Abstract
High-flow nasal cannula (HFNC) has been widely promoted during the COVID-19 pandemic to circumvent invasive mechanical ventilation. While there are several reported benefits, randomized trials demonstrate inconsistent variable success. We hypothesize that this is due to variable stopping criteria. This systematic review’s purpose is to review these criteria and investigate any associations with HFNC outcomes. We searched PubMed and EMBASE for all English-language randomized controlled trials (RCTs) published from January 1, 2007, to December 31, 2022, focusing on respiratory rate as a threshold for escalation of respiratory support. Subgroup analysis was conducted based on trial failure criteria, and intubation and mortality benefits were studied. Fisher’s exact test was performed following a 5% level of significance. Of the 22 RCTs included, 4 (18.2%) reported significant intubation benefits and 1 (0.05%) reported significant mortality benefit. The presence of objective failure criteria with a prespecified high respiratory rate threshold (35 breaths per minute or higher) had a significant effect on intubation rate reduction (P = 0.02). However, this result might be limited by the heterogeneity of the included studies. Further RCTs are required to confirm this conclusion. Given that a high respiratory rate threshold was associated with a reduction of intubation without increasing mortality, we hypothesize that among patients receiving HFNC who were eventually not intubated, the avoidance of intubation led to better clinical outcomes, while among eventually intubated patients, delays led to poorer outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
High-flow nasal cannula (HFNC) is a form of non-invasive respiratory support able to deliver humidified and heated oxygen at up to 80 L per minute, compared to traditional low-flow nasal cannulas at up to 15 L per minute. It was developed to provide high FiO2 to patients with non-hypercapnoeic hypoxemic respiratory failure. HFNC’s implementation for acute hypoxemic respiratory failure has been widely promoted during the COVID-19 pandemic to mitigate the need for invasive mechanical ventilation. Reported benefits of HFNC include improved ventilation and oxygen delivery, better removal of secretions and decreased work of breathing [1]. Clinical applications of HFNC in the context of acute hypoxemic respiratory failure include pneumonia, acute respiratory distress syndrome (ARDS) and acute cardiogenic pulmonary oedema [2].
However, randomized trials demonstrate inconsistent clinical outcomes such as intubation rates and mortality benefit. While some meta-analyses of trials report a reduction in the need for escalation of respiratory support with HFNC use, particularly intubation rates, compared to conventional oxygen therapy [3], other studies report no associated benefits for intubation, mortality or length of stay [4]. Overall, HFNC has been met with variable success. Ait Hamou et al. reported an association between HFNC failure (defined as requiring escalation to invasive mechanical ventilation) and increased intensive care unit (ICU) mortality [5]. Kang et al. reported an association between delayed intubation following HFNC use and increased mortality rates and poor clinical outcomes [6].
Differing practices regarding indications and thresholds for HFNC initiation could possibly account for the inconsistent clinical outcomes. As an example, an observational study surveying ICU practices found HFNC initiated in various clinical contexts (e.g., pneumonia, post-extubation support) using different clinical parameters [7]. By extension, these variable clinical practices are likely reflected in the designing of randomized controlled trials (RCTs) comparing HFNC with other forms of respiratory support. We hypothesize that HFNC’s variable success is in part due to variable stopping criteria in RCTs, which would lead to differing practices regarding indications and thresholds for HFNC use. We therefore aim to review these stopping criteria and investigate if these have any associations with HFNC outcomes.
2 Methods
The protocol for this systematic review was registered on PROSPERO (ID: CRD42024519794). It is available in full on the https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=519794.
2.1 Search strategy
We based our review on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [8] (Supplementary 1), and searched two databases (PubMed and EMBASE) for all English-language papers published from January 1, 2007, to December 31, 2022, using the following search terms: (("high-flow" OR "high flow") AND ("nasal" OR "oxygen" OR "therapy")) AND ("acute respiratory failure" OR "respiratory failure" OR "respiratory insufficiency" OR "acute respiratory distress" OR "hypoxemia" OR "hypoxaemia") AND ("Randomized Controlled Trial"[pt] OR "Controlled Clinical Trial"[pt] OR "Clinical Trials as Topic"[mh] OR randomized[tiab] OR placebo[tiab] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (Animals [mh] NOT Humans [mh]).
