Abstract
Background
Knowledge of clinical pharmacology concepts is essential to improve patients’ outcomes. Scarce data is available on the utilisation of these concepts in the paediatric intensive care unit (PICU). We aimed to investigate the self-perceived knowledge of clinical pharmacology concepts, educational needs and identify priorities for pharmacological research across European PICUs.
Methods
From July to November 2022 an online survey was distributed to evaluate i) the self-reported knowledge, and ii) application of key pharmacology concepts in clinical practice (using a likert scale from 1 = never apply to 10 = always apply); iii) need for additional education on them; and iv) key areas for future pharmacological research. The survey was distributed to European Society of Paediatric and Neonatal Intensive Care (ESPNIC) members and other European national PICUs societies members.
Results
Two-hundred-thirty-seven responses from 149 PICUs were collected. 54% of PICUs reported to have a clinical pharmacologist available for consultation during drug prescription and 65% of them regularly contact them during the prescribing process. Among clinical pharmacology concepts the parameter with the highest self-reported knowledge was half-life (99%) and the lowest were pharmacodynamics and volume-of-distribution (92%). The reported median application of these concepts in clinical practice ranged between 5/10 and 7/10. Most of the respondents reported the need for additional education on specific pharmacology concepts. Reported priorities for drug research mostly involved analgesics/sedatives (87%), antimicrobials (86%), and cardiovascular medications (55%).
Conclusions
Self-reported knowledge on clinical pharmacology concepts seems good, but self-perceived clinical application may improve and most of the respondents report a need for additional education. These findings call for concerted multidisciplinary efforts to streamline education and guidelines to fill this gap.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinical pharmacology is a key discipline of healthcare with significant potential to improve patients’ care and outcomes. It is based on the knowledge of pharmacology, defined as the study of drugs and their effects on the biological system. Knowledge of key clinical pharmacology principles includes the understanding of drugs’ mechanism of action, pharmacokinetics and pharmacodynamics, potential drug-drug interactions, as well as their safety profiles. The culture of pharmacology refers to beliefs, practices, and norms which characterise the field of pharmacology, and may influence the potential applications of pharmacotherapy in patients’ care across prevention and treatment. When the knowledge of pharmacology concepts is insufficient, it can lead to improper use of medications resulting in increased risks of potential adverse medication related reactions [1].
Pharmacology in the paediatric population presents several unique challenges and differs from adults due to paediatric growth and development. Changes in the glomerular filtration rate, drug metabolism capacity and body composition amongst others impact pharmacokinetics processes including absorption, distribution, metabolism and clearance. Based on developmental pharmacology, dosing recommendations for a safe and effective therapy cannot be simply derived by linear extrapolation from adult recommendations [1,2,3]. The adequate drug management and dosing in paediatric critically ill patients is even more challenging due to markedly altered and variable pharmacokinetic parameters in the context of critical illness. Examples include the increase of apparent volume of distribution following intravascular perfusion changes and fluid balance strategies; and drug clearance changes affected by cardiac output, impact on drug metabolism due to inflammatory responses and glomerular filtration rate [4]. Other comorbidities and extracorporeal therapies may also severely affect drug metabolism and dosing requirements [5].
Data from adult populations show that medication errors are frequent in the intensive care units (ICUs) and represent 78% of serious medical errors, with potentially severe consequences [6, 7]. Moreover, despite the peculiarities related to the paediatric age and critical illness, to date no international dedicated recommendations on drug dosing personalization are available for critically ill paediatric patients. In this context, the diffusion of knowledge and culture of pharmacology may be lacking and multidisciplinary collaboration among experts in the paediatric intensive care unit (PICU) becomes essential and mandatory. To date, most of the studies focused primarily on the detection of medical errors [8] rather than evaluating the self-perceived knowledge of pharmacology, identifying educational gaps, and providing suggestions for future research areas. To overcome this gap, the Pharmacology Section of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC) designed and conducted a survey to investigate the actual perceived knowledge of clinical pharmacology concepts across European PICUs, as well as to identify knowledge gaps, associated educational needs and research priorities.
