Abstract
Environmental Barrier Coatings (EBCs) are used to protect silicon carbide-based composites in the high-temperature steam combustion environment of the latest generation of high-efficiency gas turbine aero-engines. The Yb2SiO5/BSAS/Si coatings with gradient coefficient of thermal expansion were prepared using atmospheric plasma spraying (APS) method on the carbon fiber reinforced silicon carbide ceramic matrix composites (Cf/SiC) composites surface. The coating preparation process parameters were optimized by evaluating the influence of spraying power on the bonding strength, deposition efficiency, and porosity. The corrosion behaviors and mechanism of the optimized Yb2SiO5/BSAS/Si coating was investigated at 1200 ℃ by thermal cycle test in steam conditions. The results indicated that the decomposition of Yb2SiO5 and the conversion of SiO2 to Si(OH)4 were the main corrosion reactions of Yb2SiO5 top coating. Noticeably, the SiO2 component acted as an obvious sign of the reaction front diffusing, forming the clear SiO2-gradient layers. The gradient Yb2SiO5/BSAS/Si coating remained intact after thermal cycles for 500 h at 1200 ℃ in steam conditions which effectively enhanced the resistance of Cf/SiC composites to high temperature steam corrosion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
TBCs-coated superalloys have reached the limit of their temperature capability and could not meet the high thrust-to-weight ratio requirements of advanced aero-engines [1,2,3]. Carbon fiber reinforced silicon carbide ceramic matrix composites (Cf/SiC) were regarded as one of the ideal thermal structural materials to replace superalloys, because of its high temperature resistance, low density and high strength [4, 5]. However, the formed silica protective film on the surface of Cf/SiC reacted with water vapor to form volatile silica hydroxide substances in the combustion environment, which eventuated surface degradation [6, 7]. Therefore, environmental barrier coatings (EBC) were used on the surface of Cf/SiC composites to provide thermal protection and to ensure the reliability and stability of the hot-section structural components in service [8,9,10].
Over the past three decades, the EBCs systems had undergone three generations broadly [11]. The first generation was mullite/yttria-stabilized zirconia (mullite/YSZ) system, The first generation was mullite/yttria-stabilized-zirconia (mullite/YSZ) system, Unfortunately, the mismatch CTE of YSZ and mullite resulted rapid failure of coating through cracking and delamination [10]. The alkaline-earth aluminosilicate (BSAS, 1-xBaO-xSrO-Al2O3-2SiO2, 0 < x < 1) was employed as the top coat to reduce the CTE mismatch and the Si bond coat was interposed to upgrade the adhesion strength with Cf/SiC. Nevertheless, the practical application of BSAS was limited by its durability issues [12, 13]. One of the most promising materials for EBCs now was rare-earth silicates as third-generation materials, including rare-earth monosilicates (RE2SiO5, REMS) and rare-earth disilicates (RE2Si2O7, REDS) [14,15,16,17,18]. Notably, YbMS exhibited good chemical durability with inert corrosion in combustion environments [17, 19]. Moreover, structures of EBCs has gradually evolved from single-layer to multi-layer or gradient [18, 20]. A necessary condition for an ideal coating system was a good match of thermophysical properties between the coating and the substrate as well as the multilayer coating [18]. The thermal expansion coefficient of Si bonded coating is 3.5–4.5 × 10–6 K−1 [14]. Mullite with thermal expansion coefficient of 5–6 × 10–6 K−1 and BSAS with thermal expansion coefficient of 4–5 × 10–6 K−1 [21] can be used as the intermediate layer, and Yb2SiO5 with thermal expansion coefficient of 6.9–7.6 × 10–6 K−1 [15, 18] can be used as the top material to form a three-layer gradient coating structure.
