Abstract
Sleep disturbances are risk factors for postoperative delirium (POD), and sleep interventions have been proposed as potential preventive measures. However, the effectiveness of sleep interventions in preventing POD is uncertain. We performed a systematic literature search using the PubMed, Embase, and Cochrane Library databases from inception until December 24, 2022. We included randomized controlled trials on sleep interventions and POD in adult surgery patients. The screening of titles, abstracts, and full texts was performed independently by two reviewers. Another two reviewers independently performed the data extraction and assessed the risk of bias. Pooled-effect estimates were calculated with a random effect model. Our primary outcome was POD, which was assessed with the confusion assessment method (CAM), CAM for the intensive care unit (CAM-ICU), or other delirium assessment tools. We used trial sequential analysis to control for type I and II statistical errors. We also conducted prespecified subgroup analyses, according to the type of intervention, efficacy of the intervention on postoperative sleep, sample size, participant age, delirium assessment tool used, and the type of surgery. Data were obtained from 25 trials, including 4799 participants. Sleep interventions had a statistically significant difference in the incidence of POD (relative risk (RR) = 0.60; 95% confidence interval (CI), 0.46–0.77; I2 = 58%). Stratified analyses indicated that the beneficial effects of sleep interventions were evident in trials where the interventions promoted postoperative sleep (RR = 0.51; 95% CI, 0.36–0.71) as compared to trials that did not (RR = 1.01; 95% CI, 0.77–1.31) (p-value for interaction between subgroups = 0.004). Our primary analysis demonstrated that in adult patients following elective surgery, interventions that improved postoperative sleep, as compared to the standard care or placebo groups, were associated with a lower risk of POD. However, such evidences are limited by the heterogeneity among trials and the small sample sizes of some trials.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Postoperative delirium (POD) is one of the most common complications after surgery [1]. As stated in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), clinical manifestations of POD include acutely occurring disturbances in attention, thinking, and cognition that fluctuate throughout the day [2, 3]. POD affects 11–51% of patients following major elective surgery and is associated with adverse consequences, such as prolonged hospital stay, increased healthcare costs, declined cognitive and physical function, and high morbidity and mortality [4,5,6]. Therefore, prevention of POD is essential to improve postoperative outcomes in patients. There are many predisposing and precipitating risk factors for POD, including increasing age, comorbidities, preexisting cognitive impairment, and sleep disorders [7, 8]. Some of these risk factors are modifiable, which could help in reducing the incidence and severity of POD.
The prevalence of preoperative sleep disturbances is in the range of 10–30% in healthy adults (aged > 18 years) and 36–43% in home-dwelling older adults (aged > 65 years) [9]. Sleep disturbances are also common during the perioperative period, which affects up to 40% of patients who undergo major inpatient surgeries [10]. The term “sleep disturbances” comprises multiple facets of sleep, including quantitative dimensions such as sleep duration and latency and qualitative aspects such as depth or restfulness. Sleep disturbances are a combination of subjective complaints and objective evidences that include insomnia, obstructive sleep apnea, hypersomnolence, to name a few [11,12,13]. Perioperative sleep disturbances are associated with delayed postoperative recovery, higher incidence of POD or postoperative cognitive dysfunction, and poor quality of life [14, 15]. Previous studies also suggested that perioperative sleep disturbances are the main risk factors for POD [16,17,18]. Consequently, efforts were made to promote perioperative sleep to decrease the incidence of POD. Lower POD incidence has been reported after sleep interventions in some studies [19, 20] but not others [1, 21]. At the same time, meta-analyses on the reduction of the incidence of POD via sleep interventions have, thus far, focused exclusively on either pharmacological interventions or nonpharmacological interventions [22, 23] and hence, have not provided definite conclusions regarding the effect of comprehensive sleep interventions on the occurrence of POD.
Therefore, we performed an updated systematic review and meta-analysis to determine the pooled results and thus benefit clinical practice by synthesizing the available randomized controlled trials (RCTs) that assessed the efficacy of sleep interventions on preventing POD in adult surgical patients. We evaluated the existing body of research about the effects of sleep interventions on the incidence of POD.
2 Methods
We performed and reported this systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (see Supplementary 1) [24]. The protocol for this systematic review and meta-analysis was registered on INPLASY (https://doi.org/10.37766/inplasy2023.1.0083.) and is available in full on the www.inplasy.com (https://doi.org/10.37766/inplasy00000000.).
2.1 Search strategy
A systematic search of literature was conducted from inception until December 24, 2022, using electronic databases (PubMed, Embase, and Cochrane Library). The detailed electronic search strategies for each database are illustrated in Supplementary 2. In addition, we searched Google Scholar for articles related to the extracted trials.
2.2 Inclusion and exclusion criteria
Two reviewers independently assessed the retrieved studies according to predetermined inclusion and exclusion criteria. Trials were included if they (1) were RCTs; (2) included patients undergoing elective surgery; (3) applied pharmacological or nonpharmacological interventions to alleviate perioperative sleep disturbances; and (4) clearly reported the incidence of POD. Trials were excluded if they (1) included patients aged < 18 years old; (2) were published as case series, systematic review and meta-analysis, protocol or conference paper; (3) had sleep interventions as part of a comprehensive program and the independent effect of sleep interventions cannot be derived; and (4) were published in a language other than English or Chinese.
The search results from all databases were entered into Endnote X9, duplicates were removed, and the remaining publications were screened for eligibility. The screening of titles, abstracts, and full texts was performed independently by two reviewers, using the same software. Ultimately, RCTs that met the inclusion criteria were included in the final analysis. Any discrepancies that emerged were addressed through a consensus-seeking process involving a third-party investigator.
2.3 Outcome measures
The outcome was the incidence of POD, which was assessed by delirium assessment tools, including DSM-5, Confusion Assessment Method (CAM), CAM for the intensive care unit (CAM-ICU), Intensive Care Delirium Screening Checklist (ICDSC), Neelon and Champagne (NEECHAM) Confusion Scale, and Nursing Delirium Screening Scale (Nu-DESC) [7].
