Abstract
The largest freshwater and oligotrophic lake in northern Myanmar, called Indawgyi Lake, is at potential risk of eutrophication due to the phosphorus (P) export from nearby paddy fields. In this study, the P status of the soil of paddy fields and its potential mobility to the lake water to cause eutrophication have been evaluated. The Indawgyi Lake is the third largest lake in Asia and became a UNESCO biosphere reserve in June 2017 for its outstanding contribution to providing habitat for threatened birds and aquatic species. Although many other sources, e.g. high tourist interest, loss of natural forest areas, the gathering of pilgrims and lack of proper wastewater treatment plants are contributing to the P nutrients input to the lake, the increase of paddy fields around the lake area and use of synthetic fertiliser which exports to the lake is considered to the primary source of P export to the lake. In this study, the soil P was examined with different P extracted methods and P export to the lake was calculated. The extracted P differed in order and magnitude with different methods, and the order was increased in accordance with CaCl2 < DL-P < Olsen-P < BD-P < HCL-P < TP and for iron, it was HCl-Fe < TFe. All extracted Olsen-P was below 10 mg kg−1, which is below the critical level (30–172 mg kg−1) for P loss. On the other hand, the soil has a high P sorption capacity, and the maximum sorption capacity among the soil samples that were done with the Langmuir equation is 0.790 mg g−1. Our findings suggested that paddy fields are not contributing to the eutrophication of the lake at present. However, comparing the historical map around the lake area from 1944 to 2017, it has been found that the area of paddy fields around the lake has increased almost three-fold from 1944 to 2017. As a result of increasing paddy fields and synthetic fertiliser use, we can say that P export from paddy fields can cause severe eutrophication to the lake in the near future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Eutrophication in lakes has become a significant problem worldwide [1, 2]. Although it can occur naturally, it is often accelerated by human activities [1]. These sources can introduce excess nutrients into waterways. While some level of nutrients is necessary for aquatic life, an excess can lead to a series of negative consequences. The increase in plant and algae growth caused by eutrophication can lead to the depletion of oxygen in the water, as these organisms consume large amounts of oxygen during decomposition. This can result in the death of fish and other aquatic organisms that require oxygen to survive [3]. Additionally, large amounts of decaying organic matter can cause unpleasant odours and discolouration of the water [4]. It has been reported that high external nutrient input from domestic sewage, intensive agricultural activities, and pollutants from industries are responsible for breaking the terrestrial ecosystem of the catchment and causing eutrophication [1]. Among the various nutrients, phosphorus (P) input into the freshwater ecosystem is mainly responsible for the acceleration of eutrophication processes [5]. Although the cause and consequences of eutrophication are the same both in temperate and tropical regions, tropical lakes are considered more sensitive to eutrophication than temperate lakes [6, 7]. Among many other sources, agriculture has been identified as the primary P input source in most freshwater ecosystems, contributing to eutrophication [8].
Myanmar is an agro-based country, and the dominant cultivation is rice production. Before World War (ii), it was one of the largest rice-producing countries in the world [9]. It decreased afterwards. However, due to population growth, rice cultivation is increasing nationwide at a higher pace than before. Indawgyi Lake is the largest lake in Myanmar, situated in Kachin state in the northern part of the country (Fig. 1), and has been declared a UNESCO biosphere reserve since June 2017 [10].
Wetlands, intact watersheds, and subtropical moist forests surround the lake. Indawgyi Lake and its adjacent forest and wetland are the habitats for endemic and threatened birds, fish, turtles, and mammals [11]. Although the lake is both ecologically and economically important, not much research has been done to check out the lake's water ecology and risk of eutrophication. The lake's water ecology needs to be examined as its quality has been exploited by P and other nutrients imported from the surrounding paddy fields, pollution from illegal gold mining activities, and poor sanitation management of the people [10]. Other intensive uses of the area, e.g. tourist use, immigration, and pilgrim, are likely also causing the lake's P import and water quality degradation. The areas surrounding the lake area is suitable for paddy cultivation [12], which might be the primary contributor to P import to the lake. According to Grüneberg et al. [10], around 39,000 people live around the lake area, and the population is increasing daily. It has been found from the survey report of [13] that about 80% of the people in the Indawgyi region are directly engaged with agriculture for their livelihood. For rice cultivation, the farmers are already using fertilisers that contain P, Nitrogen (N) and Potassium (K) in them (Table 1).
Grüneberg et al. [10] have already found signs of eutrophication in the lake. The absence of sensitive species (Hydrilla verticillata, Potamogeton lucens, Utricularia or Chara spp.) on the west and east banks of the Indawgyi Lake could be an indication of nutrient inputs occurring predominantly in the lake areas [14]. The status of P load to the lake can be identified with proper quantification and evaluation of the amount of P export from paddy fields. The P load can be determined using the P budget calculation with the export coefficient for different fertiliser use. In this work, the characterization of P-status and the potential mobility of P based on soil P extractions and adsorption experiments have been done. With the derivation of the P export coefficient, we have tried to evaluate the contribution of rice cultivation to Indawgyi Lake's eutrophication.
