Abstract
Sericin is a highly hydrophilic macromolecular protein comprising 18 amino acids. It is considered biocompatible, nontoxic, and has antibacterial and antioxidant properties. It is applied on fabric, however, either the fabric needs to be pretreated with electrolytes or catalyst are used thereby increasing the number of processing steps. This study achieved sericin with multifunctional properties through chemical modification using cyanuric chloride. Modified sericin was applied in the dyeing stage along with the dyes on various textile substrates like wool, silk, and polyester. The functional properties were characterized by Fourier transform infrared and in modified sericin new chlorine peak at 779 cm−1 was obtained, X-ray diffractogram shows increase in crystallinity after modification of sericin, SEM showed particles of sericin on all fabrics even after 5 washes. Fabrics were analysed for antimicrobial activity and showed antimicrobial properties against gram negative and positive bacteria. Fastness properties and ultra violet protection factor of the samples were also determined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Silkworm cocoons are natural polymer fiber composites made from silk fibers and sericin binder. Raw silk generally contains around 70–75% fibroin and 30–20% sericin, which vary depending on the type of silkworm, feeding conditions, and the surrounding environment [1]. To manufacture lustrous silk from the dried cocoons of silkworms, fibroin is separated from sericin, the other major component of the cocoon, by a degumming process, and the sericin is primarily discarded in the wastewater. For a long time, sericin has been disregarded in sericulture. It is estimated that 400,000 tons of dry cocoons produce about 50,000 tons of sericin worldwide [2]. A large amount of silk sericin goes into waste from the silk industry and increases the pollution load in the industrial effluent due to increased chemical oxygen demand (COD). Particularly in nations where sericulture is prevalent, like China, India, and Brazil, sericin's removal and usage might significantly influence the economy, society, and ecology [3].
There are several methods to remove sericin in the so-called degumming process of cocoons. Extraction of silk using acid and alkali may damage the fibroin part and also are hazardous chemicals. Degumming by heat or heat under pressure has an advantage as there are no impurities, and also it is eco-friendly. Numerous researchers have reported several valuable characteristics of sericin, such as good moisture absorption and dispensation properties [4], UV resistance [5], anticoagulant [6], antioxidant, antibacterial activity [7], and inhibitory action of tyrosinase [8].
With the increased attention on environmental aspects in all walks of life, textile colourist is looking for natural biodegradable polymers, which helps impart other functional properties and increase dye uptake. Many researchers have reported using chitosan and sericin to enhance dye uptake value [5, 9]. Research has been carried out to apply sericin on jute fabric along with trisodium citrate as an esterification catalyst to improve its dyeing parameters [10]. Sericin has been applied on PET, wool, and cotton fabric using various methods for improving dye uptake. However, it does not have a direct affinity for cotton fabric [7]. Also, for applying it on wool fabric, either a crosslinker is used, or the fabric is pre-treated with sericin in the presence of an electrolyte, increasing the number of steps and chemicals [1].
The main objective of the research work is to give a multifunctional finish to the textile fabrics under the action of modified sericin. The sericin was extracted from Eri. It was modified using cyanuric chloride to introduce Cl atom on the sericin polymer, which will improve shade depth and impart antimicrobial properties and good wash fastness. Since sericin was extracted from waste silk cocoons, it will be cost-effective to produce while also reducing the effluent load produced by the silk industry.
2 Materials and methods
2.1 Materials
Waste silk cocoons were supplied by an Indian Institute of Handloom technology Assam, India. The fabric of silk, polyester, and wool was procured from Tata Mills, Mumbai. The Epi and Ppi of polyester fabric was 134 and 132 respectively having 160 GSM. The GSM of silk and wool were around 120 and 230 respectively. Acid dye CBSOL BLUE L-3G was procured from Colorband dyestuff (P) Ltd. Coralene Red disperse dye was obtained from Colourtex Industries Pvt. Ltd. Levelling agent, Saragen KDF and dispersing agent, Saragen SO were kindly provided by Sarex Chemicals India. Petroleum ether, sodium carbonate, sodium hydroxide, ethanol, methanol, chloroform, dimethyl sulfoxide (DMS), Dimethyl Formamide (DMF), sodium sulfate, tri-sodium citrate, and hydrochloric acid were purchased from SD Fine chemicals limited.
