Abstract
Background
Lung ultrasound may help to estimate extravascular lung water (EVLW). However, after paediatric cardiac surgery, the relationship of a lung ultrasound score (LUS) with subcutaneous oedema, fluid balance, or body weight is unknown.
Methods
A prospective, observational study was performed in mechanically ventilated infants following cardiac surgery. A clinical oedema score (COS), fluid balance (FB), body weight (BW) and LUS (based on a B-line scoring tool) were performed.
Results
We studied 53 infants with a median age of 8 days [IQR, 0–14]. EVLW, as estimated by the mean LUS, was 8.7 (SD 3.7) at baseline, 9.2 (SD 4.2) at 24 h, and 9.1 (SD 3.4) at 48 h, out of a maximum score of 24. However, LUS changes over time were small and infero-lateral lung zones had higher mean LUS compared to supero-anterior lung zones. Moreover, subcutaneous body oedema, as measured by a COS, change in BW, or FB, were not associated with the LUS. On multivariable, linear, mixed-effects modelling, accounting for repeated measures within children over time, there was no significant association between the COS and LUS (0.2 points [95% CI − 0.01–0.3]. Finally, in similar regression analyses, there was no significant association between each 100 gm increase in BW or 100 ml increase in FB and changes in LUS (BW, 0.3 [− 0.1–0.7] and FB, 0.1 [− 0.2–0.4]).
Conclusions
In infants following cardiac surgery, the lung ultrasound score was higher in infero-lateral lung zones compared to supero-anterior zones. However, it had no association with a subcutaneous oedema score or changes in fluid balance or body weight.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluid accumulation in children following cardiac surgery has been associated with worse oxygenation, lower cardiac output and prolonged mechanical ventilation, [1,2,3] particularly when present at extreme values [4]. The association with prolonged mechanical ventilation implies that positive fluid balance may lead to lung oedema, or increased extravascular lung water (EVLW), therefore contributing to lung injury, a recognised consequence of cardiopulmonary bypass in children. Such lung injury appears mediated by the inflammatory effects of ischemia–reperfusion injury [5,6,7] and by increased pulmonary vascular permeability. However, it may also be aggravated by a positive fluid balance. In children following cardiac surgery, subcutaneous edema is commonly observed and believed to occur because of positive fluid balance and increased microvascular permeability [8]. Physiological principles regarding the distribution of total body water throughout interstitial spaces [9] suggest that generalised subcutaneous oedema should be associated with and reflect similar changes in organ oedema.
Point-of-care ultrasound has been used to quantify EVLW, in neonates [10] and children following cardiac surgery [11], and is increasingly available to intensive care clinicians [12]. B-lines are vertical hyperechoic ultrasound artefacts generated by air–water acoustic impedance and are a suggested method for quantifying EVLW [13,14,15]. However, they may also represent other pathologies such as atelectasis [16]. Animal data using gravimetric analysis suggest that following an inflammatory stimulus, B-line quantification is associated with EVLW [17, 18]. However, neonatal studies comparing lung ultrasound to thermodilution are lacking and only a modest correlation between chest X-ray evidence of pulmonary congestion and LUS have been reported [19]. Despite this, a greater number of B-lines has been reported to be associated with weight change in children requiring dialysis, [20] and lung weight and function of donor lungs. [21] However, no data exist on children after cardiac surgery.
Accordingly, in this prospective, observational study of infants following cardiac surgery, we aimed to describe the distribution of EVLW, as determined by lung ultrasound and to investigate its relationship with subcutaneous oedema, fluid balance (determined by fluid balance charts), and body weight. We hypothesized that the magnitude of subcutaneous oedema, fluid balance or change in body weight would have a positive relationship with EVLW as expressed by the lung ultrasound score (LUS).
Methods
We performed a prospective, observational study at the Royal Children’s Hospital (RCH) PICU between May 2021 and September 2022. Permission to conduct the study was provided by the RCH Human Research Ethics Committee (HREC number HREC/65005/RCHM-2020), and procedures were followed in accordance with the Helsinki Declaration of 1975.