According to the extreme service environment of EBCs, steam and molten material corrosion has been identified as the main challenges faced by EBC and numerous scholars were committed to studying corrosion mechanisms and influencing factors of steam and melt [11, 18, 22,23,24,25,26,27]. The EBCs achieved protection against steam flowing at high temperatures by forming an airtight layer pattern that reduces the penetration of oxidizing agents into the substrate underneath it [11]. Wang et al. [28] reported the morphological characteristics of the three-layer coatings Si/mullite/Yb2SiO5 coating on the SiCf/SiC ceramic matrix composite (CMC) after water vapor corrosion at 1300 ℃ for 200 h. The Yb2SiO5 layer exhibited a gradient porous structure, which was mainly attributed to the inclusion of gasification precipitated rich SiO2 layer and the presence of a small amount of Yb2O3 alone inside the coating. The main corrosion of rare earth silicate surface materials in steam environments was derived from the volatilization of SiO2 [29]. The YbDS coatings reported by Richards et al. [30,31,32] underwent transformation from YbDS to YbMS in steam environment, while the phase transition was accompanied by changes in CTE, allowing subsequent thermal stresses induced by cyclic testing, vertical cracking, and promotion of oxidants into the silicon bond coating. Bakan et al. [22] investigated that porous YbMS reaction scale was formed on YbMS-rich coating surface attributed to very large grain boundary area and low diffusion activity at 1200 ℃, which was not conducive to the formation of large YbMS grains and crack healing. Similarly, Richards et al. [13] reported that YbMS layer was formed on the surface of a YbDS-rich coating at 1316 ℃ with a steam velocity of 4.4 cm/s, however, the continuous YbMS layer was not discovered on the surface of the YbDS rich coating. H. Ou et al. [23] identified that a bilayer structure including a thermally grown oxidized TGO layer with a diffusion layer (YbDS) was formed at the interface, derived from the oxidation by an antioxidant layer (MoSi2) and from the reaction of SiO2 with YbMS, respectively. The growth of the TGO layer was in accordance with the parabolic law, and the inward diffusion of Yb2O3 in the coatings left micropores near the interface. However, the surface of the YbDS layer with a small amount of YbMS impurities was demonstrated that the volatilization of the gaseous product Si(OH)4 not only occurred but also the evaporation of Yb(OH)3, leading to the formation of pores in YbDS, which accelerated the penetration and diffusion of oxidants [33]. In addition, N. Al Nasiri et al. [34] supposed that the formation of RE-disilicate and the solubility of RE cations/oxides resulted in a reduction in YbMS coating thickness. Therefore, the volatilisation of silica from the rare earth silicate top coat induced by flowing steam at elevated temperatures, the cracks caused by thermal stress during thermal cycling and diffusion of rare earth ions of the coating is currently the mechanism of coating failure. However, the steam corrosion mechanism of YbMS as top coating were unsystematic and require further detailed study.
The Yb2SiO5/BSAS/Si coatings were prepared on the surface of Cf/SiC composite materials through APS technology using optimized spraying parameters comparing the bonding strength, deposition efficiency, and porosity of single coatings with different spraying power coating. The steam corrosion behavior of Yb2SiO5/BSAS/Si coating was investigated at 1200 ℃ for 0 ~ 500 h to explore coating failure mechanism by analyzing the phase and structure evolution of the YbMS coatings during the cycling process in steam environment. It provided experimental parameters and theoretical basis for the development of coating systems with better performance.
2 Experiments
2.1 Coatings deposition
The Si powders with average particle size of 81 μm for bond coat deposition, the BSAS powders with average particle size of 43 μm for middle layer and Yb2SiO5 powders with average particle size of 33 μm for top coat deposition are commercial powders (Sanspriy New Material Co., Ltd., Beijing), as shown in Fig. 1. The coatings were deposited on the Cf/SiC composite (Φ 25.0 × 3.0 mm) substrates treated with alumina blasting were used for coating deposition by the APS process. The Si, BSAS and Yb2SiO5 coatings were deposited using three condition zones of low, medium and high spray power to optimize the coating preparation process. The deposition procedure and sample photo after coatings deposition were shown in Fig. 2. During the spraying process, argon (Ar) and hydrogen (H2) were used as the primary and the secondary plasma gases, respectively. The detailed spraying parameters were shown in Table 1. Moreover, the Yb2SiO5/BSAS/Si three-layer coatings prepared by the preferred coating process were used in the water vapor corrosion test.
2.2 Thermal cycle test of EBCs in steam conditions
All of the as-sprayed three-layer coatings were further annealed at 1200 ℃ for 20 h in air atmosphere before thermal cycle tests. The thermal cycle tests were performed in a self-developed system, containing a high-temperature tube furnace and liquid flow controller. The schematic diagram of the thermal cycle test equipment was shown in Fig. 3. The thermal cycle tests were conducted for a total of 100 h, 200 h, 300 h and 500 h in air and steam environments, including 50 min of holding at 1200 ℃ followed by 10 min of cooling at room temperature. Water is injected to the furnace at a rate of 1 mL/min. Water steam was formed instantaneously relying on the high temperature at the corundum tube orifice and then diffused towards the center of the corundum tube with compressed air, where the sample was placed. The samples were regulated by the sample controller to entry into or pull out high-temperature furnace.
2.3 Characterization
The phase composition of powders and as-sprayed coatings was characterized by X-ray diffractometer (XRD, D8-Advanced, Bruker AXS, Germany, CuKα radiation, λ = 0.15406 nm) in a range of 2θ = 10–90°. The microstructure and phase composition of the sample surface were observed by field emission scanning electron microscope (FE-SEM, JSM-7900F, Japan) equipped with the energy dispersive spectroscopy (EDS). The cross-section samples were fixed with epoxy resins and cut. Then smooth cross-sections obtained by grinding and polishing were used for microstructural characterization. The particle sizes of feedstock powders were measured using the laser particle size meter. The porosity of the coatings was analyzed from SEM images using Image J software. The bonding strength of coatings was tested according to the ASTMC-633-01 standard. The calculation equations is shown in Eq. 1.
where σb, Fmax and S represent the breaking strength, load undergoes tensile fracture and fracture area of the coating.