2.4 Data extraction
Two reviewers independently utilized a predesigned data extraction form to collect key information from the trials, including the first author’s name, publication year, country where the study was conducted, study design (single-center or multicenter trial), number of participants enrolled in each group, mean or median age of the participants, distribution of gender, type of surgery, intervention and comparative treatment, incidence of POD (outcome variable), measurement instrument employed to assess the occurrence of POD, and timepoint of the assessment. Any discrepancies that arose between the two reviewers were settled via discussion with a third author to ensure data accuracy and reliability.
2.5 Quality assessment of included studies
The risk of bias was assessed using the Cochrane Collaboration Risk of Bias Assessment tool [25], which covers seven domains: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting, and (7) other bias. Specifically, all these domains were evaluated, and each domain was assigned a risk classification of high, unclear, or low. Trials that exhibited one or more items associated with a high or an unclear risk of bias were ultimately classified as high risk [26]. To ensure the accuracy and reliability of the findings, any discrepancies that arose during risk assessment were resolved through careful discussion and, when necessary, by the involvement of a third-party investigator.
2.6 Data analysis
The statistical analyses were performed in RevMan (Review Manager version 5.4, Cochrane Collaboration, Oxford, United Kingdom) and R (R version 4.2.1 with the ‘meta’ package). Dichotomous outcomes were reported as the relative risk (RR) with 95% confidence interval (CI). Due to clinical and methodological heterogeneity among the trials, a conservative random-effects model was applied [27]. We identified statistical heterogeneity among included trials using the I2 statistic and Mantel‐Haenszel chi-square test (p-value for heterogeneity). Low, moderate, and substantial heterogeneities were indicated by I2 < 30%, I2 = 30–60%, and I2 = 60–90%, respectively. Similarly, p > 0.10 and p < 0.10 from the chi-square test denoted low and high heterogeneity, respectively [28]. The sources of heterogeneity were investigated in the following prespecified subgroup analysis: (1) type of interventions (bright light, dexmedetomidine, melatonin, or positive airway pressure), (2) efficacy of the interventions on postoperative sleep (positive, negative, or no evaluation), (3) sample size (< 100 or ≥ 100), (4) age (< 65 years or ≥ 65 years), (5) delirium assessment tool used (CAM/CAM-ICU, Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria, NEECHAM, or other tools/not mentioned), (6) type of surgery (noncardiac surgery, cardiac surgery, or both cardiac and noncardiac surgery). Furthermore, for dexmedetomidine, the dose administered was different in intensive care unit (ICU) patients (sedative dose or low-dose) than in non-ICU patients (low-dose or mini-dose via a patient-controlled intravenous analgesia device). We also performed a stratified analysis of the two subgroups of patients to evaluate the association between the dosage of dexmedetomidine in the postoperative period and the incidence of POD. Sensitivity analyses were performed by removing articles one by one to assess the effect of individual trials on the overall results. Publication bias was evaluated by visually inspecting a funnel plot and formally testing by Peters’ test. Two-sided statistical tests were performed, and an overall effect size with p < 0.05 indicated a significant difference.
We conducted a trial sequential analysis (TSA) using TSA software (version 0.9 beta, http://www.ctu.dk/tsa). The purpose of TSA is to adjust the statistical threshold to minimize or eliminate the risk of type I and type II errors, which have been found to affect the results of meta-analyses [29]. The required information size (RIS) was determined based on the incidence of POD in the control group (21%) and a relative risk reduction of 28.57% in the experimental group [30] with a type I error of 5% (two-tailed) and type II error of 20% (80% power). TSA generated a graph that included a cumulative Z curve, a conventional meta-analysis boundary, the estimated RIS, and the trial sequential monitoring boundary (TSMB). If the cumulative Z curve crossed both the TSMB and the conventional meta-analysis boundary, and the number of recruited patients exceeded the RIS, the evidence was considered sufficient. Otherwise, the evidence was considered insufficient, which indicated that additional trials would be required [30, 31].
3 Results
3.1 Study selection
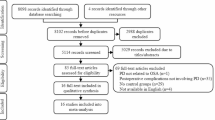
The processes of the search, screening, and selection of the trials are illustrated in Fig. 1. A total of 1267 records (1246 from the databases and 21 from other sources) were retrieved in the initial search. After removing duplicate trials (141 excluded), performing a screening of titles and abstracts (1064 excluded), and assessing the full texts (37 excluded), a total of 25 trials that met the inclusion criteria, including 4799 patients, were finally included in qualitative and quantitative analyses [1, 19,20,21, 32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52].
3.2 Study characteristics
The main characteristics of the included trials are described in detail in Table 1. These articles were published between 2007 and 2022. Among all included trials, ten trials evaluated the effect of melatonin [21, 37, 38, 41, 42, 44, 46, 48,49,50], eight assessed the effect of postoperative use of dexmedetomidine [1, 19, 20, 32, 33, 36, 47, 52], three evaluated the use of bright light in the morning [35, 39, 40], two included patients who were administered continuous positive airway pressure (CPAP) [43] or auto-titrating positive airway pressure (APAP) [34], and one used earplugs and eye masks to promote sleep-conducive environment [45]. Notably, the remaining one study used the delirium-free protocol (DFP), which involved an intramuscular injection of diazepam and a continuous intravenous infusion of flunitrazepam and pethidine [51]. Four trials enrolled patients underwent cardiac surgery [1, 44, 49, 50], nineteen trials included patients underwent noncardiac surgery [19,20,21, 32,33,34,35,36,37, 39,40,41,42,43,44,45,46,47, 51, 52], and the remaining two trials enrolled patients who underwent all type of surgeries [38, 48]. In a majority of the trials, the risk of POD was assessed using CAM or CAM-ICU [1, 19, 20, 32,33,34, 36, 38, 39, 43, 44, 46,47,48,49, 52], whereas three trials used the DSM criteria [21, 41, 51], two trials used the NEECHAM Confusion Scale [35, 40], and four trials used other tools or did not mention which tool was used [37, 42, 45, 50]. Nine trials reported statistical efficacy in improving sleep by interventions [19, 20, 36, 45, 47, 52], whereas five trials reported an insignificant effect of the sleep intervention on improvement of postoperative sleep quality [1, 33, 35, 38, 41]. The remaining eleven articles did not measure the efficacy of the intervention on postoperative sleep improvement [21, 32, 34, 37, 42,43,44, 46, 48, 49, 51].