2 Materials and methods
2.1 Study sites description
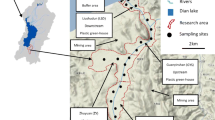
The Indawgyi Lake is in northern Myanmar's Mohnyin Township, Kachin State. It is a mountainous area where the mountains run from north to south. Above sea level, the height is 685 feet (PROCEEDING). The Indawgyi Lake flows from south to north, entering Mogaung chaung [15]. It is the main attraction of the Indawgyi Lake Wildlife Sanctuary. It is a tectonic lake measuring approximately 24 kms (km) long and 13 km wide [16]. The lake is fed by several rivers and streams, with the Mali Hka River being the main inlet and the Hka Sin River being the outlet. The average yearly temperature is 17–28 °C, rainfall is 1,789 mm, and mean relative humidity is 80–90% [17]. Being a good source of fishing, grazing and agriculture, the lake's surrounding area is inhabited by indigenous Kachin communities. It has been reported that ten villages have already been established on the fringe of the lake [18]. The north of the lake has meadow swampy soil with a pH of 6, and the lower southern area has alluvial gleysol with high clay (70%) content with a pH of 6.5 [17]. Our study soils and sediment were collected from the southern part of the lake (Fig. 2).
2.2 Sample collection and preparation
A total of six samples were collected from the nearby paddy fields at Indawgyi Lake, among which five samples (Fig. 2) were soils from nearby rice fields (Table 2) around the lake, and one was from the sediment of the lake. The sediment (Fig. 2) was collected from nearby lake area (25° 03′ 10.6" N 96° 18′ 25.4" E). Samples were collected with the help of local rangers in January 2019. The upper ~ 10 cm of soil was sampled using a stainless-steel tube of 2 cm inner diameter and was stored in airtight plastic containers. Immediately after arrival in Germany (about ten days after sampling), containers were stored at 4 °C in a refrigerator for several weeks until analysis. Additionally, one sample from surface sediment in shallow (< 0.3 m) water at the lake's south shore directly in the river's delta was also collected, stored and analyzed similarly to the soil samples. Although it was sampled in the lake, the material represents fine particulate matter imported by the river during the last rainy season.
3 Laboratory analysis
3.1 Soil properties determination
The pH was determined according to EN15933:2012(S4), which was translated and modified from the German language by the research lab. The pH for each sample was measured with a glass electrode (pH meter: WTW pH meter, pH 540 GLP, multical) and magnetic stirrer (HI 200 M) by taking 2 g of soil in a 50 ml centrifuge tube with 15 ml demineralized water. The results were saved when they became constant. Water content in the soil samples and loss of ignition (LOI) was determined with some modification of the method described by Bengtsson and Enell [19], and Heiri et al. [20]. The loss of ignition was calculated with the equation below-
Here, LOI550 represents LOI at 550 °C (as a percentage), which is the equivalent of organic matter (OM) content, DW105 represents the dry weight of the sample before combustion, and DW550 is the dry weight of the sample after heating to 550 °C.
3.2 P determination in soil
All types of P concentration in this experiment were determined colourimetrically, according to Murphy and Riley [21]. The photometric P (SRP) determination was done with the photometer (SPECORD-200, analytik jena) at extension E880 nm using a 1 cm cuvette. The Molybdenum blue method of Murphy and Riley [21] was modified to allow the detection of low-concentration P. In all P determinations, the samples were centrifuged 15 min at 5000 rpm to get a clear solution and then filtered with the filter paper (Ca- filter paper, 0.45 µm).
3.3 Soil total P determination
Total P (TP) determination was done according to Andersen [22] with some modifications. The procedure can be divided into three stages, e.g., ignition, digestion, and SRP analysis. The ignition was done with approximately 1 g of dried (105 °C) and pulverized sample at 550 °C. After cooling down in the desiccator, the contents of the crucible were quantitatively transferred into a 100 ml Erlenmeyer flask with demineralized water. Digestion was done by adding 15 ml of 2N HCl acid and proper boiling on a heat plate covered with a distillation cup filled with demineralized water. The extracted solution was neutralized with 0.5N NaOH using the p-dinitrophenol indicator and then used for TP determination.
3.4 Plant available P determination of soil
Plant available P was determined following the P determination with CaCl2-P determination, Olsen-P procedure, and double lactate (DL)-P Procedure.
3.4.1 CaCl2–P determination
Diluted CaCl2 (0.01 M) is mainly used as a substitute for water to obtain a clearer filtrate. However, the amount of soluble P in the CaCl2 is less than that in a water sample as part of the P is adsorbed with Ca2+ in soil [23]. For the preparation of the extraction solution, 0.01 M CaCl2, 1.47 g of CaCl2*2H2O were taken into a 1000 ml volumetric flask and poured deionised water up to the 1000 ml mark and mixed properly. This solution was used for P extraction from the soil. For the P extraction, approximately 2.7 g of the original wet soil were weighed for each sample into a 50 ml centrifuge tube. Then, 20 ml of 0.01 M CaCl2 was added to each sample and shaken for 2 h. When the samples were mixed properly with the solution, it was centrifuged for 15 min at 5000 rpm to get a clear solution. The sample was filtered with filter paper (Ca- filter paper, 0.45 µm), and before filtration of each sample, it was rinsed with the sample solution. The aliquot was then transferred to a labelled vial, and P was determined with SRP analysis using the ammonium molybdate-ascorbic acid method.
