment of metal contamination in groundwater and soils in the Ahangaran mining district, west of Iran. Environ Monit Assess. 2015;187(12):727. https://doi.org/10.1007/s10661-015-4864-0.
Mirzabeygi M, et al. Heavy metal contamination and health risk assessment in drinking water of Sistan and Baluchistan, Southeastern Iran. Hum Ecol Risk Assess Int J. 2017;23(8):1893–905. https://doi.org/10.1080/10807039.2017.1322895.
Ahmadi S, Jahanshahi R, Moeini V, Mali S. Assessment of hydrochemistry and heavy metals pollution in the groundwater of Ardestan mineral exploration area, Iran. Environ Earth Sci. 2018;77(5):212. https://doi.org/10.1007/s12665-018-7393-7.
Asian Development Bank. Overview of Mongolia’s water resources system and management. Ulaanbaatar: A Country Water Security Assessment; 2020.
N. Batsukh, D. Dorjsuren, and G. Batsaikhan, “The water resources, use and conservation in Mongolia (First national report),” Ministry of Nature and Environment of Mongolia, Ulaanbaatar, 2008.
Ministry of Environment and Green Development of Mongolia. Integrated Water resources management plan of Mongolia. Ulaanbaatar: Selenge Press; 2013.
TR Kamata, JA Tsevegmid, T Kim, Y Sedgewick. Mongolia-Enhancing policies and practices for ger area development in Ulaanbaatar. Disclosure, 2011.
Munkhtsengel B, Ohara M, Gerel O, Dandar S, Tsuchiya N. Preliminary study of formation mechanism of the Erdenetiin Ovoo porphyry copper-molybdenum deposit and environmental effects of erdenet mine, Northern Mongolia. AIP Conf Proc. 2006;833(1):204–7. https://doi.org/10.1063/1.2207106.
Jargalsaihan B, Orlova NA, Suzdaleva AL. Geoecological situation in the region of the Erdenet mining and processing facility (Mongolia). E3S Web Conf. 2023. https://doi.org/10.1051/e3sconf/202337103053.
Battogtokh B, Lee JM, Woo N. Contamination of water and soil by the Erdenet copper–molybdenum mine in Mongolia. Environ Earth Sci. 2014;71(8):3363–74. https://doi.org/10.1007/s12665-013-2727-y.
Solongo T, et al. Distribution and chemical speciation of molybdenum in river and pond sediments affected by mining activity in Erdenet City, Mongolia. Minerals. 2018. https://doi.org/10.3390/min8070288.
Tsetsgee S, et al. Mo contamination in rivers near the Erdenet mining area, Mongolia: Field evidence of high mobility of Mo at pH >8. ACS EST Water. 2021;1(8):1686–94. https://doi.org/10.1021/acsestwater.1c00046.
C. R. Evanko and D. A. Dzombak. Remediation of Metals-Contaminated Soils and Groundwater. Ground-Water Remediation Technologies Analysis Center, Pittsburgh, Technology Evaluation Report, 1997.
Sankhla MS, Kumari M, Nandan M, Kumar R, Agrawal P. Heavy metals contamination in water and their hazardous effect on human health-a review. Int J Curr Microbiol App Sci. 2016;5(10):759–66.
Morais S, Costa FG, de Pereira ML. Heavy metals and human health. Environ Heal Issues Pract. 2012;10(1):227–45.
Martin S, Griswold W. Human health effects of heavy metals. Environ Sci Technol Briefs Citiz. 2009;15:1–6.
Timofeev IV, Kosheleva NE, Kasimov NS, Gunin PD, Sandag E-A. Geochemical transformation of soil cover in copper–molybdenum mining areas (Erdenet, Mongolia). J Soils Sediments. 2016;16(4):1225–37. https://doi.org/10.1007/s11368-015-1126-2.
National Statistics Office of Mongolia. National Statistics Office of Mongolia. Accessed: Mar. 09, 2023. https://1212.mn/mn
Gerel O, et al. Geochemistry of granitoids and altered rocks of the Erdenet porphyry copper-molybdenum deposit, central Mongolia. In: Mao J, Bierlein FP, editors., et al., Mineral deposit research: meeting the global challenge. Heidelberg: Springer, Berlin Heidelberg; 2005. p. 1137–40.
Berzina AP, Sotnikov VI. Character of formation of the Erdenet-Ovoo porphyry Cu-Mo magmatic center (northern Mongolia) in the zone of influence of a Permo-Triassic plume. Russ Geol Geophys. 2007;48(2):141–56. https://doi.org/10.1016/j.rgg.2007.01.001.
R. Battumur, D. Khatanbaatar, T. Beejinkhuu, and T. Enkhjargal, “Report on the final results of groundwater prospecting, preliminary and detailed exploration between 2002–2003 for an independent new source of technical and domestic centralized water supply in Orkhon aimag center,” Ministry of Industry and Commerce of Mongolia, Ulaanbaatar, 2004.
Erdenet Mining Corporation. Erdenet Mining Corporation. Official website of Erdenet Mining. Accessed: Dec. 25, 2023. https://www.erdenetmc.mn
Mongolian Academy of Science, “Research on the quality of drinking water in settlements of Orkhon province, conclusions and recommendations,” Ulaanbaatar, 2019.
Mohan SV, Nithila P, Reddy SJ. Estimation of heavy metals in drinking water and development of heavy metal pollution index. J Environ Sci Health Part Environ Sci Eng Toxicol. 1996;31(2):283–9. https://doi.org/10.1080/10934529609376357.