2.2 Inclusion and exclusion
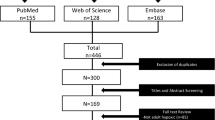
Both authors performed independent screening and selection of RCTs comparing the use of HFNC with conventional oxygen therapy/non-invasive ventilation/continuous positive airway pressure in patients with acute hypoxemic respiratory failure. Any disagreements were resolved by consensus. Decisions on inclusion were recorded on Microsoft Excel (Redmont, WA) and included in the PRISMA flow chart (Fig. 1).
2.3 Data analysis
Data extracted included trial design, study design, clinical setting, patient population, comparators, number of participants, number immunocompromised/with COVID-19/Do-Not-Resuscitate, mean age and PaO2/FiO2 (P/F) ratio, cause of hypoxemic respiratory failure, study inclusion and exclusion criteria, starting criteria, success and failure criteria, HFNC protocol, effect on need for intubation, ventilator-free days (VFDs), length of stay, mortality, desaturation and patient comfort (Tables 1, 2). The quality of included studies was assessed using the Cochrane risk of bias tool (Table 3). Preplanned subgroup analysis was done based on variability of trial stopping criteria, which was analyzed qualitatively.
Most RCTs defined HFNC treatment failure as a need for escalation of therapy to intubation and mechanical ventilation, utilizing different thresholds for escalation of oxygen therapy (e.g., vital signs, physical examination findings). In this study, we focused on respiratory rate as an objective threshold for escalation of therapy. Respiratory rate is an important objective measure for clinical severity, holding diagnostic and prognostic significance, particularly in critically ill patients. Garrido et al. found that increases in respiratory rate were significantly associated with ICU admissions, and could guide management and prevent morbidity and mortality by predicting clinical deterioration necessitating intervention [9]. Additionally, respiratory rate is used in the CURB-65 Score for Pneumonia Severity to risk stratify community-acquired pneumonia [10]. External validation of the CURB-65 demonstrated its ability to predict mortality, need for ICU admission [11] and need for intubation [12].
Subgroup analysis was conducted based on the trial failure criteria (categorical variable) used by the included studies. These criteria were designated as 'subjective criteria' (i.e., did not indicate a cut-off respiratory rate in their failure criteria), 'objective criteria with a low threshold to intubate' (cut-off respiratory rate < 35), and 'objective criteria with a high threshold to intubate' (respiratory rate ≥ 35). A respiratory rate of 35 was used as the threshold as several RCTs had used this as a marker of worsening respiratory failure for early termination [13, 14]. The studies were eventually categorized into 'high threshold' and 'low threshold or subjective'.
2.4 Outcome measures
Clinical outcomes of interest were intubation benefits (Table 4) (measured by number of patients intubated and prevalence of intubation by the end of the trial) and mortality benefit (measured by mortality rate) (Table 5). Effect measures extracted included odds ratio (OR), relative risk (RR) and hazard ratio (HR). Intubation benefits were recorded as categorical variables (designated as either 'none', ‘benefit’ and 'harm'). 'Benefit' was defined as RCTs demonstrating statistically significant reduction in intubation/ventilation/mortality rates or longer VFD, and the converse was true for 'harm'. Studies that reported no significant relationship between mode of oxygen therapy and the relevant clinical outcomes or with unknown/unreported outcomes were labeled as 'none'. Studies designated 'none' or 'harm' were ultimately categorized as 'no benefit'. Since both failure criteria and clinical outcomes were binary categorical variables, Fisher’s exact test was performed to determine the statistical significance, following a 5% level of significance (i.e., results were considered statistically significant if P < 0.05). Subgroup analysis based on the type of non-HFNC comparator used was conducted (Tables 6, 7 and 8
3 Results
3.1 Study selection
Of 1566 articles, 22 RCTs were included for review (Fig. 1) [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. RCTs concerning HFNC for treatment of acute hypoxaemic respiratory failure in an adult population were included if they reported any of the outcomes of interest (i.e., intubation rates, mortality, length of stay) and included a non-HFNC comparator (e.g., conventional oxygen therapy (COT), non-invasive ventilation (NIV) and continuous positive airway pressure (CPAP)). Non-RCTs (i.e., case reports, case series, observational, retrospective and cohort studies, and reviews), as well as RCTs concerning HFNC indicated for reasons other than acute hypoxemic respiratory failure (e.g., hypercapnoeic respiratory failure, perioperative and periprocedural support, pre-intubation and post-extubation respiratory support) were excluded from the review.
