Abstract
Background
There are limited data on the clinical characteristics and outcomes of critically ill children requiring mechanical ventilation for SARS-CoV-2 acute respiratory failure.
Methods
We performed a multicentre prospective matched cohort study of mechanically ventilated paediatric patients aged 2 weeks to 18 years with confirmed SARS-CoV-2 acute respiratory failure, excluding Multisystem Inflammatory Syndrome. Cases were matched at 1:4 ratio to a pre COVID-19 pandemic paediatric cohort. Age, paediatric acute respiratory distress (PARDS) category, and organ dysfunction on days 0–1 of mechanical ventilation were used to match patients.
Results
Of 53 COVID-19 subjects, 60% were male, median age was 11.1 years (interquartile range 1.6–15.7), and 89% had moderate to severe PARDS on days 0–1. Compared to 195 matched controls, more children with COVID-19 were obese, cognitively or functionally impaired at baseline, Hispanic/Latino, and had pre-existing respiratory and neurologic conditions. Observed 28-day mortality was not different, but the COVID-19 cohort experienced fewer ventilator-free days and, among survivors, longer PICU stays. More COVID-19 patients were transitioned to extracorporeal membrane oxygenation.
Conclusions
Children with COVID-19 related acute respiratory failure suffered disproportionately from chronic conditions and required more critical care support than children with acute respiratory failure without SARS-CoV-2 related infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
By the height of the SARS-CoV-2 (COVID-19) pandemic, it had become apparent that children would be spared the brunt of the mortality and physical morbidities associated with the worldwide infection [1, 2]. Most children infected with SARS-CoV-2 were asymptomatic or exhibited mild disease [3]. Less than 10% developed severe disease that included myocardial dysfunction, shock, paediatric acute respiratory distress syndrome (PARDS), altered mental status, and Multisystem Inflammatory Syndrome in Children (MIS-C). Initial reports in paediatrics called attention to MIS-C with few reporting on the trajectory of isolated SARS-CoV-2 related PARDS [4].
Systematic investigation of the pathophysiologic effects of SARS-CoV-2 related lung disease in children has been limited with no comprehensive multicentre studies published. In a single-center study, Perk and colleagues showed that children who tested positive for respiratory viral pathogens other than SARS-CoV-2 were more likely to be hypoxemic than those with COVID-19 infection [5]. In contrast, Chao et al. described a higher than previously appreciated rate of severe disease in children admitted to the pediatric intensive care unit (PICU) with SARS-CoV-2 infection [6]. Obesity and congenital heart disease have been associated with more severe disease [7, 8].
The infrequent occurrence of SARS-CoV-2 acute respiratory failure in children has limited our capacity to comprehensively study the disease and compare it to known problems associated with PARDS [9, 10]. Pulmonary supportive strategies center on symptomatic management of PARDS according to the Pediatric Acute Lung Injury Consensus Conference (PALICC) guidelines [11, 12]. Paediatric SARS-CoV-2 specific management evolved over the pandemic and was based on knowledge acquired in adult patients with acute respiratory distress syndrome (ARDS) [13].
To date, no study has comprehensively described children with isolated SARS-CoV-2 acute respiratory failure who required mechanical ventilation. Understanding this disease and contrasting it to what is known about PARDS is an important step in knowledge development in the field. Here, we characterize a large cohort of children with SARS-CoV-2 acute respiratory failure, without MIS-C, and compare them to a pre COVID-19 pandemic cohort of children requiring invasive mechanical ventilation for acute respiratory failure.
Methods
SARS-CoV-2 patients were prospectively enrolled in a COVID-19 observational study that was associated with the Prone and Oscillation Paediatric Clinical Trial (PROSpect), an international randomized controlled trial investigating supine versus prone positioning and conventional ventilation versus high-frequency oscillatory ventilation (HFOV) in paediatric patients with high-moderate to severe PARDS (ClinicalTrials.gov: NCT03896763). During the pandemic, PROSpect sites were well-positioned to enroll SARS-CoV-2 positive patients into an observation-only study as the research infrastructure support was in place and many sites were required to pause their non-COVID-19 related research. Thirty-three PROSpect sites were eligible to participate in the observational study, as they were institutional review board- and system-approved and open for COVID-19 patients in their unit.