Methods
Study design and ethics approval
We conducted a cross-sectional anonymous electronic survey focused on the knowledge of clinical pharmacology concepts and the associated educational needs among European PICUs. The survey was designed by the ESPNIC Pharmacology Section following the American Association for Public Opinion Research (AAPOR) guidelines [9]. Since no demographic, clinical or outcome patient data were collected in this survey, ethical approval was not deemed necessary.
Survey development and testing
The survey was developed in English and formatted using a website Google Form™ (https://www.google.com/forms/about/) instrument for distribution. Data were stored in a password protected excel file with access limited to first and last author of the manuscript. The survey was designed by the ESPNIC Pharmacology section, which is multidisciplinary in nature as it is composed by physicians, nurses, pharmacologists and clinical pharmacists. Among the 15 authors of the survey, 12 are PICU intensivists, one physician with both specialties in intensive care and pharmacology and two authors are pharmacologists. The question domains and specific questions were constructed through an extensive review of the literature focusing on significant studies concerning the most contentious aspects of pharmacology knowledge and medication errors in both pediatric and adult literature. Subsequently, the survey authors evaluated this review during Zoom meetings, where theoretical knowledge intersected with experiential multidisciplinary insights from practice. Subsequently, the survey was piloted by 10 healthcare professionals, paediatric intensivists or clinical pharmacologists, in order to test it for clarity and face validity, with a following adaptation of the questions [10]. The survey consisted of 33 questions, divided into three sections, and required 15 min on average to be completed (Supplemental file 1): Part A) ICUs and respondent’s characteristics, for the definition of ICU location, type, and size and clinical pharmacologist availability for drug prescription; Part B) a. knowledge and application of key clinical pharmacology concepts such as clearance, volume of distribution, half-life time, pharmacokinetics and pharmacodynamics expressed as a Likert scale from 1 to 10 (from 1 = never apply to 10 = always apply), and the b. need for more education on each of them; Part C) questions regarding the knowledge on the drug prescription adjustment in some clinical scenarios (e.g. Continuous Renal Replacement Therapy [CRRT], ExtraCorporeal Membrane Oxygenation [ECMO], sepsis, burns), and what drug/class of drugs should be considered a priority for drug research. We used single- and multiple-choice closed-ended questions to facilitate analyses and comparisons, as well as free-text ones to allow the extrapolation of more detailed information on each topic. It was not compulsory to answer to all the questions and in the manuscript’s tables we reported the number of responses in round brackets.
Recruitment of European PICUs and data collection
This survey targeted prescribing staff working in PICUs in Europe (i.e. exclusive paediatric ICUs, mixed neonatal and paediatric ICUs, and mixed adult and paediatric ICUs). Using the ESPNIC network, as well as the ESPNIC dedicated official newsletters and social media account (official handle of ESPNIC: @ESPNIC_Society) and other European national PICU societies members, the questionnaire was spread across Europe using a dedicated link created from the Google Form instrument. No identifying data regarding staff or patients was collected and consent was implied by completing the survey. We accepted more than one response from each ICU, in order to highlight the variability in knowledge and the application of pharmacology concepts in the daily clinical practice. All responses received between July and November 2022 were included in the analysis. Responses from non-prescribing staff and incomplete responses were excluded. To maximise the response rate, reminders were sent every 14 days (6 in total) through the ESPNIC network, the official ESPNIC social media account (@ESPNIC_Society in Twitter) and newsletter every month.
Data analysis
Raw data downloaded from Google Form were checked for data completeness. Data were analysed using STATA (version 17.0, StataCorp LP, College Station, TX, USA). Descriptive data were reported as number and frequency for categorical variables, and median and interquartile ranges (IQR) for continuous variables, given their non-parametric distribution. Pharmacology knowledge and need for further education were subsequently compared between two groups of PICUs, based on the availability of a clinical pharmacologist during the prescribing process, and comparing junior (< 40 years) and senior (> 40 years) prescribers. The Pearson Chi-square test or the Fisher-exact test when appropriate (n < 5 in > 20% cells) were used for comparison of categorical variables. The non-parametric U Mann–Whitney test was used for comparison of continuous or ordinal variables.