3.2 Study characteristics
Among the included studies, 12 studies (54.5%) were conducted in an ICU setting, followed by 7 (31.5%) in an Emergency Department setting (Table 1). Non-HFNC comparators used included COT, NIV and CPAP (Tables 1, 2).
3.3 Study analysis
Of the 22 RCTs included, 5 (22.7%) had high threshold failure criteria, while 17 (77.3%) did not have a high threshold failure criterion (of which 2 (9.1%) were objective with a low respiratory rate threshold, and 15 (68.2%) had subjective criteria). 4 trials (18.2%) reported significant intubation reduction. The presence of objective failure criteria with a prespecified high respiratory rate was found to have a significant effect on intubation rate reduction (P = 0.024). Subgroup analysis by non-HFNC comparator showed non-significant results with the same direction of benefit: COT comparators (P = 0.055) (Table 7); NIV and CPAP comparators (P = 0.378) (Table 8). The study by Frat et al. was excluded from this subgroup analysis due to the presence of multiple treatment arms in the study design. In addition, a secondary analysis excluding studies on COVID-19 patients showed non-significant results with the same direction of benefit (P = 0.097) (Table 9).
While the study by Frat et al. reported a significant reduction in mortality when using HFNC, all 21 (95.5%) other RCTs did not report any significant mortality benefits associated with HFNC use. Thus, mortality was not included in the statistical testing.
4 Discussion
When HFNC is applied as a means for acute hypoxemic respiratory failure support, having objective trial failure criteria with a high respiratory rate threshold for intubation confers significant benefits in intubation reduction, but without any significant reduction in overall mortality, as compared to subjective or less stringent trial failure criteria, which were neither associated with intubation or mortality benefit.
An objective high respiratory rate threshold may serve as a double-edged sword, resulting in no net mortality benefit despite reducing the risk of intubation. In this review, high thresholds were associated with a reduction of intubation without increasing mortality. We hypothesize that among patients given HFNC who were eventually not intubated, the avoidance of intubation led to better clinical outcomes. Higher intubation thresholds allow the benefits of HFNC to be actualized, and serve as a resource-saving measure, especially in the ICU setting. Conversely, among patients who are eventually intubated, delays in intubation can lead to worse outcomes. This was demonstrated in the trial by Andino et al., which reported intubation benefits with HFNC using objective failure criteria. Despite identical intubation criteria, intubation occurred significantly later in patients treated with HFNC as compared to COT, leading to a lack of an improvement trend in P/F ratio from the first 8 h among HFNC-treated patients who were eventually intubated [27]. Prospective cohort studies (e.g., Kang et al.) have similarly observed an association between delayed intubation post-HFNC and increased mortality [6]. Gonzales et al. also found that increased time to intubation from first respiratory support (HFNC and NIV) was associated with an increased risk of in-hospital mortality and worsened diffusion capacity for carbon monoxide (DLCO) during subsequent follow-up of surviving patient [35]. The proposed mechanism for this is patient self-induced lung injury, where spontaneous vigorous inspiratory effort results in high transpulmonary pressures that damage the lungs [36]. By extension, sedation and endotracheal intubation minimizes the progression of lung injury [37]. However, this was not consistently observed across all the included RCTs. For instance, Elagamy et al. reported intubation benefits with HFNC using objective failure criteria, but no significant difference (P = 0.785) in time to intubation was noted between HFNC-treated patients (51.17 ± 14.94 h) and NIV-treated patients (52.18 ± 6.67 h) [30].
This review’s data suggest that to optimize clinical outcomes for patients on HFNC, clinicians must reconcile adherence to a high respiratory rate threshold for intubation and not delaying necessary escalation to invasive mechanical ventilation. This requires identification of patients at risk of poor outcomes following delayed intubation. In a propensity score analysis of the RISC-19-ICU cohort of critically-ill COVID-19 patients, the authors suggested a closely monitored trial of HFNC therapy, followed by rapid intubation in patients with a high risk of failure and mortality (identified with prognostic scores) [38]. Since this did not account for prior respiratory support initiation in general wards, which has proven to be feasible [39], Gonzalez et al. suggested intubation within the first 48 h from the first respiratory support (e.g., HFNC) [35].