We enrolled paediatric patients ≥ 2 weeks of age (≥ 42 weeks post gestational age) and < 18 years of age who were mechanically ventilated with chest imaging consistent with acute pulmonary parenchymal disease and a confirmed positive SARS-CoV-2 polymerase chain reaction or antibody test, who did not exhibit MIS-C associated with COVID-19 [4, 14] and were not enrolled into PROSpect.
The matched cohort of non-COVID-19 patients was derived from the RESTORE (Randomized Evaluation of Sedation Titration for Respiratory Failure) study of protocolized sedation versus usual care in paediatric patients mechanically ventilated for acute respiratory failure (ClinicalTrials.gov: NCT00814099) [15]. While RESTORE enrolled subjects in 2009 through 2013, it is well documented that clinical management of PARDS has not changed during this time [12, 16, 17].
The COVID-19 observational study was approved by the University of Pennsylvania institutional review board (IRB) for all U.S. sites (‘Prone Oscillation Pediatric Clinical Trial’; IRB #831295; annual approval, most recent August 8, 2023) and at the local institutional level for international sites. Data collected during the RESTORE study was with IRB approval at each participating site. Written informed consent from the legal guardian of each enrolled patient was obtained for both cohorts. All aspects of the PROSpect and RESTORE trials were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975.
Functional status in the COVID-19 cohort was assessed using the Paediatric Cerebral Performance Category (PCPC), Paediatric Overall Performance Category (POPC), and Functional Status Scale (FSS) [18, 19]. Quality of life was assessed using the chronological age-appropriate Paediatric Quality of Life Inventory (PedsQL™; Version 4.0 Generic Core Scales for subjects ≥ 2 years; Infant Scales for subjects < 2 years) [20, 21] as reported by parent/guardian at 12 months post-PICU discharge. Functional status in the RESTORE cohort was assessed using the PCPC and POPC.
Statistical analysis
Each COVID-19 patient was matched with up to four unique, randomly selected patients from the RESTORE study based on age category (< 4.99, 5–11.99, 12–15.99, and 16–17.99 years of age), PARDS category based on worst oxygenation index (OI) or oxygen saturation index (OSI) on days 0–1 of mechanical ventilation (severe (OI ≥ 16.0 or OSI ≥ 12.3), moderate (OI of 8.0–15.9 or OSI of 7.5–12.2), mild (OI of 4.0–7.9 or OSI of 5.0–7.4), or at risk (OI < 4.0 or OSI < 5.0)) [12], and presence of organ dysfunctions (respiratory, cardiovascular, hematologic, neurologic, and renal) on days 0–1. Matching was performed using these variables to control for severity of illness. Categorical age groups were chosen based on differences in COVID-19 vaccine eligibility. As all matching variables were categorical, exact matching was performed.
The primary outcomes were ventilator-free days (VFD) through day 28 and in-hospital mortality at day 28. Patients who died by day 28 were assigned zero VFD. Comparisons of baseline characteristics, PICU course, and outcomes between the two cohorts (COVID-19 and RESTORE) were performed using stratified Wilcoxon rank-sum tests for continuous and ordinal variables and stratified exact tests for binary and nominal variables, adjusting for the matching factors. For the primary outcomes, we report the unstratified Hodges-Lehmann location shift for VFD and the stratified odds ratio for 28-day mortality with 95% confidence intervals. Patients with the same combinations of age, PARDS, and organ dysfunction categories were combined into the same strata for more efficient analyses. As we considered this matched cohort study descriptive in nature, we did not adjust for multiple comparisons. We explored whether obesity in mechanically ventilated paediatric patients with SARS-CoV-2 infection is associated with worse outcomes by comparing the primary outcomes between obese and non-obese patients within the COVID-19 and RESTORE cohorts separately. Weight categories were determined using weight-for-height z-scores based on World Health Organization growth charts for children aged < 2 years and body mass index-for-age z-scores based on Centers for Disease Control and Prevention growth charts for children aged 2 years and older. Statistical analyses were performed with SAS software, Version 9.4 (SAS Institute, Cary, NC) using two-sided 0.05 level tests.
