Abstract
This study present the factors controlling the aldehyde emission (formaldehyde, acetaldehyde, propionaldehyde, etc.) from plant species such as Pinus densiflora, Pinus koraiensis, Quercus acutissima, Quercus variabilis, Ginkgo biloba, and Oryza sativa. Even in the same tree species illustrate the emission rate variation of as much as 30 ~ 40%. The β-value, a parameter quantifying the temperature and emission correlation, were assessed as 0.07, 0.05 for formaldehyde and acetaldehyde, respectively. The coefficient of correlation was 0.4 and 0.5. for Quercus variabilis. In addition, the correlation between PAR (photosynthetically active radiation) and the emission is assessed close to the temperature impact on the emission. The emission of formaldehyde and acetaldehyde from P. densiflora, P. koraiensis, and Q. variabilis also depends on temperature and PAR. The ERs of total aldehydes of P. koraiensis are assessed at the level of 357.2 ng gdw−1 h−1, followed by propionaldehyde (110.6 ng gdw−1 h−1), acetaldehyde (102.5 ng gdw−1 h−1), and formaldehyde (73.66 ng gdw−1 h−1).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Over the past three decades, the importance of biogenic volatile organic compounds (BVOCs) for tropospheric photochemistry has been intensively studied. The studies demonstrate that BVOC emissions are an order of magnitude higher than those from anthropogenic sectors (AVOCs) (Goldstein & Galbally, 2007; Guenther et al., 2006). Furthermore, in general, BVOCs are more reactive to the tropospheric oxidant such as hydroxyl radical (OH), ozone, and nitrate radical (NO3) than AVOCs, which has tremendous implications of their photochemistry in regional to global air quality and climate by producing ozone and secondary organic aerosols (SOA).
Although terpenoids such as isoprene, monoterpenes, and sesquiterpenes (Monson et al., 1995; Ling et al., 2011) are the major BVOC species, oxygenated VOCs such as carbonyl compounds are also known to be emitted from certain tree species. In particular, aldehydes are known to be emitted from various plants. Stotzky and Schenk (1976) presented experimental findings that formaldehyde and acetaldehyde emission from young trees about to be germinated. In addition, acetaldehyde emissions tend to increase when plants experience environmental stress (Kimmer & Kozlowski, 1982; Kimmer & MacDonald, 1987). Nondek et al. (1992) reported that formaldehyde and acetaldehyde were emitted from forests, and Martin et al. (1991) measured the daily change of formaldehyde in coniferous forests, showing the highest concentration at about 10 ppb at noon likely from isoprene oxidation processes. Kesselmeier et al. (1997a) demonstrated that significant amounts of formaldehyde and acetaldehyde are emitted from Quercus ilex (holmoak) and Pinus pinea (Italian stone pine). In addition, there are studies demonstrating acetaldehyde emission from crops. Arey et al. (1991) reported that hexanal and (E)-2-hexenal are emitted from cotton and alfalfa, and Jork (1996) presented formaldehyde and acetaldehyde emission from some crops.
Just like any other BVOCs, acetaldehyde and formaldehyde emissions are controlled by environmental factors. The acetaldehyde emission in general illustrates positive correlations with temperature and PAR (Calfapietra et al., 2013; Kim et al., 2020; Loreto & Schnitzler, 2010). In addition to the physical environmental factors, environmental stress factors such as air pollution, herbivores can also enhance carbonyl emissions (Ülo Niinemets, 2004).
Although terpenoid species have been the target for the studies to investigate the correlations between emission rates and environmental factors (Lim et al., 2008; Lüpke et al., 2016; Son et al., 2015; Vedel-Petersen et al., 2015), there have been limited studies investigating the controlling factors of the carbonyl emissions (Mellouki et al., 2015; Millet et al., 2010; Kesselmeier et al., 1997a).
This study presents the emission factors and the correlations of environmental factors such as temperature and PAR and emission rates for the tree species of Pinus densiflora, Pinus koraiensis, Quercus acutissima, Quercus variabilis, Ginkgo biloba, and Oryza sativa which accounts for about 13% of tree species of South Korea.
