Abstract
Purpose
Pediatric acute respiratory distress syndrome (PARDS) is underrecognized in the pediatric intensive care unit and the interpretation of chest radiographs is a key step in identification. We sought to test the performance of a machine learning model to detect PARDS in a cohort of children with respiratory failure.
Materials and methods
A convolutional neural network (CNN) model previously developed to detect ARDS on adult chest radiographs was applied to a cohort of children age 7 days to 18 years, admitted to the PICU, and mechanically ventilated through a tracheostomy, endotracheal tube or full-face non-invasive positive pressure mask between May 2016 and January 2017. Two pediatric critical care physicians and a pediatric radiologist reviewed chest radiographs to evaluate if the chest radiographs were consistent with ARDS (bilateral airspace disease) and PARDS (any airspace disease) and the CNN model was tested against clinicians.
Results
A total of 328 chest radiographs were evaluated from 66 patients. Clinicians identified 84% (276/328) of the radiographs as potentially consistent with PARDS. Inter-rater reliability between individual clinicians and between the model and clinicians was similar (Cohen’s kappa 0.48 [95% CI 0.37–0.59] and 0.45 [95% CI 0.33–0.57], respectively). The model was better at identifying PARDS (AUC 0.882, F1 0.897) than ARDS (AUC 0.842, F1 0.742) and had equivalent or better performance to individual clinicians.
Conclusions
An ARDS detection model trained on adults performed well in detecting PARDS in children. Computer-assisted identification of PARDS on chest radiographs could improve the diagnosis of PARDS for enrollment in clinical trials and application of PARDS guidelines through improved diagnosis.
Similar content being viewed by others
Introduction
Pediatric acute respiratory distress syndrome (PARDS) is a rapid-onset and potentially life-threatening lung injury [1]. Diagnosis of PARDS requires an assessment of the disease’s etiology, onset, a measure of oxygenation defect, and acute pulmonary parenchymal infiltrates on chest radiography per Pediatric Acute Lung Injury Consensus Conference (PALICC) [2]. The PALICC chest radiograph criteria for PARDS are distinct from the pre-existing Berlin Acute Respiratory Distress Syndrome (ARDS) criteria by allowing for the presence of unilateral disease. PALICC authors justified this distinction based on three factors—the lack of evidence on the sensitivity of chest radiograph to detect pulmonary parenchymal infiltrates, the heterogeneity present within the diagnosis, and question of whether bilateral infiltrates impart additional risk [2]. Regardless, interpreting clinicians and researchers have notably poor agreement when evaluating if chest radiography meets ARDS or PARDS criteria [3,4,5].
ARDS is underrecognized in both adults and children [6, 7]. Disease onset and measures of oxygen defect are readily obtainable from electronic medical records; however, clinician screening of chest radiographs is time-consuming and highly variable. Using computer-aided detection of findings consistent with PARDS on chest radiographs has the potential to improve the clinical care and research of pediatric acute respiratory failure. At the bedside, adherence to lung-protective principles in PARDS is associated with improved patient outcomes, and recognition of the disease is a key step in applying consensus guidelines [8,9,10]. Automated screening for inclusion in PARDS research studies could also remove a barrier for the inclusion of more patients and research sites into multicenter research studies and minimize center-level variation in patient inclusion.
A deep convolutional neural network (CNN) developed to identify findings of ARDS on chest radiographs was trained using 600,000 chest radiographs from adult patients and demonstrated excellent performance in an external cohort [11, 12]. However, when this model incorrectly assigned a high probability of ARDS, but clinicians did not assign ARDS, it was observed that unilateral airspace disease was present on some radiographs. Because unilateral disease is included in the definition of PARDS, it is possible that this CNN could be applied to children to detect PARDS. In this study, we sought to test the performance of the model trained and validated on adult patients in a cohort of children.