Results
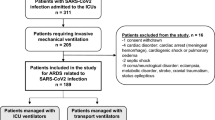
From May 29, 2020 to June 6, 2022, 325 COVID-19 positive patients were identified at 31 sites participating in the COVID-19 observational study. Of the 272 COVID-19 positive patients not enrolled, 98 were not intubated because of COVID-19 related acute respiratory failure, 37 had parent/guardian decline consent, 22 were not in the study age range, 21 were enrolled in the PROSpect trial, 18 had parent/guardian unavailable, 3 exhibited MIS-C, and 73 were not enrolled for other reasons (e.g., language barrier, system issue). Fifty-three patients were enrolled in the COVID-19 observational study across 21 sites in the United States, Canada, and Australia. These patients were matched with 195 patients from the RESTORE trial, including 102 in the RESTORE sedation protocol group and 93 in the usual care group. Of the COVID-19 patients, 42 matched exactly with 4 RESTORE patients, 6 matched with 3 RESTORE patients, 4 matched with 2 RESTORE patients, and 1 matched with only 1 RESTORE patient.
Cohort characteristics
Baseline demographic and medical history information for the PROSpect COVID-19 observational cohort and the RESTORE cohort are shown in Table 1. Both cohorts had predominantly direct PARDS with pneumonia as the leading diagnosis. Significantly more patients in the COVID-19 cohort were obese as compared to the RESTORE cohort (43% vs 19%; P = 0.001). More COVID-19 patients were Hispanic/Latino (37% vs 21%; P = 0.048). Significantly more COVID-19 patients were cognitively or functionally impaired at baseline as assessed by PCPC > 1 (57% vs 33%; P = 0.004) or POPC > 1 (64% vs 36%; P < 0.001). The paediatric risk of mortality was lower in the COVID-19 cohort (median 1.2% vs 4.9%; P < 0.001). Additionally, those with COVID-19 induced PARDS were more likely to have an underlying respiratory condition (e.g., asthma, obstructive sleep apnoea) or neurologic condition (e.g., seizure, neuromuscular disorder, cerebral palsy) or Trisomy 21.
PICU course
The PICU course for the COVID-19 and RESTORE cohorts is displayed in Table 2. COVID-19 patients had more severe PARDS during hospitalization compared to RESTORE patients (severe PARDS 75% vs 67%; P = 0.01). Only 4% of the COVID-19 cohort were managed with HFOV as compared with 17% of the RESTORE cohort (P = 0.01). Those with COVID-19 related PARDS were more likely to receive neuromuscular blockade for the entire duration of days 1 and 2 (36% vs 17%; P = 0.002), receive vasoactive medications (62% vs 48%; P = 0.008), and be cannulated for extracorporeal membrane oxygenation (ECMO; 15% vs 4%; P = 0.006). Sedative use was higher in the COVID-19 cohort with an increased mean daily opioid dose (median; 2.1 vs 1.3 mg/kg; P < 0.001), cumulative opioid dose (26.3 vs 14.9 mg/kg; P = 0.02), and number of different sedative classes received (4 vs 3; P < 0.001) (Table 2). In the COVID-19 cohort, administration of dexmedetomidine, propofol, ketamine, and barbiturates were increased, but benzodiazepine use was lower compared to the RESTORE cohort.
Management strategies
Specific management of the COVID-19 cohort is summarized in Table 2. Common interventions included the use of anti-infective agents (81%), anticoagulants (68%), and steroids (53%). One COVID-19 patient received one vaccine dose prior to admission. On enrolment, static compliance was measured during an inspiratory hold in 29 patients: median 15.0 mL/cm H2O (interquartile (IQR) 8.1–23.4) or 0.37 mL/cm H2O/kg (0.31–0.43). Of these, 28 patients (97%) had direct lung injury.