2 Experiment and analysis methodology
2.1 Target tree species and sampling sites
The studied tree species and the experimental methods associated with quantifying emission rates of aldehyde and formaldehyde are thoroughly discussed in Kim et al. (2020). The discussion presented in this paper is based on the results, presented in Kim et al. (2020). While the previous paper focused on comparing the emission rate of aldehyde according to tree species and the emission characteristics of terpene and aldehyde emitted from trees, this paper focused on how aldehyde emission correlates with temperature, PAR, and habitat environment.
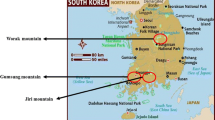
The emission rate observations were carried out in the forests on Worak Mountain, Chungbuk Province (P. densiflora, P. koraiensis, Q. acutissima, and Q. variabilis) and trees planted (G. biloba) at Konkuk University, Seoul. In addition, the emission rates for O. sativa were characterized in the paddy fields of Hwaseong, Gyeonggi Province over an extended period (May 5 to October 29, 2004). Worak Mountain is located in the central part of the country (36°52′06.7′′N, 128°05′41.0′′E), as can be seen in Fig. 1. The more detailed information on Worak Mountain is presented by Kim et al. (2020). Konkuk University is located in southeastern Seoul (37°32′24.8′′N, 127°04′39.7′′E). Hwaseong is located in the southwestern rural area of the Gyeonggi Province (37°13′12.5′′N, 126°43′20.4′′E). More detailed sampling sites and meteorological conditions are summarized in Table 1.
Sampling sites of vegetation-emitted biogenic volatile organic compound. This figure is a quote from a previous paper (Kim et al., 2020), and the sampling sites are the same
2.2 Sampling device
The emission measurements were conducted using a dynamic flow-through vegetation chamber designed to minimize the loss or contamination of reactive BVOCs (Lim et al., 2008; Ling et al., 2011; Son et al., 2015). More detailed experimental configurations are described by Kim (2001a, 2001b), Kim et al. (2005), and Lim et al. (2008). The measurement system consists of the chamber, zero-air system, vacuum pump, mass flow controller, 2,4-dinitrophenylhrdrazine (DNPH) cartridge for the aldehyde (LpDNPH10L, Supelco, USA), and O3 scrubber. We used 20 L Tedlar bag (SKC, U.S.) for the enclosure chamber. The Model 701 zero-air generator (API, U.S.) was used to supply air into the chamber. The N 035.1.2 AN.18 vacuum pump (KNF Neuberger, USA), at a flow rate equal to the rate of the inflowing zero air, was used to discharge the air inside the chamber (Kim, 2001a, 2001b). The system flow rate was controlled at 5 L min−1 by a mass flow controller (2000-5CH, GMATE, Germany). We characterize how long it takes to reach steady state by injecting hexane (Sigma-Aldrich, U.S.A.) using Dynacalibrator (model# 230, VICI, Texas, USA) which illustrate that a constant hexane concentration in the chamber was reached after 20 min from the experimental initiation.
After well passed 20 min of the initiation of the experiment, a part of the exhaust-air stream was passed onto the DNPH cartridge (aldehyde: 1.5–2.0 L min−1, 20 min), and the remaining-air flow was vented into the atmosphere.
The sampling flow rates and times were determined according to the breakpoints of the DNPH cartridge, depending on the tree species. Furthermore, an ozone scrubber filled with KI crystals in a polypropylene tube (L: 40 mm; i.d.: 10 mm) was used to prevent the interruption of the O3 during the aldehyde sampling. All the samples were stored at 4 °C in a refrigerator and analyzed within 48 h.
All the temperatures from both inside and outside the chamber were measured. In addition, the leaf temperature was measured directly by touching the leaf backside using a thermocouple (SDT 25, Type-K, Summit, South Korea. PAR was measured at the top surface of the chamber using a PAR sensor (BabucM BM-BRS, LSI, Italy).