3.3 Study quality
Many of the included trials were considered to have low risk of bias, given that they clearly reported random sequence generation (20 trials, 80%), allocation concealment (17 trials, 68%), blinding of personnel and participants (18 trials, 72%), blinding of the outcome assessment (22 trials, 88%), incomplete outcome data (0 trials, 0%), selective reporting (0 trials, 0%), and other bias (0 trials, 0%). Figure 2 presents a comprehensive depiction of the risk of bias in each study, as determined by the Cochrane criteria.
3.4 Pooled analysis
There was significant heterogeneity among the 25 trials (p = 0.0001; I2 = 58%). Meta-analysis using the random-effects model demonstrated that interventions aimed at promoting postoperative sleep were associated with a reduced occurrence of POD compared with the control group. The pooled RR for POD was 0.60 (95% CI, 0.46–0.77; p < 0.0001) (Fig. 3). As demonstrated in Fig. 4, the RIS was 4002 participants. The number of recruited patients reached the RIS and the cumulative Z curve crossed the TSMB for benefit, indicating a statistically significant beneficial effect of the sleep interventions on POD.
Forest plots of the pooled incidences of postoperative delirium for the sleep intervention group and the control group. Statistical analyses were conducted using a random-effects model. The size of the squares for risk ratio (RR) reflects the weight of the trial in the pooled analyses. Horizontal bars = 95% confidence intervals (CIs)
Trial sequential analysis (TSA) of the sleep interventions for decreasing the incidence of postoperative delirium (POD). A required information size (RIS) of 4002 patients was calculated using the predefined alpha = 0.05 (two-sided), beta = 0.20 (80% power), an anticipated relative risk reduction of 28.57%, and an event proportion of 21% in the control arm. The blue cumulative Z curve was constructed using a random-effects model. The horizontal brown lines represent the conventional meta-analysis boundary. The horizontal red lines represent the trial sequential monitoring boundary. The cumulative Z curve crosses both conventional meta-analysis boundary and the trial sequential monitoring boundary, and the number of recruited participants is more than the required information size, which represents that the pooled evidence of sleep intervention in decreasing the incidence of POD was sufficient
3.5 Subgroup analysis
We explored heterogeneity with prespecified subgroup analyses (Supplementary Figs. 1, 2, 3, 4, 5, 6 and 7). The forest plot revealed significant difference in the POD incidence in trials investigating the effects of bright light therapy (RR = 0.24; 95% CI, 0.09–0.65) or dexmedetomidine (RR = 0.53; 95% CI, 0.39–0.72). However, the pooled results did not demonstrate a significantly low incidence of POD after melatonin administration (RR = 0.79; 95% CI, 0.58–1.08) or positive airway pressure (PAP) therapy (RR = 0.69; 95% CI, 0.13–3.82) (Supplementary Fig. 1). Regarding the efficacy of the sleep interventions on postoperative sleep, the subgroup analysis showed that the interventions that were effective in promoting postoperative sleep were also effective in preventing POD (RR = 0.51; 95% CI, 0.36–0.71). Likewise, trials that did not assess perioperative sleep reported a reduction in POD risk among patients who received sleep interventions (RR = 0.59; 95% CI, 0.39–0.91). However, the trials that did not find improvements in postoperative sleep among those receiving sleep interventions similarly did not report a reduction in POD incidence among study subjects (RR = 1.01; 95% CI, 0.77–1.31) (Supplementary Fig. 2). Additional subgroup analyses were performed for different delirium assessment tools. Three trials used the DSM criteria (RR = 0.83; 95% CI, 0.27–2.57), two trials used the NEECHAM criteria (RR = 0.31; 95% CI, 0.07–1.31) for delirium assessment, both did not demonstrate a significant reduction in the incidence of POD. Sixteen trials used CAM or CAM-ICU (RR = 0.61; 95% CI, 0.46–0.82) for the POD diagnosis, four trials used other tools or did not mention which tool was used (RR = 0.34; 95% CI, 0.17–0.66) for the POD diagnosis, both subgroups demonstrated a comparable reduction in POD risk (Supplementary Fig. 3). Subgroup analyses also demonstrated significant difference in the POD incidence following noncardiac surgery (RR = 0.55; 95% CI, 0.40–0.76) but not cardiac surgery (RR = 0.68; 95% CI, 0.41–1.11) (Supplementary Fig. 4). There was a significant decrease in the incidence of POD by the sleep interventions, irrespective of the sample size, age, and dosage of dexmedetomidine (Supplementary Figs. 5, 6 and 7). We also performed a post-hoc subgroup analysis according to the timepoint of administration of the interventions, whether preoperatively (RR = 0.40; 95% CI, 0.17–0.90), postoperatively (RR = 0.49; 95% CI, 0.34–0.71), or both (RR = 0.92; 95% CI, 0.68–1.24) (Supplementary Fig. 8).
3.6 Sensitivity analysis
We used a one-by-one literature removal method to identify potential outlier trials responsible for the observed heterogeneity and to evaluate the stability of the results. We found no individual trials significantly reducing the heterogeneity after elimination. At the same time, we observed that no single study had a noteworthy impact on the pooled RR, which suggested that our meta-analysis results were robust (Fig. 5).