3.4.1.1 Olsen-P procedure
The method was developed by Olsen et al. [24], where alkaline sodium bicarbonate is used as an extraction solution. This method is applicable in the pH range of mildly acidic to alkaline range. So, the solution's pH should be adjusted at pH 8.5 with the use of NaOH alkali. The main principle of this method is that the OH− and the CO32− in the NaHCO3 solution decrease the activity of Ca2+ and Al3+ in the solution. As a result, the Al, Ca, and Fe bound P get released, increasing P in the solution. To prepare the extract solution (0.5 M NaHCO3 and pH 8.5), around 200 ml of demineralised water was taken into a volumetric flask. Then, 42 g of NaHCO3 was dissolved into it and mixed properly. The pH of the solution was adjusted to 8.5 by using 50% NaOH or 1N NaOH. Then the flask was filled up to 1L. To prepare the soil sample, approximately 1 g of wet soil was taken from each sample into a 50 ml Erlenmeyer flask. Then 20 ml of extracting solution was added into it and shaken for 30 min at room temperature. After proper mixing, the samples were taken for centrifuge for 15 min at 5000 rpm. Then, the solution was filtered with filter paper (Ca- 0.45 µm), and the aliquot was prepared for SRP analysis for P determination (after neutralisation with 0.5N NaOH using p-Dinitrophenol indicator).
3.4.1.2 DL-P procedure
The double lactate procedure [25] is a popular method for determining plant-available P in Europe, especially in Germany. In this method, calcium lactate is used for the preparation of the stock solution. To prepare the stock solution 120 g of calcium lactate pentahydrate (C6H10CaO6*5H2O) was taken into 1 L of volumetric flask containing 0.8 L boiling water and stirred until dissolved. Then 40 ml HCl (10 mol/L) was added to lower the pH and then filled up to 1 L. This can be used as a stock solution. 25 ml of DL stock solution was taken into 0.5 L water with pH 3.6 for the extraction solution. For P extraction, approximately 1 g of fresh soil was taken from each sample into a 50 ml centrifuge tube, and 40 ml of extraction solution was added. After shaking for 90 min, the sample was centrifuged for 15 min at 5000 rpm. Then, the solution was filtered with filter paper (Ca- 0.45 µm), and the aliquot was transferred into a levelled vial for SRP analysis. The aliquot was used for P determination using the ammonium molybdate-ascorbic acid method.
3.5 0.5N Fe-HCl extraction and redox-sensitive P determination
Inorganic P can be analyzed in 0.5N or 1N HCl. In this experiment, 0.5N HCl was used to analyze inorganic P and the amount of reactive Fe in the solution. The extraction solution was prepared by adding 25 ml of 0.5N HCl with approximately 0.5 g of fresh soil into a 50 ml centrifuge tube. According to Psenner et al. [26], Hupfer [27] and Grüneberg [28], redox-sensitive P with bicarbonate dithionite (BD) extraction is the second step of sequential P extraction. The BD is mainly reactive for Fe-bound P. The experiment was carried out by adding 20 ml of 0.11 M BD (prepared with NaHCO3 and Na2S2O4) solution into approximately 0.8 g of fresh soil. After extraction and filtration with filter paper (Ca- 0.45 µm), 3.5 ml Ethylenediaminetetraacetic acid (EDTA) and 15 ml filtrate solution was added, and the air was bubbled through the sample for one hour to expel excess dithionite before SRP analysis.
3.6 P adsorption experiment
Generally, adsorption experiments are carried out by making an equilibrium of an adsorptive solution of known composition, concentration, and volume and a known amount of adsorbent at a constant temperature and pressure for a while such that equilibrium is attained. The P adsorption experiment was done according to Nair and Graetz [29] for four soil samples with 10 sub-samples from each soil sample. The known concentration of P was 0, 0.25, 0.5, 1.0, 2.0, 5.0, 10, 20, 50 and 100 mg P L−1 that were prepared from KH2PO4 in 0.01 M CaCl2 solution. Approximately 0.7 g of fresh soil from 10 sub-samples from each soil was taken into a 50 ml centrifuge tube and treated with 20 ml of the known concentration of prepared P solution. After 3 h of centrifuge, it was filtered, and the procedure for P determination was followed through SRP analysis. The amount of P adsorbed (denoted by q) by the soil can be determined from the equation below [30]:
Here, q is the amount of adsorption (adsorbate per unit mass of adsorbent) in mg kg−1, Ci and Cf are the initial and final concentrations of adsorptive, V is the adsorptive volume, and EW is the mass of the adsorbent in kg. This quantity of adsorbent can be used to determine the adsorption isotherm.