2.3 Carbonyl quantifications
The sampled DNPH cartridges were analyzed using the Waters 2695 high-performance liquid chromatography (HPLC) instrument (Waters, USA). The aldehydes collected in the DNPH cartridge were extracted for the HPLC analysis using 2 mL of acetonitrile (Sigma-Aldrich, USA). The lower detection limit (MDL) and the repeatability were assessed using the relative standard deviation (RSD), of the method regarding the 10 target aldehydes, which are formaldehyde, acetaldehyde, propionaldehyde, crotionaldehyde, methacrolein, butyraldehyde, benzaldehyde, valeraldehyde, m-tolualdehyde, and hexaldehyde, are from 16–27 ppt and 0.085–0.295% (n = 10), respectively (Table 2). The coefficients of determination (r2) of the calibration curve regarding all the target compounds are higher than 0.99.
3 Results and discussion
3.1 Comparison of ER of aldehydes according to habitat environment for each species
We found that even within the same tree species, the rates and the chemical speciation of emissions can substantially vary as illustrated in Table 3. The results illustrate formaldehyde and acetaldehyde emissions from two different species of Pinus densiflora, Ginkgo biloba, Quercus acutissima, and Quercus variabilis, using two experimental units (Set 1 and Set 2) during the experiment. For P. densiflora, most emission rates of aldehyde were 40% lower in Set 1 than in Set 2. During this experiment, the temperatures were set at 31 °C for Set 1 and at 32 °C for Set 2 with little temperature difference, showing that emissions are more affected by other characteristics of trees. It can be translated that the same tree species could have different leaf structures depending on other environmental factors such as solar radiation, other environmental factors, or genetic differences. Normally, sun leaves that bask in the sun have a dense palisade layer and a thick layer of cubicles and leaves to inhibit transpiration, making it difficult for BVOCs in the lipid layer to evaporate and diffuse into the air. Meanwhile, shade leaves located in a place with less sunshine tend to have broader leaves and contain more chlorophyll for more efficient photosynthesis even at low intensity of light, and their palisade layer has been loosely developed and the cuticle layer and leaves are thin. Thus, substances contained in leaves can easily evaporate and diffuse.
Q. acutissima (summer) also illustrated different emission rates (35% to 40%) between two trees depending on emitted species as shown in Table 3. During the experiment, the temperature was almost similar at 30 °C and 31 °C and the PAR was higher for Set 2 with 437 μmol(m2)−1 s−1 than Set 1 with 161 μmol(m2)−1 s−1. It is appeared that the intrinsic differences for those sample trees cause the differences in emissions rather than environmental factors. This conclusion is warranted by the quality assurance and quality control measures. As indicated by He et al. (2000) who conducted a study on 15 Eucalyptus trees and concluded that a difference in emission rates between terpene and isoprene could be as high as 10 times depending on trees, many researchers also reiterated that there could be a noticeable difference in the amount and types of BVOCs including aldehydes emitted from each tree even from the same species. Such a difference could occur due to different tree age, environments of habitats, or the quality of soil. In the previous paper (Kim et al., 2020), the difference in emission rate was mainly investigated depending on the age of the tree, but in addition to age, habitat environment can also affect the emission rate.
3.2 Correlation coefficients between ERs of aldehydes emitted from each tree and temperature
Monoterpenoids in coniferous trees are normally stored in a specific organ called resin ducts and is emitted according to temperature (Kesselmeier, 1999).
The following formula was used to quantify the temperature dependency of the emissions from the presented dataset.
ER refers to an emission rate (ng(gdw)−1 h−1) and β is a constant according to characterize aldehyde emissions in each experimental dataset (Kim, 2001a, 2001b; Tingey et.al., 1981).
The formula above can be expressed as follows (Kim, 2001a, 2001b; Tingey et.al., 1981) and it can be used for standardizing the amounts of aldehydes measured in various temperature conditions as of 30 °C.
ER refers to an emission rate of aldehydes at the temperature T (K) while ERs is an emission rate of aldehydes at the standard temperature Ts(K).