Materials and methods
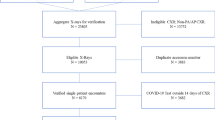
This study was approved by the University of Michigan Institutional Review Board (HUM00129801) and all ethical principles for research involving human subjects were followed. Consent for study inclusion was waived. Chest radiographs from eligible patients were analyzed from 3 weeks coinciding with the Pediatric Acute Respiratory Distress Syndrome Incidence and Epidemiology (PARDIE) study from May 2016 to January 2017. Patients aged 7 days to 18 years were eligible for inclusion if they were admitted to the pediatric intensive care unit and mechanically ventilated through a tracheostomy or endotracheal tube or full-face non-invasive positive pressure mask. The sample was enriched with a convenience sample of 12 children who received extracorporeal membrane oxygenation (ECMO), but only radiographs prior to ECMO cannulation were included in the current analysis.
All chest radiographs were assessed for the presence of infiltrates consistent with the PALICC definition of PARDS (unilateral or bilateral airspace disease) and the Berlin definition of ARDS (bilateral airspace disease only) by a pediatric intensivist (JGK) and a pediatric radiologist (MGM). Chest radiographs from the same patient were interpreted independently. If there was agreement on the presence or absence of infiltrates consistent with the PALICC PARDS definition or Berlin ARDS definition, the radiograph was classified as such. If there was disagreement, a third pediatric intensivist (RPB) independently reviewed the radiograph. Chest radiographs were classified as clinician-identified PARDS or ARDS if 2/2 or 2/3 reading clinicians determined that the image met the specified criteria.
We calculated pairwise inter-rater reliability between the initial reading clinician (JGK), pediatric radiologist (MGM), and the CNN model. We assess inter-rater reliability using Cohen’s kappa. We determined the model’s ability to identify Berlin ARDS and PALICC PARDS separately by calculating the area under the receiver-operator curve (AUROC), and F1 score. To calculate F1, we selected a model probability threshold to classify chest radiographs as positive that matched the average sensitivity of the reading intensivist and radiologist and fixed this threshold to enforce this sensitivity. Based on this approach, probability threshold values of 0.195 and 0.362 were used as the CNN output score to identify PALICC PARDS and Berlin ARDS, respectively. We used gradient-weighted class activation mapping (Grad-CAM) to qualitatively understand which areas of the chest radiograph image the CNN model focused on when it correctly and incorrectly classified images. Representative images were presented as demonstrative examples of CNN focus.
Results
There were 328 chest radiographs from 66 patients analyzed (Table 1). There were 59 of 66 patients (89%) with at least one chest radiograph with findings consistent with PARDS. The number of chest radiographs per patient ranged from 1 to 22. 84% (276/328) of the chest radiographs had radiographic findings consistent with PARDS and 48% (158/328) had findings consistent with Berlin ARDS. There were 28 of 66 patients (42%) who were classified as PARDS by the PARDIE investigators because they had both a consistent chest radiograph and met all other clinical criteria without exclusions.
When applied to the pediatric cohort, the CNN previously trained to detect ARDS in adults had an AUROC of 0.882 (95% 0.84–0.92) for the identification of PALICC PARDS (any airspace disease) on chest radiograph (Table 2). The same model also had an AUROC of 0.84 (95% CI 0.8–0.883) for the identification of Berlin ARDS (bilateral airspace disease) on radiographs. Among chest radiographs with bilateral airspace disease, the CNN model generated a higher output probability score (median 0.470, IQR 0.338–0.716) compared to chest radiographs with unilateral disease (median 0.276, IQR 0.194–0.393) (Fig. 1). Chest radiographs without any airspace disease had the lowest probability outputs (median 0.170, IQR 0.108–0.238).
The inter-rater reliability was similar between the clinicians and the deep learning model. Cohen’s kappa in detecting PARDS was 0.48 (95% CI 0.372–0.585) between the two initial reading clinicians, 0.46 (95% CI 0.35–0.57) between the deep model and the pediatric intensivist and 0.47 (95% CI 0.36–0.57) between the deep model and the pediatric radiologist (Table 3).