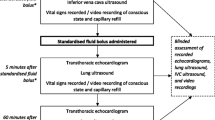
Infants were eligible if they were within 72 h following cardiac surgery, were mechanically ventilated, and were expected to remain so for 48 h. Only those nursed in an Atom Infant warmer cot (Parker Healthcare, AU) were eligible because infants could be accurately weighed using built-in scales [22]. Infants less than 7 kg can be nursed in such cots. We used change in body weight (BW) as a surrogate for change in total body water. Infants were excluded if they were deemed unsafe to weigh, previously participated in the study or had localised oedema as a result of other pathologies, for example, central venous thrombosis or trauma (Supplementary Table 1 for full list of inclusions and exclusions). In addition, infants with anatomical or physiological reasons that could independently cause increased EVLW were excluded such as left ventricular failure, persistent or unrepaired anomalous pulmonary venous drainage or congenital lung anomalies. Following written consent to participate, infants had the following measurements performed at enrolment and two subsequent 24-h periods: weight measurement, daily fluid balance, a clinical oedema score (COS) and lung ultrasound. Data were entered into a case report form and subsequently to a database managed using REDCap electronic data capture tools [23].
Demographic and clinical characteristics were recorded from the PICU database including age, sex, weight, admission diagnosis (ANZPICR diagnostic codes) [24], Risk adjustment for congenital heart surgery (RACHS), Paediatric index of mortality (PIM3) score [25], duration of invasive MV, use of ECMO and renal replacement therapy (RRT), intensive care LOS and intensive care mortality.
Lung ultrasound
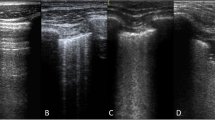
Lung ultrasound (LUS) was performed at 3-time points; baseline (at enrolment), 24 h and 48 h and timed with other measurements. Scans were performed using the Venue™ Ultrasound System (GE Healthcare) with the L8-18i linear array hockey stick, ultrasound probe transducer (5-9 MHz) and a lung pre-set focused at the level of the pleural line. The depth of penetration was standardized to 4–8 cm. Video images were recorded from 4 regions for each hemithorax: zone 1, superior in the mid-clavicular line; zone 2, inferior in the mid clavicular line; zone 3, superior in the mid-axillary line; and zone 4 inferior in the mid axillary line capturing the costo-diaphragmatic angle. A B-line was identified as a continuous echogenic line arising from the pleural margin that obliterated an A-line and extended the length of the image. The most perpendicular intercostal space to the ultrasound beam was chosen and then visualised throughout a respiratory cycle. If more than one intercostal space met these criteria, the space with the greatest number of B-lines was included. The number of B-lines was counted in one intercostal space per image and the lung ultrasound score (LUS) for each region was calculated as follows (Supplementary Fig. 1):
-
0: A-pattern (defined by the presence of A-lines only);
-
1: B-pattern (defined as the presence of 1 or 2 well-spaced B-lines);
-
2: severe B pattern (defined as the presence of ≥ 3 well-spaced B-lines or coalescent B-lines; and.
-
3: extended consolidation is defined as the presence of consolidation limited to the subpleural space or any pleural effusion. (similar to other scoring methodologies) [26].
Ultrasound imaging was performed by BG, SK or VM who were trained by sonographers prior to the study commencement. The LUS scores were attributed by a clinician with expertise in lung ultrasound (AOB) who was blinded to other measurements. A maximum total LUS for each period was 24. If a lung zone was considered not assessable, or not available, the median score for that hemithorax was manually imputed. If more than two segments were not assessable, then the score was not analysed.
Clinical oedema score
At each timepoint, a clinical oedema score (COS) was performed. The technique, inter-observer agreement and validity of the COS have previously been described [27]. In short, the 19-point COS was developed using a composite grading of oedema in six body regions including the periorbital region, upper limb, hand, torso, lower limb and foot. Each region was assessed as having either none (score = 0), mild (1), moderate (2), or severe (3) oedema based on descriptive criteria. The COS is shown in Supplementary Fig. 2. At each timepoint, the COS was performed by the primary investigator (BG) or either of two trained doctors (VM/SK).
