Abstract
This study aims to analyze the sensitivity of volatile organic compounds (VOCs) to ambient concentrations of fine particles (PM2.5) in the representative industrial city of Ulsan, Korea. For the calculation of sensitivity coefficients between VOCs and PM2.5 (SVOCs-PM2.5), PM2.5 data were obtained from an air quality monitoring station, and their corresponding 6-h average concentrations of VOCs (alkanes, alkenes, aromatics, and total VOCs) were measured at the Yeongnam intensive air monitoring station. The air monitoring period was divided into the warm-hot season (May–October 2020) and the cold season (November 2020–January 2021). The sensitivity coefficients in the low pollution period of PM2.5 (5 < PM2.5 ≤ 15 μg/m3) were higher and much higher than those in the medium pollution period (15 < PM2.5 ≤ 35 μg/m3) and high pollution period (35 < PM2.5 ≤ 50 μg/m3), respectively. This result indicates that the change ratios of PM2.5 concentrations to the background (PM2.5 ≤ 5 μg/m3) per unit concentration change of VOCs (particularly alkenes) in the high PM2.5 pollution period were much higher than those in the low pollution period. This also indicates that PM2.5 concentrations above 35 μg/m3 were more easily affected by the unit concentration change of VOCs (particularly alkenes) than those below 15 μg/m3. The average sensitivity coefficients during the cold season increased in a range of 23–125% as compared to those during the warm-hot season, except the alkenes-PM2.5 sensitivity with a decrease of 7%. It means that the impact of VOCs (except alkenes) on PM2.5 concentrations was relatively low in the cold season. However, in the cold season, the alkenes might contribute more to PM2.5 formation, particularly over the high pollution period, having PM2.5 > 35 μg/m3, than other VOC groups. The result of this study can be a basis for establishing PM2.5 management plans in industrial cities with large VOC emissions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Most people in the world are exposed to ambient and indoor particulate matter (PM) every day throughout their entire life, which can result in adverse health effects (EEA, 2017; WHO, 2018). Particularly, the exposure to fine particles, such as PM1.0 and/or PM2.5, and/or respirable PM, having a 50% cut point at 4.0 μm particles, brings a much higher lung deposition rate than that the exposure to coarse particles (PM10-2.5) (NIOSH, 1998; Phillips & Oh, 2020; Vallero, 2014). Fine particles usually have increased surface areas per unit mass and high mobility compared to coarse particles and thus can carry more toxic or hazardous air pollutants (Apte et al., 2015; Brown et al., 2013; EEA, 2014; Lelieveld et al., 2015), such as heavy metals and polychlorinated dibenzo-p-dioxins/furans, which can trigger various mutagenicity or cancers (Bari & Kindzierski, 2017; Delfino, 2002; Goodkind et al., 2019). In order to minimize the adverse health effects of fine particles, therefore, it is very important not only to decrease the exposure to PM2.5 (including PM1.0) but also to figure out their detailed composition and formation mechanism (Heo et al., 2016; Saini & Sharma, 2020). PM2.5 is defined as particulate matter with an aerodynamic diameter less than or equal to a nominal 2.5 μm. PM2.5 is also composed of primary and secondary PM. Primary PM means particulate matter directly emitted from sources, such as vehicles or mobile tailpipes, re-entrained road dust or fugitive dust and industrial stacks and processes (Querol et al., 2001). Secondary PM represents particulate matter formed via chemical reactions between primary PM components and other chemical species, including moisture and ozone (O3) in the atmosphere or under sunlight irradiation (Luo et al., 2018; Yan et al., 2021). Typical secondary PM is generated from emissions of precursor compounds, including sulfur oxides (SOX: SO2 + SO3), nitrogen oxides (NOX: NO + NO2), volatile organic compounds (VOCs), and ammonia (NH3), released from various sources of air emissions such as traffic emissions, industrial emissions, port and ship emissions, and natural emissions (Park et al., 2013; Sun et al., 2006).
The national ambient air quality standards (NAAQS) on PM2.5 enforced from January 1, 2015, in Korea are 35 μg/m3 (24-h average) and 15 μg/m3 (annual average; its 99 percentile must not be exceeded in the annual standard value). The US Environmental Protection Agency (EPA) strengthened its primary annual standard of PM2.5 from 15 to 12 μg/m3 on December 14, 2012, requiring the 3-year average of its annual average should be less than or equal to 12.0 μg/m3 (US EPA, 2012). These strengthening trends of NAAQS on fine particles indicate that reducing PM2.5 concentrations are crucial for public health protection, which also has brought major public interest or agenda to reduce ambient PM2.5 levels in Korea. Therefore, there have been a lot of studies to identify PM2.5 components and find secondary PM formation mechanisms, particularly in Korea. Secondary PM2.5 mainly consists of both inorganic matter of sulfates and nitrates, including ammonium sulfate ((NH4)2SO4), ammonium bisulfate ((NH4)HSO4), and ammonium nitrate (NH4NO3), formed from chemical reactions of primary emissions of SOX, NOX, and NH4, and organic matter including organic aerosols formed from coagulation through condensation/nucleation and/or photochemical oxidation of VOCs (Kim et al., 2022; Lee et al., 2023; Park et al., 2013, 2018).