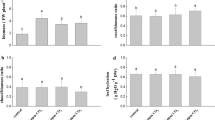
Tables 4, 5, and 6 and Fig. 2 show ERs, β values, and correlation coefficients to express the temperature dependence of aldehydes emissions of each tree investigated in this study.
For P. densiflora (spring), the β value of acetaldehyde was 0.094 with a correlation coefficient of 0.788, indicating a high correlation with temperature. Formaldehyde and total aldehydes posted their β values at 0.073 and 0.072 respectively with correlation coefficients of 0.820 and 0.708, also revealing positive relevance. However, with a β value of 0.043 and a correlation coefficient of 0.261, propionaldehyde showed significantly less temperature dependence compared with other aldehydes. When compared with values measured from Pinus ponderosa by Ignacio et al. (2004), their results were quite similar to those of formaldehyde (β = 0.07, r2 = 0.33) but acetaldehyde (β = 0.04, r2 = 0.07) showed higher correlation with temperature in this study.
The ERs of total aldehydes stood at 357.2, followed by propionaldehyde (110.6), acetaldehyde (102.5), and formaldehyde (73.66) for the P. koraiensis (spring) experiments. As seen in P. densiflora, acetaldehyde had the β value (0.075) and the highest correlation with temperature (0.788). With the β value of 0.039 and a correlation coefficient of 0.550, formaldehyde had lower correlation coefficient than acetaldehyde, still showing relevance with temperature to some extent. Propionaldehyde did not show any obvious correlation with temperature. For P. koraiensis measured during summer, the β values of aldehydes ranged from − 0.002 to 0.023 with relatively low correlation with temperature (0.000 to 0.106). This observation could be caused by the fact that the temperature range (26 ~ 34 °C) in the summer time was narrower than those from the spring (23 ~ 48 °C). Therefore, there is a lack of the wider temperature coverage that warrants a meaningful statistical analysis.
The ERs value of the total aldehydes for P. koraiensis (fall) assessed as 299.4, followed by acetaldehyde (161.3), formaldehyde (46.1), and propionaldehyde (27.9). Like P. densiflora, acetaldehyde recorded the highest correlation coefficient (0.538) with a β value of 0.054 while both of formaldehyde and propionaldehyde had correlation coefficients below 0.5. During the Fall even with a wider distribution of temperatures from 15 to 36 °C, the formaldehyde and acetaldehyde emissions were considerably low compared to other seasons and tended to maintain a certain level rather than increasing or decreasing the emission rate despite a change in temperatures, resulting a weak correlation with temperature.
It may not be possible to clearly explain the different levels of correlation with temperature depending on seasons; however, it could be attributed to different weather conditions at the time of measurement, or it could be due to individual differences or differences in abiotic conditions of trees as the experiment was not conducted on the identical trees in each season.
Regarding G. biloba, the β values measured during spring were negative for all substances with no apparent correlation with temperature (below 0.5), whereas during fall, formaldehyde had the β value of 0.082 with a correlation coefficient of 0.539, indicating a statistically relevant correlation. However, all other substances failed to show any correlation with temperature below 0.5.
In summary, it was found that, for P. densiflora and P. koraiensis, aldehydes’ emission characteristics resembled terpene emitted from coniferous trees. Although it varies depending on tree species, aldehyde emissions from coniferous trees are characterized by substances in resin ducts that slowly evaporate. While G. biloba belongs to coniferous trees, it showed different emission patterns.
Based on correlation analysis with temperature for each aldehyde emitted from Q. acutissima, Q. variabilis, and O. sativa, it was found that most of these substances except for propionaldehyde showed a meaningful correlation coefficient of 0.5 or higher.
3.3 Comparison of the aldehydes ERs according to temperature and PAR
The following is the most frequently used equation to express the temperature dependency of BVOC emission (Guenther et al., 1991, 1993).
Again, ER refers to an actual emission while ERS means an emission factor at the standard condition of 30 °C and 1000 μmol(m2)−1 s−1. CT and CL are constants for temperature and solar radiation that are expressed as follows.