Results
Survey respondents and PICUs characteristics
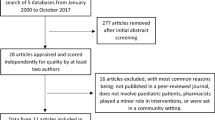
After applying exclusion criteria, a total of 237 responses from 149 PICUs and 24 countries were collected during the study period (Fig. 1). PICUs and respondents’ characteristics are reported in Table 1. All the respondents of the survey were physicians, most of them specialised in Anaesthesia (106, 45%) or Paediatrics (96, 41%), primarily employed by academic/teaching hospitals (219, 92%). Most of the units are exclusively paediatric PICUs (151, 64%), while a minority are mixed adult and paediatric ICUs (9, 4%). Clinical pharmacologists are available for consultation during drug prescription in 54% of the PICUs, and 65% (53/81) of the respondents regularly contact them during the prescribing process. Hospital pharmacists are regularly contacted by 45% of the respondents.
Pharmacology knowledge, application and educational needs
Table 2 reports data on knowledge of pharmacology concepts, their application and associated educational needs, comparing PICUs according to clinical pharmacologist availability. Self-reported knowledge on pharmacology concepts varies from 99% for the half-life concept to 92% for the volume of distribution and pharmacodynamics. PICUs with a clinical pharmacologist available demonstrate a higher knowledge on volume of distribution (95% vs 88%, p = 0.020) and pharmacodynamics (96% vs 87%, p = 0.005). Application of these concepts in the clinical practice ranges from 5/10 (IQR 3–7) for volume of distribution to 7/10 for half-life (IQR 5–8) and pharmacokinetics (IQR 5–8), with no difference if a clinical pharmacologist is available onsite (p = 0.605 for clearance, p = 0.624 for volume of distribution, p = 0.197 for half-life, p = 0.348 for pharmacokinetics, p = 0.119 for pharmacodynamics). Most of the respondents reported the need for additional education to increase their knowledge on all the pharmacology concepts, with volume of distribution (88%), clearance (86%) and pharmacodynamics (86%) concepts being the most reported. Half-life is the most often evaluated parameter during prescription and with less need for additional education (21%). Junior prescribers reported a significantly higher need for additional education in multiple concepts (clearance 94% vs 82%, p = 0.038; volume of distribution 96% vs 84%, p = 0.016, half-life 83% vs 72%, p = 0.048; and pharmacodynamics 94% vs 82%, p = 0.030) and a significant lower application of these concepts in clinical practice (volume of distribution, p = 0.016; half-life, p = 0.001; pharmacokinetics, p = 0.026) (Supplemental File 2).
Evaluated parameters during drug prescription and dose adjustment
Most of the respondents declare the need for additional education on drugs’ pharmacokinetic characteristics to understand the correct timing to prescribe a bolus to achieve the optimal drug concentration (80%), to increase the rate of continuous infusion (81%) and the pharmacokinetics changes during some diseases (80%). A similar need for more education was identified regarding the modality to adjust drug prescription in patients treated with CRRT (74%) and ECMO (87%). Overall, most of the respondents reported always using therapeutic drug monitoring (65%) and state that they consider the use of pharmacokinetics models when appropriate (68%) for drug dose adjustment. Respondents indicated that they tend to regularly adjust drug dosing according to renal function (95%), patient’s age (92%) and actual daily body weight (87%). Respondents reported that they consider clinical pharmacology less in ECMO (37%) or according to serum albumin level (27%), with no difference depending on the availability of a pharmacologist except for the presence of ECMO (p = 0.037). Senior prescribers regularly consider, significantly more than juniors, drug-drug interactions (p = 0.006), presence of obesity (p = 0.035) and serum albumin levels (p = 0.005) (Table 3 and Supplemental File 3).
Research priorities
Respondents research priorities in pharmacology mainly focus on drug-specific topics such as analgesics/sedatives (87%), antimicrobials (86%), and cardiovascular medications (55%), less on the general clinical pharmacology questions, e.g. impact of disease, age, obesity, treatment modality; (Fig. 2).
Discussion
Our multinational European survey on pharmacology and associated educational needs in PICUs across Europe surveyed 237 prescribing staff members from 24 countries and demonstrated an overall good self-perceived knowledge on clinical pharmacology concepts in PICU. However, prescribers reported a need for additional education on most of the domains, especially on pharmacokinetics, and drug-dosing adjustment during extracorporeal treatments like CRRT and ECMO. We may suppose that optimal pharmacological treatment in the PICU is paramount for providing patients with the highest standard of care and preventing conceptual errors. Our data underscore the significance of this aspect, highlighting the need for implementing educational activities within the pediatric critical care setting. Junior prescribers reported less application of pharmacological concepts and a higher need for education on them. Therefore, educational efforts should be made at all stages of medical training, given that juniors are the main prescribers of drugs in PICUs.