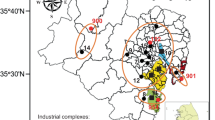
One of the major environmental policies in Korea is to reduce ambient levels of PM2.5 to improve urban air quality and minimize the adverse health effects associated with PM2.5 exposures (Kim & Lee, 2018). In particular, many people who live in the metropolitan city of Ulsan, which is a typical and largest industrial city in Korea, have been so much concerned about the ambient PM2.5 level. In Ulsan, large amounts of air pollutants, such as SOx, NOx, NH4, and VOCs, are emitted from world scale-industrial complexes of petrochemical, non-ferrous, shipbuilding, and automobile industries (NIER, 2022). In addition, emissions from ports and ships cannot be neglected. The air emissions of toxic or hazardous chemicals including VOCs from industrial activities in Ulsan are much larger than those in other industrial cities in Korea (Kim et al., 2019; Nguyen et al., 2018; Vuong et al., 2022). Therefore, the Korean Government and the Ulsan Metropolitan Government have been greatly interested in reducing VOC emissions in terms of public health. Furthermore, the ambient levels of secondary organic aerosol (SOA) can be declined by reducing VOC emissions because large fractions of PM2.5 are generated from VOCs through condensation, aggregation, nucleation, condensation growth of nuclei, and coagulation (Lee et al., 2023; Yang et al., 2020). To evaluate the impacts of VOCs on the level of PM2.5, information on the formation of SOA is required. Recently, the concept of sensitivity coefficients between VOCs and PM2.5 (or O3) values has been introduced to find more detailed information on secondary PM2.5. There have been only a few studies in China on sensitivity analysis of VOC concentration changes that affect ambient PM2.5 concentrations (Han et al., 2017, 2018; Zhang et al., 2020). Yang et al. (2020) first conducted numerical sensitivity simulations of VOCs on PM2.5 concentrations in Busan and Ulsan, Korea, during the heat wave period in 2018. They found that the increase in VOCs emission does not result in increasing SOA generation in a linear manner. They also reported that the tenfold increase in VOCs emission seems to increase 30% in SOA formation. However, the information on the change of PM2.5 concentrations by unit concentration change of VOCs considering their background concentrations has not been clearly found yet world around. In particular, tremendous amounts of VOCs are released into the ambient environment from industrial activities such as world-scale petrochemical refinery and production, automobile manufacturing, shipbuilding, etc., in Ulsan, the largest industrial city in Korea. Thus, this study aims to evaluate how much sensitive the ambient PM2.5 concentrations are to VOCs measured in Ulsan.
2 Basic theory
Han et al. (2017) proposed a concept of sensitivity or sensitivity coefficients between ambient VOC and fine particle (PM2.5) concentrations and also between ambient VOC and ozone (O3) concentrations (SVOCs-O3) based on a similarity or close relationship between VOC and PM2.5 values and also between VOC and O3 values. The sensitivity analysis is based on the sensitivity coefficients of VOC to PM2.5 concentrations. The sensitivity coefficient (SVOCs-PM2.5) of measured VOC concentrations to measured PM2.5 concentrations is defined as follows (Han et al., 2017, 2018):
where SVOCs-PM2.5 is the sensitivity coefficient between ambient VOC and PM2.5 concentrations. BPM2.5 is the background value defined as an average of the PM2.5 concentrations, which is equal to or less than 5 μg/m3 out of the PM2.5 values measured at the interest site during the study period. BVOCs is the background value defined as an average of the VOC concentrations obtained at the study site during the background period of PM2.5 (BPM2.5 period). ΔVOCs represent the VOC concentration difference between the obtained BVOCs and the measured VOC values during the study period. Similarly, ΔPM2.5 indicates the PM2.5 concentration difference between the obtained BPM2.5 and the measured PM2.5 values.
The sensitivity information on VOC concentrations to PM2.5 concentrations means how much PM2.5 values are affected by the change in VOC values. The sensitivity analysis between VOCs and PM2.5 is based on the information on the sensitivity coefficients of interested ambient VOCs and their corresponding PM2.5 concentrations. The information on the sensitivity coefficients of VOCs to PM2.5 (SVOCs-PM2.5) can help to figure out the impacts of PM2.5 concentration change to the background PM2.5 value associated with the VOC concentration change to the background VOC value. Based on the definition of the sensitivity coefficient, such a high coefficient much higher than 1.0 means that a large change in VOC concentrations against the background value of VOCs is required to get a unit concentration change of PM2.5. Such a low coefficient, much lower than 1.0, represents that PM2.5 concentrations are easily affected by a small change of VOC concentrations against the background value of VOCs. From the sensitivity information on VOCs-PM2.5 concentrations, we can estimate or evaluate how much PM2.5 concentrations can be affected by the change of VOC concentrations at the site of interest. The sensitivity coefficients of different types of VOCs can be classified into alkanes, alkenes, aromatics, and TVOCs based on their main chemical structures and concentrations, which can be used to analyze the detailed impacts on PM2.5 formation or concentrations of VOCs with different structures. Their sensitivity coefficients can provide relative numerical information on different types of VOCs toward PM2.5 concentrations or formation. Thus, their relative sensitivity information can be used for deciding priority VOCs to cut their emissions at specific sources to decrease the PM2.5 levels. If the emission profiles of VOCs are known for major industries, the sensitivity coefficients of the VOCs could be useful information to cut the emissions of VOCs, particularly during high PM events. However, this sensitivity coefficient information does not provide the solid relationship between VOCs and PM2.5 concentrations of an interest area. Also, this sensitivity approach does not also differentiate transboundary inflow or local-generated PM2.5 or VOCs concentrations, which would lead to some limitation to use the sensitivity coefficients.