Respiratory support and outcomes
Ventilator-free days through day 28 were median 17.6 (IQR 3.3–22.3) in the COVID-19 cohort and 20.3 (14.7–23.7) in the RESTORE cohort (unstratified Hodges-Lehmann location shift –2.4 days [95% CI, –4.9 to –0.5]; stratified Wilcoxon P = 0.003; Table 3). Thirteen patients (25%) in the COVID-19 cohort were assigned zero VFD compared to 21 (11%) in the RESTORE cohort (P = 0.02). The duration of assisted breathing (through 90 days) was longer in the COVID-19 cohort with a median of 14.0 days (7.8–52.8) vs 9.5 days (5.6–16.9) in the RESTORE cohort (P = 0.004). A higher proportion of COVID-19 PARDS patients received non-invasive respiratory support pre-intubation and post-extubation (66% and 74% vs 45% and 43%; P = 0.003 and P < 0.001, respectively).
No in-hospital mortality differences were seen at 28 days (8% in the COVID-19 cohort vs 6% in the RESTORE cohort; stratified odds ratio 1.14 [exact 95% CI, 0.24–4.20]; P > 0.99; Table 3) or 90 days. Among survivors, COVID-19 patients were ventilated longer through day 28 (median 9.5 vs 7.3 days; P = 0.003) and had longer PICU (12.6 vs 11.2 days; P = 0.02) and hospital (19 vs 17 days; P = 0.02) lengths of stay. No difference was seen in change in cognitive or functional outcome between cohorts.
Obesity status
Compared to 30 non-obese COVID-19 patients, 23 obese patients with SARS-CoV-2 infection did not experience significantly fewer VFD (median 18.0 (IQR 0–22.2) vs 17.2 (9.8–22.4) days; P = 0.50) or higher in-hospital mortality at 28 days (3 (13%) vs 1 (3%); P = 0.31). Similarly, 37 obese patients without SARS-CoV-2 infection did not experience significantly fewer VFD (median 19.3 (IQR 14.0–23.0) vs 20.5 (16.0–23.8); P = 0.68) or higher 28-day hospital mortality (2 (5%) vs 10 (6%); P > 0.99) compared to 158 non-obese patients in the RESTORE cohort. In both cohorts, there was no difference in race and ethnicity by obesity status.
Post-hospitalization follow-up
Among survivors in the COVID-19 cohort (n = 47), 17 parents/guardians were not contacted for follow-up, including 12 for whom contact information could not be obtained, 3 outside the United States, 1 with guardianship issues, and 1 who refused follow-up. Nineteen parents/guardians provided follow-up data at 12-months post-PICU discharge, while 11 did not respond to multiple contact attempts.
Of the 19 patients with follow-up data, 11 (58%) had cognitive impairment (PCPC > 1) and 12 (63%) had functional impairment (POPC > 1) at baseline per medical record review, 14 (74%) had cognitive and functional impairment at follow-up per parent-report, and 10 (53%) had a worsening of cognitive and functional outcome at follow-up from baseline. At follow-up, median FSS score was 12 (IQR 8–15) for 17 patients, suggesting mild to moderate dysfunction per domain. Mean PedsQL total score at follow-up was 69.1 (standard deviation (SD) 22.6), mean physical health summary score was 75.3 (SD 26.6), and mean psychosocial health summary score was 66.1 (SD 23.8). Total scores in healthy children average 82.7 (SD 15.4), physical health summary scores average 84.5 (SD 19.5), and psychosocial health summary scores average 81.7 (SD 15.2) [22]. PedsQL total and physical health summary scores could not be calculated for 5 COVID-19 patients (4 with PCPC of 4 or 5 (severe disability or coma/vegetative state)) and psychosocial health summary score could not be calculated for 2 patients (1 with PCPC of 5) due to parents responding “does not apply to my child” or not answering more than half of the relevant questions.