R refers to a gas constant (8.134 JK−1 mol−1), CT1 to 95,000 J−1 mol−1, CT2 to 230,000 J−1 mol−1, TM to 314 K or 41 °C, α to 0.0027 and CL1 to 1.06.6. These values were suggested by several researchers including Guenther et al. (1993) which mostly assessed from empirical datasets. However, these values can be variable depending on tree species and experimental conditions.
Figures 3 and 4 show PAR and emission rates of aldehydes for each tree species.
In coniferous trees, P. densiflora also positively correlates with PAR, as seen with temperature, since the correlation coefficient ranged from 0.4 to 0.5 for entire substances. Normally, P. densiflora shows distinct features depending on seasons. Like temperature, during spring, propionaldehyde’s correlation coefficient with PAR was assessed as less than 0.5, which is low, but formaldehyde and acetaldehyde had a positive correlation at 0.4 to 0.5.
However, the correlation with PAR was all below 0.5 during summer and fall. It may not be possible to fully explain the reason for such low correlations with temperature and PAR during summer compared to spring, but the range of temperature and PAR was relatively narrower at the time of measurement, vis-a-vis spring. During spring, the temperature ranged from 23 to 48 °C and PAR from 55 to 1500 μmol (m2)−1 s−1 while temperature was from 26 to 35 °C and PAR from 13 to 1000 μmol (m2)−1 s−1 in summer. Thus, it was found that the correlation coefficients varied between emission rates and meteorological factors due to a difference in the distribution of meteorological factors in two different seasons. For G. biloba, the correlation coefficient with PAR was equal to below 0.5 both in summer and fall. It showed no apparent correlation with temperature and PAR during summer.
However, in autumn, except for propionaldehyde, the correlation with temperature was favorable with a coefficient of 0.5 or higher. In contrast, the correlation with PAR was less than 0.5, showing a different result between temperature and PAR. In terms of Q. acutissima and Q. variabilis, formaldehyde and acetaldehyde had a positive correlation with temperature and PAR at 0.4 to 0.5.
Figures 5 and 6 illustrate the relationship between CLCT and emission rates. P. densiflora had a positive correlation (0.5 or higher) between emission rates and CLCT for formaldehyde, acetaldehyde, and propionaldehyde, whereas P. koraiensis and G. biloba showed weak correlation.
For broad-leaved trees, Q. acutissima recorded a favorable correlation coefficient at 0.4 to 0.5 between the emission rates and CLCT for three aldehydes. While Q. variabilis showed differences in emission species and seasonality. In summer, all quantified emissions had weak correlations, but during autumn, acetaldehyde and total aldehydes had a good correlation between emission rates and CLCT with a coefficient of 0.5 or higher.
In addition, Table 7 lists correlation coefficients of predicted values with actual measurements of P. densiflora in coniferous trees and Q. variabilis in broad-leaved trees that showed a relatively good correlation with temperature and PAR among various tree species, to compare which model is more suitable for aldehydes between T80, so-called terpene model considering only temperature (Tingey et al., 1981) and G93 also known as ‘isoprene model’ in consideration of temperature and PAR (Guenther et al., 1993). As a result, P. densiflora showed similar correlation coefficients obtained by the two models, but for Q. variabilis, correlation coefficients obtained by the isoprene model are higher. Given the outcome above, it was found that using these models, emissions of aldehydes released from trees should be calculated considering only temperature for P. densiflora and considering both temperature and PAR for Q. variabilis, so as to obtain more accurate data. More detailed results can be found in the author’ preceding research (Kim et al., 2020).
Propionaldehyde had a considerably low correlation in all tree species compared to other substances, suggesting a need to develop a novel emission modeling.