3 Results and discussion
3.1 Phase composition and microstructure of the coatings after spraying
Figure 4 displayed the XRD patterns of the Si, BSAS, and Yb2SiO5 powders and as-sprayed coatings. It was revealed that the broadened XRD diffraction peaks of the Si coatings indicated that their crystallinity becomes worse. This result was thought to be a peak produced by phase transformation and refinement of the ingredient powder due to the high plasma energy and rapid cooling [18, 23, 35, 36]. The XRD patterns of as-sprayed BSAS, and Yb2SiO5 coatings exhibited obvious amorphous characteristic peaks compared with the initial powders, especially the as-sprayed BSAS coating [36]. There an obvious amorphous SiO2 peak packet was presented at around 30°, which also indicated that BSAS undergoes significant decomposition during the spraying process [13]. In addition, peaks of Yb2O3 (PDF# 43-1037) were also found in as-sprayed Yb2SiO5 coatings, which was attributed to the decomposition of Yb2SiO5 particles accompanied by the volatilization of silica during the plasma spraying process [19, 23].
The cross-sectional morphology of as-sprayed single-layer Si, BSAS and Yb2SiO5 coatings on the Cf/SiC composite substrates prepared with different powers was shown in Fig. 5. The as-sprayed coatings prepared with different powers exhibit a typical thermal spray structure with a clear interface between the coating and the substrate [37]. Moreover, the coatings were relatively dense tightly and tightly bonded to the substrate, while the microcracks or pores as defects were left in the coating. The thickness of coating was identified in Fig. 5, and the deposition efficiency of coating spraying was reflected by the thickness of the coating. The maximum thickness of Si coating prepared at 24 kW reached up to 650 μm, and the thickness of Si coating is around 304 μm with increase in spraying power up to 40 kW. Higher spraying power improved the spray gun temperature at which thermal ablation of particles was increased. Therefore, high power induces a decrease in deposition efficiency. For as-spraying BSAS coatings, the thickness of the layers deposited from 35 to 45 kW were dwindled from ~401 μm to ~233 μm. BSAS with a low glass transition temperature was prone to overheating in the APS flame stream around 3000 ℃, resulting in ablation and decomposition. Elevated spraying power exacerbated the ablation and decomposition of BSAS, resulting in a lower deposition efficiency of the coating. The deposition efficiency of the as-sprayed Yb2SiO5 coating followed a similar trend with power as that of Si and BSAS coatings. The difference was that the Yb2SiO5 reduction index is smaller. It was attributed to the degradation rate of the Yb2SiO5 during the APS process to generate Yb2O3, which was proven by the XRD results.
3.2 Mechanical properties of the coatings after spraying
Figure 6 displayed the porosity and bond strength with substrates of as-sprayed coatings at different powers. The porosities of the as-sprayed Si layers with 24 kW, 30 kW and 40 kW were calculated to be roughly 16.45%, 15.41% and 13.76% by Image J software, as shown in Fig. 6a. The reason for the densification of Si coating was that Si particles were well spread after melting at high temperature. However, the Si coating sprayed by 24 kW demonstrated the highest bonding strength (4.03 MPa) with the Cf/SiC substrate, as shown in Fig. 6b. It was speculated that the loss of Si on the surface of the substrate due to high temperature oxidation [38] was detrimental to the bond strength with the coating. Then, as the spraying power increases, the bond strength of the substrate surface and the Si coating decreased based on more pronounced oxidation.
The lowest porosity of the BSAS coating was prepared at 40 kW is 6.52%, which was closely correlated with the melting state of powders and process parameters [5, 13]. Further, the BSAS particles were well melted at 40 kW and the as-deposited BSAS coating had the best bond strength with the substrate. It was related to factors such as the viscous flow of BSAS glass and the migration rate of molten droplets [39]. Overheating of the BSAS particles and increased oxidation of the substrate surface were induced by the higher power (45 kW), resulting in the attenuation of the bonding strength of the BSAS coating to the substrate. The BSAS coating sprayed at 40 kW had sufficient contact with the substrate, good interfacial occlusion, tight interlayer bonding with the substrate and low porosity.
The porosity of the as-sprayed Yb2SiO5 coating tended downwards smaller with the increase of power, as well as the deposition efficiency. Lower porosity was beneficial to improve the steam corrosion resistance of Yb2SiO5 coatings. Overall, the effect of spraying power on the porosity and bond strength of Yb2SiO5 coatings was relatively mild. The overheating phenomenon of the Yb2SiO5 particles with the high melting point, was not obvious and the particle melting degree was increased in pace with the increment of spraying power, which was conducive to the increase in bonding strength with the substrate. Still, more severe oxidation was occurring on the counter-substrate, which was detrimental to the coating bond strength. The bonding strength between as-sprayed Yb2SiO5 coating and Cf-SiC substrate was determined by the combined effect of the fusion of coating particles and the oxidation of the substrate.
The three-layer coatings were composed of Si, BSAS, and Yb2SiO5 layers with approximately 110 μm, 110 μm, and 90 μm, which were deposited by Si, BSAS and Yb feedstock powders at a rate of 800 mm/s under 24 kW, 40 kW and 40 W of spraying power, respectively. Other parameters were the same as in Table 1.