3 Methods
3.1 Data collection of VOCs and PM2.5
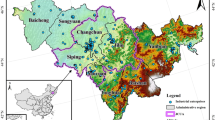
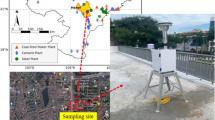
For the analysis of the sensitivity coefficients (SVOCs-PM2.5 of VOCs-PM2.5), this study used the PM2.5 data obtained from an air quality monitoring station (AQMS) at an urban residential site and their corresponding VOC concentrations (6-h average) measured at the Yeongnam (YN) intensive air monitoring station (Fig. 1). At the AQMS, hourly concentrations of ambient PM2.5 were measured using a particulate monitor (BAM-1020, Met One, USA). Six-h-averaged VOC samples were collected using a sequential tube sampler (STS-25, Perkin Elmer, USA) with a pump (MP-Σ30KNII, Sibata, Japan) at a flow rate of 50 mL/min. Adsorbent tubes (Carbotrap 300, Supelco, USA) were filled with Carbotrap C, Carbotrap B, and Carbosieve S-III. Target VOCs (29 alkanes, 9 alkenes, and 16 aromatics) were analyzed using a thermal desorption instrument (TD; UNITY Series 2, Markes, UK) coupled with a gas chromatograph/mass spectrometer (GC/MS) (7890B/5975A, Agilent, USA). For quality assurance/quality control (QA/QC), the method detection limits (MDLs) were calculated by multiplying the Student’s t-value (ts) at 99% confidence (3.14) with the standard deviation (SD) of the concentrations of the target VOCs in the spiked seven replicates using a gaseous standard mixture (0.2 μg/m3). The gaseous standard mixture was prepared by combining the Custom Terpene Standard (S-94000–02, AccuStandard, USA) and Wisconsin DNR Mix (GRH-003S, AccuStandard, USA). Details on instrumental conditions and quality assurance/quality control (QA/QC) can be found elsewhere (Kim et al., 2022). The VOC sampling site was located in an urban-background residential area at a distance of < 1 km from the AQMS. They are located downwind from the petrochemical and automobile ICs between late spring and mid-summer. Thus, the PM2.5 and VOC sampling sites can be affected by emissions from industrial activities, depending upon prevailing seasonal winds, rather than the traffic emissions from the metropolitan areas of Ulsan.
3.2 Calculations of sensitivity coefficients of VOCs toward PM2.5
The sensitivity coefficients of VOCs toward corresponding PM2.5 (SVOCs-PM2.5) were computed according to the calculation formula proposed by Han et al. (2017), as shown above. For calculating the sensitivity coefficient of each VOC, its concentration needs to be matched with its corresponding PM2.5 concentration. In this study, the individual concentrations of all the target VOCs in each sample showed high variability mostly due to the variability of detection and level of each VOC identified for the 6-h-sampling period. To calculate the sensitivity coefficients of the classified VOC groups, it is also necessary to obtain all the VOC concentrations matched with the PM2.5 concentrations. In particular, it is essential to find the average background value (BVOCs) of all the VOC groups matched with the average background value of PM2.5 (BPM2.5: PM2.5 ≤ 5.0 μg/m3). If each VOC is not detected or its concentration is less than the BVOCs, it is impossible to compute the difference (△VOCs) between the measured and background VOC concentrations, resulting in 0 or no information on the sensitivity coefficient of the corresponding VOC-PM2.5 pair, which makes troubles for this study. In this study, therefore, 6-h averaged values of BPM2.5 and △PM2.5 were used by adding all the 1-h data and then dividing it by 6, based on the hourly concentrations of the PM2.5 measured for 6 h of the VOC sampling periods. The BVOCs and △VOCs, which should be matched with the BPM2.5 and △PM2.5, respectively, were obtained to calculate the sensitivity coefficient of the corresponding VOCs-PM2.5 pair.