As a confirmation of the importance of clinical pharmacology within the healthcare system, our survey showed that dedicated trained clinical pharmacologists are available for consultation in more than half of the PICUs, and that 2/3 of the surveyed health professionals queried them during the prescribing process. Our data demonstrated minimal impact of the presence of a clinical pharmacologist on the proficiency of personnel in pharmacokinetics knowledge (eg. on the knowledge of volume of distribution and pharmacodynamics). Moreover, our survey did not explore the role of the clinical pharmacologist in both educating the healthcare personnel in the PICUs on pharmacology concepts and verifying their correct application in daily clinical practice. We therefore believe that it is essential for the clinical pharmacologist to assume the role of educator in this area to fill this gap and improve the clinical management of patients in PICU. Furthermore, clinical practices frequently diverge from evidence-based treatment protocols in children, accentuating the critical role of clinical pharmacologists in ensuring optimal therapeutic approaches [11].
An important milestone in the realm of clinical pharmacology has been the realisation that pharmacology, and by extension pharmacotherapy, exhibits notable specific needs when applied to paediatric patients1. The rapid physiological changes accompanying growth, including the maturation of liver and kidney function and organ-specific growth, a process known under the definition of developmental pharmacology, profoundly influence drug pharmacokinetics and pharmacodynamics, particularly in the neonatal period and the first year of life1. Similar distinctive characteristics and considerations have been identified and validated among pediatric patients admitted to the ICU but the presence of critical illness instigates significant alterations in systemic and organ-specific homeostasis, leading to further altered changes in dosing requirements and making the clinical picture even more complex [12,13,14]. Moreover, the use of extracorporeal organ support equipment, such as CRRT and ECMO, may further alter drug pharmacokinetics and pharmacodynamics [12, 13]. Finally, the problem of the use of off-label drugs in pediatrics and the low level of evidence for these drugs increase the risk of potential lack of efficacy and toxicity [15]. In this intricate landscape, the paediatric critically ill patient receiving intensive care emerges as a unique and multifaceted entity from a pharmacological standpoint.
For this reason, it seems essential that additional and dedicated education is required for neonatologists and paediatric critical care prescribers. Particularly the knowledge of pharmacokinetics and pharmacodynamics developmental pharmacology and the impact of covariates related to environmental issues (e.g. critical illness) or new treatments such as ECMO, CRRT should be guaranteed in PICUs. Clinical trials with physiologically based pharmacokinetics models in different settings should be promoted in pediatric ages to increase the understanding of these drugs and their application in PICU [16].
Considering our data, the respondents reported the use of therapeutic drug monitoring when available and underlined the importance of using physiologically based pharmacokinetics models when appropriate for drug dose adjustment. This is an area mainly developed recently, usually starting from existing models created for adults [17].
A known difficult setting for dose adjustment is the presence of extracorporeal treatments. Pharmacokinetics is modified, particularly the volume of distribution. The presence of drug absorption by circuit component and the possible patient’s organ failure altered pharmacokinetics in a significant way making necessary the therapeutic drug monitoring, if available. Otherwise, drug efficacy and toxicity should be regularly assessed monitoring the patient and laboratory analysis [18].