Body weight and fluid balance
Changes in body weight (BW) were calculated between baseline and 24 h and, 24 to 48 h. The technique and the precision of BW measurements have been previously described. (cite Gelbart in press—agreement). Fluid balance was calculated as the absolute net difference between fluid input and output timed with BW and oedema measurement. Net fluid input included all intravenous and enteral fluids. Net fluid output included urine, stool, drain and peritoneal dialysis effluent. The absolute change in fluid balance during the observation period was recorded.
Outcomes
The primary outcome was EVLW expressed as the summarised total LUS score. The secondary outcome was the change in LUS between time intervals.
Statistical analysis plan
Demographic and clinical characteristics were described using frequencies and proportions and for continuous data, mean (+ / − standard deviation (SD)) or median and the interquartile range (IQR), if skewed. Firstly, the symmetry of the distribution of LUS was inspected by histogram plots. We described the LUS scores for each lung zone at each timepoint. For the whole cohort, LUS scores were summarised as mean (+ / − SD) or median (IQR) for each zone and as a total daily score and displayed graphically. The lung ultrasound score was explored in a linear mixed effects model to investigate differences in mean scores between lung zones and within lung zones over time. Fixed effects incorporated into the model were the lung zone, the lung (left/right) and time points, along with their interaction terms. Pairwise comparisons of the estimated marginal means between lung zones and between time intervals were performed.
The relationships between the COS and changes in BW, FB and LUS for each child at each time point were visualised using scatterplots. Pearson’s correlation coefficient was calculated for the relationship between the COS and LUS for each time point. Subsequently, the LUS score was explored within a multivariable, mixed effects, linear regression model, with a random intercept accounting for repeated measures within infants. Explanatory variables included the COS, change in BW, and change in FB. Covariates with potential influence incorporated into the model were the RACHS (risk of death as a percentage), duration of cardiopulmonary bypass (hour) and whether peritoneal dialysis was provided during the study period. For changes in FB and BW, the LUS at baseline was incorporated into the model to account for its change over time. Analyses were performed with R software, version 4.1.2 (The R Foundation for Statistical Computing, Vienna, Austria) with the specific packages listed in the Supplemental Digital Content. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist for cohort studies was completed and is found in the Supplemental Digital Content file. A p value < 0.05 was considered statistically significant.
Results
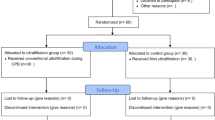
Between May 2021 and September 2022, 53 infants were enrolled following cardiac surgery. The consort diagram is shown in Fig. 1. Declined consent accounted for six infants who were excluded from the analysis and missing ultrasound images accounted for the loss of seven images over the study period. The demographic and clinical characteristics of all infants are shown in Table 1. The median time from surgery to baseline measurements was 26 h [IQR, 23–29]. The median positive end-expiratory pressure (PEEP) at baseline, 24 and 48 h were 5 cmH2O (IQR: 5,6), 5 cmH2O (IQR: 5,6) and 6 cmH2O (IQR: 5,6), respectively. One child was extubated shortly after enrolment, seven on day 2 and nine on day 3.
Patterns of extravascular lung water
At baseline, 53 infants had a lung ultrasound;:52 at 24 h and, 46 at 48 h. Therefore, changes in LUS between baseline and 24 h, and 24 to 48 h, were available for 52 and 46 infants, respectively. The median score was imputed for 7/1272 (0.6%) lung zones. The absolute LUS scores for each timepoint are summarised in Supplementary Table 2, and the distribution of values are visualised in Supplementary Fig. 4. The mean changes in LUS score at each time interval were less than 1 point and are shown in Supplementary Table 3 and their distribution is visualised in Supplementary Figs. 5. In a linear mixed effects model, higher mean LUS scores were found infero-laterally compared to supero-anterior regions (Fig. 2 and Supplementary Figs. 6 and 7). In a similar model, within each independent lung zone, there were no differences in mean LUS between baseline, 24 and 48 h (Fig. 3). The mean LUS by lung zone for all infants during the study period is shown in Supplementary Table 4.