When reviewing the Grad-CAM of chest radiographs both correctly and incorrectly classified by the CNN as PARDS (Fig. 2), we found that the model generally focused on areas of lung airspace disease when it correctly classified chest radiographs as PARDS (Fig. 2a). When the model incorrectly assigned a chest radiograph as PARDS, it often focused on areas outside the lung fields (Fig. 2b). When clinicians identified the radiograph as PARDS and the CNN model did not, the radiographs seemed to either have very subtle infiltrates or there were larger, homogenous-appearing infiltrates that may have been difficult for the model to recognize as the lung field (Fig. 2c).
Example Grad-CAM images demonstrating areas of CNN model focus in correctly and incorrectly assigned images. The Grad-CAM image shows a heat map of the area on the image where the model focuses, with areas of green/yellow/red demonstrating the most focus. The CNN probability output score for each image is presented on the images. a is the chest radiograph and Grad-CAM image of a patient that clinicians and the CNN model both identified as PALICC PARDS. b shows the chest radiograph and Grad-CAM image of a patient where the clinician identified the patient as not having PARDS and the model identified PARDS (false positive). c shows the chest radiograph of a patient where the model did not identify PARDS but the clinicians did (false negative)
Discussion
In this analysis, we applied a CNN model trained and validated to detect findings of ARDS in adult patients to a cohort of children with respiratory failure. The model performed as well as clinicians in the identification of chest imaging findings consistent with pediatric ARDS. Despite being trained and validated in adults, the deep learning model performed better in the identification of PALICC PARDS than Berlin ARDS in a pediatric cohort.
When the model was trained on adult patients, the machine learning model trained on features in images that were labeled as ARDS through multiple clinician evaluations. This approach means that the factors clinicians weigh when determining if a chest radiograph is consistent with ARDS (bilateral vs. unilateral disease, presence of atelectasis, etc.) may not be the features the model weighs when assigning a probability of ARDS. We speculate several possible reasons the model performed better in the identification of PARDS, despite never being trained to identify PARDS. The first possible explanation is the misclassification of unilateral disease as ARDS that was anecdotally observed in the adult ARDS model validation [12]. This misclassification issue in the adult model may be a beneficial feature in the effort to identify pediatric ARDS which allows for unilateral disease. The CNN model is a continuous function of the input image, so it is possible that the larger the infiltrate area is in the image, the higher the output score. Since the overall infiltrate area is (most likely) larger in bilateral ARDS than unilateral (on average), the model may fail when there are large infiltrate areas in one lung field. In the case where the model “missed” clinician-identified PARDS cases, the chest imaging findings were either subtle, or more homogeneous, as in Fig. 2c. It is possible that the model is using textural image features and “patchy” airspace disease is more readily identified.
Machine learning has great potential in a data-rich environment like the pediatric intensive care unit [13, 14]. The algorithm in this study was developed using a methodology in machine learning called transfer learning, where the model was pre-trained to identify common chest radiograph findings in a large dataset before being trained to label ARDS [12, 15]. This process of transfer learning can support model development when there is a small dataset, which is a common issue in pediatric critical care. This study, while not trained on pediatric data, demonstrates the potential of either directly applying adult-trained machine learning models or to support the development of pediatric models. The concept of transfer learning may allow for the more rapid development and deployment of machine learning models in pediatric critical care.
This study has several important limitations. The study population included only children invasively or non-invasively mechanically ventilated with a high incidence of PARDS, so it is possible that the model performance would change when applied to more general critical care population. The positive predictive value and negative predictive value presented should be interpreted with this context. However, the primary measures of model performance including AUROC should be independent of the event rate. Importantly, this single-center study requires validation in a larger multicenter cohort with more clinicians interpreting chest radiographs.
While identification of features of PARDS on a chest radiograph is an important advance, there are other potential applications of this technology. The allowance for unilateral disease in the definition for PARDS opens the door to segment the chest radiograph into the left and right lung and determine disease laterality. Furthermore, we have previously shown that there are important clinical and pathophysiological differences associated with chest radiograph findings, so the ability of a machine learning model to detect findings on a chest radiograph that are associated with clinical outcomes would be meaningful for clinicians [16].