3 Methods
3.1 Extraction of sericin powder
The manually cleaned cocoon shells were cut into small pieces for sericin extraction. The aqueous sericin extraction was carried out in an autoclave at 120℃ for 2 h. The obtained solution containing sericin was stored in a deep freezer (Eltek Equipment Pvt Ltd, Mumbai) at -20℃ for 24 h and then lyophilized for 48 h (BioEra Life Sciences Pvt Ltd). The resulting fine powder was used for modification.
3.2 Modification of sericin
2 gm of sericin and 2 gm of cyanuric chloride were added in a mixture of 8 ml of DMF and 60 ml of DCM in a round bottom flask. It was stirred at room temperature for 8 to 10 h [11]. After the reaction the Sericin chloride (modified sericin) gets precipitated out which is then filtered to get the crude brown powder. It was purified by multiple washing with ethanol, acetone, and ether. Purified sericin powder was used for further application.
3.3 Application of modified sericin
To investigate the multifunctional finishing property of modified sericin. It was applied at the dyeing stage along with the dye on various textile substrates.
For the dyeing of wool and silk with acid dye, an MLR (material-to-liquor ratio) of 1:30 and1% shade was selected. Dyeing was done in a rota dyeing machine at 90℃ for 45 m in presence of 1% ammonium acetate. Four samples each of silk and wool were taken and named as S1, S2, S3, and S4, and W1, W2, W3, and W4 respectively. S1 and W1 underwent the standard acid dyeing process without using modified sericin. S2, S3, S4, W2, W3 and W4 were dyed with the same dyeing procedure using 0.5%, 1% and 2% modified sericin in the same dye bath.
For disperse dyeing, MLR was maintained at 1:30, and a dyeing shade of 2%, 0.5 gpl Saragen SO, 0.5 gpl Saragen KDF, 1 gpl ammonium sulfate, and modified sericin in varying concentration as that for silk and wool was employed. Fabrics were dyed in HTHP (High temperature high pressure) machine at 130℃ for 45 min. Formic acid and ammonium sulfate are added to the dye bath to maintain pH 5–6. The unfixed disperse dye after dyeing was removed from the fabric by subjecting the dyed fabric to 2gpl of caustic soda, 2gpl of sodium hydrosulfite and 2 gpl soap at 700C.
4 Characterization
4.1 Fourier transform infrared (FTIR)
FT-IR analysis of the sericin, modified sericin, and dyed fabrics was carried out with an FTIR-8400S (Shimadzu Corporation, Japan). The IR spectra were recorded using a pike miracle ATR module with diamond/ZnSe crystal with 45 consecutive scans ranging from 650 to 4000 cm−1 at a 4 cm−1 resolution.
4.2 X-ray diffraction (XRD) analysis
The XRD pattern of sericin, modified sericin powder was tested using an X-ray diffractometer, XRD-6100 (Shimadzu, Japan), having Cu-Kα with λ = 0.15406 nm in the range of 5° to 40° at a scanning rate of 2°/min.
4.3 Color strength evaluation of dyed fabric
The dyed samples were evaluated for color strength using a computer color matching system (SpectraScan 5100 + , Premier Colourscan Instrument Pvt. Ltd.) 5 scans of each sample were taken at different positions. The color strength of dyed samples was measured in the Kubelka Munk function and expressed as K/S values at maximum wavelength.
4.4 Fastness properties
Wash Fastness of the dyed samples was carried out as per ISO 105-C06 wherein the dyed fabrics were treated with commercial laundering at 30 °C for 30 min. Light Fastness Tester GT-D02A-1 was used to determine the light fastness properties of the samples as per standard ISO 105-B02 using artificial light i.e. Xenon arc [12].
4.5 Scanning electron microscopy (SEM)
The surface morphology of dyed fabrics was studied using SEM analysis XL 30 (Philips, The Netherlands). Prior to SEM testing, the samples were sputter coated with gold to dissipate the static charges occurring due to electron bombardment with an accelerated electrons having 12 kV energy.
4.6 Antimicrobial test
Evaluation of the antimicrobial activity of the sericin, modified sericin, and modified sericin treated textile substrates was studied according to AATCC 147 standard method using S.aureus and K. pneumoniae using parallel streak test.
4.7 UV-protection
“UV spectrophotometer (UV-2600, Shimadzu, Japan)” with an integrated sphere load at an interval of 5 nm in the region of 290 to 400 nm, was used for the determination of UPF of the fabrics (Test method: AS/NZ 4399). The % blockage of UV-A and UV-B, ranging from 315—400 nm and 280—315 nm, respectively, [13].