The classified groups of alkanes, alkenes, aromatics, and TVOCs were composed of 29, 9, 16, and 52 VOCs, respectively. However, not all the VOCs in each classified group were identified in the samples, which have variability in terms of the detection frequency of individual compounds among the collected samples. For this reason, it was difficult to keep good consistency among different samples for their sensitivity evaluation. In order to minimize the variability existing among the different VOC samples in keeping with a sort of representativity of the classified groups, this study only used the VOC samples identified more than 68% (using a concept of μ ± 1σ in statistics) out of the total number (29, 9, 16, and 52 components in alkanes, alkenes, aromatics, and TVOCs, respectively) of the VOC groups. Also, only the PM2.5 data matching with the VOC samples (having more than 19, 7, 11, and 36 components in alkanes, alkenes, aromatics, and TVOCs, respectively) were used to calculate the sensitivity coefficients (SVOCs-PM2.5) of the VOCs-PM2.5 pairs.
4 Results and discussion
4.1 Levels and patterns of VOCs
Table 1 provides descriptive statistics of the target alkanes, akenes, and aromatics, respectively, detected from 319 samples collected at the YN station during the study period. The concentrations of total VOCs (TVOCs) by adding their geo-mean and arithmetic mean values of alkanes, alkenes, and aromatics were 15.77 and 44.78 μg/m3, respectively. This large difference in the TVOC concentration is due to large concentration variations of individual compounds among the air samples. The major compounds with relatively high concentrations were iso-pentane, n-pentane, propane, and other pentanes in alkanes; 1-pentene, 1-hexene, and propylene in alkenes; and toluene, ethylbenzene, m,p-xylenes, benzene, and o-xylene in aromatics. The relative fractions of alkanes, alkenes, and aromatics to the TVOC concentrations were 55.6, 7.8, and 35.6% in geo-mean values and 49.5, 13.1, and 37.4% in arithmetic mean values, respectively (Fig. 2). Based on the information on the TVOC concentrations identified at the site, the emission reduction priority of VOCs could be in the following order: alkanes > aromatics > alkenes. However, in order to make a decision on the emission reduction policy or priority of the VOCs for reducing PM2.5 concentrations, it is necessary to consider many factors, including emission amounts and strength considering seasonal variations or characteristics in production amounts, facilities, and processes at sources, control technology maturity and application feasibility, meteorological conditions, and socio-economical situations. However, most of these factors could not be feasible options to be selectable at emission sources because they are given as unchangeable or fixed forms or conditions in the actual field. Therefore, this study focuses on the VOCs-PM2.5 sensitivity coefficients (SVOCs-PM2.5), which may provide basic ideas for VOC emission reduction or control measures for PM2.5 reduction through better understanding the VOC sensitivity, considering their chemical structures, toward PM2.5 concentrations.
4.2 Analysis of VOCs-PM2.5 sensitivity coefficients in warm-hot seasons
The ambient PM2.5 concentrations at the YN station are affected by many factors, including direct emissions from primary sources, scattering dust near the sampling site, short- and long-range transport, and secondary formation of inorganic and organic aerosols. The ambient PM2.5 concentrations are also greatly affected by environmental factors (e.g., topography and terrain) and meteorological parameters (e.g., relative humidity, wind speed and direction, and intensity of solar radiation). However, this study focused on the impacts of levels and patterns of VOCs with different chemical structures, including alkanes, alkenes, and aromatics, on the ambient concentrations of PM2.5 in two seasonal periods, including the warm-hot season (May–October 2020) and the cold season (November 2020–January 2021). The VOCs-PM2.5 sensitivity coefficients (SVOCs-PM2.5) were obtained using the Eq. (1) for the different VOC groups of alkanes, alkenes, aromatics (Table 1), and TVOCs, respectively, matched with the 6-h-averaged PM2.5 data. During the warm-hot season, the arithmetic mean sensitivity coefficients, excluded in the background period (PM2.5 ≤ 5 μg/m3), were obtained in the following order: aromatics (1.34 ± 2.04) > TVOCs (0.91 ± 0.67) > alkenes (0.82 ± 0.78) ≈ alkanes (0.82 ± 0.54). The obtained averaged sensitivity coefficients were separated into the three concentration periods, classified based on the 6-h-averaged PM2.5 values, including low pollution (5 < PM2.5 ≤ 15 μg/m3), medium pollution (15 < PM2.5 ≤ 35 μg/m3), and high pollution (35 < PM2.5 ≤ 50 μg/m3). The low pollution period showed the highest average values of the sensitivity coefficients in the following order (Fig. 3): aromatics (2.64 ± 3.17) > TVOCs (2.34 ± 0.77) > alkanes (2.09 ± 0.00) > alkenes (1.49 ± 1.27). As the PM2.5 concentrations increased up to 25 μg/m3 (up to 20 μg/m3 in PM2.5 value for aromatics), the sensitivity coefficients rapidly decreased and, after that, slowly decreased or not even significantly changed.