Discussion
While much has been learned about COVID-19 illness since early 2020, knowledge of severe COVID-19 infection in children remains limited. Our study imparts new information regarding the effects of severe SARS-CoV-2 infection in a largely unvaccinated paediatric population as compared to a matched cohort of mechanically ventilated patients with acute respiratory failure. Paediatric patients with COVID-19 related acute respiratory failure were more likely to have a comorbid respiratory or neurologic condition, be obese, be Hispanic/Latino, and have cognitive and/or functional impairment on PICU admission. Compared to children with non-COVID-19 related acute respiratory failure, their course of illness was also more severe. They experienced longer durations of mechanical ventilation and length of PICU stay.
It is unclear whether the course of critical illness was primarily associated with direct effects of COVID-19 infection or whether management strategies, including the need for strict airborne isolation, especially pre-healthcare worker vaccination, including limited entry into patient rooms contributed. In the current study, patients with COVID-19 were more likely to receive neuromuscular blockade for the first two days of mechanical ventilation and received double the mean daily and cumulative doses of opioids compared to patients with non-COVID-19 related acute respiratory failure. The decreased use of HFOV in the COVID-19 cohort may be related to concern about environmental contamination; specifically, without a closed circuit or scavenging system, exhaled gases are released into the patient’s room. While the decreased use of HFOV could be related to a general trend in paediatric critical care management, there are no data to support such a speculation.
Static compliance (Crs) was measured on enrollment in our COVID-19 cohort. Early in the pandemic, it was suggested that adults with COVID-19 ARDS showed a different clinical phenotype than non-COVID-19 ARDS patients [23,24,25]. In small observational studies published early during the pandemic, it was observed that the severity of hypoxemia was out of proportion to impairment in respiratory system mechanics with patients having a relatively well-preserved respiratory system compliance [26,27,28,29,30]. However, in later studies, these observations were not confirmed, and, in fact, the relationship between Crs and ARDS severity quantified by the PaO2/FiO2 ratio was found to be similar between COVID-19 and non-COVID-19 ARDS patients [29, 30]. Such observations have not been reported before in children. Our study is the first to report lung mechanics in COVID-19 paediatric acute respiratory failure, and we found Crs normalized to bodyweight (Crs/kg) to be compatible with values previously reported for moderate PARDS [31].
Interestingly, those patients in the COVID-19 cohort were more likely to have abnormal neurocognitive functioning at baseline as assessed by PCPC and POPC scoring. The explanation for this association is unknown, but it may be speculated that neurologic sequelae of COVID-19 infection may have been a factor. At follow-up per parent report, cognitive and functional impairment increased to 74% in the COVID-19 cohort, and just over half of the children had a worsening of cognitive and functional outcome at follow-up as compared to pre-COVID-19 illness baseline. As the study was not designed or powered to assess long-term outcomes, we can only speculate as to the aetiology of the worsening of cognitive and functional outcome after discharge. While the number of patients with follow-up (n = 19) is limited, their follow-up cognitive and functional outcome and quality of life scores are low as compared to a healthy paediatric population.
Our study demonstrates that obesity in mechanically ventilated paediatric patients with SARS-CoV-2 infection does not affect outcomes which is consistent with the similarly matched cohort without COVID-19. However, our study is not designed or powered to assess a causal relationship between obesity and outcome. Variable findings have been reported regarding the relationship of obesity, severity of COVID-19 infections, and outcome with inconsistent explanations [25,26,27, 31,32,33,34]. Interestingly, Bouzid and colleagues noted that obesity was less common in adult patients presenting to the emergency department with the omicron variant as compared to those presenting during the delta variant wave [34].
The increased representation of the Hispanic/Latino population in the COVID-19 cohort is noteworthy. It is unclear whether this finding is related to a genetic predisposition, pattern of spread of COVID-19 in the communities served by the enrolling PICUs, or baseline differences in the PICUs included in the two cohorts analysed. Of note, there was no difference in race and ethnicity by obesity status. Our limited sample size precludes additional analysis or speculation.