4 Conclusion
Emission rates of aldehydes emitted from trees were 30% to 40% different depending on what tree we sampled, even within the same species. Especially for P. densiflora, P. koraiensis (spring), Q. acutissima, and Q. variabilis, their β values (the slope between emission rates and temperature) ranged from 0.3 to 1.3 depending on sampled chemical species. Excluding propionaldehyde, most of them also showed positive correlation (0.5 or higher) with temperature. The comparison with the ERs (30 °C) and average emission rate (31–32 °C) revealed that P. densiflora and P. koraiensis had higher average emission rates by about 40%, whereas Q. acutissima and Q. variabilis also had higher average emission rates (30–31 °C) by 40% to 50% compared to the ERs.
G. biloba and O. sativa showed no obvious correlation between emission rates and temperature and PAR. However, P. densiflora, P. koraiensis, Q. acutissima, and Q. variabilis had a positive correlation coefficient of 0.5 or higher. In addition, the results of T80 (temperature only) and G93 (both temperature and PAR) showed that P. densiflora had similar correlation of observed and predicted values in both models while Q. variabilis had higher correlation in G93 than in T80.
Availability of data and materials
The datasets generated during and analyzed during the current study are available in from the corresponding author on reasonable request.
References
Arey, J., et al. (1991). The emission of (Z)-3-hexen-1-ol, (Z)-3-hexenylacetate and other oxygenated hydrocarbons from agricultural plant species. Atmospheric Environment, 25A, 1063–1075.
Calfapietra, C., Fares, S., Manes, F., Morani, A., Sgrigna, G., & Loreto, F. (2013). Role of biogenic volatile organic compounds (BVOC) emitted by urban trees on ozone concentration in cities: a review. Environmental Pollution, 183, 71–80.
Goldstein, A. H., & Galbally, I. E. (2007). Known and unexplored organic constituents in the earth’s atmosphere. Environmental Science & Technology, 41, 1514e1521.
Guenther, A., Karl, T., Harley, P., Wiedinmyer, C., Palmer, P. I., & Geron, C. (2006). Estimates of global terrestrial isopreneemissions usingMEGAN (model ofemissions of gases and aerosols from nature). Atmospheric Chemistry and Physics, 6, 3181e3210.
Guenther, A. B., Monson, R. K., & Fall, R. (1991). Isoprene and monoterpene emission rate variability: Observations with Eucalyptus and emission rate algorithm development. Journal of Geophysical Research, 96(D6), 10799–10808.
Guenther, A. B., Zimmerman, P. R., Harley, P. C., Monson, R. K., & Fall, R. (1993). Isoprene and monoterpene emission rate variability: model evaluations and sensitivity analyses. Journal of Geophysical Research, 98, 12609–12618.
He, Congrong, Murray, Frank, & Lyons, Tom. (2000). Monoterpene and isoprene emissions from 15 Eucalyptus species in Australia. Atmospheric Environment, 34, 645–655.
Jork, E. M. (1996). Laboruntersuchungen zum Austausch organischer Sauren und Aldehyde zwischen landwirtschaftlichen Pflanzen und der Atmosphye. Diplomarbeit.
Kesselmeier, J., Bode, K., Hofmann, U., Miler, H., Scher, L., Wolf, A., Ciccioli, P., Brancaleoni, E., Cecinato, A., Frattoni, M., Foster, P., Ferrari, C., Jacob, V., Fugit, J. L., Dutaur, L., Simon, V., & Torres, L. (1997a). The BEMA-Project: Emission of short chained organic acids, formaldehyde와 acetaldehyde and monoterpenes from Quercus ilex L. and Pinus pinea L. in relation to physiological activities, carbon budget and emission algorithms Atmos. Environ, 31(1), 119–134.
Kim, J. C. (2001a). Development of a novel sampling technique for natural VOC emissions. J. Korean Society for Atmospheric Environment, 17(E2), 61–70.
Kim, J. C. (2001b). Factors controlling natural VOC emissions in a southeastern US pine forest. Atmospheric Environment, 35, 3279–3292.
Kim, J. C., Kim, K. J., Kim, D. S., & Han, J. S. (2005). Seasonal variations of monoterpene emissions from coniferous trees of different ages in Korea. Chemosphere, 59, 1685–1696.