The adsorption isotherm was adjusted with the Langmuir isotherm equation. The linear form of the equation is:
By plotting C/q vs C produces a linear line where the slope of the linear equation is 1/b, and the intercept is 1/kb. By solving the linear equation with slope and intercept, the maximum adsorption capacity (b) can be determined. The equilibrium P concentration (EPC0) refers to the concentration of phosphorus in the soil solution at which the net movement of phosphorus between the solid soil phase and the soil solution is near equilibrium. In other words, it's the point at which the rate of phosphorus release from the soil solid phase (e.g., minerals and organic matter) into the soil solution is roughly equal to the rate of phosphorus uptake by plants or the rate of phosphorus loss from the soil through various processes [31]. It can be calculated from the Freundlich sorption isotherm. The Freundlich equation can be expressed as:
where Kd is the distribution coefficient, and n is a correction factor [30]. The linear form of the equation can be written as-
A plot log C vs log q gives a linear equation which is used to determine the equilibrium P concentration, where log Kd is the intercept and 1/n is the slope.
3.7 P budget calculation
The OECD [32] empirical model was used to calculate the in-lake P concentration (Plake) and critical load (Lcrit) calculation. According to this method, the annual mean P (Plake) calculation is done with the equation:
where tR is the theoretical water retention time. The critical external load, Lcrit (g m−2 year−1), was calculated with the equation:
where Pcrit is the annual mean in-lake P concentration in mg L−1 for the good ecological status of the lake, qs is the hydraulic loading rate to the lake in m year−1, and τ is the theoretical water retention time in a year. The P export coefficient was calculated using the P export for without fertiliser uses, low fertiliser uses (fertiliser use < 30 kg per hectares per year (ha−1 year−1)) and high fertiliser uses (fertiliser use > 30 kg ha−1 year−1) from the literature [36,37,38,39,40,41,42].
4 Results
4.1 Soil properties determination
From the visual standpoint, the colour of the soil appeared reddish-brown to black. The fine texture of the soil was also felt in touch, indicating a high amount of clay content. Grüneberg et al. [10] found that the soil in the paddy fields around the lake is humus alluvial soils (gleysols). The pH of the soil (Table 3) indicates that the soil is slightly acidic, which matches the properties of gleysols [33].
4.2 P forms and adsorption properties
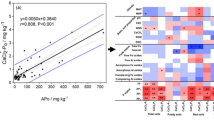
The extracted P with different methods (mean of all soils) increased in the order of CaCl2-P < DL-P < Olsen-P < BD-P < HCl-P < TP (Fig. 3). This sequence matches other researchers' findings [34] and reflects different extraction mechanisms. The P obtained from the soil samples with the CaCl2-P extraction method was below the detection limit. It has been found that the DL-P is roughly half of Olsen-P. The Olsen-P is still ~ 1% of TP and 8.5% of inorganic P. From the value of TP and HCl-P (Table 4), it can be said that organic P is the dominating P (~ 87% of TP) in the soil TP.
The adsorption of P (Table 5) was increased with the increased amount of P added to the solution. The graphical representation of Cf vs q (Fig. 4) is the sorption properties of soil from different paddy fields around Indawgyi Lake. The maximum sorption capacity (qmax) was calculated from the Langmuir sorption isotherm. The average maximum sorption capacity found for soil samples is 0.594 ± 0.145 mg g−1, which mainly depends not on the soil P content but the soil's properties (Fe and Al content). This amount of P adsorption is expected for tropical soil, which matches the result of De Campos et al. [35].
The equilibrium P concentration (Table 6) was calculated from the Freundlich sorption isotherm graph (Cf vs q), and the average equilibrium P concentration was 0.0021 ± 0.0013 mg L−1. The measured mean total iron content in the experimental soil was 48.2 ± 10.19 mg g−1.
4.3 P export from paddy fields
The actual P export from the paddy fields was calculated with different P export coefficient depending on the rate of fertiliser used (all calculation has been done in this experiment from the measured data values provided by the research lab). Three different cases were considered for paddy fields regarding the fertiliser used (no fertiliser used, low fertiliser used and high fertiliser used), where other scenarios (P export from the catchment, atmospheric deposition, tourist, population and washing detergent) were considered to be the same. As no export coefficient for the development of Indawgyi Lake is available, the export coefficient has been calculated using the mean value from different literatures. As a result, the export coefficient sometimes does not reflect the original characteristics of the considered area. The calculated mean export coefficient for the above conditions is provided in Table 7.
Based on the historical map of 1944, using GIS (Geographic Information System) mapping, it has been calculated that the rice cultivation area in the lowland around the lake has increased almost threefold in 2017 (Fig. 5). The geometric calculation shows that around 13.97% of the catchment area was used for rice farming in 2017. In 1944, the calculated area of paddy fields around the lake was 5041 ha, and during that time, farmers did not use any fertiliser for rice cultivation [10]. In that case, the P export from paddy fields has been calculated at 1.56 t year−1, and the total P export is 29 t year−1. In 2017, it was found that the paddy fields had increased to 15,642 ha [10]. Flora and Fauna International in Loneton, Myanmar, has informed the sample collection team that 4.0 kg P ha−1 year−1 is already being used for rice cultivation in the fields around the lake. Using the export coefficient of P, Grüneberg et al. [10] quantified an annual P import of 40–85 mg m−2 year−1 from paddy fields, which amounts to about 17% of the total annual P import. In that case, the P export from paddy fields is 4.8 t year−1, and the total P load to the lake is 32.3 t year−1. From the calculation, it has been found that if the use of P fertiliser increases by more than 30 kg ha−1 year−1 in future (assuming that the paddy fields and all other conditions described above will be the same as of 2017), the export of P from paddy fields will increase to 29.71 t year−1 and the total export will be 57.12 t year−1 (Table 8). Under the condition of no agricultural activities and sewages, the minimum P export is 9.43 t year−1 (Catchment forest and atmospheric deposition are considered).