5 Result and discussion
5.1 Structural modification of the sericin
In general; hydroxyl group (OH) of alkyl alcohols gets substituted with chlorine (Cl) when treated with haloarenes in presence of the suitable solvents [11]. Sericin was modified accordingly which involves substitution of one of its OH group with the Cl of the cyanuric chloride as shown in the Fig. 1. There are some other functional groups like COOH, NH2, and OH are also present in the sericin. These groups can react with the cyanuric chloride (Cy-Cl) in the mentioned (Fig. 1) manner and can yield amide (1), ester (2), and anhydride (3) including reaction intermediates 1’, 2’, and 3’. These above mentioned product formed are as per the literature [14]. In this structural modification of sericin; cyanuric chloride is used as a limiting reagent so it will not remain unreacted after completion of reaction to nullify any possible health issues. It is shown in the mechanism that end products of the targeted reaction are Cy-OH and Sericn-Cl where Cy-OH is water soluble and can be easily washed out during post treatment of the application.
5.2 FTIR
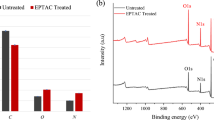
The IR spectrum of the extracted and modified sericin are presented in Fig. 2. The peptide group of proteins gives rise to eight characteristic bands called amides A, I, II, III, IV, V, VI, and VII [15]. Among these, amide I is the strongest absorption band of the protein. The intensity of the amide A band is mainly due to the N–H stretching vibration. Amide I is mainly controlled by stretching vibration of C = O (70–85%) and C-N group (10–20%). Its frequency is initiated in the range of 1600–1700 cm-1. Amide II originates in the region 1510–1580 cm-1 and is more intricate than amide I. It derives mainly from in-plane N–H bending. The remaining potential energy ascends from the C-N and C–C stretching vibrations [15, 16].
The spectra of extracted and modified sericin were nearly similar. The obtained spectra of modified sericin confirmed the introduction of the Cl group by the appearance of a new peak at 779 cm−1 after the modification reaction [17].
5.3 XRD Analysis
Figure 3 shows the XRD patterns of sericin and modified sericin treated with cyanuric chloride and shows the changes in crystallinity and amorphous region after modification with cyanuric chloride. The diffractogram of sericin showed lower crystallinity (11.77%) than the modified sericin film (14.03%). The slight increase in crystallinity can be attributed to the crystallization characterization of sericin on dissolution and regeneration at lower temperatures [18].
The X-ray diffraction (XRD) analysis of sericin extracted from cocoon shells shows a broad diffraction peak at 2θ = 19 (Fig. 3) [19] Previous studies by Silva et al. and Jo & Um reported the highest diffraction peak at 2θ = 19.2. This peak suggests the transformation of the random coil structure into a β-sheet structure through intermolecular hydrogen bonding between the hydroxyl groups of the amino acids present in sericin [20, 21]. This transformation shows the structural transformation of sericin powder obtained from degumming and freeze-drying processes.
Due to sericin modification by cyanuric chloride, the modified sericin powder showed additional narrow diffraction peaks at 20, 26, and 29°. The diffraction intensity of the modified samples on XRD curves increases, which indicates that the content of β -structure increases to some extent. This confirmed that prepared modified sericin possessed a crystalline structure.
5.4 Color strength evaluation of dyed fabric
The K/S value of the dyed samples, and their optical image, are shwon in Table 1. It can be observed that as compared to dyeing without modified sericin, the depth, i.e., K/S value, has increased with the increase in the concentration of modified sericin from 0.5% to 2%. The higher K/S value indicated that the exhaustion rate rises with increased concentration of modified sericin. The increase in depth for wool and silk might be due to an increase in positively charged amine groups due to the bonded sericin present on the fabric which has greater affinity for dyes than silk fibre [1]. For polyester, it could be due to the enhancement in the affinity imparted by the introduction of Cl group in the modified sericin with the dye as well as with the fabric [22].
5.5 Fastness properties of dyed fabric
The result of various fastness properties like wash and light fastness are mentioned in Table 2. In the case of wash fastness, there was no staining on adjacent fabric in silk and polyester, and both showed excellent wash fastness, whereas, in wool, the results are good with little tinting and slight shade variation. Similar results were obtained for light fastness also.