Lung ultrasound score by lung zone across three time points. CI confidence interval: the point estimate represents the mean lung ultrasound score, and the error bars represent the individual 95% CI around the mean. In a linear mixed effects model, there was no statistically significant difference in the pairwise comparison of the estimated marginal means of LUS scores for each zone for either time interval (24 h—baseline; p = 0.15, 48 to 24 h; p = 0.30). There was no evidence of interaction between zone, lung and time (not reported)
Relationship between LUS and the clinical oedema score
The COS at each time point is summarised in Supplementary Table 2. The relationship between total COS and total LUS, for each child at each time point, is shown in Fig. 4. In a multivariable, linear, mixed effects model, accounting for repeated measurements within infants, there was no significant association between the clinical oedema score and the LUS; coefficient 0.2 [95% CI, − 0.01–0.3] (Supplementary Table 5).
Relationship between change in EVLW, body weight and fluid balance
In a multivariable, linear mixed effects model, after accounting for the LUS at baseline, there was no significant association between a change in either BW or fluid balance and a change in LUS (BW coefficient 0.3 [95% CI, − 0.1–0.7], FB coefficient 0.1 [95% CI, − 0.2–0.4] (Supplementary Table 6).
Body weight, fluid balance and clinical oedema scores when no change in LUS occurred
For 15 time intervals in 14 infants, there was no change in LUS between time intervals. The mean change in BW, when no change in LUS, was detected ranged from − 1.4% (SD 3.2) to − 3.0% (SD 2.9). Similarly, the mean change in the COS ranged from 1.6 points (SD 3.3) to − 2.0 points (SD 4.4) (Supplementary Table 7).
Discussion
Key findings
In this prospective study of infants following cardiac surgery, EVLW, as estimated by a LUS, was moderate, with little change across time points. In multivariable, mixed effects, linear regression analyses, there was no significant association between clinically detected oedema and LUS. Similarly, changes in fluid balance or changes in BW were not associated with changes in LUS. Inferolateral lung zones had higher mean LUS compared to supero-anterior lung zones. The changes in LUS between time intervals were small. In a small proportion of time intervals, when no change in LUS was detected, the mean change in BW was as great as 3%, and the mean change in COS was as great as 2 points.
Relationship to previous studies
Lung ultrasound is used to assess EVLW in children in intensive care [10]. However, in children following cardiac surgery, there are few data reporting the validity of LUS as a measure of EVLW. Weak to moderate correlation between LUS and chest X-ray appearance of pulmonary oedema has been reported [19] as well as only moderate correlation between LUS and EVLW, measured by transpulmonary thermodilution [28]. Some studies have reported EVLW, or LUS, to be associated with duration of cardiopulmonary bypass, duration of MV and PICU LOS [11, 19, 29]; however, they are mostly small observational cohort studies [11, 29] with either a single postoperative LUS assessment [29] or no investigation of the effect of fluid balance or oedema on LUS and little consideration of the possibility that B-lines after cardiac surgery may reflect areas of atelectasis. In addition, preoperative factors, rather than fluid balance, have been reported to be associated with post operative LUS [19]. A randomized trial of ultrafiltration during cardiopulmonary bypass on EVLW in children reported LUS changes before and after, but did not investigate fluid removal and changes in LUS [30]. Therefore, limited data exist that investigate the association between clinical measurement of fluid accumulation or fluid loss and estimates of EVLW using LUS.