The high sensitivity coefficient in the low pollution period (or zone) below PM2.5 ≤ 15 μg/m3 indicates that a more change in VOC concentrations against its background value (BVOCs) is required to make a unit change of PM2.5 concentration against its background value (BPM2.5: PM2.5 ≤ 5 μg/m3). This result implies that PM2.5 concentrations could be more easily or greatly affected by other factors rather than changes in VOC concentrations and their physicochemical properties in the low pollution period. Instead, PM2.5 concentrations may be more influenced by meteorological conditions (e.g., relative humidity, wind speed, and wind direction) and/or the secondary formation of PM2.5, mostly affected by inorganic ions such as sulfates, nitrates, and ammonium. The result of the sensitivity coefficients also suggests that PM2.5 concentrations are more easily affected by VOCs in the medium and high pollution periods above 15 μg/m3 in PM2.5 values than those in the low pollution period (5 < PM2.5 ≤ 15 μg/m3) less than or equal to 15 μg/m3, which is the NAAQS on PM2.5 in Korea. In particular, alkenes showed the lowest sensitivity coefficients in all the pollution periods (5 < PM2.5 ≤ 50 μg/m3). This result means that PM2.5 concentrations were affected the most easily by the small concentration change of alkenes against their background value at the YN station. Then, the alkanes in the low and high pollution periods of PM2.5 (5–15 μg/m3 and 35–50 μg/m3) or the TVOCs in the medium pollution period (15–35 μg/m3) were the next following VOC groups which easily affect the concentration change of PM2.5 at the YN station.
Figure 4 shows the sensitivity coefficients (SVOCs-PM2.5) in the Y-axis against PM2.5 concentrations in the X-axis over the warm-hot season. In the regression analysis of their relationship (SVOCs-PM2.5 vs. PM2.5) for VOC groups, the alkanes showed the highest determination coefficient (R2 = 0.60), followed by the alkenes (R2 = 0.45) and the aromatics (R2 = 0.19), which is an opposite trend to the order in the sensitivity coefficients: aromatics (2.64 ± 3.17) > alkanes (2.09 ± 0.00) > alkenes (1.49 ± 1.27). In the regression analysis of the polynomial (trigonal) equations on the sensitivity coefficients (SVOCs-PM2.5) versus PM2.5 concentrations, the determination coefficients (R2) were in the following order: alkanes (R2 = 0.83) > alkenes (R2 = 0.71) > aromatics (R2 = 0.27), which are much higher than their corresponding natural log-scaled R2 values. The determination coefficients (SVOCs-PM2.5 vs. PM2.5 concentrations) of the TVOCs, which were determined from the concentrations of all the detected VOCs, showed high values of R2 = 0.54 (log-scaled) and R2 = 0.84 (polynomial (trigonal) equation). However, the R2 values of the aromatics, which are mostly emitted from the national industrial complexes, were much lower than those of the alkanes, alkenes, and TVOCs (NICS, 2022). This result represents that the concentration changes of alkanes, alkenes, and TVOCs greatly affect changes in PM2.5 concentrations. Therefore, the emission reduction of TVOCs can be a good strategy for reducing PM2.5 levels in urban areas of Ulsan. In particular, focusing on the emission reduction of alkanes and alkenes rather than aromatics would be more effective for PM2.5 reduction. However, this conjecture does not mean that the emission reduction of aromatics is not necessary to reduce PM2.5 levels in Ulsan. The higher values of R2 on the log-scaled sensitivity coefficients of alkanes and alkenes vs. PM2.5 concentrations were also identified in a ferrous-metal industrial area in China (Zhang et al., 2020). In the current study, however, the R2 value on the log-scaled or polynomial (trigonal) equation for the sensitivity coefficient of the aromatics vs. PM2.5 values was much lower than that of alkanes and alkenes vs. PM2.5 concentrations. This result is somewhat different from the higher R2 value, given in log-scaled equations, identified in a petrochemical industrial area in China (Han et al., 2018). However, there is a limitation in directly comparing their R2 values between the relevant studies in China and the current study in Ulsan, Korea, because there is a large difference in the concentration ranges of PM2.5 investigated among the studies. For example, the investigated PM2.5 data in Ulsan, Korea, were below 50 μg/m3, which are relatively low concentrations; however, they were below 300 μg/m3 in the Chinese studies, which are extremely high concentrations.
4.3 Analysis of VOCs-PM2.5 sensitivity coefficients in the cold season
During the cold season (November 2022–January 2022), the arithmetic mean sensitivity coefficients (SVOCs-PM2.5) in the PM2.5 concentration range (from 5 to 50 μg/m3) were as follows: alkanes (1.84 ± 2.85) > TVOCs (1.78 ± 2.00) > aromatics (1.66 ± 2.29) > alkenes (0.76 ± 0.39). The average sensitivity coefficients obtained in the cold season were higher (125% increase in alkanes, 95% increase in TVOCs, and 23% increase in aromatics) than those in the warm-hot season, except for a 7% decrease in alkenes. This result represents that the unit concentration change of VOCs (alkanes, aromatics, and TVOCs except for alkenes) leads to more minor changes in PM2.5 concentrations in the cold season than in the warm-hot season. In other words, the PM2.5 concentrations in the cold period were less sensitive to the concentration change of VOCs except alkenes than those in the warm-hot season (Table 2).