5.6 SEM analysis
Figure 4 shows the photographs of modified sericin-treated silk, wool, and polyester fabrics. As is seen from the micrographs, the application of modified sericin onto silk, wool, and polyester fiber affected the surface morphology of fiber. The treated silk fibre revealed fine coating and micro-particles of modified sericin resulting in the rougher surface (Fig. 4a and d). Similar observations were made for the wool and polyester fabrics.
The increased surface roughness and the introduction of polar groups such as hydroxyl and amide group facilitated physical adsorption and chemical bonding of the dyes on the sericin modified silk, wool and polyester fabrics.
5.7 Antimicrobial test
The antibacterial test for the dyed as well as extracted and modified sericin is shown in Fig. 5. The figure shows the sample after 24 h incubation with gram positive and gram-negative bacteria. The modified sericin powder, as well as fabrics dyed using modified sericin showed antimicrobial activity. The zone of inhibition for modified sericin for gram-positive and negative bacteria was 0.7 mm and 0.9 mm, respectively (a,b). Silk fabric showed a 1 mm zone of inhibition for both gram-positive and negative bacteria. Both wool and polyester fabrics showed a 1 mm zone of inhibition for gram-negative bacteria; however, for gram-positive, no zone of inhibition was seen, but there was no growth below the specimen.
5.8 Ultra violet protection factor
The Ultra protection factor (UPF) of the dyed fabrics is mentioned in Table 3. With the increased modified sericin concentration, the UPF value of treated fabrics has increased. This might be because as sericin concentration increases, the depth of shade has also increased, resulting in higher K/S values. Aravin reported a similar observation in which UPF increases with increasing shade depth [23]. Furthermore, UPF values are affected by various fabric construction factors such as pores, thickness, and weight, as well as processing parameters such as dyeing and finishing.
6 Conclusion
We have successfully modified sericin using cyanuric chloride. The modified sericin was applied to the fabric without using any mordant or chemical during the dyeing process. The fabric shows an increase in the depth of the shade. The wash fastness to colour was also good and light fastness values were also excellent even after 10 washes. There was an increase in the UPF value of all dyed fabrics due to the incorporation of sericin. The fabrics showed antimicrobial activity against gram-positive and negative bacteria. The modified sericin acted as a dye-exhausting agent for dyeing wool and silk using acid dye and polyester using disperse dye simultaneously imparting other functional properties.
Availability of data and materials
Data will be made available on request.
Change history
30 May 2024
A Correction to this paper has been published: https://doi.org/10.1007/s44251-024-00043-8
References
Eser CO, Yavas A (2021) Effect of silk sericin pre-treatment on dyeability of woollen fabric. Ind Textila 72(2):203–209. https://doi.org/10.35530/IT.072.02.1771
Aramwit P, Siritientong T, Srichana T (2012) Potential applications of silk sericin, a natural protein from textile industry by-products. Waste Manag Res 30(3):217–224. https://doi.org/10.1177/0734242X11404733
Kamalraj D, Subramaniam V (2015) Application of Sericin on Polyester Fabric. Appl Mech Mater 813–814:156–160. https://doi.org/10.4028/WWW.SCIENTIFIC.NET/AMM.813-814.156
Kunz RI, Brancalhão RMC, de Ribeiro FCL, Natali MRM (2016) Silkworm Sericin Properties and Biomedical Applications. Biomed Res Int 2016:1–19. https://doi.org/10.1155/2016/8175701
Chaudhary H, Gupta D, Gupta C (2017) Multifunctional dyeing and finishing of polyester with Sericin and Basic dyes. J Text Inst 108(3):314–324. https://doi.org/10.1080/00405000.2016.1165401
Tamada Y, Sano M, Niwa K, Imai T, Yoshino G (2004) Sulfation of silk sericin and anticoagulant activity of sulfated sericin. J Biomater Sci Polym Ed 15(8):971–980. https://doi.org/10.1163/1568562041526469
BelhajKhalifa I, Ladhari N, Touay M (2012) Application of sericin to modify textile supports. J Text Inst 103(4):370–377. https://doi.org/10.1080/00405000.2011.580539
Aramwit P, Damrongsakkul S, Kanokpanont S, Srichana T (2010) Properties and antityrosinase activity of sericin from various extraction methods. Biotechnol Appl Biochem 55(2):91–98. https://doi.org/10.1042/ba20090186
A. R. Shirvan, M. Shakeri, and A. Bashari, “Recent advances in application of chitosan and its derivatives in functional finishing of textiles,” Impact Prospect. Green Chem. Text. Technol., 2019 107–133, https://doi.org/10.1016/B978-0-08-102491-1.00005-8.