From our survey, the main priorities in drug research highlighted by the respondents are related to analgesics/sedatives, antibiotics, and cardiovascular drugs. Practices related to analgesia and sedation in European PICUs are known to be highly variable, and efforts should be made to standardize and offer high-level evidence based education in this field [19,20,21,22]. Similarly, strategies to optimise antimicrobial dosing (amongst others antimicrobial stewardship programs) must be prioritised, due to the increase of multidrug resistance bacteria in recent years, which is recognized as a major threat worldwide [23,24,25]. In addition, several studies demonstrated that paediatric critical patients are frequently underexposed to antimicrobial therapies, which could have an important impact on patients´outcomes [26]. Although a recent ESPNIC survey has shown that 88% of the 60 European PICUs included had an antibiotic stewardship program implemented, there seems to be a need to further expand this effort and involve PICU physicians within these programs [27]. Knowledge on cardiovascular medications also stood up as a research priority, reflecting how the field of paediatric cardiac critical care has developed rapidly in the last thirty years [28]. Outcomes of neonates and children with congenital heart diseases have significantly improved during this timeframe, mainly due to exponential advances in technologies and an expanding collection of pharmacotherapies [28]. However, a recent proof-of- concept study on the level of scientific evidence to support dosing requirements for cardiovascular drugs in children showed that only low-quality efficacy studies were available for the majority of the off-label cardiovascular drugs [29]. Overall, collaborative research and education efforts are needed in all these fields, and these should include not only novel investigations, but also the development of expert-based consensus guidelines and an effective and equal distribution of this achieved knowledge worldwide. Antiepileptic drugs, which have shown the highest risk related to drug-drug interactions, were not included in our list of priorities as an option and we acknowledge this as a limit of our study [30]. Nevertheless, in the survey it was allowed the possibility to choose “other” within the research priorities, but only a minority of the respondents reported this class of drugs and not less important, the literature is scarce if we search similar studies related to this topic. In a previous survey conducted by Keijsers et al., pharmacists and general practitioners were evaluated for their pharmacology knowledge and pharmacotherapy skills using a written assessment comprising 51 questions. The study reported higher scores among the pharmacist group, but the results were opposite for physicians. Unlike our survey, the design of this study aimed to measure effective knowledge rather than self-perceived knowledge. However, the final message remains similar, as the authors emphasized the importance of continuous education throughout one's professional career [31].
Our survey has some limitations. First, we could not collect data from each European country, partly due to lack of contacts, limited knowledge about the respective infrastructures, and language barriers. Furthermore, we could not calculate the response rate, since we did not have the denominator of prescribing staff across Europe. Nevertheless, we had a high number of responses, and it should be noted that it would have been particularly challenging to retrieve the exact number of these features in all the European PICUs. Second, we could not explore all aspects of pharmacology knowledge and we could have missed questions on some specific drug class due to constraints of how extensive the survey would have become. Third, since our survey was limited to European centres, we cannot extrapolate our findings to other PICUs globally, where the resources available may differ. Fourth, the possibility of respondent bias could not be avoided, being inherent to every survey such as the misinterpretation of some words (eg. we did not specify the frequency of “regular contact with the pharmacologist”) or we did not specify if a pharmacist may be also a pharmacologist and viceversa. Fifth, we did not include in our list of research priorities the antiepileptics, a class of drugs which may have high potential of interactions. Last, we did not collect data regarding the type of care within the hospitals (i.e. primary vs secondary vs tertiary level of care), therefore we could not compare results according to this difference.
Conclusions
Self-perceived knowledge of clinical pharmacology concepts seems overall good. However, the self-perceived clinical application of pharmacology knowledge is not reported as optimal and most of the respondents report a crucial need for additional education. These findings call for concerted efforts from the paediatric critical care and pharmacology communities to engage in strong global initiatives for education, training, research, and guidelines development on pharmacology in the PICU.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Smits A, Annaert P, Cavallaro G, De Cock PAJG, de Wildt SN, Kindblom JM et al (2022) Current knowledge, challenges and innovations in developmental pharmacology: A combined conect4children Expert Group and European Society for Developmental, Perinatal and Paediatric Pharmacology White Paper. Br J Clin Pharmacol 88(12):4965–4984
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE (2003) Developmental pharmacology–drug disposition, action, and therapy in infants and children. N Engl J Med 349(12):1157–1167
Rodieux F, Wilbaux M, van den Anker JN, Pfister M (2015) Effect of Kidney Function on Drug Kinetics and Dosing in Neonates, Infants, and Children. Clin Pharmacokinet 54(12):1183–1204
Dhont E, Van Der Heggen T, De Jaeger A, Vande Walle J, De Paepe P, De Cock PA (2020) Augmented renal clearance in pediatric intensive care: are we undertreating our sickest patients? Pediatr Nephrol 35(1):25–39
Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW et al (2014) Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 14(6):498–509
Tully AP, Hammond DA, Li C, Jarrell AS, Kruer RM (2019) Evaluation of Medication Errors at the Transition of Care From an ICU to Non-ICU Location. Crit Care Med 47(4):543–549
Escrivá Gracia J, Brage Serrano R, Fernández GJ (2019) Medication errors and drug knowledge gaps among critical-care nurses: a mixed multi-method study. BMC Health Serv Res 19(1):640
Alghamdi AA, Keers RN, Sutherland A, Ashcroft DM (2019) Prevalence and Nature of Medication Errors and Preventable Adverse Drug Events in Paediatric and Neonatal Intensive Care Settings: A Systematic Review. Drug Saf 42(12):1423–1436
American Association for Public Opinion Research. Standard definitions report. Accessed February 5, 2024. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://aapor.org/wp-content/uploads/2023/05/Standards-Definitions-10th-edition.pdf.