Through the Empirical models (32, deep lake), the annual mean P concentration (Plake) can be calculated, which is essentially a model of sediment net retention of P. According to it, the annual mean P concentration (Pcrit) should not exceed 0.01 mg L−1 to ensure oligotrophic conditions. Using the mean P inflow concentration for 2017, the calculated P in-lake concentration (Plake) is 0.011 mg L−1. So, comparing these two values, it can be said that at present, the in-lake P concentration does not exceed the Pcrit value to retain the oligotrophic state of the lake. Using the value of total P export in 2017 mentioned above (32.3 t year−1), the calculated total external areal load to the lake, Lext is 0.261 g m−2 year−1. The critical load of P defines the maximum external P load into the lake for not exceeding the defined target value of Plake. The calculated critical load Lcrit is 0.238 g m−2 year−1.
5 Discussion
As the soil properties in the Indawgyi region are suitable for rice cultivation, paddy fields are rapidly increasing around the lake area. From the soil P extraction results, it has been found that the Olsen-P content in our soil is relatively low than the findings of Hua et al. [8] and Zhang et al. [43]. This may be the reason for using a low amount of fertilisers in Indawgyi paddy fields compared to the fertiliser used by Hua et al. [8]. Another reason for low plant-available P in the soils might be the low pH and high Fe content. The weather conditions and topography are also responsible for P loss from the paddy fields. For example, P is adsorbed by the soil or precipitated on the soil after the application of P fertiliser to the paddy fields. But, frequent drought and flooding can result in the desorbtion of P from the soil that was adsorbed into it [44]. It has been found that due to the flooding of paddy field's soil, the oxidation status of the soil decreases quickly, and pH goes to the neutrality [45]. For plant-available P, Thomason [46] has reported that below pH 5.5, aluminum becomes more abundant in soil, and most of the phosphates react with it and form the most stable aluminum phosphates. On the other hand, the adsorption properties of the soil increased with the increased amount of P added to the solution. The calculated low EPC0 also denotes that the soil would absorb further P from the soil solution even at a low P concentration. This might be because the soil contains a relative amount of iron, which can bind more P, increase P adsorption, and make them less available for plant uptake [35, 47]. In this experiment, the actual P export from the paddy fields was calculated considering three different scenarios. Calculating P runoff from paddy fields is important because it impacts the freshwater ecosystem and causes eutrophication. However, not much research has been conducted on P management and loss in paddy production [8]. The loss and export of P from paddy fields depend not on a single factor but on many correlated factors. The surface runoff from paddy fields, rate of fertiliser application, the form of fertiliser, amount of time after fertiliser application, rainfall, slope, temperature, soil P content, amount of metal oxides (mainly Fe oxides and Al oxides), the content in the soil, rate of precipitation and favourable hydrological conditions to move P from soil to water are important factors that contribute to P export from paddy fields. The rice cropping system is quite different from the upland cropping system as they are prepared with deep, water-impermeable plough pans, and the field berms are constructed on the soil surface (called field ponding water, FPW) [8]. So, during the rice-growing season, when excess rainfall happens, and the field berms exceed their capacity to hold water anymore, surface runoff occurs from paddy fields and mixes with the nearby water bodies. The concentration of total and dissolved P in the surface runoff from nearby paddy fields significantly correlates with the respective P forms in the FPW [8]. Several studies have reported that the P fertilization rate significantly affected P concentration in the runoff, especially for dissolved phosphorus (DP) [39, 43, 48]. Olsen-P and soil solution P, which are extractable with CaCl2, have a relationship. There is a "change point" of Olsen-P above which the CaCl2-P increases rapidly. This soil solution P can easily be removed with surface runoff when the water percolates into the soil profile [8]. With the increasing rate of P fertilization, the soil may be saturated with P. So, the long-term application of P exceeding the crop uptake and accumulation of Olsen P surplus above a certain point may severely increase the P loss to a greater extent [43, 49]. Although the change point of soil Olsen-P was not determined in Indawgyi soil, from the low Olsen-P value and very low CaCl2-P value (as it was below the detection limit), it indicates the low risk of P loss and export from the paddy fields to the Indawgyi Lake. In this experiment, the soil was acidic in nature, and the P adsorption was relatively high. Our experimented soil also has low CaCO3 content (5.3 ± 0.83% of the dry weight) that matches the acidic soil's characteristics. Zhai et al. [50] have reported that P binds more strongly in acidic soil containing (Fe or Al) oxides than in alkaline soil containing Ca and Mg. In that case, the export of P from alkaline soil is more than that of acidic soil containing Fe oxides and Al oxides.