More recently, the respiratory oxygenation (ROX) index, which incorporates respiratory rate, SpO2 and FiO2, was shown to predict the need for escalation of respiratory support to intubation post-HFNC [40]. Patients started on HFNC were reassessed at 2-, 6- and 12-h intervals after HFNC initiation, and ROX scores ≥ 4.88 correlated with lower intubation risk. Conversely, lower ROX scores (< 2.85, < 3.47, and < 3.85 at 2, 6, and 12 h respectively) consistently predicted HFNC failure. However, the ROX index requires measurement of SpO2, which may differ from SaO2, particularly in patients with dark skin pigmentation [41]. The respiratory rate itself could be more objective than the ROX index as a stopping criterion for HFNC, and both could be prospectively compared in future HFNC trials.
4.1 Strengths and limitations
The strength of this review is the inclusion of trials that report both HFNC benefits and no benefits across various clinical outcomes. At the same time, we included RCTs that studied patient populations with acute hypoxemic respiratory failure secondary to COVID-19, which is one of the main areas of interest for HFNC use. While COVID-19 associated ARDS has been shown to be substantially similar to non-COVID associated ARDS in terms of alveolar damage and mechanisms of hypoxaemia [42], a secondary analysis of trials not involving COVID-19 patients showed the effect of objective failure criteria was not significant. This is likely due to a lack of sufficient studies. Respiratory mechanics, response to treatment and prognosis are similar in both COVID-19 and non-COVID-19 associated ARDS [43, 44], and thus they were included in the main analysis. A limitation of the study is that non-English databases were not studied, however PubMed and EMBASE contained most of the important HFNC trials. Due to the lack of consistently reported continuous data in the included trials, other parameters such as P/F ratio, SF ratio and PaCO2 could not be included in our analysis. However, there is merit to examining these clinical outcomes in future studies. Regression analysis was considered, but was not feasible as the failure thresholds analysed were discrete criteria as opposed to continuous variables, as well as due to a lack of sufficient trials. While the time to intubation will be a useful clinical outcome to ascertain the association between delays to intubation post-HFNC and poor clinical outcomes, this was not consistently reported across the included RCTs, albeit an interesting outcome to evaluate in future. The effect of objective failure criteria was not significant when subgroup analysis was performed by type of non-HFNC comparator and is likely due to lack of sufficient studies. While high respiratory rate thresholds might be associated with a reduction of intubation, this result may be limited by the heterogeneity of the included studies and a lack of consistency in the data reported. As such, further RCTs are required to ascertain this association.
5 Conclusion
In order to actualise the benefits of HFNC, a balance must be struck between adhering to high thresholds for intubation and avoiding unnecessary delays to escalation of respiratory support. The respiratory rate is an important objective threshold to reduce intubation rates without increasing mortality, and could potentially be used as a simple but objective stopping criterion for HFNC.
Availability of data and materials
Data that supports the study findings are available upon reasonable request.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- COT:
-
Conventional oxygen therapy
- CPAP:
-
Continuous positive airway pressure
- COPD:
-
Chronic obstructive pulmonary disease
- HFNC:
-
High-flow nasal cannula
- HR:
-
Hazard ratio
- ICU:
-
Intensive Care Unit
- NIV:
-
Non-invasive ventilation
- OR:
-
Odds ratio
- P/F ratio:
-
PaO2/FiO2
- RCT:
-
Randomized controlled trial
- ROX:
-
Respiratory oxygenation
- RR:
-
Relative risk
- S/F ratio:
-
SpO2/FiO2
- VFD:
-
Ventilator-free day
References
Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respir Med. 2009;103(10):1400–5. https://doi.org/10.1016/j.rmed.2009.04.007.
See KC. Approach to acute respiratory failure for frontline clinicians. Singapore Med J. 2022;63(12):740–5. https://doi.org/10.4103/singaporemedj.SMJ-2022-002.