The finding of increased use of rescue ECMO is not surprising given the outcome differences between the two cohorts. However, this difference may be related to changes in clinical practice patterns over time (2020–2022 for COVID-19 vs 2009–2013 for RESTORE). Additionally, the severity of the various COVID-19 variants may have played a role in the need for ECMO cannulation at various points of the pandemic.
While we report the largest prospective cohort of paediatric patients with severe COVID-19 infection requiring mechanical ventilation across international sites, our study has limitations. The number of COVID-19 patients with follow-up data is particularly low as contact information could not be obtained for parents who could not visit the hospital because they were COVID-19 positive and/or due to restrictive visitation policies. For those with contact information, the non-response rate was high. An uncontrolled confounding variable is the various strains of SARS-CoV-2 virus that circulated throughout the pandemic. Also, natural and vaccine-induced immunity has changed the picture for COVID-19 infection in adults and children. It must be noted that the matched cohort design can only yield associations, not causative conclusions. Furthermore, the incidence and potentially implications of obesity may have varied pre- and post-pandemic. Lastly, the period of data collection for the two studies (2020–2022 vs. 2009–2013) may reflect different clinical practice patterns, for example, use of pre- and post-intubation noninvasive respiratory support, administration of neuromuscular blockade, and use of HFOV, rather than intrinsic differences in patients with and without COVID-19 infection. However, PALICC-2 and PARDIE publications note that overall PARDS management has not changed in a clinically significant manner during this timeframe [12, 16, 17].
Conclusion
Children with acute respiratory failure due to COVID-19, compared with matched controls without COVID-19 infection, were more likely to be obese, Hispanic/Latino, and cognitively or functionally impaired at baseline. More children with COVID-19 also had pre-existing respiratory and neurologic conditions. While the risk of mortality was lower in the COVID-19 cohort, observed mortality was not different between groups. However, children with COVID-19 experienced fewer ventilator-free days and, among survivors, longer PICU stays.
Children with COVID-19 related acute respiratory failure suffered disproportionately from chronic conditions and required more critical care support than children with acute respiratory failure without SARS-CoV-2 related infection. Mid- and long-term outcome for children with acute respiratory failure related to COVID-19 infection requires continued investigation.
Availability of data and materials
Currently not publicly available.
References
Kim L, Whitaker M, O’Halloran A et al (2020) Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19 - COVID-NET, 14 States, March 1-July 25, 2020. MMWR Morb Mortal Wkly Rep 69(32):1081–1088
Lu X, Zhang L, Du H, Zhang J et al (2020) SARS-CoV-2 infection in children. N Engl J Med 382(17):1663–1665
Shekerdemian LS, Mahmood NR, Wolfe KK et al (2020) Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to us and canadian pediatric intensive care units. JAMA Pediatr 174(9):868–873
Feldstein LR, Tenforde MW, Friedman KG et al (2021) Characteristics and outcomes of US children and adolescents with Multisystem Inflammatory Syndrome in Children (MIS-C) compared with severe acute COVID-19. JAMA 325(11):1074–1087
Perk O, Ozcan S, Emeksiz S et al (2021) Comparison of clinical findings in SARS-CoV-2 with other respiratory viruses in critically Ill children during the COVID-19 pandemic. J Trop Pediatr 67(6):367–74
Chao JY, Derespina KR, Herold BC et al (2020) Clinical characteristics and outcomes of hospitalized and critically Ill children and adolescents with coronavirus disease 2019 at a tertiary care medical center in New York City. J Pediatr 223:14–19.e2
Zachariah P, Johnson CL, Halabi KC et al (2020) Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children’s hospital in New York City. New York JAMA Pediatr 174(10):e202430
Akcay N, Kihtir HS, Durak C et al (2022) Mortality risk factors among critically ill children with acute COVID-19 in PICUs: a multicenter study from Turkish pediatric critical COVID-19 and MIS-C study group. Pediatr Infect Dis J 41(9):742–750