The average sensitivity coefficients toward the PM2.5 concentrations (5 < PM2.5 ≤ 50 μg/m3) during the cold season were separated into three concentration periods: low, medium, and high pollution periods (Fig. 5). The low pollution period (5 < PM2.5 ≤ 15 μg/m3) showed the highest average values of the sensitivity coefficients in the following order: alkanes (2.85 ± 4.41) > TVOCs (2.79 ± 2.97) > aromatics (2.24 ± 3.26) > alkenes (0.83 ± 0.07). The order of the average sensitivity coefficients in the cold season was quite different from that in the warm-hot season [aromatics (2.64 ± 3.17) > TVOCs (2.34 ± 0.77) > alkanes (2.09 ± 0.00) > alkenes (1.49 ± 1.27)]. During the entire pollution period (5 < PM2.5 ≤ 50 μg/m3), a large change in PM2.5 concentration was observed by a change in the unit concentration of alkenes in the cold season (Fig. 5). The concentration change of all the VOC groups in the cold season greatly affected the change of PM2.5 concentrations during the high pollution period (35 < PM2.5 ≤ 50 μg/m3). However, the concentration change of other VOCs (alkanes, aromatics, and TVOCs) in the low pollution period resulted in a relatively small change in PM2.5 concentrations compared to the case of the high pollution period of PM2.5 concentrations.
Compared to the results of the average sensitivity coefficients in the warm-hot season, the cold season in the low pollution period (5 < PM2.5 ≤ 15 μg/m3) showed two distinct characteristics depending upon their chemical structures. The sensitivity coefficients for alkanes and TVOCs were increased by 36% and 19%, respectively (Table 3). On the other hand, alkenes and aromatics showed a reverse trend of sensitivity coefficients, decreased by 7% and 16%.
The average sensitivity coefficient of only alkenes during the medium pollution period (15 < PM2.5 ≤ 35 μg/m3) in the cold season was 0.82 (vs. 0.62 in the warm-hot season) lower than 1. However, the values of other VOCs (alkanes, aromatics, and TVOCs) were 1.30–1.31 (0.70–0.79 in the warm-hot season) and were higher than 1. This result means that PM2.5 concentrations over the medium pollution period in the cold season were less sensitive to the concentration change of VOCs than those in the warm-hot season. This result also indicates that other factors, such as meteorological and/or environmental conditions, were more important in affecting the PM2.5 levels. However, over the high pollution period (35 < PM2.5 ≤ 50 μg/m3), the average value of sensitivity coefficients of the alkenes was 0.16 (vs. 0.57 in the warm-hot season), which was much lower than 1. In addition, the values of other groups of VOCs were 0.50–0.59 (vs. 0.63–0.93 in the warm-hot season). These low sensitivity coefficients imply that PM2.5 concentrations over the high pollution period in the cold season would be greatly affected by the concentration change of VOCs, in particular by the concentration change of the alkenes. Based on these results, the emission reduction of alkenes should be the top priority strategy for controlling VOCs to reduce PM2.5 levels in the cold season over all the pollution periods (5 < PM2.5 ≤ 50 μg/m3), in particular, over the high pollution period.
Figure 6 shows the log-scaled sensitivity coefficients (SVOCs-PM2.5) in the Y-axis against PM2.5 concentrations in the X-axis over the cold season. In the regression analysis of their relationship (SVOCs-PM2.5 vs. PM2.5), the determination coefficient (R2) values of the VOC groups were in the following order: TVOCs (0.25) > alkenes (0.22) > alkanes (0.15) > aromatics (0.11). The R2 values in the regression analysis of their polynomial (trigonal) equations were in the following order: alkenes (0.35) > TVOCs (0.34) > alkanes (0.25) > aromatics (0.15), which were much lower than those over the warm-hot season. However, their low determination coefficients do not represent that controlling VOC emissions would not effectively reduce PM2.5 levels in the cold season. It is because their sensitivity coefficients over the high pollution period were much lower than those over the warm-hot season. Therefore, the emission reduction strategies of all the VOCs, focusing on alkenes at major emission sources, could somehow contribute to decreasing the PM2.5 levels in the cold season, particularly over the high pollution period.