Interpretation of findings
In this study, increased EVLW, as estimated by a LUS, was evident in all infants in the post operative period. However, only small changes were detected between time intervals. Changes in measurements of subcutaneous oedema or fluid balance charts or body weight were not associated with changes in LUS. Several possibilities may explain these findings. First, factors that contribute to the accumulation of EVLW after cardiac surgery might persist despite changes in total body water. Thus, direct lung inflammation and increased permeability may explain persistent EVLW [31]. Therefore, resolution of EVLW may occur more slowly, align with the recovery from the inflammatory injury, and be dissociated from changes in subcutaneous oedema or fluid balance or body weight. Of note, however, 21% of our cohort did not receive cardiopulmonary bypass. Moreover, it would seem unlikely that an inflammatory lung injury would distribute preferentially in inferolateral lung zones. Second, lung ultrasound scoring systems may be insufficiently specific to detect changes in EVLW and may also reflect the presence of areas of atelectasis, consolidation or changes in pulmonary blood flow. Residual cardiac anomalies (left to right shunts) or left ventricular dysfunction might be responsible for a component of EVLW and shifts in total body water may not have equivalent effects in the lung. Third mean changes in LUS and changes in weight or fluid balance may be too small within two 24-h periods to detect a true difference in this population. Finally, changes in mean airway pressure will influence post operative atelectasis and such changes will impact LUS. In this study, the median PEEP on each day were similar; however, the number that remained ventilated each day decreased.
Strength and limitations
The strength of this study is the prospective, standardized collection of synchronized measurements of tissue oedema, fluid balance, body weight and LUS over three time points in infants following cardiac surgery. We used a LUS based on previously described scoring systems and our images were analysed by a clinician with expertise in lung ultrasound, who was blinded to other measurements. Our study is unique in that the relationship between subcutaneous oedema, and LUS was investigated in addition to fluid balance and body weight. However, we acknowledge some limitations. The study was single-centre and focused on a specific subgroup of children, thus limiting the generalizability of these findings to the broader population of critically ill children. We did not account for infants with residual left to right shunts, changes in mean airway pressure or left ventricular function, which may be confounding factors for the appearance of B-lines. We used lung ultrasound and specifically, the number of B-lines to estimate EVLW. Several limitations apply to this technique. B-lines may be non-specific in the detection of EVLW and can reflect atelectasis as well as lung water [32]. Moreover, quantifying B-lines is reported to have only moderate correlation when compared to thermodilution techniques [28]. Lung ultrasound scores were based on an arbitrary ordinal scale based on the number of B-lines. However, we used a previously reported scoring system that accounted for the number and coalescence of B-lines. Only four zones in each lung were analysed to estimate EVLW and therefore may underestimate total EVLW, particularly from posterior regions. However, we selected zones from supero-anterior to inferolateral segments of the lung to account for the potential wide distribution of LUS. Inter-observer variability was not investigated. However, clinicians performing lung ultrasound undertook focused training from a sonographer and a clinician with expertise in ultrasound prior to the commencement of the study. Many confounding factors could contribute to increased EVLW or their detection by quantifying B-lines. However, we accounted for important, potentially influential covariates such as the duration of cardiopulmonary bypass, RACHS and whether peritoneal dialysis was administered during the study period. Moreover, we were pragmatic about excluding infants with left ventricular failure or anatomical reasons for increased EVLW.
Conclusions
Infants following cardiac surgery were found to have a moderate amount of EVLW as determined by lung ultrasound. However, oedema determined by clinicians, changes in body weight or fluid balance was not associated with the degree of lung water as estimated by a LUS. The LUS was greater in inferolateral compared to supero-anterior lung regions and changes across three 24-h time points were small. These findings suggest that either lung water is not influenced by changes in total body water or that lung ultrasound may be insufficiently specific for detecting EVLW.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request at the discretion of the corresponding author.