Ferreyro BL, Angriman F, Munshi L, et al. Association of Noninvasive Oxygenation Strategies With All-Cause Mortality in Adults With Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-analysis. JAMA. 2020;324(1):57–67. https://doi.org/10.1001/jama.2020.9524.
Baldomero AK, Melzer AC, Greer N, et al. Effectiveness and Harms of High-Flow Nasal Oxygen for Acute Respiratory Failure: An Evidence Report for a Clinical Guideline From the American College of Physicians. Ann Intern Med. 2021;174(7):952–66. https://doi.org/10.7326/m20-4675.
Ait Hamou Z, Levy N, Charpentier J, Mira JP, Jamme M, Jozwiak M. Use of high-flow nasal cannula oxygen and risk factors for high-flow nasal cannula oxygen failure in critically-ill patients with COVID-19. Respir Res. 2022;23(1):329. https://doi.org/10.1186/s12931-022-02231-2
Kang BJ, Koh Y, Lim CM, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015;41(4):623–32. https://doi.org/10.1007/s00134-015-3693-5.
Puah SH, Li A, Cove ME, et al. High-flow nasal cannula therapy: A multicentred survey of the practices among physicians and respiratory therapists in Singapore. Aust Crit Care. 2022;35(5):520–6. https://doi.org/10.1016/j.aucc.2021.08.001.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. Mar 29 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Garrido D, Assioun JJ, Keshishyan A, Sanchez-Gonzalez MA, Goubran B. Respiratory Rate Variability as a Prognostic Factor in Hospitalized Patients Transferred to the Intensive Care Unit. Cureus. Jan 23 2018;10(1):e2100. https://doi.org/10.7759/cureus.2100.
Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–82. https://doi.org/10.1136/thorax.58.5.377.
Shah BA, Ahmed W, Dhobi GN, Shah NN, Khursheed SQ, Haq I. Validity of pneumonia severity index and CURB-65 severity scoring systems in community acquired pneumonia in an Indian setting. Indian J Chest Dis Allied Sci. 2022;52(1):9–17.
Capelastegui A, España PP, Quintana JM, et al. Validation of a predictive rule for the management of community-acquired pneumonia. Eur Respir J. 2006;27(1):151–7. https://doi.org/10.1183/09031936.06.00062505.
Makdee O, Monsomboon A, Surabenjawong U, et al. High-Flow Nasal Cannula Versus Conventional Oxygen Therapy in Emergency Department Patients With Cardiogenic Pulmonary Edema: A Randomized Controlled Trial. Ann Emerg Med. 2017;70(4):465-472.e2. https://doi.org/10.1016/j.annemergmed.2017.03.028.
Osman A, Via G, Sallehuddin RM, et al. Helmet continuous positive airway pressure vs. high flow nasal cannula oxygen in acute cardiogenic pulmonary oedema: a randomized controlled trial. Eur Heart J Acute Cardiovasc Care. Dec 18 2021;10(10):1103–1111. https://doi.org/10.1093/ehjacc/zuab078
Parke RL, McGuinness SP, Eccleston ML. A preliminary randomized controlled trial to assess effectiveness of nasal high-flow oxygen in intensive care patients. Respir Care. 2011;56(3):265–70. https://doi.org/10.4187/respcare.00801.
Bell N, Hutchinson CL, Green TC, Rogan E, Bein KJ, Dinh MM. Randomised control trial of humidified high flow nasal cannulae versus standard oxygen in the emergency department. Emerg Med Australas. 2015;27(6):537–41. https://doi.org/10.1111/1742-6723.12490.
Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–96. https://doi.org/10.1056/NEJMoa1503326.
Rittayamai N, Tscheikuna J, Praphruetkit N, Kijpinyochai S. Use of High-Flow Nasal Cannula for Acute Dyspnea and Hypoxemia in the Emergency Department. Respir Care. 2015;60(10):1377–82. https://doi.org/10.4187/respcare.03837.
Jones PG, Kamona S, Doran O, Sawtell F, Wilsher M. Randomized Controlled Trial of Humidified High-Flow Nasal Oxygen for Acute Respiratory Distress in the Emergency Department: The HOT-ER Study. Respir Care. 2016;61(3):291–9. https://doi.org/10.4187/respcare.04252.