3.7 Publication bias
Publication bias was evaluated through visual examination of a funnel plot and formal testing by Peters’ test using R statistical software. The funnel plot for the overall incidence of POD showed no obvious asymmetry (Supplementary Fig. 9A and B). Additionally, Peters’ tests were conducted to further assess for publication bias, and no significant bias was found in this meta-analysis (p = 0.1043) (Supplementary 3).
4 Discussion
We identified 25 RCTs with a total of 4799 participants that compared the incidence of POD between the sleep intervention group and the control group. Despite there was significant heterogeneity among the included trials, we showed that sleep interventions in surgical patients was associated with a decreased risk of POD. Notably, the reduction in POD risk was evident in trials that reported significant improvements in postoperative sleep. TSA confirmed the beneficial effect of sleep interventions. Leave-one-out meta-analysis demonstrated that our results were not driven by an individual study and further confirmed the robustness of the results.
According to the pooled results, we observed that the use of dexmedetomidine was associated with as high as a 50% reduction in the incidence of POD. Dexmedetomidine is a potent and highly selective agonist of the α2-adrenoceptor with analgesic, anxiolytic and sedative properties, which is widely used in clinical anesthesia and postoperative sedation [53, 54]. Previous studies have demonstrated that dexmedetomidine can induce nonrapid eye movement (NREM) sleep-like electroencephalogram (EEG) features and consolidate sleep after surgery, leading to an increase in stage 2 of NREM sleep, decrease in stage 1 of NREM sleep, prolonged total sleep time, lower nocturnal fragmented sleep, and eventually better subjective sleep quality for patients [55]. Our present finding of dexmedetomidine effectively reducing the incidence of POD in patients is consistent with the results of a previous meta-analysis, in which Duan et al. [56] had demonstrated that dexmedetomidine had positive actions on diminishing the risk of POD in adult patients. Notably, we excluded trials in which the patients received dexmedetomidine only during the intraoperative period. The reason for this exclusion was that the objective of this meta-analysis was to investigate the role of sleep interventions on POD incidence, and dexmedetomidine usage only in the intraoperative period was mainly intended for intraprocedural sedation and may not have been associated with sleep interventions. Similarly, we observed that the occurrence of POD was comparatively lower among patients who received bright light therapy, a nonpharmacological method of sleep intervention that helped adjust the circadian rhythm and has a therapeutic effect on sleep disturbances in patients with mental disorders [57, 58]. However, our subgroup analysis revealed that melatonin or melatonin receptor agonists did not significantly affect the incidence of POD. In fact, a study by Wang et al. [59] concluded that the use of melatonin or melatonin receptor agonists during the perioperative period did not reduce the risk of POD (RR = 0.93; 95% CI, 0.70–1.24), which was consistent with our current findings. However, this was not consistent with results from a previous meta-analysis that observed beneficial effects of melatonin and melatonin receptor agonists on perioperative sleep and reduction in the risk of POD (pooled RR = 0.49; 95% CI, 0.28–0.88; p = 0.017) [22]. This inconsistency may be attributed to the heterogeneous design of the included trials (different dosages and timings of melatonin administration, etc.) and the different methods used for assessing the outcome (CAM/CAM-ICU, DSM criteria, etc.). Furthermore, seven trials did not assess the efficacy of intervention on postoperative sleep in patients who received melatonin or melatonin receptor agonists, [21, 37, 42, 44, 46, 48, 49], only one trial reported a statistically positive effect [50], and two trials did not report a significant improvement in sleep between the intervention and control groups [38, 41]. The absence of a formal sleep assessment may have led to an underestimation of the effectiveness of interventions for patients receiving melatonin or melatonin receptor agonists, further adding to the uncertainty of these interventions in reducing the occurrence of POD. Therefore, larger RCTs are required in future to investigate whether melatonin or melatonin receptor agonists have a preventive effect on POD.
A substantial body of research has considered obstructive sleep apnea (OSA) as a main risk factor for POD [7, 60,61,62]. There is a plausible biological link between OSA and POD, as OSA is likely to trigger inflammation, hypoxia, and disrupted sleep patterns, which could contribute to the development of POD [63]. It has been demonstrated that PAP could alleviate these possible triggers and significantly improves the symptoms of OSA [64]. However, the pooled finding in the subgroup analysis of our study did not suggest a protective effect of PAP therapy on decreasing the occurrence of POD in surgical patients who were at risk for OSA. Therefore, sleep interventions targeting OSA (PAP in short-term treatment) may have no role in decreasing postoperative incident delirium. The present meta-analysis only included two trials [34, 43] with a total of 334 participants, and there was significant heterogeneity between the two trials. Thus, due to the limited sample size and significant heterogeneity, the conclusions should be interpreted with caution. Because only one RCT [45] involved the use of earplugs and eye masks, and only one trial [51] included patients who were administered DFP using diazepam, flunitrazepam, and pethidine to maintain nocturnal sleep rhythm in the perioperative period, we refrained from performing subgroup analysis in both of them. Moreover, our analysis revealed that only the trials that demonstrated effective sleep interventions on postoperative sleep reported a lower incidence of POD. Furthermore, this was true in adult patients undergoing noncardiac surgery, but not in those undergoing cardiac surgery. Additionally, irrespective of the sample size, age, and dosage of dexmedetomidine, sleep interventions appear to be effective in reducing the incidence of POD.