Considering three different scenarios, the calculated P exports from the paddy fields will increase with the increase in rice cultivation and fertiliser use. From the Empirical models OECD [32], the calculated P in-lake concentration (Plake) is 0.011 mg L−1, which did not exceed the Pcrit value (0.01 mg L−1) to ensure oligotrophic conditions. Through this calculated value, it can also be assumed that the lake might still be in the oligotrophic state, but the actual trophic state can only be determined by water sampling and analysis. Nevertheless, the P budget calculation for Indawgyi Lake represents that increasing P fertiliser uses more than 30 kg ha−1 year−1 (in the future) will increase the Plake to 0.017 g m−3 (Table 9) that is for the mesotrophic state within the trophic category.
External measures are necessary when the external load exceeds the critical external load. By comparing the value of critical external load and calculated total external load, there is not too much P import to the lake according to the OECD [32] model. However, it has been mentioned above that Grüneberg et al. [10] have already found some signs of eutrophication, which indicates that the P load into the lake is much higher than the calculated external load. The critical load calculation with the empirical models can indicate the lake's trophic state, but it may have significant uncertainties. As a result, large errors may occur in determining the lake's trophic state by using only empirical models. However, the main drawback of the OECD [32] model is that this model has been prepared for temperate lakes and is not entirely appropriate for tropical lakes, which are known to be more sensitive to nutrient import. So, the oligotrophic state of the Indawgyi Lake with the present P import is at risk. Thus, external measures are necessary to retain the lake's trophic state. Without proper external treatment, eutrophication may become a severe problem for the lake. With time, the use of fertilisers in paddy fields are also increasing slowly [10]. If the farmers start to use fertilisers containing P more than 30 kg ha−1 year−1, then the total external areal load will increase to two times higher than the critical external load of the lake. At present, the trophic state of the lake is at risk with the present P import. The high external P load to the lake with increased fertiliser uses may cause hyper-eutrophication and deteriorate the lake's ecology. As the rice cultivation around the Indawgyi Lake area is increasing rapidly [10], the export of P from the paddy fields may increase simultaneously, which will degrade the lake's water quality and significantly impact the surrounding ecological environment.
6 Conclusion
As one of the biggest inland lakes in southeast Asia, Indawgyi Lake and the surrounding wetland are excessively used by humans. Although some signs of eutrophication have already been seen in the Indawgyi Lake [10], and it has been assumed that rice agriculture is a significant contributor to P export into the lake, a different result has been obtained from the soil P analysis of paddy fields of Indawgyi catchment area. The export of P from the paddy fields is still relatively low; nevertheless, rice cultivation can become a major contributing factor to changing the lake from its original oligotrophic state to a higher state. Furthermore, with the increase in fertiliser usage, P export from the paddy fields will also increase, which will eventually cause severe eutrophication to the lake. To protect the lake from severe eutrophication in the future, farmers should be educated on sustainable farming, such as using more organic fertilisers instead of chemical fertilisers. The population around the lake should also be controlled, and awareness among tourists needs to be increased to protect the current lake ecosystem and surrounding environment.
Data availability
Data is available upon request to the authors.
References
Beklioglu M, Meerfhoff M, Søndergaard M, Jeppesen E. Eutrophication and restoration of shallow lakes from a cold temperate to a warm Mediterranean and a (sub)tropical climate. In: Ansari, A. A., Gill, S. S., Lanza, G. R., Rast, W. (Eds). Eutrophication: Causes, Consequences and Control. 1970. Doi: https://doi.org/10.1007/978-90-481-9625-8_4
Le C, Zha Y, Li Y, et al. Eutrophication of Lake Waters in China: cost, causes, and control. Environ Manage. 2010. https://doi.org/10.1007/s00267-010-9440-3.
Chislock MF, Doster E, Zitomer RA, Wilson AE. Eutrophication: causes, consequences, and controls in aquatic ecosystems. Nat Educat Knowl. 2013;4(4):10.
George S. Hunt, the direct effects on some plants and animals of pollution in the great Lakes. Bioscience. 1965. https://doi.org/10.2307/1293295.
Sharpley A. Managing agricultural phosphorus to minimize water quality impacts. Sci Agric. 2016. https://doi.org/10.1590/0103-9016-2015-0107.
Lewis WM Jr. Basis for the protection and management of tropical lakes. Lakes Reserv Res Manage. 2000. https://doi.org/10.1046/j.1440-1770.2000.00091.x.
Mendiondo EM. Global Review of Lake and Reservoir Eutrophication and Associated Management Challenges. 2009. https://www.researchgate.net
Hua L, Liu J, Zhai L, Xi B, Zhang F, Wang H, Lu H, Chen A, Fu B. Risk of Phosphorus run off losses from five Chinese paddy soils under conventional management practice. Agriculture, ecosystem and environment. 2017. Doi: https://doi.org/10.1016/j.agee.2017.05.015
Naing TAA, Kingsbury AJ. Buerkert A, Finckh MR. A Survey of Myanmar Rice Production and Constraints. Journal of Agriculture and Rural Development in the Tropics and Subtropics. 2008; 109(2): 151–168
Grüneberg B, Rücker J, Kreber N, Leßmann D. Ausbreitung von submersen Makrophyten und Eutrophierungsproblematik im Indawgyi-See in Myanmar. Ergebnisse der Jahrestagung, 2018 in Kamp-Lintfort, Eigenverlag der DGL Essen. 2018;2019:125–37.