Lemiale V, Mokart D, Mayaux J, et al. The effects of a 2-h trial of high-flow oxygen by nasal cannula versus Venturi mask in immunocompromised patients with hypoxemic acute respiratory failure: a multicenter randomized trial. Crit Care. Nov 2 2015;19:380. https://doi.org/10.1186/s13054-015-1097-0.
Lemiale V, Resche-Rigon M, Mokart D, et al. High-Flow Nasal Cannula Oxygenation in Immunocompromised Patients With Acute Hypoxemic Respiratory Failure: A Groupe de Recherche Respiratoire en Réanimation Onco-Hématologique Study. Crit Care Med. 2017;45(3):e274–80. https://doi.org/10.1097/ccm.0000000000002085.
Azoulay E, Lemiale V, Mokart D, et al. Effect of High-Flow Nasal Oxygen vs Standard Oxygen on 28-Day Mortality in Immunocompromised Patients With Acute Respiratory Failure: The HIGH Randomized Clinical Trial. JAMA. 2018;320(20):2099–107. https://doi.org/10.1001/jama.2018.14282.
Shebl E, Embarak S. High-flow nasal oxygen therapy versus noninvasive ventilation in chronic interstitial lung disease patients with acute respiratory failure. Article. Egyptian Journal of Chest Diseases and Tuberculosis. 2018;67(3):270–275. https://doi.org/10.4103/ejcdt.ejcdt_33_18
Azevedo JR, Montenegro WS, Leitao AL, Silva MM, Prazeres JS, Maranhao JP. High flow nasal cannula oxygen (HFNC) versus non-invasive positive pressure ventilation (NIPPV) in acute hypoxemic respiratory failure. A pilot randomized controlled trial. Note. Intensive Care Medicine Experimental. 2015;3 Suppl 1:A166. https://doi.org/10.1186/2197-425X-3-S1-A166.
Menga LS, Raggi V, Bongiovanni F, et al. Helmet non-invasive ventilation versus high flow nasal oxygen in acute hypoxemic respiratory failure: Physiological effects. Conference Abstract. Critical Care. 2019;23. https://doi.org/10.1186/s13054-019-2358-0.
Ruangsomboon O, Dorongthom T, Chakorn T, et al. High-Flow Nasal Cannula Versus Conventional Oxygen Therapy in Relieving Dyspnea in Emergency Palliative Patients With Do-Not-Intubate Status: A Randomized Crossover Study. Ann Emerg Med. 2020;75(5):615–26. https://doi.org/10.1016/j.annemergmed.2019.09.009.
Andino R, Vega G, Pacheco SK, et al. High-flow nasal oxygen reduces endotracheal intubation: a randomized clinical trial. Ther Adv Respir Dis Jan-Dec. 2020;14:1753466620956459. https://doi.org/10.1177/1753466620956459.
Aksakal A, Sağlam L, Kerget B, Yilmazel UE. Comparison of the effectiveness of high-flow and conventional nasal cannula oxygen therapy in pulmonary embolism patients with acute hypoxemic respiratory failure. Article Tuberkuloz ve Toraks. 2021;69(4):469–76. https://doi.org/10.5578/tt.20219604.
Grieco DL, Menga LS, Cesarano M, et al. Effect of Helmet Noninvasive Ventilation vs High-Flow Nasal Oxygen on Days Free of Respiratory Support in Patients With COVID-19 and Moderate to Severe Hypoxemic Respiratory Failure: The HENIVOT Randomized Clinical Trial. JAMA. 2021;325(17):1731–43. https://doi.org/10.1001/jama.2021.4682.
Elagamy AE, Taha SS, Elfawy DM. High flow nasal cannula versus non- invasive ventilation in prevention of intubation in immunocompromised patient with acute hypoxemic respiratory failure. Egypt J Anaesth. 2021;37(1):432–9. https://doi.org/10.1080/11101849.2021.1978744.
Ospina-Tascón GA, Calderón-Tapia LE, García AF, et al. Effect of High-Flow Oxygen Therapy vs Conventional Oxygen Therapy on Invasive Mechanical Ventilation and Clinical Recovery in Patients With Severe COVID-19: A Randomized Clinical Trial. JAMA. 2021;326(21):2161–71. https://doi.org/10.1001/jama.2021.20714.