References
Sampaio TZ, O’Hearn K, Reddy D, Menon K (2015) The influence of fluid overload on the length of mechanical ventilation in pediatric congenital heart surgery. Pediatr Cardiol 36(8):1692–9. https://doi.org/10.1007/s00246-015-1219-0
Lex DJ, Tóth R, Czobor NR et al (2016) Fluid overload is associated with higher mortality and morbidity in pediatric patients undergoing cardiac surgery*. Pediatr Crit Care Med 17:307–314
Hassinger AB, Wald EL, Goodman DM (2014) Early postoperative fluid overload precedes acute kidney injury and is associated with higher morbidity in pediatric cardiac surgery patients. Pediatr Crit Care Med 15:131–138
Gelbart B, Serpa Neto A, Stephens D et al (2022) Fluid accumulation in mechanically ventilated, critically ill children: retrospective cohort study of prevalence and outcome. Pediatr Crit Care Med 23:990–998
Hirleman E, Larson D (2008) Cardiopulmonary bypass and edema: physiology and pathophysiology. Perfusion 23:311–322
Stiller B, Sonntag J, Dahnert I et al (2001) Capillary leak syndrome in children who undergo cardiopulmonary bypass: clinical outcome in comparison with complement activation and C1 inhibitor. Intensive Care Med 27:193–200
Maehara T, Novak I, Wyse RK et al (1991) Perioperative monitoring of total body water by bio-electrical impedance in children undergoing open heart surgery. Eur J Cardiothorac Surg 5:258–264 (discussion 265)
Kottke MA, Walters TJ (2016) Where’s the leak in vascular barriers? A review Shock 46:20–36
Levick JR, Michel CC (2010) Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res 87:198–210
Lichtenstein AD, Mauriat P (2012) Lung ultrasound in the critically Ill neonate. Curr Pediatr Rev 8:217–223
Girona-Alarcón M, Cuaresma-González A, Rodríguez-Fanjul J et al (2022) LUCAS (lung ultrasonography in cardiac surgery) score to monitor pulmonary edema after congenital cardiac surgery in children. J Matern Fetal Neonatal Med 35:1213–1218
Lovrenski J (2020) Pediatric lung ultrasound -- pros and potentials. Pediatr Radiol (3):306. https://doi.org/10.1007/s00247-019-04525-y. https://search.ebscohost.com/login.aspx?direct=true&AuthType=sso&db=edsgao&AN=edsgcl.628500714&site=eds-live&scope=site&custid=s2775460
Lichtenstein DA (2009) Ultrasound examination of the lungs in the intensive care unit. Pediatr Crit Care Med 10:693–698
Lichtenstein D, Mézière G, Biderman P et al (1997) The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med 156:1640–1646
Grune J, Beyhoff N, Hegemann N et al (2020) From bedside to bench: lung ultrasound for the assessment of pulmonary edema in animal models. Cell Tissue Res 380:379–392
Volpicelli G, Elbarbary M, Blaivas M et al (2012) International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 38:577–591
Jambrik Z, Gargani L, Adamicza A et al (2010) B-lines quantify the lung water content: a lung ultrasound versus lung gravimetry study in acute lung injury. Ultrasound Med Biol 36:2004–2010
Ma H, Huang D, Zhang M et al (2015) Lung ultrasound is a reliable method for evaluating extravascular lung water volume in rodents. BMC Anesthesiol 15:162
Kaskinen AK, Martelius L, Kirjavainen T et al (2017) Assessment of extravascular lung water by ultrasound after congenital cardiac surgery. Pediatr Pulmonol 52:345–352
Allinovi M, Saleem M, Romagnani P et al (2017) Lung ultrasound: a novel technique for detecting fluid overload in children on dialysis. Nephrol Dialysis Transpl 32:541–547
Ayyat KS, Okamoto T, Niikawa H et al (2019) DireCt lung ultrasound evaluation (CLUE): a novel technique for monitoring extravascular lung water in donor lungs. J Heart Lung Transplant 38:757–766
Gelbart B, Masterson K, Serratore A et al (2021) Precision of weight measurement in critically ill infants: a technical report. Crit Care Resusc 23:414–417
Harris PA, Taylor R, Minor BL et al (2019) The REDCap consortium: building an international community of software platform partners. J Biomed Inform 95:103208
Australian and New Zealand Paediatric Intensive Care Registry https://www.anzics.com.au/wp-content/uploads/2018/08/ANZPICR-Data-Dictionary.pdf. Accessed 1 Oct 2022