3.3 Corrosion behavior of Yb2SiO5/BSAS/Si coating in steam conditions
The Yb2SiO5/BSAS/Si coatings were heat-treated at 1200 °C in an air atmosphere for 20 h to eliminate morphological differences caused by spraying processes and improve the cyclic performance of the coatings [17] before steam corrosion. The heat treatment temperature is determined by the subsequent experimental temperature. Figure 7 was the XRD patterns of Yb2SiO5/BSAS/Si coatings after thermal cycling for different times in steam conditions at 1200 ℃. It could been seen that the major phases of the three-layer coatings before and after steam corrosion are Yb2SiO5 [20]. Moreover, trace amounts of Yb2O3 appeared on the surface of the original coating, attributed to the decomposition of Yb2SiO5 during the APS process, as shown in Eq. (2). After thermal cycling for 100 h, the diffraction peak intensity of the Yb2SiO5 phase weakened and the peak width increased after steam corrosion. Meanwhile, the content of Yb2O3 on the surface of the Yb2SiO5/BSAS/Si coating increased significantly compared to the uncorroded sample, and trace amounts of Yb2Si2O7 phase appeared as a new phase in the XRD patterns. The formation reaction of Yb2Si2O7 might come from three reactions, which are the reaction of Yb2SiO5 with steam (Eq. 3), the reaction of Yb2SiO5 with SiO2 (Eq. 5), and the reaction of Yb2O3 with SiO2 (Eq. 4). Among them, SiO2 originated from the decomposition reaction of Yb2SiO5. Extending thermal cycling time, Yb2O3 content maintained a continuous slow increase, while Yb2Si2O7 content showed a cyclic variation with an increase followed by a decrease. The decrease of Yb2Si2O7 content and the increase of Yb2O3 content might be attributed to decomposition of Yb2Si2O7 at high temperatures (Eq. 6). Moreover, the diffraction peaks of the Yb2SiO5 improved after 200 h. Based on the higher SiO2 activity of Yb2Si2O7 compared to Yb2SiO5, Yb2Si2O7 was more likely to react with steam to generate volatile Si(OH)4 and Yb2SiO5 (Eq. 8). Then, Yb2SiO5 crystallinity deteriorated again when the thermal cycling time reached 300 h, and improved again after 500 h. It is noteworthy that no obvious SiO2 diffraction peaks were observed in the XRD spectrum of the three-layer coatings surface which was related to the volatilisation of Si(OH)4 [21] (Eq. 7) or the formation of Yb2Si2O7. It can be guessed that the following reaction process takes place on the surface of the coating [24, 27, 40,41,42].
Or
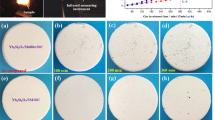
Figure 8 was surface morphology of as-sprayed Yb2SiO5/BSAS/Si coatings corroded for 0 h(a), 100 h(b), 300 h(c) and 500 h(d) in steam conditions at 1200 ℃. The coating still was kept integral and no significant flaking was observed, which indicated that the three-layer exhibits great corrosion resistance in steam conditions at 1200 ℃, as shown in Fig. 8a. The initial coating surface exhibited typical coating material accumulation and particle sintering. The coating surface became rougher by longer thermal cycling time. Corroded Yb2SiO5/BSAS/Si coatings in steam environment revealed the presence of light contrast raised ridges located at the grain boundaries on the surface, in Fig. 8b-e. Ridges dominated the microstructure and increased in density with corrosion time, which was consistent with the results observed by N. Al Nasiri et al. [43]. However, darker small particles were observed on the surface of the three-layer coatings after corrosion. The surface morphology of corroded Yb2SiO5/BSAS/Si coatings in steam environment was divided into darker grain regions, lighter regions of accumulated ridge structure and darker small particles. The lighter ridge structure was the Yb2O3. The elemental contents of the surface structures by EDS were showed in Table 2. The Si content of the formed darker small particles (Spot 1 and Spot 2) was significantly larger than that of Yb2SiO5 (Yb2O3·SiO2) and Yb2Si2O7 (0.5Yb2O3·SiO2). The Yb/Si molar ratio of the Spot 3 was significantly larger than that of Yb2SiO5(Yb/Si = 2:1), thus the surface of three-layer coating corroded for 100 h was consist of Yb2SiO5 and Yb2O3. That is, Yb2O3, as the decomposition product of Yb2SiO5, was still distributed in the surface coating, whereas SiO2 with Yb2SiO5/Yb2O3 was precipitated to form the small granular particles (Spot 1 and Spot 2). After corrosion by thermal cycling for 300 h, the Yb/Si molar ratio of formed small particles (Spot 4) was larger than that of corroded coating for 100 h, and the Yb/Si molar ratio proceeded to increase as the corrosion time extended to 500 h (Spot 6). It can also be demonstrated that the corrosion process persisted with the loss of Si, which was occurred by chemical reactions such as those shown in reactions (1 ~ 8) [21]. Additionally, the corrosion holes appeared on the coating surface (Fig. 8d) after corrosion for 300 h. The reason was that the Yb2O3 phase reacted with steam to form the volatile ytterbium hydroxide (Yb(OH)3), which induced the formation of corrosion voids and destroyed the integrity of the Yb2SiO5 coating [27], as shown in reaction Eq. (9).