Sleep is a natural state of reduced arousal that plays a crucial role in learning, memory, and cognitive function [65]. It has been reported that sleep disturbances are independent risk factors for POD, which can lead to long-term cognitive dysfunction after surgery [66, 67]. Disruptions in sleep patterns may trigger a process of neuronal apoptosis in regions of the brain that are closely associated with cognitive function [68, 69]. Therefore, neuronal apoptosis is considered one of the pathological factors of sleep disturbance-related cognitive dysfunction. As reported earlier, sleep disturbances are associated with neuroinflammation, alterations in neurotransmitter activity, and cerebral hypoxic and hypoperfusion injury [68, 70]. Because the possible pathophysiological mechanisms of POD also include neuroinflammation and changes in neurotransmitters [71], sleep disturbances may be associated with POD. Therefore, improving perioperative sleep can be an effective method for attenuating the incidence of POD. Sleep improvement interventions include both pharmacological and nonpharmacological measures. In a meta-analysis, Hu et al. [23] sought and recommended nonpharmacological interventions, such as music therapy, noise reduction, and social support, to enhance sleep in critically ill patients. Compared with a prior meta-analysis of thirteen RCTs, which demonstrated that interventions targeting sleep and circadian health reduced the risk of POD [30], the current meta-analysis included as many trials as possible that used multiple sleep interventions, thus enabling us to analyze the effect of sleep interventions on POD incidence more comprehensively.
The advantages of this meta-analysis are as follows. First, it incorporated a comprehensive collection of trials. Second, both nonpharmacological and pharmacological interventions that are widely used and recommended during the perioperative period were included, resulting in pooled results with high significance. Third, our meta-analysis exhibited no significant publication bias. Further sensitivity analyses also confirmed the consistency and robustness of our findings.
The present study exhibits certain limitations. First, most of the analyses manifested a high degree of heterogeneity, which is an anticipated outcome due to the diverse sleep interventions administered to the participants in the included trials. Second, our meta-analysis did not entail stratification by the type of sleep disturbance. In fact, there are different phenotypes of perioperative sleep disturbances, such as OSA and psychologically based sleep deprivation. These different types of sleep disturbances may have diverse effects on POD, so this could be a potential confounder and may have influenced the current results. Additionally, some interventions like oral melatonin were administered preoperatively, whereas some interventions like earplugs and masks were administered postoperatively. The focus should also be on the effect of the timing of sleep interventions on POD. Therefore, performing a high-quality meta-analysis with a large sample size is necessary to further determine whether the phenotype of sleep disturbances or the timing of interventions in the perioperative period is related to the incidence of POD. Third, considerable variability in the methods utilized to evaluate both POD and sleep disturbances was observed, which may have also affected the validity and reliability of our pooled results. Fourth, several subgroups analyzed in this meta-analysis exhibited a limited sample size, thereby potentially attenuating the reliability of the synthesized results. Finally, while a significant disparity in the incidence of POD was observed between the group that received sleep interventions and the control group, a substantial proportion of patients did not receive any type of assessment pertaining to perioperative sleep. Moreover, including only articles written in English or Chinese may have affected the internal and external validity of our findings.
5 Conclusions
For adult patients undergoing elective surgery, interventions for improving postoperative sleep were associated with a lower incidence of POD, especially when the intervention effectively improved sleep quality. Considering the heterogeneity of included trials, we should interpret the results with caution. Large-scale RCTs in future are still warranted to confirm the current results.
Availability of data and materials
The data and materials generated and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Abbreviations
- AMT:
-
Abbreviated Mental Test
- APAP:
-
Auto-titrating positive airway pressure
- BLT:
-
Bright light therapy
- CABG:
-
Coronary artery bypass graft
- CAM:
-
Confusion Assessment Method
- CAM-ICU:
-
Confusion Assessment Method for the intensive care unit
- CI:
-
Confidence interval
- CPAP:
-
Continuous positive airway pressure
- DFP:
-
Delirium-free protocol
- DSM:
-
Diagnostic and Statistical Manual of Mental Disorders
- DSM-5:
-
The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders
- EEG:
-
Electroencephalogram
- GSQS:
-
Groningen Sleep Quality Score
- ICU:
-
Intensive care unit
- ICDSC:
-
Intensive Care Delirium Screening Checklist
- ISI:
-
Insomnia Severity Index
- NEECHAM:
-
Neelon and Champagne Confusion Scale
- NREM:
-
Nonrapid eye movement
- NRS:
-
Numeric Rating Scale
- Nu-DESC:
-
Nursing Delirium Screening Scale
- OSA:
-
Obstructive sleep apnea
- PAP:
-
Positive airway pressure
- POD:
-
Postoperative delirium
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RASS:
-
Richmond Agitation and Sedation Scale
- RCSQ:
-
Richards-Campbell Sleep Questionnaire
- RCTs:
-
Randomized controlled trials
- RIS:
-
Required information size
- RR:
-
Relative risk
- SICU:
-
Surgical intensive care unit
- TSA:
-
Trial sequential analysis
- TSMB:
-
Trial sequential monitoring boundary
References
Li X, Yang J, Nie X, Zhang Y, Li X, Li L, et al. Impact of dexmedetomidine on the incidence of delirium in elderly patients after cardiac surgery: a randomized controlled trial. PLoS One. 2017;12(2):e170757.
Hshieh TT, Inouye SK, Oh ES. Delirium in the elderly. Psychiat Clin N Am. 2018;41(1):1–17.
Tang X, Zhang X, Dong H, Zhao G. Electroencephalogram features of perioperative neurocognitive disorders in elderly patients: a narrative review of the clinical literature. Brain Sci. 2022;12(8):1073.
Vlisides P, Avidan M. Recent advances in preventing and managing postoperative delirium [version 1; peer review: 2 approved]. F1000 Res. 2019;8:607.
Avidan MS, Maybrier HR, Abdallah AB, Jacobsohn E, Vlisides PE, Pryor KO, et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet. 2017;390(10091):267–75.
Luger MF, Müller S, Kammerlander C, Gosch M, Luger TJ. Predictors of postoperative cognitive decline in very old patients with hip fracture. Geriatr Orthop Surg. 2014;5(4):165–72.
Aldecoa C, Bettelli G, Bilotta F, Sanders RD, Audisio R, Borozdina A, et al. European society of anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesth. 2017;34(4):192–214.