MONREC. Management Plan for the Multi-Internationally Designated Indawgyi Lake Basin. Ministry of Natural Resource and Environmental Conservation, Myanmar. 2017.
Frauke K, Than ZM. Socio-Economic developments of Indawgyi Lake Area, Kachin State, Myanmar. J Myanmar Acad Arts Sci Yangon Myanmar. 2011;5:281–99.
Bhangari BB, Nakamura H, Suzuki S. Indawgyi Lake: The One and Only Tectonic Lake in Myanmar-Experimental report of reconnaissance survey on 4–13 January 2015, Tokyo, Ramsar Center Japan. 2015
Kreber N. Gewässerökologischer Zustand und Eutrophierungsgefährdung des Indawgyi Sees in Myanmar. A master thesis submitted to Brandenburg University of Technology Cottbus-Senftenberg, Germany. 2018
Aung S. Agriculture and human impact on environment in indawgyi area: case study in mohnyin township, kachin state, myanmar. 9th Neu-kku International conference (On socio-economic and environmental issues in development). 2018;11:701–17
Ng PK, Mar W, Shih HT. Indawmon thanayei, a new genus and new species of freshwater crab (Crustacea: Brachyura: Potamidae) from Indawgyi Lake, Kachin State Myanmar. Raffles Bull Zoo. 2020. https://doi.org/10.26107/RBZ-2020-0069.
Than ZM. Socioeconomic Analysis of the Indawgyi Lake Area, Mohnyin Township. A thesis submitted to University of Cologne, Germany. 2011.
Ko CO, Haputta P, Gheewala SH. Estimation of the value of direct use ecosystem services of Indawgyi Lake Wildlife Sanctuary in Myanmar. J Sustain Energy Environ. 2020;11:11–20.
Bengtsson L, Enell M. Chemical analysis. In Berglund, B. E. (ed.), Handbook of Holocene Palaeoecology and Palaeohydrology. Wiley Ldt., Chichester. 1986; 423–451. ISBN 0 471 90691 3
Heiri O, Lotter AF, Lemcke G. Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J Paleolimnol. 2001. https://doi.org/10.1023/A:1008119611481.
Murphy J, Riley JP. A modified single solution method for the determination of phosphate in natural waters. Analyt Chim. 1962. https://doi.org/10.1016/S0003-2670(00)88444-5.
Andersen JM. An Ignition method for determination of total phosphorus in lake sediments. Water Res. 1976. https://doi.org/10.1016/0043-1354(76)90175-5.
Bigham JM, Schwertmann U, Traina SJ, Winland RL, Wolf M. Schwertmannite and the chemical modeling of iron in acid sulfate waters. Geochim Cosmochim Acta. 1996;60(12):2111–21. https://doi.org/10.1016/0016-7037(96)00091-9.
Olsen SR, Cole CV, Watanabe FS, Dean LA. Estimation of Available Phosphorus in Soils by extraction with Sodium Bicarbonate. U. S. Department of Agriculture. 1954; Circular No. 939
Kreimeyer M. Vergleichende Betrachtung unterschiedlicher Phosphor-Extraktionsverfahren für landwirtschaftliche Nutzböden. A thesis submitted to Ostfalia Hochschule für angewandte Wissenschaften, Germany. 2013
Psenner RV, Pucsko P, Sager M. Die Fraktionierung organischer und anorganischer Phosphorverbindungen von Sediment. Arch Hydrobiol. 1984;70:111–55.
Hupfer M, Gächter R, Giovanoli R. Transformation of phosphorus species in settling seston and during early sediment diagenesis. Aquat Sci. 1995. https://doi.org/10.1007/BF00878395.
Grüneberg B. Akkumulation, Transformation und Remobilisierung von Phosphor aus Sedimenten saurer Tagebauseen. PhD thesis, Brandenburg Univ. of Technology, Cottbus, Germany; 2006
Nair VD, Graetz DA. Phosphorus saturation in spodosols impacted by manure. J Environ Qual. 2002. https://doi.org/10.2134/jeq2002.1279.
Sparks DL, Page AL, Helmke PA, Loeppert RH. In Donald LS (Ed.): Methods of soil analysis. Madison, Wis., American Society of Agronomy (Soil Science Society of America book series, no. 5). 1996.
Jehangir HB, Samira HD, Timothy AL. Effect of kinetic control, soil:solution ratio, electrolyte cation, and others, on equilibrium phosphorus concentration. Geoderma. 2012;174:209–14. https://doi.org/10.1016/j.geoderma.2011.12.027.
Organization for Economic Co-operation and Development (OECD). Eutrophication of waters: monitoring, assessment and control. Paris: [Washington, DC: Organisation for Economic Co-operation and Development; Sold by OECD Publications and Information Center]; 1982
Mansfeldt T, Overesch M. Arsenic mobility and speciation in a gleysol with petrogleyic properties: a field and laboratory approach. J Environ Qual. 2014;42:1130–41.
Wuenscher R, Unterfrauner H, Peticzka R, Zehetner F. A comparison of 14 soil phosphorus extraction methods applied to 50 agricultural soils from Central Europe. Plant Soil Environ. 2015. https://doi.org/10.17221/932/2014-PSE.