Kim, S. Y., Kim, J. C., Park, C. Y., & Son, Y. S. (2020). Emission characteristic of aldehydes from representative plant species of the Korean peninsula. Atmospheric Research, 236, 104840.
Kimmerer, T. W., & Kozlowski, T. T. (1982). Ethylene, ethane, acetaldehyde, and ethanol production by plants under stress. Plant Physiology, 69, 840–847.
Kimmerer, T. W., & MacDonald, R. C. (1987). Acetaldehyde and ethanol biosynthesis in leaves of plants. Plant Physiology, 84, 1204–1209.
Lim, J. H., Kim, J. C., Kim, K. J., Son, Y. S., Sunwoo, Y., & Han, J. S. (2008). Seasonal variations of monoterpene emissions from Pinus densiflora in East Asia. Chemosphere, 73, 470–478.
Ling, Z. H., Guo, H., & Yu, Y. F. (2011). Sources of ambient volatile organic compounds and their contributions to photochemical ozone formation at a site in the Pearl River Delta, southern China. Environmental Pollution, 159, 2310–2319.
Loreto, F., & Schnitzler, J. P. (2010). Abiotic stresses and induced BVOCs. Trends in Plant Science, 15, 154–166.
Lüpke, M., Leuchner, M., Steinbrecher, R., & Menzel, A. (2016). Impact of summer drought on isoprenoid emissions and carbon sink of three Scots pine provenances. Tree Physiology, 36, 1382–1399.
Martin, R. S., Westberg, H., Allwine, E., Ashman, L., Farmer, J. C., & Lamb, B. (1991). Measurement of isoprene and its atmospheric oxidation products in a central Pennsylvania deciduous forest. Journal of Atmospheric Chemistry, 13, 1–32.
Mellouki, A., Wallington, T. J., & Chen, J. (2015). Atmospheric chemistry of oxygenated volatile organic compounds: Impacts on air quality and climate. Chemical Reviews, 115, 3984–4014.
Millet, D. B., Guenther, A., Siegel, D. A., Nelson, N. B., Singh, H. B., de Gouw, J. A., Warneke, C., Williams, J., Eerdekens, G., Sinha, V., Karl, T., Flocke, F., Apel, E., Riemer, D. D., Palmer, P. I., & Barkley, M. (2010). Global atmospheric budget of acetaldehyde: 3-D model analysis and constraints from in-situ and satellite observations. Atmospheric Chemistry and Physics, 10, 3405–3425.
Niinemetstingey, Ü., Loreto, F., & Reichstein, M. (2004). Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends in Plant Science, 9(4), 180–186.
Nondek, L., Rodler, D. R., & Birks, J. W. (1992). Measurement of sub-ppbv concentrations of aldehydes in a forest atmosphere using a new HPLC technique. Environmental Science and Technology, 26, 1174–1178.
Son, Y. S., Kim, K. J., Jung, I. H., Lee, S. J., & Kim, J. C. (2015). Seasonal variations and emission fluxes of monoterpene emitted from coniferous trees in East Asia: Focused on Pinus rigida and Pinus koraiensis. Journal of Atmospheric Chemistry, 72, 27–41.
Stotzky, G., & Schenk, S. (1976). Observations on organic volatiles from germinating seeds and seedlings. Amer. J. Bot., 63, 798–805.
Tingey, D. T., Manning, M., Grothaus, L. C., & Burns, W. F. (1980). Insuence of light and temperature on monoterpene emission rates from slash pine. Plant Physiology, 65, 797–801.
Vedel-Petersen, I., Schollert, M., Nymand, J., & Rinnan, R. (2015). Volatile organic compound emission profiles of four common arctic plant. Atmospheric Environment, 120, 117–126.
Funding
The author did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
This paper has a sole author.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, SY. Factors influencing the emissions of aldehydes from plant species of the Korean peninsula. Asian J. Atmos. Environ 17, 16 (2023). https://doi.org/10.1007/s44273-023-00015-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44273-023-00015-8