The cross-sectional morphology of the as-sprayed Yb2SiO5/BSAS/Si coatings before and after steam corrosion at 1200 ℃ was shown in Fig. 9. After heat treatment, the three-layer coating had a dense structure without obvious longitudinal cracks, as shown in Fig. 9a. Few small pores were distributed in the interior of the coatings, which was caused by the plasma spraying process. The small pore area in the Yb2SiO5 coating corroded for 100 h significantly magnified, which was attributed to the space provided by microcracks and pore areas for the infiltration of corrosive media, and the surrounding Yb2SiO2 was decomposed in Fig. 9b. As the steam corrosion reached 300 h, the corrosion spread from the cavity to the periphery, and the corrosion network had gradually been penetrated into the entire Yb2SiO5 layer, as shown in Fig. 9c. The BSAS layer has already begun to corrode after 500 h corrosion, in Fig. 9d.
Figure 10 was the cross-sectional morphology and element mapping of as-sprayed Yb2SiO5 coatings before steam corrosion. Three areas with obvious contrast in the Yb2SiO5 coating, bright white, light gray and dark gray contrast in Fig. 10b. Wherein light gray was the main component, which was the main phase Yb2SiO5. The dark regions contained higher Si content than the brighter ones [44]. Bright white was the Yb2O3 and the dark gray was the Yb2O3·SiO2(0 < Yb/Si < 2:1), as shown in Fig. 10c. The result was from the decomposition phenomenon of rare earth silicates and migration of internal Si elements during deposition processes [45].
Figure 11 was the cross-sectional morphology and element mapping of Yb2SiO5 coatings corroded for 300 h in steam conditions at 1200 ℃. The interior of the corrosion cavities was filled by the corrosion product SiO2, and a nYb2O3·SiO2 (n > 1) layer was formed at the edges. Meanwhile, the uncorroded areas was composed of Yb2O3. Therefore, the SiO2 component inside the Yb2SiO5 coating would diffuse towards the corrosion front, and enriched at the edges of corrosion network. It was more likely to react with H2O to form volatile Si(OH)4, with the reaction equations e of Eqs. (1), (5), (7), and (8). As the SiO2 content gradually decreases, the residual part was composed of Yb2O3 and the Yb/Si ratio of corrosion front was significantly less than 1:1 and 2:1, as shown in Fig. 11a.
Figure 12 was the cross-sectional morphology and element mapping of Yb2SiO5 coatings corroded for 500 h in steam conditions at 1200 ℃. The corrosion at the Yb2SiO5/BSAS interface was also distributed along the cavities and the Si elemental segregation was appeared at the edge of the cavities, as shown in the orange dashed frame. That is, the Yb2SiO5/BSAS interface was corroded through the plundering of SiO2.
Figure 13 illustrated the failure mechanism of Yb2SiO5/BSAS/Si coating on Cf/SiC composites. The small pores and microcracks in YbMS coatings prepared by atmospheric plasma spraying technology provided diffusion channels for steam infiltration. The YbMS on the surface layer were decomposed into metal oxides (Yb2O3) and silica (SiO2) in steam environment. The decomposed SiO2 component was precipitated to form small Si-rich particles (nYb2O3·SiO2, 0 < n < 0.5). Nearby, Yb-rich ridge like structures (mYb2O3·SiO2, m > 1) were formed on the surface. Furthermore, the SiO2 on the surface could react with steam to form Si(OH)4, which was then evaporated. The depletion of the SiO2 component of the surface by corrosion process causes the internal SiO2 component replenishment to the surface and the SiO2 accumulated on the surface to form kYb2O3·SiO2(0.5 < k < 1). When the coating was uneven, more contact surface with steam were provided by small hollow and a gradient of Si-content products was formed at the interface. SiO2 layer was formed on the outermost layer and a Si-rich layer was formed in the middle, which was consistent with surface reactions from the diffusion and aggregation of SiO2 components inside the coating. As the corrosion reaction proceeded, distinct reaction areas were formed along the diffusion pathway of steam in the microcracks of the YbMS coating. Whereby, pathway holes were more pronounced corrosion reactions for large contact surfaces. Moreover, the corrosion network structure inside the coating was progressively determined by the diffusion paths. When corrosion diffused to the YbMS/BSAS interface, it brought the aggregation of SiO2 components to the interface and the corrosion expanded on the YbMS/BSAS interface which ultimately induced the Yb2SiO5 layer to exfoliate. Significantly, the gradient Si structure at the interface was not formed, which was attribute to the high SiO2 activity of BSAS.