Ong JSM, Tosoni A, Kim Y et al (2020) Coronavirus disease 2019 in critically Ill children: a narrative review of the literature. Pediatr Crit Care Med 21(7):662–666
Martin B, DeWitt PE, Russell S et al (2022) Characteristics, outcomes, and severity risk factors associated with SARS-CoV-2 infection among children in the US national COVID cohort collaborative. JAMA Netw Open 5(2):e2143151
Derespina KR, Kaushik S, Plichta A et al (2020) Clinical manifestations and outcomes of critically Ill children and adolescents with coronavirus disease 2019 in New York City. J Pediatr 226:55–63.e2
Pediatric Acute Lung Injury Consensus Conference Group (2015) Pediatric acute respiratory distress syndrome: consensus recommendations from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med 16(5):428–439
Rimensberger PC, Kneyber MCJ, Deep A et al (2021) Caring for critically Ill children with suspected or proven coronavirus disease 2019 infection: recommendations by the scientific sections’ collaborative of the European Society of Pediatric and Neonatal Intensive Care. Pediatr Crit Care Med 22(1):56–67
Feldstein LR, Rose EB, Horwitz SM et al (2020) Multisystem Inflammatory Syndrome in U.S. Children and adolescents. N Engl J Med 383(4):334–346
Curley MA, Wypij D, Matthay MA et al (2015) Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA 313(4):379–389
Rowan CM, Klein MJ, Hsing DD et al (2020) Early use of adjunctive therapies for pediatric acute respiratory distress syndrome: a PARDIE study. Am J Respir Crit Care Med 201(11):1389–1397
Emeriaud G, López-Fernández YM, Iyer NP et al (2023) Executive summary of the second international guidelines for the diagnosis and management of pediatric acute respiratory distress syndrome (PALICC-2). Pediatr Crit Care Med 24(2):143–168
Fiser DH (1992) Assessing the outcome of pediatric intensive care. J Pediatr 121(1):68–74
Pollack MM, Holubkov R, Funai T et al (2014) Relationship between the functional status scale and the pediatric overall performance category and pediatric cerebral performance category scales. JAMA Pediatr 168(7):671–676
Varni JW, Burwinkle TM, Seid M, Skarr D (2003) The PedsQL™* 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Acad Pediatr 3(6):329–341
Varni JW, Limbers CA, Sorensen LG et al (2011) The PedsQL™ infant scales: feasibility, internal consistency reliability, and validity in healthy and ill infants. Qual Life Res 20(1):45–55
Varni JW, Limbers CA, Burwinkle TM (2007) Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL 4.0 generic core scales. Health and Qual Life Outcomes 5:43
Gattinoni L, Coppola S, Cressoni M et al (2020) COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med 201(10):1299–1300
Gattinoni L, Chiumello D, Rossi S (2020) COVID-19 pneumonia: ARDS or not? Crit Care 24(1):154
Schenck EJ, Hoffman K, Goyal P et al (2020) Respiratory mechanics and gas exchange in COVID-19-associated respiratory failure. Ann Am Thorac Soc 17(9):1158–1161
Grasselli G, Tonetti T, Protti A et al (2020) Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med 8(12):1201–1208
Beloncle FM, Pavlovsky B, Desprez C et al (2020) Recruitability and effect of PEEP in SARS-Cov-2-associated acute respiratory distress syndrome. Ann Intensive Care 10(1):55
Pan C, Chen L, Lu C et al (2020) Lung recruitability in COVID-19-associated acute respiratory distress syndrome: a single-center observational study. Am J Respir Crit Care Med 201(10):1294–1297
Chiumello D, Busana M, Coppola S et al (2020) Physiological and quantitative CT-scan characterization of COVID-19 and typical ARDS: a matched cohort study. Intensive Care Med 46(12):2187–2196
Ferrando C, Suarez-Sipmann F, Mellado-Artigas R et al (2020) Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med 46(12):2200–2211
Ilia S, van Schelven PD, Koopman AA et al (2020) Effect of endotracheal tube size, respiratory system mechanics, and ventilator settings on driving pressure. Pediatr Crit Care Med 1(1):e47–e51
Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet 395(10223):497–506
Vassilopoulou E, Bumbacea RS, Pappa AK et al (2022) Obesity and infection: what have we learned from the COVID-19 pandemic. Front Nutr 9:931313
Bouzid D, Visseaux B, Kassasseya C et al (2022) Comparison of patients infected with delta versus omicron COVID-19 variants presenting to paris emergency departments : a retrospective cohort study. Ann Intern Med 175(6):831–837
Acknowledgements
None.