Burns KEA, Duffett M, Kho ME, Meade MO, Adhikari NKJ, Sinuff T et al (2008) A guide for the design and conduct of self-administered surveys of clinicians. CMAJ 179(3):245–252
Allegaert K, Langhendries JP, van den Anker JN (2013) Educational paper: do we need neonatal clinical pharmacologists? Eur J Pediatr 172(4):429–435
Zagli G, Tarantini F, Bonizzoli M, Di Filippo A, Peris A, De Gaudio AR et al (2008) Altered pharmacology in the Intensive Care Unit patient. Fundam Clin Pharmacol 22(5):493–501
Valentine K, Kummick J (2022) PICU Pharmacology. Pediatr Clin North Am 69(3):509–529
Brussee JM, Vet NJ, Krekels EHJ, Valkenburg AJ, Jacqz-Aigrain E, van Gerven JMA et al (2018) Predicting CYP3A-mediated midazolam metabolism in critically ill neonates, infants, children and adults with inflammation and organ failure. Br J Clin Pharmacol 84(2):358–368
van der Zanden TM, Smeets NJL, de Hoop-Sommen M, Schwerzel MFT, Huang HJ, Barten LJC et al (2022) Off-Label, but on-Evidence? A Review of the Level of Evidence for Pediatric Pharmacotherapy. Clin Pharmacol Ther 112(6):1243–1253
Allegaert K (2014) Tailored tools to improve pharmacotherapy in infants. Expert Opin Drug Metab Toxicol 10(8):1069–1078
van der Heijden JEM, Freriksen JJM, de Hoop-Sommen MA, van Bussel LPM, Driessen SHP, Orlebeke AEM et al (2022) Feasibility of a Pragmatic PBPK Modeling Approach: Towards Model-Informed Dosing in Pediatric Clinical Care. Clin Pharmacokinet 61(12):1705–1717
Yalcin N, Sürmelioğlu N, Allegaert K (2022) Population pharmacokinetics in critically ill neonates and infants undergoing extracorporeal membrane oxygenation: a literature review. BMJ Paediatr Open 6(1):e001512
Daverio M, von Borell F, Ramelet AS, Sperotto F, Pokorna P, Brenner S et al (2022) Pain and sedation management and monitoring in pediatric intensive care units across Europe: an ESPNIC survey. Crit Care 26(1):88
Mondardini MC, Sperotto F, Daverio M, Amigoni A (2023) Analgesia and sedation in critically ill pediatric patients: an update from the recent guidelines and point of view. Eur J Pediatr 182(5):2013–2026
Amigoni A, Conti G, Conio A, Corno M, Fazio PC, Ferrero F et al (2022) Recommendations for analgesia and sedation in critically ill children admitted to intensive care unit. J Anesth Analg Crit Care 2(1):9
Smith HAB, Besunder JB, Betters KA, Johnson PN, Srinivasan V, Stormorken A et al (2022) 2022 Society of Critical Care Medicine Clinical Practice Guidelines on Prevention and Management of Pain, Agitation, Neuromuscular Blockade, and Delirium in Critically Ill Pediatric Patients With Consideration of the ICU Environment and Early Mobility. Pediatr Crit Care Med 23(2):e74-110
Donà D, Barbieri E, Daverio M, Lundin R, Giaquinto C, Zaoutis T et al (2020) Implementation and impact of pediatric antimicrobial stewardship programs: a systematic scoping review. Antimicrob Resist Infect Control 9(1):3
Charani E, McKee M, Ahmad R, Balasegaram M, Bonaconsa C, Merrett GB et al (2021) Optimising antimicrobial use in humans – review of current evidence and an interdisciplinary consensus on key priorities for research. The Lancet Regional Health - Europe 1(7):100161
Willems J, Hermans E, Schelstraete P, Depuydt P, De Cock P (2021) Optimizing the Use of Antibiotic Agents in the Pediatric Intensive Care Unit: A Narrative Review. Paediatr Drugs 23(1):39–53
Bassetti M, Vena A, Croxatto A, Righi E, Guery B (2018) How to manage Pseudomonas aeruginosa infections. Drugs Context 7:212527