Swarbrick CJ, Partridge JSL. Evidence-based strategies to reduce the incidence of postoperative delirium: a narrative review. Anaesthesia. 2022;77 Suppl 1:92–101.
Butris N, Tang E, He D, Wang D, Chung F. Sleep disruption in older surgical patients and its important implications. Int Anesthesiol Clin. 2023;61(2):47–54.
Lu L, Wang S, Rao W, Zhang Q, Ungvari GS, Ng CH, et al. The prevalence of sleep disturbances and sleep quality in older Chinese adults: a comprehensive meta-analysis. Behav Sleep Med. 2018;17(6):683–97.
Madsen MT, Rosenberg J, Gögenur I. Actigraphy for Measurement of sleep and sleep-wake rhythms in relation to surgery. J Clin Sleep Med. 2013;9(4):387–94.
Rhon DI, Snodgrass SJ, Cleland JA, Cook CE. Comorbid Insomnia and sleep apnea are associated with greater downstream health care utilization and chronic opioid use after arthroscopic hip surgery. Pain Physician. 2019;22(4):E351–60.
Cok OY, Seet E, Kumar CM, Joshi GP. Perioperative considerations and anesthesia management in patients with obstructive sleep apnea undergoing ophthalmic surgery. J Cataract Refr Surg. 2019;45(7):1026–31.
Evans JL, Nadler JW, Preud’Homme XA, Fang E, Daughtry RL, Chapman JB, et al. Pilot prospective study of post-surgery sleep and EEG predictors of post-operative delirium. Clin Neurophysiol. 2017;128(8):1421–5.
Song J, Chu S, Cui Y, Qian Y, Li X, Xu F, et al. Circadian rhythm resynchronization improved isoflurane-induced cognitive dysfunction in aged mice. Exp Neurol. 2018;306:45–54.
Leung JM, Sands LP, Newman S, Meckler G, Xie Y, Gay C, et al. Preoperative sleep disruption and postoperative delirium. J Clin Sleep Med. 2015;11(8):907–13.
Wang H, Zhang L, Luo Q, Li Y, Yan F. Effect of sleep disorder on delirium in post-cardiac surgery patients. Can J Neurol Sci. 2020;47(5):627–33.
Ibala R, Mekonnen J, Gitlin J, Hahm EY, Ethridge BR, et al. A polysomnography study examining the association between sleep and postoperative delirium in older hospitalized cardiac surgical patients. J Sleep Res. 2021;30(5):322.
Su X, Meng Z, Wu X, Cui F, Li H, Wang D, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10054):1893–902.
Wu X, Cui F, Zhang C, Meng Z, Wang D, Ma J, et al. Low-dose dexmedetomidine improves sleep quality pattern in elderly patients after noncardiac surgery in the intensive care unit. Anesthesiology. 2016;125(5):979–91.
de Jonghe A, van Munster BC, Goslings JC, Kloen P, van Rees C, Wolvius R, et al. Effect of melatonin on incidence of delirium among patients with hip fracture: a multicentre, double-blind randomized controlled trial. CMAJ. 2014;186(14):E547–56.
Zhang Q, Gao F, Zhang S, Sun W, Li Z. Prophylactic use of exogenous melatonin and melatonin receptor agonists to improve sleep and delirium in the intensive care units: a systematic review and meta-analysis of randomized controlled trials. Sleep Breath. 2019;23(4):1059–70.
Hu RF, Jiang XY, Chen J, Zeng Z, Chen XY, Li Y, et al. Non-pharmacological interventions for sleep promotion in the intensive care unit. Cochrane Database Syst Rev. 2015;2015(10):D8808.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ-Brit Med J. 2021;372:n71.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Koster G, Wetterslev J, Gluud C, Zijlstra JG, Scheeren TWL, van der Horst ICC, et al. Effects of levosimendan for low cardiac output syndrome in critically ill patients: systematic review with meta-analysis and trial sequential analysis. Intens Care Med. 2015;41(2):203–21.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Imberger G, Thorlund K, Gluud C, Wetterslev J. False-positive findings in Cochrane meta-analyses with and without application of trial sequential analysis: an empirical review. BMJ Open. 2016;6(8):e11890.
Lu Y, Li Y, Wang L, Lydic R, Baghdoyan HA, Shi X, et al. Promoting sleep and circadian health may prevent postoperative delirium: a systematic review and meta-analysis of randomized clinical trials. Sleep Med Rev. 2019;48:101207.
Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017;17(1):39.
Guo Y, Sun LL, Chen ZF, Li QF, Jiang H. [Preventive effect of dexmedetomidine on postoperative delirium in elderly patients with oral cancer]. Shanghai J Stomatol. 2015;24(2):236–9. Chinese.
Yang X, Li Z, Gao C, Liu R. Effect of dexmedetomidine on preventing agitation and delirium after microvascular free flap surgery: a randomized, double-blind, control study. J Oral Maxil Surg. 2015;73(6):1065–72.
Wong J, Doherty HR, Singh M, Choi S, Siddiqui N, Lam D, et al. The prevention of delirium in elderly surgical patients with obstructive sleep apnea (PODESA): a randomized controlled trial. BMC Anesthesiol. 2022;22(1):290.
Taguchi T, Yano M, Kido Y. Influence of bright light therapy on postoperative patients: a pilot study. Intens Crit Care Nur. 2007;23(5):289–97.
Sun Y, Jiang M, Ji Y, Sun Y, Liu Y, Shen W. Impact of postoperative dexmedetomidine infusion on incidence of delirium in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. Drug Des Devel Ther. 2019;13:2911–22.
Sultan SS. Assessment of role of perioperative melatonin in prevention and treatment of postoperative delirium after hip arthroplasty under spinal anesthesia in the elderly. Saudi J Anaesth. 2010;4(3):169–73.
Robinson TN, Dunn CL, Adams JC, Hawkins CL, Tran ZV, Raeburn CD, et al. Tryptophan supplementation and postoperative delirium-A randomized controlled trial. J Am Geriatr Soc. 2014;62(9):1764–71.