An algorithm trained and validated to detect ARDS in adult chest radiographs performs well in children for the detection of findings consistent with PARDS. The application of this model can support the clinical diagnosis of PARDS and eligibility for research enrollment. This study also highlights the potential for application of models developed in adult critical care to a pediatric critical care population.
Availability of data and materials
A limited, de-identified version of these data could be made available to other researchers from accredited research institutions after entering into a data use agreement with the University of Michigan. Requests will be reviewed on an individual basis by the University of Michigan Medicine Data Office for Clinical and Translation Research.
Code availability
A web demonstration tool of the model is available at https://ardsdetect.com/.
References
Khemani RG, Smith L, Lopez-Fernandez YM, Kwok J, Morzov R, Klein MJ, Yehya N, Willson D, Kneyber MCJ, Lillie J, Fernandez A, Newth CJL, Jouvet P, Thomas NJ, Abaleke E, Ackerman KG, Acuña C, Adu-Darko M, Affolter JT, Agbeko R, Al Amoudi A, Alahmadti A, Aldairi N, Alibrahim O, Allen K, Allen C, Al-Subu A, Althabe M, Alvear J, Anil AB, Anthony H, Aramburo A, Arjona Villanueva D, Ashtari N, Ávila Vera A, Baines P, Bales M, Barr S, Barry D, Baudin F, Beca J, Belfield H, Beltramo F, Benken L, Bhalla A, Blom A, Botta P, Bourgoin P, Brezmes M, Briassoulis G, Bridier A, Brierley J, Brio Sanagustin S, Broden E, Butt W, Bysani K, Camilo C, Camporesi A, Campos-Miño S, Can FK, Capocasa P, Caro I D, Carroll C, Castellani P, Castillo AE, Chen Y, Chima RS, Chiusolo F, Cinquegrani K, Coates B, Coronado-Munoz A, Cortéz A, Cruces Romero P, Cullimore M, Cvijanovich N, Dahmer MK, Deep A, Delzoppo C, Di Nardo M, Díaz F, Dijkstra S, Dockery WK, Dominguez TE, Dumitrascu M, Dursun O, Dwarakanathan B, Elghuwael I, Emeriaud G, Erickson S, Español SF, Estil JB, Feather C, Feinstein Y, Fernández A, Ferreyra M, Flori H, Fortini YV, Fortune P-M, French ME, Gaboli M, Gale H, García Casas P, García González M, Gautam R, Gedeit R, Genuini M, Gertz S, Giampieri M, Gil Escobar C, Giuliano Jr JS, Godoy Mundaca L, Goni Orayen C, Gonzalez Gomez JM, Govantes B, Guichoux J, Guzman Rivera GA, Haileselassie B, Han YY, Harrell A, Hartmann S, Hazwani T, Hefley G, Henderson G, Hsing DD, Hughes-Schalk A, Hume J, Ilia S, Inwald D, Iolster T, Izquierdo LM, Jafari-Namin S, Jaimon N, Jarillo Quijada AE, Jarvis JD, Jayachandran C, Jennings C, Jeyapalan AS, Jimenez Rivera NJ, Jones D, Jouvet P, Kasch M, Keary JT, Kelley C, Kessel A, Khemani R, Kida Y, King C, Kneyber M, Kniola A, Krallman K, Kubis S, Kustka L, Kwok J, Kyo M, Landry LM, Latifi S, Lawton-Woodhall A, Lillie J, Lin JC, Llorente De La Fuente AM, Lopez Alarcón YP, López Fernández Y, Lopez-Herce J, Lum LCS, Macrae D, Maddux AB, Madurga Revilla P, Mahapatra S, Maria M, Martínez L, Martinez De Azagra A, Martínez León AF, Mazzillo Vega L, Mccorkell J, Mcintyre K, Medina T, Medina A, Mellish C, Mendizabal M, Merritt C, Mildner R, Milesi C, Modesto I Alapont V, Monjes C, Monjure T, Montes MJ, Morales Martinez A, Morgan R, Morzov R, Mourani PM, Murkowski K, Murphy M, Napolitano N, Nerheim D, Nett ST, Newth C, Nofziger R, Nunez MJ, Ohshimo S, Onate Vergara E, Ongun EA, Orqueda D, Oruganti S, Pagowska-Klimek I, Palanca Arias D, Pappachan J, Pardo Carrero R, Parker