Alptekinoğlu Mendil N, Temel Ş, Yüksel RC, et al. The use of high-flow nasal oxygen vs. standard oxygen therapy in hematological malignancy patients with acute respiratory failure in hematology wards. Turk J Med Sci. Aug 30 2021;51(4):1756–1763. https://doi.org/10.3906/sag-2007-228.
Agmy G, Adam M, Hsanen E, Mahmoud MA. High-flow nasal cannula versus noninvasive ventilation in the prevention of escalation to invasive mechanical ventilation in patients with acute hypoxemic respiratory failure. Article. Egyptian Journal of Chest Diseases and Tuberculosis. 2022;71(1):81–87. https://doi.org/10.4103/ecdt.ecdt_12_20.
Perkins GD, Ji C, Connolly BA, et al. Effect of Noninvasive Respiratory Strategies on Intubation or Mortality Among Patients With Acute Hypoxemic Respiratory Failure and COVID-19: The RECOVERY-RS Randomized Clinical Trial. JAMA. 2022;327(6):546–58. https://doi.org/10.1001/jama.2022.0028.
González J, Benítez ID, de Gonzalo-Calvo D, et al. Impact of time to intubation on mortality and pulmonary sequelae in critically ill patients with COVID-19: a prospective cohort study. Crit Care. 2022;26(1):18. https://doi.org/10.1186/s13054-021-03882-1.
Marini JJ, Gattinoni L. Management of COVID-19 Respiratory Distress. JAMA. 2020;323(22):2329–30. https://doi.org/10.1001/jama.2020.6825.
Brochard L, Slutsky A, Pesenti A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am J Respir Crit Care Med. 2017;195(4):438–42. https://doi.org/10.1164/rccm.201605-1081CP.
Wendel Garcia PD, Aguirre-Bermeo H, Buehler PK, et al. Implications of early respiratory support strategies on disease progression in critical COVID-19: a matched subanalysis of the prospective RISC-19-ICU cohort. Crit Care. 2021;25(1):175. https://doi.org/10.1186/s13054-021-03580-y
Colombo SM, Scaravilli V, Cortegiani A, et al. Use of high flow nasal cannula in patients with acute respiratory failure in general wards under intensivists supervision: a single center observational study. Respir Res. 2022;23(1):171. https://doi.org/10.1186/s12931-022-02090-x.
Roca O, Caralt B, Messika J, et al. An Index Combining Respiratory Rate and Oxygenation to Predict Outcome of Nasal High-Flow Therapy. Am J Respir Crit Care Med. 2019;199(11):1368–76. https://doi.org/10.1164/rccm.201803-0589OC.
Valbuena VSM, Seelye S, Sjoding MW, et al. Racial bias and reproducibility in pulse oximetry among medical and surgical inpatients in general care in the Veterans Health Administration 2013–19: multicenter, retrospective cohort study. Bmj. 2022;378:e069775. https://doi.org/10.1136/bmj-2021-069775.
Beloncle FM. Is COVID-19 different from other causes of acute respiratory distress syndrome? J Intensive Med. 2023;3(3):212–9. https://doi.org/10.1016/j.jointm.2023.02.003.
Brault C, Zerbib Y, Kontar L, et al. COVID-19- versus non-COVID-19-related Acute Respiratory Distress Syndrome: Differences and Similarities. Am J Respir Crit Care Med. 2020;202(9):1301–4. https://doi.org/10.1164/rccm.202005-2025LE.
Haudebourg AF, Perier F, Tuffet S, et al. Respiratory Mechanics of COVID-19- versus Non-COVID-19-associated Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2020;202(2):287–90. https://doi.org/10.1164/rccm.202004-1226LE.
Acknowledgements
Not applicable.
Funding
There has been no financial support for this work that could influence its outcome.
Author information
Authors and Affiliations
Contributions
All authors contributed to the screening and selection, data extraction, data analysis and interpretation and writing of the manuscript. All authors had full access to the data in the study and take responsibility for the integrity and accuracy of the data analysis.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors gave their content for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pan, J.T., See, K.C. Criteria for stopping high-flow nasal oxygen for acute hypoxemic respiratory failure: a systematic review of randomized controlled trials. APS 2, 23 (2024). https://doi.org/10.1007/s44254-024-00060-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44254-024-00060-8