Potharajaroen S, Tangwongchai S, Tayjasanant T, Thawitsri T, Anderson G, Maes M. Bright light and oxygen therapies decrease delirium risk in critically ill surgical patients by targeting sleep and acid-base disturbances. Psychiat Res. 2018;261:21–7.
Ono H, Taguchi T, Kido Y, Fujino Y, Doki Y. The usefulness of bright light therapy for patients after oesophagectomy. Intens Crit Care Nur. 2011;27(3):158–66.
Oh ES, Leoutsakos JM, Rosenberg PB, Pletnikova AM, Khanuja HS, Sterling RS, et al. Effects of Ramelteon on the prevention of postoperative delirium in older patients undergoing orthopedic surgery: the RECOVER randomized controlled trial. Am J Geriat Psychiat. 2021;29(1):90–100.
Nickkholgh A, Schneider H, Sobirey M, Venetz WP, Hinz U, Pelzl LH, et al. The use of high-dose melatonin in liver resection is safe: first clinical experience. J Pineal Res. 2011;50(4):381–8.
Nadler JW, Evans JL, Fang E, Preud’Homme XA, Daughtry RL, Chapman JB, et al. A randomised trial of peri-operative positive airway pressure for postoperative delirium in patients at risk for obstructive sleep apnoea after regional anaesthesia with sedation or general anaesthesia for joint arthroplasty. Anaesthesia. 2017;72(6):729–36.
Mahrose R, ElSerwi H, Maurice A, Elsersi M. Postoperative delirium after coronary artery bypass graft surgery: dexmedetomidine infusion alone or with the addition of oral melatonin. Egypt J Anaesth. 2021;37(1):62–8.
Le Guen M, Nicolas-Robin A, Lebard C, Arnulf I, Langeron O. Earplugs and eye masks vs routine care prevent sleep impairment in post-anaesthesia care unit: a randomized study. Brit J Anaesth. 2014;112(1):89–95.
Jaiswal SJ, Vyas AD, Heisel AJ, Ackula H, Aggarwal A, Kim NH, et al. Ramelteon for prevention of postoperative delirium. Crit Care Med. 2019;47(12):1751–8.
Hong H, Zhang D, Li M, Wang G, Zhu S, Zhang Y, et al. Impact of dexmedetomidine supplemented analgesia on delirium in patients recovering from orthopedic surgery: a randomized controlled trial. BMC Anesthesiol. 2021;21(1):1–3.
Gupta PK, Verma R, Kohli M, Shukla N, Kannaujia S. The effect of ramelteon on postoperative delirium in elderly patients: a randomised double-blind study. J Clin Diagn Res. 2019;13(12):UC15–9.
Ford AH, Flicker L, Kelly R, Patel H, Passage J, Wibrow B, et al. The healthy heart-mind trial: randomized controlled trial of melatonin for prevention of delirium. J Am Geriatr Soc. 2019;68(1):112–9.
Dianatkhah M, Ghaeli P, Hajhossein TA, Karimi A, Salehiomran A, Bina P, et al. Evaluating the potential effect of melatonin on the post-cardiac surgery sleep disorder. J Tehran Heart Cent. 2015;10(3):122–8.
Aizawa K, Kanai T, Saikawa Y, Takabayashi T, Kawano Y, Miyazawa N, et al. A novel approach to the prevention of postoperative delirium in the elderly after gastrointestinal surgery. Surg Today. 2002;32(4):310–4.
Xiaolin W, Wang Jia Mu, Dongliang WD. [Dexmedetomidine combined with ropivacaine for continuous femoral nerve block improved postoperative sleep quality in elderly patients after total knee arthroplasty]. Natl Med J China. 2018;98(10):728–32. Chinese.
Chuan A, Sanders RD. The use of dexmedetomidine to prevent delirium after major cardiac and non-cardiac surgery. Anaesthesia. 2021;76(10):1296–9.
Mo Y, Zimmermann AE. Role of dexmedetomidine for the prevention and treatment of delirium in intensive care unit patients. Ann Pharmacother. 2013;47(6):869–76.
Guldenmund P, Vanhaudenhuyse A, Sanders RD, Sleigh J, Bruno MA, Demertzi A, et al. Brain functional connectivity differentiates dexmedetomidine from propofol and natural sleep. Brit J Anaesth. 2017;119(4):674–84.
Duan X, Coburn M, Rossaint R, Sanders RD, Waesberghe JV, Kowark A. Efficacy of perioperative dexmedetomidine on postoperative delirium: systematic review and meta-analysis with trial sequential analysis of randomised controlled trials. Brit J Anaesth. 2018;121(2):384–97.
Cohen SR, Steiner W, Mount BM. Phototherapy in the treatment of depression in the terminally ill. J Pain Symptom Manag. 1994;9(8):534–6.
Moyce Z, Rodseth RN, Biccard BM. The efficacy of peri-operative interventions to decrease postoperative delirium in non-cardiac surgery: a systematic review and meta-analysis. Anaesthesia. 2014;69(3):259–69.
Wang C, Zhou L. Melatonin and melatonergic agents for the prevention of postoperative delirium: a meta-analysis of randomized placebo-controlled trials. Asian J Surg. 2022;45(1):27–32.
Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc. 2001;76(9):897–905.
Walsh TS, Palmer J, Watson D, Biggin K, Seretny M, Davidson H, et al. Multicentre cohort study of red blood cell use for revision hip arthroplasty and factors associated with greater risk of allogeneic blood transfusion. Surv Anesthesiol. 2012;108(1):63–71.
Roggenbach J, Klamann M, von Haken R, Bruckner T, Karck M, Hofer S. Sleep-disordered breathing is a risk factor for delirium after cardiac surgery: a prospective cohort study. Crit Care. 2014;18(5):477.