Edet A, Offiong O. Evaluation of water quality pollution indices for heavy metal contamination monitoring. A study case from Akpabuyo-Odukpani area, Lower Cross River Basin (southeastern Nigeria). GeoJournal. 2002;57(4):295–304.
Prasanna M, Praveena S, Chidambaram S, Nagarajan R, Elayaraja A. Evaluation of water quality pollution indices for heavy metal contamination monitoring: a case study from Curtin Lake, Miri City, East Malaysia. Environ Earth Sci. 2012;67(7):1987–2001.
Biswas PK, Uddin N, Alam S, Sultana S, Ahmed T. Evaluation of heavy metal pollution indices in irrigation and drinking water systems of Barapukuria coal mine area, Bangladesh. Am J Water Resour. 2017;5(5):146–51.
Singh R, Venkatesh A, Syed TH, Reddy A, Kumar M, Kurakalva RM. Assessment of potentially toxic trace elements contamination in groundwater resources of the coal mining area of the Korba Coalfield, Central India. Environ Earth Sci. 2017;76(16):1–17.
World Health Organization. Guidelines for drinking-water quality. Geneva: World health organization; 2004.
Chandrasekar N, Selvakumar S, Yasala S, Wilson J, Peter T, M S. Hydrogeochemical assessment of groundwater quality along the coastal aquifers of southern Tamil Nadu, India. Environ Earth Sci. 2013. https://doi.org/10.1007/s12665-013-2864-3.
Utom AU, Odoh BI, Egboka BC. Assessment of hydrogeochemical characteristics of groundwater quality in the vicinity of Okpara coal and Obwetti fireclay mines, near Enugu town, Nigeria. Appl Water Sci. 2013;3(1):271–83.
Wang Y, Dong R, Zhou Y, Luo X. Characteristics of groundwater discharge to river and related heavy metal transportation in a mountain mining area of Dabaoshan, Southern China. Sci Total Environ. 2019;679:346–58.
Smedley PL, Kinniburgh DG. Molybdenum in natural waters: a review of occurrence, distributions and controls. Appl Geochem. 2017;84:387–432. https://doi.org/10.1016/j.apgeochem.2017.05.008.
Zhang S, Liu G, Sun R, Wu D. Health risk assessment of heavy metals in groundwater of coal mining area: a case study in Dingji coal mine, Huainan coalfield, China. Hum Ecol Risk Assess Int J. 2016;22(7):1469–79. https://doi.org/10.1080/10807039.2016.1185689.
Peng H, Yao F, Xiong S, Wu Z, Niu G, Lu T. Strontium in public drinking water and associated public health risks in Chinese cities. Environ Sci Pollut Res. 2021;28(18):23048–59. https://doi.org/10.1007/s11356-021-12378-y.
Stamatis G, Voudouris K, Karefilakis F. Groundwater pollution by heavy metals in historical mining area of Lavrio, Attica, Greece. Water Air Soil Pollut. 2001;128(1):61–83. https://doi.org/10.1023/A:1010337718104.
Karbassi S, Nasrabadi T, Shahriari T. Metallic pollution of soil in the vicinity of national Iranian lead and zinc (NILZ) company. Environ Earth Sci. 2016;75(22):1433. https://doi.org/10.1007/s12665-016-6244-7.
Nasrabadi T, Abbasimaedeh P. Groundwater quality degradation of urban areas (case study: Tehran city, Iran). Environ Sci Technol. 2013. https://doi.org/10.1007/s13762-013-0340-y.
Karbassi AR. Anthropogenic share of metal contents in soils of urban areas. Pollution. 2018. https://doi.org/10.2205/poll.2018.261506.474.
Kosheleva N, Kasimov NS, Timofeev I. Potentially toxic elements in urban soil catenas of W-Mo (Zakamensk, Russia) and Cu-Mo (Erdenet, Mongolia) mining areas. J Soils Sediments. 2018. https://doi.org/10.1007/s11368-017-1897-8.
Sharma R, Al-Busaidi T. Groundwater pollution due to a tailings dam. Eng Geol. 2001;60(1–4):235–44.
Pichler T, Koopmann S. Should monitoring of molybdenum (Mo) in groundwater, drinking water and well permitting made mandatory? Environ Sci Technol. 2020;54(1):1–2. https://doi.org/10.1021/acs.est.9b06869.
Funding
Financial support was provided by the Japan Society for the Promotion of Science (Grant No. JP22KK0166 and JPJSBP120219915) the Higher Engineering Education Development Project, Functional material based on Mongolian Natural Minerals for Environmental Engineering, Cementitious and Float Process (No. J11A15) and PhD Student Support Program by Institute of Nature and Environmental Technology, Kanazawa University (Grant No. 22070).
Author information
Authors and Affiliations
Contributions
Conceptualization: EM, KF, TM, AO. Methodology: KF, EM, BG. Fieldwork and sampling: EM, AO, BG, TB. Laboratory analysis: EM, BG, KF. Writing—original draft preparation: EM. Writing—review and editing: KF, TM, AO, SN. Supervision: TM, KF, AO, SN.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Neither animal nor human data nor experiments are reported or used in this manuscript. Due to this, it is not applicable to this part. All authors have read, understood, and have complied as applicable with the statement on "Ethical responsibilities of Authors" as found in the Instructions for Authors.
Consent for publication
The final version of the manuscript was reviewed and approved by all authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Munkhsuld, E., Murayama, T., Fukushi, K. et al. Heavy metal concentrations and water quality assessment of different types of drinking water wells in the Erdenet Cu–Mo mining area. Discov Environ 2, 12 (2024). https://doi.org/10.1007/s44274-024-00037-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44274-024-00037-1