Das Debasish, Bakshi Sumantra, and Bhattacharya Pinaki, “Modification of jute with sericin for improvement in dyeing,” Int. J. Latest Trends Eng. Technol., vol. 12, no. 2, 2018, doi: https://doi.org/10.21172/1.122.02.
De Luca L, Giacomelli G, Porcheddu A (2002) An efficient route to alkyl chlorides from alcohols using the complex TCT/DMF. Org Lett 4(4):553–555. https://doi.org/10.1021/ol017168p
Jung JS, Kim SH (2019) Application of smectite for textile dyeing and fastness improvement. RSC Adv 9(63):36631–36639. https://doi.org/10.1039/C9RA05768D
Kale RD, Gorade VG, Madye N, Chaudhary B, Bangde PS, Dandekar PP (2018) Preparation and characterization of biocomposite packaging film from poly(lactic acid) and acylated microcrystalline cellulose using rice bran oil. Int J Biol Macromol 118:1090–1102. https://doi.org/10.1016/j.ijbiomac.2018.06.076
G. Giacomelli and A. Porcheddu, “1,3,5-Triazines,” in Comprehensive Heterocyclic Chemistry III, Elsevier, 2008, pp. 197–290. doi: https://doi.org/10.1016/B978-008044992-0.00803-8
Gulrajani ML, Brahma KP, Kumar PS, Purwar R (2008) Application of silk sericin to polyester fabric. J Appl Polym Sci 109(1):314–321. https://doi.org/10.1002/app.28061
Kale RD, Gorade VG, Parmaj O (2020) Novel Sericin/Viscose Rayon-Based Biocomposite: Preparation and Characterization. J Nat Fibers 17(4):532–541. https://doi.org/10.1080/15440478.2018.1503131
“IR Spectrum Table.” https://www.sigmaaldrich.com/IN/en/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (accessed Jan. 18, 2023).
Lee HG, Jang MJ, Park BD, Um IC (2023) Structural Characteristics and Properties of Redissolved Silk Sericin. Polymers (Basel) 15(16):3405. https://doi.org/10.3390/polym15163405
Tao W, Li M, Xie R (2005) Preparation and Structure of Porous Silk Sericin Materials. Macromol Mater Eng 290(3):188–194. https://doi.org/10.1002/mame.200400306
Jo YN, Um IC (2015) Effects of solvent on the solution properties, structural characteristics and properties of silk sericin. Int J Biol Macromol 78:287–295. https://doi.org/10.1016/j.ijbiomac.2015.04.004
Silva VR, Hamerski F, Weschenfelder TA, Ribani M, Gimenes ML, Scheer AP (2016) Equilibrium, kinetic, and thermodynamic studies on the biosorption of Bordeaux S dye by sericin powder derived from cocoons of the silkworm Bombyx mori. Desalination Water Treat 57(11):5119–5129. https://doi.org/10.1080/19443994.2014.996776
Lin FY, Mackerell AD (2017) Do Halogen-Hydrogen Bond Donor Interactions Dominate the Favorable Contribution of Halogens to Ligand-Protein Binding? J Phys Chem B 121(28):6813–6821. https://doi.org/10.1021/acs.jpcb.7b04198
A. P. Periyasamy, “Natural dyeing of cellulose fibers using syzygium cumini fruit extracts and a bio-mordant: A step toward sustainable dyeing,” Sustain. Mater. Technol., vol. 33, Sep. 2022, doi: https://doi.org/10.1016/j.susmat.2022.e00472.
Author information
Authors and Affiliations
Contributions
Jasam Pattanaik: methodology, data collection, draft manuscript preparation; Babita U. Chaudhary; data analysis, interpretation of results, manuscript preparation, review and final manuscript submission; Zahir Ali Siddiqui: study conceptualization, manuscript preparation; Shristi Tewari: final manuscript preparation, Rohan Meshram: Interpretation of result and review of final manuscript; Ravindra D. Kale: Supervision, study design and conceptualization, final manuscript review. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The Authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The first author's family name has been corrected.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pattanaik, J., Chaudhary, B., Siddiqui, Z.A. et al. Modification of sericin using cyanuric chloride and its application on the textile substrate to impart multifunctional properties. Surf. Sci. Tech. 2, 12 (2024). https://doi.org/10.1007/s44251-024-00041-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44251-024-00041-w