Straney L, Clements A, Parslow RC et al (2013) Paediatric index of mortality 3: an updated model for predicting mortality in pediatric intensive care*. Pediatr Crit Care Med 14:673–681
Brat R, Yousef N, Klifa R et al (2015) Lung ultrasonography score to evaluate oxygenation and surfactant need in neonates treated with continuous positive airway pressure. Jama, Pediatr 169:e151797–e151797
Gelbart B, Kapalavai SK, Marchesini V et al (2023) A clinical score for quantifying edema in mechanically ventilated children with congenital heart disease in intensive care. Critical Care Explorations 5:e0924
Niyogi SG, Kumar B, Puri GD et al (2022) Utility of lung ultrasound in the estimation of extravascular lung water in a pediatric population—a prospective observational study. J Cardiothorac Vasc Anesth 36:2385–2392
Vitale V, Ricci Z, Gaddi S et al (2017) Lung ultrasound profile after cardiopulmonary bypass in paediatric cardiac surgery: first experience in a simple cohort. Interact Cardiovasc Thorac Surg 24:598–602
Elayashy M, Madkour MA, Mahmoud AAA et al (2019) Effect of ultrafiltration on extravascular lung water assessed by lung ultrasound in children undergoing cardiac surgery: a randomized prospective study. BMC Anesthesiol 19:93
Bronicki RA, Hall M (2016) Cardiopulmonary bypass-induced inflammatory response: pathophysiology and treatment. Pediatr Crit Care Med 17:S272–S278
Volpicelli G, Skurzak S, Boero E et al (2014) Lung ultrasound predicts well extravascular lung water but is of limited usefulness in the prediction of wedge pressure. Anesthesiology 121:320–327
Acknowledgements
The authors acknowledge senior sonographer Tania Griffiths, Department of Radiology, Royal Children’s Hospital, for sharing her expertise and for providing training in lung ultrasound and Cameron Patrick from the Department of Mathematics and Statistics, University of Melbourne, for statistical advice.
Code availability
Custom code.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
The study was initially conceived and designed by BG. All authors contributed to the study conception and design. Material preparation and data collection were performed by BG, AV, AS, JA, VM, and SKK. AO’B measured lung ultrasound scores. Analyses were performed by BG. The first draft of the manuscript was written by BG, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Inclusion and Exclusion criteria. Supplementary Figure 1. Lung Ultrasound Scoring tool. Supplementary Figure 2. The clinical edema scoring tool. Supplementary Table 2. Summarised lung ultrasound score and clinical edema score at each time point. Supplementary Table 3. Summarised changes in lung ultrasound score, body weight, clinical edema scores, and fluid balance at each time interval. Supplementary Figure 4. Distribution of Lung Ultrasound Scores at Baseline, 24 hours and 48 hours. Supplementary Figure 5. Distribution of the change in Lung Ultrasound Scores between baseline and 24 hours and 24 and 48 hours. Supplementary Figure 6. Comparison of the lung ultrasound score for each lung zone at baseline, 24 and 48 hours. Supplementary Figure 7. Lung Ultrasound Score for each lung zone within each lung at all time points. Supplementary Table 4. Mean lung ultrasound scores for each lung zone over the study period (n = 302 lung zones). Supplementary Table 5. Linear mixed effects regression analysis for the association between a clinical oedema score and lung ultrasound score. Supplementary Table 6. Mixed linear effects regression analysis for the association between changes in body weight, fluid balance and lung ultrasound score. Supplementary Table 7. Change in the clinical edema score, body weight and fluid balance when there was no change in lung ultrasound score (n = 15 time intervals)
Additional file 2.
STROBE Statement—Checklist of items that should be included in reports of cohort studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gelbart, B., Marchesini, V., Kapalavai, S.K. et al. Lung ultrasound scores are not associated with subcutaneous oedema or fluid balance in infants after cardiac surgery. Intensive Care Med. Paediatr. Neonatal 1, 21 (2023). https://doi.org/10.1007/s44253-023-00020-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44253-023-00020-3