4 Conclusions
Yb2SiO5/BSAS/Si, Yb2SiO5, BSAS, and Si coatings were prepared on the surface of Cf/SiC composites by atmospheric plasma spraying method. Yb2SiO5, BSAS, and Si coatings with good interface bonding were obtained by optimizing spraying power. The steam corrosion behavior of Yb2SiO5/BSAS/Si coating was investigated at 1200 ℃after thermal cycle for 0 ~ 500 h. The main conclusions are as follows:
-
(1)
Yb2SiO5 and BSAS were decomposed into amorphous states during the spraying process. Elevated spraying power exacerbated the ablation and decomposition of particles, resulting in a lower deposition efficiency of the coating. The BSAS coating and Yb2SiO5 coating with good interfacial bonding were obtained only at 40 kW.
-
(2)
The deposition efficiency and coating bonding strength of Si were reduced at higher power result from the fester thermal erosion of Si powders and substrate oxidation, the 24 kW was considered as the deposition of Si particles.
-
(3)
Typical ridges were formed on the surface of the Yb2SiO5/BSAS/Si coating and small Si-rich particles are precipitated, which are subsequently volatilized by the reaction with steam.
-
(4)
The internal SiO2 component of YbMS in three-layer coating as top coating would diffuse to the corrosion interface and a Si gradient layer was formed at the reaction front.
-
(5)
After a cumulative heat treatment of 300 h the YbMS layer was completely corroded in steam conditions. Significant corrosion is present at the YbMS/BSAS interface and extends along the interface by corroded 500 h.
References
Evans AG, Mumm DR, Hutchinson JW, Meier GH, Pettit FS (2001) Mechanisms controlling the durability of thermal barrier coatings. Prog Mater Sci 46:505–553. https://doi.org/10.1016/S0079-6425(00)00020-7
Clarke DR (2003) Materials selection guidelines for low thermal conductivity thermal barrier coatings. Surf Coat Technol 163–164:67–74. https://doi.org/10.1016/S0257-8972(02)00593-5
Anandh S, Kuppusami P, Kirubaharan AM, Rajasekaramoorthy M, Subramanian R (2023) Advanced thermal barrier coatings for aerospace gas turbine engine applications. In: Ram KG (ed) Advanced ceramic coatings for emerging applications, 8th edn. Elsevier, pp 151–183. https://doi.org/10.1016/B978-0-323-99624-2.00010-3
Padture NP (2016) Advanced structural ceramics in aerospace propulsion. Nat Mater 15:804–809. https://doi.org/10.1038/nmat4687
Fellet M, Rossner W (2015) Ceramic-matrix composites take the heat. MRS Bull 40:916–918. https://doi.org/10.1557/mrs.2015.283
Jacobson NS (1993) Corrosion of silicon-based ceramics in combustion environments. J Am Ceram Soc 76:3–28. https://doi.org/10.1111/j.1151-2916.1993.tb03684.x
More KL, Tortorelli PF, Ferber MK, Walker LR, Keiser JR, Miriyala N, Brentnall WD, Price JR (2000) Exposure of ceramics and ceramic matrix composites in simulated and actual combustor environments. J Eng Gas Turbines Power 122:212–218. https://doi.org/10.1115/1.483197
Lee KN (2000) Current status of environmental barrier coatings for Si-Based ceramics. Surf Coat Technol 133–134:1–7. https://doi.org/10.1016/S0257-8972(00)00889-6
Lee KN (2005) Protective coatings for gas turbines. DOE-National Energy Technology Laboratory. http://www.netl.doe.gov/File%20Library/Research/Coal/energy%20systems/turbines/handbook/4-4-2.pdf/
Lee KN (2000) Key durability issues with mullite-based environmental barrier coatings for Si-based ceramics. J Am Ceram Soc 122:632–636. https://doi.org/10.1115/99-GT-443
Tejero MD, Bennett C, Hussain T (2021) A review on environmental barrier coatings: history, current state of the art and future developments. J Eur Ceram Soc 41:1747–1768. https://doi.org/10.1016/j.jeurceramsoc.2020.10.057
Lee KN, Fox DS, Eldridge JI, Zhu D, Robinson RC, Bansal NP, Miller RA (2003) Upper temperature limit of environmental barrier coatings based on mullite and BSAS. J Am Ceram Soc 86:1299–1306. https://doi.org/10.1111/j.1151-2916.2003.tb03466.x
Cui Y, Guo M, Wang C, Jiao J, Cheng L (2022) Preparation and water-vapour corrosion behaviour of BSAS environmental barrier coatings fabricated on ceramic matrix composites. Surf Coat Technol 449:128953. https://doi.org/10.1016/j.surfcoat.2022.128953
Lee KN, Fox DS, Bansal NP (2005) Rare earth silicate environmental barrier coatings for SiC/SiC composites and Si3N4 ceramics. J Eur Ceram Soc 25:1705–1715. https://doi.org/10.1016/j.jeurceramsoc.2004.12.013