MM, Parrilla J, Patankar N, Pávez Madrid P, Payen V, Paziencia F, Pedraza C, Perez Lozano G, Pilar Orive J, Piñeres Olave BE, Pintimalla A, Pinto N, Plunkett A, Pon S, Pons Odena M, Poterala R, Qiao H, Quiñonez Lopez D, Ralston K, Ramirez Cortez G, Ratiu A, Rea M, Reyes Dominguez S, Rodgers C, Rodriguez Campoy P, Ronan L, Rosemary D, Rowan C, Sadasivam K, Sanchez Diaz JI, Sanders R, Santanelli J, Sapru A, Schneider J, Sforza J, Shea S, Shein SL, Sherring C, Sheward V, Shime N, Shukla A, Siaba Serrate A, Sierra Y, Sikora L, Silvestre C, Singleton M, Sloniewsky D, Smith R, Smith L, Song H, Sousa Moniz M, Spaeder M, Spear D, Spinella P, Starck J, Stoneman E, Su F, Subramanian G, Sullivan E, Sundararajan S, Sweberg T, Sykes K, Tabata Y, Tai CW, Tala J, Tang SF, Tantalean J, Taylor R, Thomas N, Tibby S, Tieves KS, Torero L, Torres SF, Totapally B, Travert B, Truemper E, Turón G, Typpo K, Valle JR, Vargas G SI, Vasquez Hoyos P, Vasquez Miranda D, Vavrina M, Vidal NÁ, Virk M, Walsh L, Wegner Araya A, Weitz J, Wellisch L, Wellman P, Willson D, Woods K, Yehya N, Yerovi R, Yunger T, Zuluaga Orrego C, Zurek J, (2019) Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. Lancet Respir Med 7: 115-128
Khemani RG, Smith LS, Zimmerman JJ, Erickson S, Conference PALIC, G, (2015) Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 16:S23-40
Lopez-Fernandez YM, Smith LS, Kohne JG, Weinman JP, Modesto-Alapont V, Reyes-Dominguez SB, Medina A, Pineres-Olave BE, Mahieu N, Klein MJ, Flori HR, Jouvet P, Khemani RG, Syndrome PARD, I, Epidemiology VI, the Pediatric Acute Lung I, Sepsis Investigators N, (2020) Prognostic relevance and inter-observer reliability of chest-imaging in pediatric ARDS: a pediatric acute respiratory distress incidence and epidemiology (PARDIE) study. Intensive Care Med 46:1382–1393
Sjoding MW, Hofer TP, Co I, Mcsparron JI, Iwashyna TJ (2019) Differences between patients in whom physicians agree and disagree about the diagnosis of acute respiratory distress syndrome. Ann Am Thorac Soc 16:258–264
Peng J-M et al (2017) Does training improve diagnostic accuracy and inter-rater agreement in applying the Berlin radiographic definition of acute respiratory distress syndrome? A multicenter prospective study. Critical Care 21(1). https://europepmc.org/backend/ptpmcrender.fcgi?accid=PMC5251343&blobtype=pdf
McCrory MC, Woodruff AG, Saha AK, Evans JK, Halvorson EE, Bass AL (2022) Nonadherence to appropriate tidal volume and PEEP in children with pARDS at a single center. Pediatr Pulmonol 57(10):2464–2473. https://doi.org/10.1002/ppul.26060
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A, Investigators LS, Group ET, (2016) Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315: 788-800
Bhalla AK, Klein MJ, Emeriaud G, Lopez-Fernandez YM, Napolitano N, Fernandez A, Al-Subu AM, Gedeit R, Shein SL, Nofziger R, Hsing DD, Briassoulis G, Ilia S, Baudin F, Pineres-Olave BE, Maria Izquierdo L, Lin JC, Cheifetz IM, Kneyber MCJ, Smith L, Khemani RG, Newth CJL, Syndrome PARD, I, Epidemiology VI, Pediatric Acute Lung I, Sepsis Investigators N, (2021) Adherence to lung-protective ventilation principles in pediatric acute respiratory distress syndrome: a pediatric acute respiratory distress syndrome incidence and epidemiology study. Crit Care Med 49:1779–1789