5 Conclusion
In the analysis of VOCs-PM2.5 sensitivity coefficients (SVOCs-PM2.5) for VOCs (≤ 14 μg/m3) and PM2.5 (≤ 50 μg/m3) measured at the sampling site located in a less-busy urban area of Ulsan, Korea, the PM2.5 concentrations change ratio compared to background values was evaluated. The influence of VOC unit concentration change was found to be greater during the high pollution period (35 < PM2.5 ≤ 50 μg/m3) than the low pollution period (5 < PM2.5 ≤ 15 μg/m3). This result represents that the PM2.5 concentrations against the unit concentration change of the VOCs were more sensitively changed as the PM2.5 levels were higher than 35 μg/m3. Over the low to the medium pollution periods (5 < PM2.5 ≤ 35 μg/m3), the highest ratio was observed for the alkenes, indicating that the PM2.5 concentrations were more sensitively changed by the unit concentration change of the alkenes than other VOC groups. In the seasonal comparison of the VOCs-PM2.5 sensitivity coefficients, the change ratio of the PM2.5 concentrations by the unit concentration change of the VOCs, except the alkenes, over the medium pollution period (15 < PM2.5 ≤ 35 μg/m3) was lower in the cold season than those in the warm-hot season. In particular, the PM2.5 concentrations were easily changed by the unit concentration change of the alkenes over the high pollution period in the cold season rather than those in the warm-hot season. The current study outcome can be used to evaluate the potential or sensitivity to changes (or reduction) in ambient PM2.5 concentrations by changing (or reducing) VOC emissions, and then, it can be helpful to make a policy decision to reduce ambient PM2.5 levels in Ulsan, Korea, and around the world.
References
Apte, J. S., Marshall, J. D., Cohen, A. J., & Brauer, M. (2015). Addressing global mortality from ambient PM2.5. Environmental Science & Technology, 49, 8057–8066.
Bari, M. A., & Kindzierski, W. B. (2017). Concentrations, sources and human health risk of inhalation exposure to air toxics in Edmonton, Canada. Chemosphere, 173, 160–171.
Brown, J. S., Gordon, T., Price, O., & Asgharian, B. (2013). Thoracic and respirable particle definitions for human health risk assessment. Particle and Fibre Toxicology, 10, 12.
Delfino, R. J. (2002). Epidemiologic evidence for asthma and exposure to air toxics: Linkages between occupational, indoor, and community air pollution research. Environmental Health Perspectives, 110, 573–589.
EEA. (2014). Premature deaths attributable to PM2.5, NO2 and O3 exposure in 41 European countries and the EU-28, 2014. European Environment Agency, pp. 1–5. https://www.eea.europa.eu/highlights/improving-air-quality-in-european/premature-deaths-2014.
EEA. (2017). Air quality in Europe — 2017 report. European Environment Agency, pp. 57–58. https://doi.org/10.2800/850018.
Goodkind, A. L., Tessum, C. W., Coggins, J. S., Hill, J. D., & Marshall, J. D. (2019). Fine-scale damage estimates of particulate matter air pollution reveal opportunities for location-specific mitigation of emissions. Proceedings of the National Academy of Sciences, 116, 8775–8780.
Han, D., Gao, S., Fu, Q., Cheng, J., Chen, X., Xu, H., Liang, S., Zhou, Y., & Ma, Y. (2018). Do volatile organic compounds (VOCs) emitted from petrochemical industries affect regional PM2.5? Atmospheric Research, 209, 123–130.
Han, D., Wang, Z., Cheng, J., Wang, Q., Chen, X., & Wang, H. (2017). Volatile organic compounds (VOCs) during non-haze and haze days in Shanghai: Characterization and secondary organic aerosol (SOA) formation. Environmental Science and Pollution Research, 24, 18619–18629.
Heo, J., Adams, P. J., & Gao, H. O. (2016). Public health costs of primary PM2.5 and inorganic PM2.5 precursor emissions in the United States. Environmental Science & Technology, 50, 6061–6070.
Kim, S.-J., Kwon, H.-O., Lee, M.-I., Seo, Y., & Choi, S.-D. (2019). Spatial and temporal variations of volatile organic compounds using passive air samplers in the multi-industrial city of Ulsan, Korea. Environmental Science and Pollution Research, 26, 5831–5841.
Kim, S.-J., Lee, S.-J., Lee, H.-Y., Son, J.-M., Lim, H.-B., Kim, H.-W., Shin, H.-J., Lee, J. Y., & Choi, S.-D. (2022). Characteristics of volatile organic compounds in the metropolitan city of Seoul, South Korea: Diurnal variation, source identification, secondary formation of organic aerosol, and health risk. Science of the Total Environment, 838, 156344.
Kim, Y. P., & Lee, G. (2018). Trend of air quality in Seoul: Policy and science. Aerosol and Air Quality Research, 18, 2141–2156.
Lee, S.-J., Lee, H.-Y., Kim, S.-J., Kang, H.-J., Kim, H., Seo, Y.-K., Shin, H.-J., Ghim, Y. S., Song, C.-K., & Choi, S.-D. (2023). Pollution characteristics of PM2.5 during high concentration periods in summer and winter in Ulsan, the largest industrial city in South Korea. Atmospheric Environment, 292, 119418.
Lelieveld, J., Evans, J. S., Fnais, M., Giannadaki, D., & Pozzer, A. (2015). The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature, 525, 367–371.
Luo, Y., Zhou, X., Zhang, J., Xiao, Y., Wang, Z., Zhou, Y., & Wang, W. (2018). PM2.5 pollution in a petrochemical industry city of northern China: Seasonal variation and source apportionment. Atmospheric Research, 212, 285–295.