Campos MD, Antonangelo A, Alleoni LRF. Phosphorus sorption index in humid tropical soils. Soil Tillage Res. 2015. https://doi.org/10.1016/j.still.2015.09.020.
Zhuo D, Liu L, Yu H, Yuan C. A national assessment of the effect of intensive agro-land use practices on nonpoint source pollution using emission scenarios and geo-spatial data. Environ Sci Pollut Res. 2018. https://doi.org/10.1007/s11356-017-0118-8.
Hua L, Zhai L, Liu J, Liu H, Zhang F, Fan X. Effect of irrigation-drainage unit on phosphorus interception in paddy field system. J Env Manage. 2019. https://doi.org/10.1016/j.jenvman.2019.01.059.
Guo HY, Zhu JG, Wang XR, Wu ZH, Zhang Z. Case study on nitrogen and phosphorus emissions from paddy field in Taihu region. Environ Geochem Health. 2004. https://doi.org/10.1023/B:EGAH.0000039583.71423.b4.
Liu J, Zuo Q, Zhai LM, Luo CY, Liu HB, Wang HY, Liu S, Zou GY, Ren TZ. Phosphorus losses via surface runoff in rice-wheat cropping systems as impacted by rainfall regimes and fertiliser applications. J Integr Agric. 2016. https://doi.org/10.1016/S2095-3119(15)61087-5.
Huang DF, Li WH, Wang LM, Lin XJ, Fan P, Qiu XX. Study on water and fertiliser managements and evaluation of nitrogen and phosphorus non-point source pollution from paddy field. Env Energy Sustain Develop. 2013;4:335.
Huang J, Gao J, Jiang Y, Yin H, Amiri BJ. Sources, distribution and export coefficient of phosphorus in lowland polders of Lake Taihu Basin China. Env Pollution. 2017. https://doi.org/10.1016/j.envpol.2017.08.089.
Leon A, Kohyama K. Estimating nitrogen and phosphorus losses from lowland paddy rice fields during cropping seasons and its application for life cycle assessment. J Clean Prod. 2017. https://doi.org/10.1016/j.jclepro.2017.06.116.
Zhang Z, Zhang JY, He R, Wang ZD, Zhu YM. Phosphorus interception in floodwater of paddy field during the rice growing season in TaiHu Lake Basin. Environ Pollut. 2007. https://doi.org/10.1016/j.envpol.2006.05.031.
Song KY, Zoh KD, Kang H. Release of phosphate in a wetland by changes in hydrological regime. Sci Total Environ. 2007. https://doi.org/10.1016/j.scitotenv.2006.11.035.
Zhang Z, Zhu Y, Cheng J, Bailey JS. Phosphorus export from a paddy rice field during flood events. Soil Use Manag. 2002. https://doi.org/10.1111/j.1475-2743.2002.tb00247.x.
Thomason W. Understanding Phosphorus Behavior in Soils. Noble research institute. 2002. https://www.noble.org/news/publications/ag-news-and-views/2002/january/understandingphosphorus-behavior-in-soils/ Accessed 18 March 2023
Fink JR, Inda AV, Tiecher T, Barrón V. Iron oxides and organic matter on soil phosphorus availability. Soil Tillage Res. 2016. https://doi.org/10.1590/1413-70542016404023016.
Smith DR, Owens PR, Leytem AB, Warnemuende EA. Nutrient losses from manure and fertiliser applications as impacted by time to first runoff event. Environ Pollut. 2007. https://doi.org/10.1016/j.envpol.2006.08.021.
Kleinman P, Sullivan D, Wolf A, Brandt R, Dou ZX, Elliot H, et al. Selection of water extractable Phosphorus test for manures and bio solids as an indicator of runoff potential. J Environ Qual. 2007. https://doi.org/10.2134/jeq2006.0450.
Zhai LM, CaiJi ZM, Liu J, Wang HY, Ren TZ, Gai XP, et al. Short-term effects of maize residue bio char on phosphorus availability in two soils with different phosphorus sorption capacities. Biol Fertl Soils. 2015. https://doi.org/10.1007/s00374-014-0954-3.
Acknowledgements
We would like to express our deepest gratitude and special thanks to our supervisor Dr. rer. nat. Björn Grüneberg, Department of Freshwater Conservation, Faculty of Environment and Natural Science at the Brandenburg University of Technology Cottbus-Senftenberg, Cottbus, Germany, who, despite being extraordinarily busy with his duties, took time out to listen, guide and keep us on the correct path and allow us to carry out our research. We also thank Ingo Henschke and Heike Garbe for collecting the samples with the help of local rangers.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors did not receive funding from funding agencies or organizations for the submitted work.
Author information
Authors and Affiliations
Contributions
MRH and SN, both worked in methodology, data analysis, manuscript preparation. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
There were no study participants, and ethics approval was waived.
Competing interests
We declare that all authors do not have any competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hashar, M.R., Nasrin, S. Phosphorus forms and adsorption properties of paddy field soils and potential risk to Indawgyi Lake, Myanmar. Discov Environ 2, 2 (2024). https://doi.org/10.1007/s44274-024-00028-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44274-024-00028-2