Clos M, Schlapbach LJ, Arata-Bardet J, Javouhey E, Mortamet G (2022) European Society of Neonatal and Pediatric Intensive Care (ESPNIC) Section on Infection, Inflammation, and Sepsis. Antimicrobial stewardship programs in European pediatric intensive care units: an international survey of practices. Eur J Pediatr. 181(7):2873–7
Pollak U, Feinstein Y, Mannarino CN, McBride ME, Mendonca M, Keizman E et al (2022) The horizon of pediatric cardiac critical care. Front Pediatr 10:863868
Smeets NJL, Raaijmakers LPM, van der Zanden TM, Male C, de Wildt SN (2023) Guiding future paediatric drug studies based on existing pharmacokinetic and efficacy data: Cardiovascular drugs as a proof of concept. Br J Clin Pharmacol 89(9):2888–2901
Lebowitz MB, Olson KL, Burns M, Harper MB, Bourgeois F (2016) Drug-Drug Interactions Among Hospitalized Children Receiving Chronic Antiepileptic Drug Therapy. Hosp Pediatr 6(5):282–289
Keijsers CJPW, Leendertse AJ, Faber A, Brouwers JRBJ, de Wildt DJ, Jansen PAF (2015) Pharmacists’ and general practitioners’ pharmacology knowledge and pharmacotherapy skills. J Clin Pharmacol 55(8):936–943
Acknowledgements
Authors would like to thank all members of the Pharmacology Section of ESPNIC as well as respondents who spared their time to answer the survey.
Collaborators (helped with the validation process)
Saima Ashar: saimaasghar87@hotmail.com
Katerina Ntouzepi: papkat@gmail.com
Kostas Tziouvas: ktziouvas@yahoo.com
Vanessa Guy Viterbo: evydorian@gmail.com
Guilaime Mortamet: GMortamet@chu-grenoble.fr
Michael Levy: michael.levy@aphp.fr
Raquel Cieza: raquelcieza@hotmail.es
Laura Herrera Castillo: laura.herreracastillo@gmail.com
Daria Gaffo: daria.gaffo@aopd.veneto.it
Carolina Birolo: carolina.birolo@aulss8.veneto.it
Jef Willems: JEF.WILLEMS@ugent.be
Funding
None to disclose.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Marco Daverio and Angela Amigoni. The first draft of the manuscript was written by Marco Daverio, Francesca Sperotto, Elisa Poletto, Stefania Bianzina, and Angela Amigoni and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Marco Daverio and Angela Amigoni had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Marco Daverio is responsible for the data analysis.
Corresponding author
Ethics declarations
Ethical approval and consent to participate.
Consent was implied by completing the survey.
Consent for publication
All listed authors have approved the manuscript before submission, including the names and order of authors.
Competing interests
The authors report no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
44253_2024_44_MOESM2_ESM.docx
Supplementary Material 2: Supplementary file 2. Knowledge of pharmacology concepts, their application and educational needs comparing junior (< 40 years) vs senior (> 40 years) prescribers.
44253_2024_44_MOESM3_ESM.docx
Supplementary Material 3: Supplementary file 3. Material 3:Supplemental File 3. Parameters considered during drug prescription comparing junior (< 40 years) vs senior (> 40 years) prescribers.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Daverio, M., Sperotto, F., Poletto, E. et al. Assessment of self-perceived knowledge of key clinical pharmacology concepts and educational needs among European Paediatric Intensive Care Units: an ESPNIC survey. Intensive Care Med. Paediatr. Neonatal 2, 21 (2024). https://doi.org/10.1007/s44253-024-00044-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44253-024-00044-3