Nguyen, T. N. T., Jung, K.-S., Son, J. M., Kwon, H.-O., & Choi, S.-D. (2018). Seasonal variation, phase distribution, and source identification of atmospheric polycyclic aromatic hydrocarbons at a semi-rural site in Ulsan, South Korea. Environmental Pollution, 236, 529–539.
NICS. (2022). Pollutant release and transfer register (PRTR). National Institute of Chemical Safety, https://icis.me.go.kr/prtr/main.do.
NIER. (2022). 2019 National air pollutants emission. National Institute of Environmental Research, pp. 123–143. https://www.air.go.kr/main.do.
NIOSH. (1998). NIOSH manual of analytical methods (NMAM), fourth edition: Particulates not otherwise regulated, respirable. National Institute for Occupational Safety & Health, pp. 2–6. NIOSH Manual of Analytical Methods (NMAM), Department of Health and Human Services, Cincinnati
Park, S.-S., Jung, S.-A., Gong, B.-J., Cho, S.-Y., & Lee, S.-J. (2013). Characteristics of PM2.5 haze episodes revealed by highly time-resolved measurements at an air pollution monitoring supersite in Korea. Aerosol and Air Quality Research, 13, 957–976.
Park, Y.-M., Park, K.-S., Kim, H., Yu, S.-M., Noh, S., Kim, M.-S., Kim, J.-Y., Ahn, J.-Y., Lee, M.-D., Seok, K.-S., & Kim, Y.-H. (2018). Characterizing isotopic compositions of TC-C, NO3−-N, and NH4+-N in PM2.5 in South Korea: Impact of China’s winter heating. Environmental Pollution, 233, 735–744.
Phillips, H., & Oh, J. (2020). Evaluation of aldehydes, polycyclic aromatic hydrocarbons, and PM2.5 levels in food trucks: A pilot study. Workplace Health & Safety, 68, 443–451.
Querol, X., Alastuey, A., Rodriguez, S., Plana, F., Ruiz, C. R., Cots, N., Massagué, G., & Puig, O. (2001). PM10 and PM2.5 source apportionment in the Barcelona Metropolitan Area, Catalonia. Spain. Atmospheric Environment, 35, 6407–6419.
Saini, P., & Sharma, M. (2020). Cause and age-specific premature mortality attributable to PM2.5 exposure: An analysis for million-plus Indian cities. Science of the Total Environment, 710, 135230.
Sun, Y., Zhuang, G., Tang, A., Wang, Y., & An, Z. (2006). Chemical characteristics of PM2.5 and PM10 in haze−fog episodes in Beijing. Environmental Science & Technology, 40, 3148–3155.
US EPA. (2012). Revised air quality standards for particle pollution and updates to the air quality index (AQI). United States Environmental Protection Agency, pp. 1–5. https://www.epa.gov/sites/default/files/2016-04/documents/2012_aqi_factsheet.pdf.
Vallero, D. (2014). Fundamentals of air pollution. Academic press. Elsevier Inc, San Diego.
Vuong, Q. T., Park, M.-K., Do, T. V., Thang, P. Q., & Choi, S.-D. (2022). Driving factors to air pollutant reductions during the implementation of intensive controlling policies in 2020 in Ulsan. South Korea. Environmental Pollution, 292, 118380.
WHO. (2018). WHO issues latest global air quality report: Some progress, but more attention needed to avoid dangerously high levels of air pollution. World Health Organization, https://www.who.int/china/news/detail/02-05-2018-who-issues-latest-global-air-quality-report-some-progress-but-more-attention-needed-to-avoid-dangerously-high-levels-of-air-pollution.
Yan, G., Zhang, P., Yang, J., Zhang, J., Zhu, G., Cao, Z., Fan, J., Liu, Z., & Wang, Y. (2021). Chemical characteristics and source apportionment of PM2.5 in a petrochemical city: Implications for primary and secondary carbonaceous component. Journal of Environmental Sciences, 103, 322–335.
Yang, G. H., Jo, Y. J., Lee, H. J., Song, C. K., & Kim, C. H. (2020). Numerical sensitivity tests of volatile organic compounds emission to PM2.5 formation during heat wave period in 2018 in two southeast Korean cities. Atmosphere, 11, 331.
Zhang, X., Gao, S., Fu, Q., Han, D., Chen, X., Fu, S., Huang, X., & Cheng, J. (2020). Impact of VOCs emission from iron and steel industry on regional O3 and PM2.5 pollutions. Environmental Science and Pollution Research, 27, 28853–28866.
Acknowledgements
This study was supported by the National Institute of Environmental Research (NIER-2021-03-03-001).
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
The authors read and approved the final manuscript. The researcher claims no conflicts of interest
Corresponding authors
Ethics declarations
Competing interests
The researcher claims no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, BK., Choi, SD., Shin, B. et al. Sensitivity analysis of volatile organic compounds to PM2.5 concentrations in a representative industrial city of Korea. Asian J. Atmos. Environ 17, 3 (2023). https://doi.org/10.1007/s44273-023-00003-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s44273-023-00003-y