Nasiri NA, Patra N, Horlait D, Jayasselan DD, Lee WE (2016) Thermal properties of rare earth monoslicates for EBC on Si-based ceramic composites. J Am Ceram Soc 99:589–596. https://doi.org/10.1111/jace.13982
Tian Z, Zheng L, Wang J, Wan P, Li J, Wang J (2016) Theoretical and experimental determination of the major thermo-mechanical properties of RE2SiO5 (RE=Tb, Dy, Ho, Er, Tm, Yb, Lu, and Y) for environmental and thermal barrier coating applications. J Eur Ceram Soc 36:189–202. https://doi.org/10.1016/j.jeurceramsoc.2015.09.013
Kassem R, Nasiri NA (2021) A comprehensive study on the mechanical properties of Yb2SiO5 as a potential environmental barrier coating. Surf Coat Technol 426:127783. https://doi.org/10.1016/j.surfcoat.2021.127783
Xiao S, Li J, Huang P, Zhang A, Tian Y, Zhang X, Zhang J, Ryu J, Han G (2023) Evaluation of environmental barrier coatings: a review. Int J Appl Ceram 20:2055–2076. https://doi.org/10.1111/ijac.14370
Klemm H (2010) Silicon nitride for high-temperature applications. J Am Ceram Soc 93:1501–1522. https://doi.org/10.1111/j.1551-2916.2010.03839.x
Lee KN (2020) Special issue: environmental barrier coatings. Coatings 10(6). https://doi.org/10.3390/coatings10060512
Lu MH, Feng ZH, Zhou YC (2015) Recent research progress on environmental barrier coatings for non-oxide ceramics. J Ceram 36:107–118. https://doi.org/10.13957/j.cnki.tcxb.2015.02.001
Bakan E, Sohn YJ, Kunz W, Klemm H, Vaßen R (2019) Effect of processing on high-velocity water vapor recession behavior of Yb-silicate environmental barrier coatings. J Eur Ceram Soc 39:1507–1513. https://doi.org/10.1016/j.jeurceramsoc.2018.11.048
Ou H, Fan K, Guo L, Zhang Y, Wang Y, Sun J, Fu Q (2024) Corrosion behavior of Al2O3-modified Yb2SiO5 environmental barrier coating under water vapor conditions at 1500 ℃. J Eur Ceram Soc 44:1202–1216. https://doi.org/10.1016/j.jeurceramsoc.2023.09.063
Zhuo X, Wang C, Wang M, Wu J, Zhang X, Cui J, Mei X, Fan Z (2023) Al-modified environmental barrier coatings for protection against water vapor corrosion. Corros Sci 217:111123. https://doi.org/10.1016/j.corsci.2023.111123
Zhou W, Niu Z, Chen X, Xiao P, Li Y (2023) Synergistic effect of water vapour on the thermal corrosion of CFAS melt to Yb2Si2O7 environmental barrier coating material. Corros Sci 225:111625. https://doi.org/10.1016/j.corsci.2023.111625
Zhao M, Hu X, He J, Li Y, Song WJ (2023) Corrosion behavior and mechanism of ytterbium monosilicate by molten calcium-magnesium-alumino-silicate melts at 1400 ℃ and 1500 ℃. Ceram Int 49:23756–23764. https://doi.org/10.1016/j.ceramint.2023.04.213
Zhang Z, Xue Z, Park Y, Park H, Qiu Z, Qiao L, Feng P, Wierzba B, Byon E, Zhang S (2023) High-temperature performance of thermal environmental barrier coatings in 90%H2O-10%O2 conditions at 1475 ℃. Corros Sci 224:111535. https://doi.org/10.1016/j.corsci.2023.111535
Wang C, Liu M, Feng J, Zhang X, Deng C, Zhou K, Zeng D, Guo S, Zhao R, Li S (2020) Water vapor corrosion behavior of Yb2SiO5 environmental barrier coatings prepared by plasma spray-physical vapor deposition. Coatings 10(1–15):392. https://doi.org/10.3390/coatings10040392
Ridley M, Opila E (2021) Thermochemical stability and microstructural evolution of Yb2Si2O7 in high-velocity high-temperature water vapor. J Eur Ceram Soc 41:3141–3149. https://doi.org/10.1016/j.jeurceramsoc.2020.05.071
Richards BT, Young KA, de Francqueville F, Sehr S, Begley MR, Wadley H (2016) Response of ytterbium disilicate-silicon environmental barrier coatings to thermal cycling in water vapor. Acta Mater 106:1–14. https://doi.org/10.1016/j.actamat.2015.12.053
Richards BT, Begley MR, Wadley HNG, Smialek JL (2015) Mechanisms of ytterbium monosilicate/mullite/silicon coating failure during thermal cycling in water vapor. J Am Ceram Soc 98:4066–4075. https://doi.org/10.1111/jace.13792
Richards BT, Sehr S, de Franqueville F, Begley MR, Wadley HNG (2016) Fracture mechanisms of ytterbium monosilicate environmental barrier coatings during cyclic thermal exposure. Acta Mater 103:448–460. https://doi.org/10.1016/j.actamat.2015.10.019
Zhang Z, Park Y, Park H, Kang Y, Xue Z, Zhang S, Byon E, Koo BH (2023) Thermal cycle test performances of environmental barrier coatings of HfO2-SiO2/Yb2Si2O7 in the steam environment at 1475 ℃. J Eur Ceram Soc 43:5004–5013. https://doi.org/10.1016/j.jeurceramsoc.2023.04.030