King CR, Fritz BA, Escallier K, Ju YS, Lin N, McKinnon S, et al. Association between preoperative obstructive sleep apnea and preoperative positive airway pressure with postoperative intensive care unit delirium. JAMA Netw Open. 2020;3(4):e203125.
Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;3:CD001106.
Franks NP, Wisden W. The inescapable drive to sleep: overlapping mechanisms of sleep and sedation. Science. 2021;374(6567):556–9.
Wang H, Zhang L, Zhang Z, Li Y, Luo Q, Yuan S, et al. Perioperative sleep disturbances and postoperative delirium in adult patients: a systematic review and meta-analysis of clinical trials. Front Psychiatry. 2020;11:570362.
O’Gara BP, Gao L, Marcantonio ER, Subramaniam B. Sleep, pain, and cognition: modifiable targets for optimal perioperative brain health. Anesthesiology. 2021;135(6):1132–52.
Boonstra TW, Stins JF, Daffertshofer A, Beek PJ. Effects of sleep deprivation on neural functioning: an integrative review. Cell Mol Life Sci. 2007;64(7–8):934–46.
Yin M, Chen Y, Zheng H, Pu T, Marshall C, Wu T, et al. Assessment of mouse cognitive and anxiety-like behaviors and hippocampal inflammation following a repeated and intermittent paradoxical sleep deprivation procedure. Behav Brain Res. 2017;321:69–78.
Daulatzai MA. Cerebral hypoperfusion and glucose hypometabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer’s disease. J Neurosci Res. 2017;95(4):943–72.
Jin Z, Hu J, Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Brit J Anaesth. 2020;125(4):492–504.
Acknowledgements
Not applicable.
Funding
This research was funded by the National Natural Science Foundation of China (81970448 to C.L. and 82030038 to H.D.) and the University and Hospital Funded Clinical Research Projects (XJZT21L17 and 2021LC2202 to C.L.).
Author information
Authors and Affiliations
Contributions
Design of meta-analysis: Hailong Dong, Chong Lei, and Xuemiao Tang. Search strategy: Xuemiao Tang and Jia Li. Screening of results: Xuemiao Tang and Jia Li. Data extraction: Bo Yang and Xuemiao Tang. Risk of bias: Jia Li and Bo Yang. Statistical analysis: Bo Yang and Jia Li. Drafting of manuscript: Xuemiao Tang. Revision of manuscript: Hailong Dong, Chong Lei, and Xuemiao Tang.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors listed above approved the submission of final manuscript.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 2.
Search strategy.
Additional file 4: Supplemental Fig. 1.
Forest plot showing pooled analysis of studies categorized into bright light group, dexmedetomidine group, melatonin group, and PAP group. Statistical analyses were conducted using the random-effects model. Size of squares for risk ratio (RR) reflects the weight of the trial in the pooled analyses. Horizontal bars 95 % confidence intervals (CI). PAP, positive airway pressure.
Additional file 5: Supplemental Fig. 2.
Forest plot showing pooled analysis of studies with different effect of sleep interventions. Statistical analyses were conducted using the random-effects model. Size of squares for risk ratio (RR) reflects the weight of the trial in the pooled analyses. Horizontal bars 95 % confidence intervals (CI).
Additional file 6: Supplemental Fig. 3.
Forest plot showing pooled analysis of studies with different delirium diagnosis tools. Statistical analyses were conducted using the random-effects model. Size of squares for risk ratio (RR) reflects the weight of the trial in the pooled analyses. Horizontal bars 95 % confidence intervals (CI).
Additional file 7: Supplemental Fig. 4.
Forest plot showing pooled analysis of studies categorized into non-cardiac surgery, cardiac surgery, or both cardiac and non-cardiac surgery. Statistical analyses were conducted using the random-effects model. Size of squares for risk ratio (RR) reflects the weight of the trial in the pooled analyses. Horizontal bars 95 % confidence intervals (CI).
Additional file 8: Supplemental Fig. 5.
Forest plot showing pooled analysis of studies categorized into studies with sample size less than 100 and greater than or equal to 100. Statistical analyses were conducted using the random-effects model. Size of squares for risk ratio (RR) reflects the weight of the trial in the pooled analyses. Horizontal bars 95 % confidence intervals (CI).
Additional file 9: Supplemental Fig. 6.
Forest plot showing pooled analysis of studies categorized into studies with median/mean age less than 65 years old and greater than or equal to 65 years old. Statistical analyses were conducted using the random-effects model. Size of squares for risk ratio (RR) reflects the weight of the trial in the pooled analyses. Horizontal bars 95 % confidence intervals (CI).
Additional file 10: Supplemental Fig. 7.
Forest plot showing pooled analysis of studies categorized into ICU patients (sedative dose or low-dose of dexmedetomidine) and non-ICU patients (low-dose or mini-dose via PCA of dexmedetomidine). Statistical analyses were conducted using the random-effects model. Size of squares for risk ratio (RR) reflects the weight of the trial in the pooled analyses. Horizontal bars 95 % confidence intervals (CI). PCA, patients-controlled analgesia.
Additional file 11: Supplemental Fig. 8.
Forest plot with subgroup analysis ‘preoperatively’, ‘postoperatively’, ‘preoperatively and postoperatively’. Statistical analyses were conducted using the random-effects model. Size of squares for risk ratio (RR) reflects the weight of the trial in the pooled analyses. Horizontal bars 95 % confidence intervals (CI). PCA, patients-controlled analgesia.
Additional file 12: Supplemental Fig. 9.
A Funnel plot with pseudo 95% confidence interval. Random-effects meta-analysis. B Funnel plot with pseudo 95% confidence interval. Fixed-effects meta-analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, X., Li, J., Yang, B. et al. Efficacy of sleep interventions on postoperative delirium: a systematic review and meta-analysis of randomized controlled trials. APS 1, 29 (2023). https://doi.org/10.1007/s44254-023-00027-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44254-023-00027-1