Khemani RG, Parvathaneni K, Yehya N, Bhalla AK, Thomas NJ, Newth CJL (2018) Positive end-expiratory pressure lower than the ARDS network protocol is associated with higher pediatric acute respiratory distress syndrome mortality. Am J Respir Crit Care Med 198:77–89
Rubenfeld GD, Cooper C, Carter G, Thompson BT, Hudson LD (2004) Barriers to providing lung-protective ventilation to patients with acute lung injury. Crit Care Med 32:1289–1293
Farzaneh N, Ansari S, Lee E et al (2023) Collaborative strategies for deploying artificial intelligence to complement physician diagnoses of acute respiratory distress syndrome. NPJ Digit Med 6:62. https://doi.org/10.1038/s41746-023-00797-9
Sjoding MW, Taylor D, Motyka J, Lee E, Co I, Claar D, McSparron JI, Ansari S, Kerlin MP, Reilly JP, Shashaty MGS, Anderson BJ, Jones TK, Drebin HM, Ittner CAG, Meyer NJ, Iwashyna TJ, Ward KR, Gillies CE (2021) Deep learning to detect acute respiratory distress syndrome on chest radiographs: a retrospective study with external validation. Lancet Digit Health 3:e340–e348
Gutierrez G (2020) Artificial intelligence in the intensive care unit. Crit Care 24:101
Wiens J, Fackler J (2018) Striking the right balance-applying machine learning to pediatric critical care data. Pediatr Crit Care Med 19:672–673
Yosinski J, Clune J, Bengio Y, Lipson H (2014) How transferable are features in deep neural networks? In: Advances in Neural Information Processing Systems 27 (NIPS ’14). NIPS Foundation
Kohne JG, Dahmer MK, Weeks HM, Kaciroti N, Quasney MW, Sapru A, Curley MAQ, Matthay M, Flori H (2020) Impact of bilateral infiltrates on inflammatory biomarker levels and clinical outcomes of children with oxygenation defect. Crit Care Med 48:e498–e504
Funding
Support for this study came from a Charles Woodson Pilot Research Award from the University of Michigan.
Author information
Authors and Affiliations
Contributions
Conceptualization: R.P.B. and M.W.S.; data curation: J.G.K., N.F., R.P.B., and M.G.; formal analysis: N.F. and S.A.; funding acquisition: R.P.B. and M.W.S.; methodology: J.G.K., N.F., R.P.B., and M.W.S.; supervision: R.P.B. and M.W.S.; writing—original draft: J.G.K. and N.F.; writing—review and editing: J.G.K., N.F., R.P.B., M.G., S.A., and M.W.S.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the University of Michigan Institutional Review Board (HUM00129801) and all ethical principles for research involving human subjects were followed. Consent for study inclusion was waived.
Competing interests
The University of Michigan has filed a US Utility Patent application (No. 17/082,145) for the invention, UM IR # 2020–026 “Computer Vision Technologies for Rapid Disease Detection,” which uses software technology to process chest radiographs to detect acute diseases, of which Dr Sjoding reports being an inventor. Dr. Sjoding reports funding from the National Institute of Health (NIH) (K01 HL136687) and Department of Defense (W81XWH2010496). Dr. Barbaro reports funding from the NIH (K12 HL138039; R01 HL153519). Dr. Ansari reports funding from the NIH (K01HL155404). Drs. Kohne and Farzaneh report funding from the NIH (K12TR004374).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kohne, J.G., Farzaneh, N., Barbaro, R.P. et al. Deep learning model performance for identifying pediatric acute respiratory distress syndrome on chest radiographs. Intensive Care Med. Paediatr. Neonatal 2, 5 (2024). https://doi.org/10.1007/s44253-024-00034-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44253-024-00034-5