Nasiri NA, Patra N, Pezoldt M, Colas J, Lee WE (2019) Investigation of a single-layer EBC deposited on SiC/SiC CMCs: processing and corrosion behaviour in high-temperature steam. J Eur Ceram Soc 39:2703–3271. https://doi.org/10.1016/j.jeurceramsoc.2018.12.019
Ryu H, Lee SM, Han YS, Choi K, An GS, Nahm S, Oh YS (2019) Preparation of crystalline ytterbium disilicate environmental barrier coatings using suspension plasma spray. Ceram Int 45:5801–5807. https://doi.org/10.1016/j.ceramint.2018.12.048
Guo M, Cui Y, Wang C, Jiao J, Bi X, Tao C (2023) Design and characterization of BSAS-polyester abradable environmental barrier coatings (A/EBCs) on SiC/SiC composites. Surf Coat Technol 465:129617. https://doi.org/10.1016/j.surfcoat.2023.129617
Chen D, Harmon R, Dwivedi G, Dambra C, Dorfman M (2021) In-flight particle states and coating properties of air plasma sprayed ytterbium disilicates. Surf Coat Technol 417:127186. https://doi.org/10.1016/j.surfcoat.2021.127186
Chen PJ, Xiao P, Li Z, Li JW, Pu DM, Deng PY, Li Y (2020) Effect of residual silicon on the oxidation resistance of bilayer Yb2Si2O7/SiC coated C/SiC composites at high temperature. Corros Sci 170:108676. https://doi.org/10.1016/j.corsci.2020.108676
Cojocaru CV, Lévesque D, Moreau C, Lima RS (2013) Performance of thermally sprayed Si/mullite/BSAS environmental barrier coatings exposed to thermal cycling in water vapor environment. Surf Coat Technol 216:215–223. https://doi.org/10.1016/j.surfcoat.2012.11.043
Ueno S, Jayaseelan DD, Ohji T (2005) Development of oxide-based EBC for silicon nitride. Int J Appl Ceram Technol 1:362–373. https://doi.org/10.1111/j.1744-7402.2004.tb00187.x
Maier N, Nickel KG, Rixecker G (2007) High temperature water vapour corrosion of rare earth disilicates (Y, Yb, Lu)2Si2O7 in the presence of Al(OH)3 impurities. J Eur Ceram Soc 27:2705–2713. https://doi.org/10.1016/j.jeurceramsoc.2006.09.013
Ueno S, Ohji T, Lin HT (2008) Recession behavior of Yb2Si2O7 phase under high speed steam jet at high temperatures. Corros Sci 50:178–182. https://doi.org/10.1016/j.corsci.2007.06.014
Al Nasiri N, Patra N, Jayaseelan DD, Lee WE (2017) Water vapour corrosion of rare earth monosilicates for environmental barrier coating application. Ceram Int 43:7393–7400. https://doi.org/10.1016/j.ceramint.2017.02.123
Wu NN, Wang YL, Tong YL, Tan Y, Liu HF, Xiong X, Liu R (2023) Interaction of ytterbium monosilicate environmental barrier coating material with molten calcium-magnesium-aluminosilicate (CMAS). Corros Sci 211:110864. https://doi.org/10.1016/j.corsci.2022.110864
Zhong X, Wang Y, Niu Y, Huang L, Li Q, Zheng X (2021) Corrosion behaviors and mechanisms of ytterbium silicate environmental barrier coatings by molten calcium-magnesium-alumino-silicate melts. Corros Sci 191:109718. https://doi.org/10.1016/j.corsci.2021.109718
Acknowledgements
This work was supported by National Key Research and Development Plan (No. 2021YFB3702304-4) and National Science and Technology Major Special Basic Research Project (No. J2019-VI-0017-0132).
Statements and declarations
1. All data in this manuscript is true and reliable. All contents have not been copyrighted or published previously and is not under consideration for publication elsewhere.
2. The first author (Weiguo Mao) in this manuscript is a member of the editorial board of this journal. He was not involved in the editorial review or the decision to publish this article. All authors declare that there are no competing interests.
Author information
Authors and Affiliations
Contributions
Weiguo Mao (First author): Conceptualization, Funding Acquisition, Resources, Supervision, Writing - Review & Editing. Wenjie Ye: Conceptualization, Methodology, Software, Investigation, Formal Analysis, Data Curation, Writing - Original Draft, Xizhi Fan: Preparation, Characterization. Zhe Zhou: Equipment Regulation, Experimental Application. Cuiying Dai: Resources, Supervision. Nannan Wu (Corresponding Author): Investigation, Formal Analysis, Data Curation, Writing - Review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mao, W., Ye, W., Fan, X. et al. Investigation of Yb2SiO5/BSAS/Si coating on Cf/SiC composites: processing and steam corrosion behavior. Surf. Sci. Tech. 2, 10 (2024). https://doi.org/10.1007/s44251-024-00039-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44251-024-00039-4