The PROSpect COVID-19 Investigative Team
• Peter M. Luckett MD, University of Texas Southwestern Medical Center and Children’s Health, Dallas, TX.
• Michele Kong MD, Children’s of Alabama; Heersink School of Medicine, The University of Alabama at Birmingham, Birmingham, AL.
• Palen Mallory MD, Duke Children’s Hospital, Duke University, Durham, NC.
• Nadir Yehya MD, Children’s Hospital of Philadelphia; Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA.
• Erin Kreml MD, Phoenix Children’s Hospital, Phoenix, AZ.
• Adam Schwartz MD, Children’s Hospital of Orange County Children's Hospital; University of California, Irvine School of Medicine; Orange, CA.
• Kari Wellnitz MD, Stead Family Department of Paediatrics, University of Iowa Hospitals & Clinics, Iowa City, IA.
• Katherine Clement MD, University of North Carolina Children’s Hospital, University of North Carolina at Chapel Hill, Chapel Hill, NC.
• Timothy Cornell MD, Lucille Packard Children’s Hospital Stanford, Stanford University, Palo Alto, CA.
• Emilie Henry MD, The Children’s Hospital at Oklahoma University Medical Center, Oklahoma City, OK.
• Laurie Lee MN, Alberta Children’s Hospital, Alberta, Canada.
• Sidharth Mahapatra MD, Children’s Hospital & Medical Center, The University of Nebraska Medical Center, Omaha, NE.
• Melissa B. Porter MD, Norton Children’s Hospital, University of Louisville, Louisville, KY.
• Courtney Rowan MD, Riley Hospital for Children; Indiana University School of Medicine, Indianapolis, IN.
• Neal J. Thomas MD, Penn State Children’s Hospital; Pennsylvania State University College of Medicine, Hershey, PA.
• Shan Ward MD, UCSF Benioff Children's Hospitals, San Francisco and Oakland, San Francisco, CA.
• Himanshu Aneja MD, The Children's Hospital at Westmead, The Sydney Children’s Hospital Network, New South Wales, Australia.
• Jessica Asencio MD, Cohen Children’s Medical Center; Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Queens, NY.
• Jasmine Dowell MD, Children’s Wisconsin, Medical College of Wisconsin, Milwaukee, WI.
• Kim McMahon MD, AI DuPont Children’s Hospital, Wilmington, DE.
• Matei Petrescu MD, The Children's Hospital of San Antonio, Baylor College of Medicine, San Antonio, TX.
Code availability
Not applicable.
Funding
University of Pennsylvania Investing in the Future Fund (Dr. Curley); National Heart, Lung, and Blood Institute and the National Institute of Nursing Research, National Institutes of Health (for RESTORE; grant U01 HL086622 to Dr. Curley and grant U01 HL086649 to Dr. Wypij).
Author information
Authors and Affiliations
Consortia
Contributions
Authors provided substantial contributions to the conception (IMC, MCK, DW, MAQC) or design of the work (IMC, LAA, NN, DW, MCK, MAQC); or the acquisition (MK, PML), analysis (OSD, LAA, DW), or interpretation of data (IMC, MK, NN, MAPE, PML, MCK, MAQC) for the work; All drafted the work or reviewed it critically for important intellectual content; All provided final approval of the manuscript; and All agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheifetz, I.M., Dawkins-Henry, O.S., Kong, M. et al. Characteristics and outcomes of children with SARS-CoV-2 respiratory failure: a matched cohort study. Intensive Care Med. Paediatr. Neonatal 2, 18 (2024). https://doi.org/10.1007/s44253-024-00041-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44253-024-00041-6