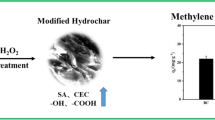
Abstract
The application of hydrochar as a cost-effective solution has received much attention for the remediation of contaminated water. An economical and environmental approach to enhancing the physicochemical and adsorption performance of hydrochar is essential. In this study, the green technology of ball-milling was firstly employed to improve the adsorption capacity of hydrochar for the typical antibiotics norfloxacin. Aqueous batch adsorption experiment using both pristine and ball milled hydrochar derived from water hyacinth, prepared by hydrothermal carbonization at three temperatures (180, 200, 220 °C) was conducted. The results showed that ball-milling decreased the specific surface area of hydrochar, but still greatly enhanced their performance on the adsorption of norfloxacin. Surface functional groups, aromatization degree, and hydrophobicity of hydrochar were increased after ball-milling, as evidenced by measurements of Boehm titration, Raman spectra, and contact angle, respectively. With these changes, all the ball-milled water hyacinth hydrochar exhibited a better performance on the adsorption of norfloxacin than pristine hydrochar. Ball-milled 220 °C water hyacinth hydrochar showed the greatest norfloxacin adsorption (68.53 mg g−1) compared to unmilled hydrochar (24.29 mg g−1), and the enhancement was effective in a wide pH range (5–9) in aqueous solutions. The thermodynamics study indicated that the norfloxacin adsorption on ball-milled hydrochar was both physically spontaneous and exothermic. Combined physicochemical characterization of hydrochar and batch experiment results suggest that the enhanced adsorption capacity was owing to boosting H-bonds, π-π electron-donor–acceptor, and hydrophobic interaction. This study suggested that ball-milling can be served as a facile, green, and cost-effective method to obtain modified hydrochar for the removal of pollutants in water.
Highlights
• Surface functional groups and hydrophobicity of hydrochar were increased after ball-milling.
• Ball-milling was firstly used to modify hydrochar (BMHC) to remove antibiotics.
• BMHC had a high adsorption capacity of 68.53 mg g-1 for norfloxacin (NOR).
• NOR adsorption on BMHC was enhanced by increased H-bonds, π-π EDA, and hydrophobic interaction.
AbstractSection Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Antibiotics such as quinolones are widely used in medicine, animal husbandry, and agriculture. It was reported that up to 70% of them are released into the environment due to poor metabolization (Van Doorslaer et al. 2014). Norfloxacin (NOR), a typical quinolone antibiotic, is frequently detected in drinking water, ground water, and surface water at concentrations amounting to 0.52 mg L−1 (Fick et al. 2009). The NOR residues in water may accelerate the development of antibiotic resistance, leading to a possible risk to human health and the environment (Liang et al. 2023). Therefore, it is urgent to develop a sustainable and effective method of removing NOR from the water environment.
Scientists have successfully remediated NOR in water by advanced oxidation processes, biological treatments, physical sorption, etc. (Monisha et al. 2021; Li et al. 2022; Guy Laurent Zanli et al. 2022). Particularly, sorption is attractive owing to its simplicity, high removal efficiency, and low cost. Especially in recent years, turning waste biomass such as agricultural residues, livestock manure, and water plants into the economic adsorbent ‘hydrochar’ has attracted enormous attention (Huang et al. 2023; Jalilian et al. 2024). Hydrochar is a type of carbonization material prepared by hydrothermal carbonization (HTC), wherein the subcritical water is used to treat biomass at 180–300 °C of temperature, 10–20 MPa of pressure, and 0.5–10 h of holding time (Sevilla and Fuertes 2009; Zhang et al. 2024). This is a green and energy-saving way to treat biomass without the extra energy consumption of the per-drying process, especially for high-moisture biomass like fruit peel, kitchen garbage, water plants, etc.
Water hyacinth is a noxious invasive aquatic plant with a high moisture content that can reduce the oxygen and nutrients in the water and may clog rivers (Mitan 2019). Water hyacinth derived hydrochar could be used to adsorb heavy metals, volatile organic compounds, dyes, antibiotics, etc. (Saning et al. 2019; Yu et al. 2024), and the adsorption capacity could be improved by different modification methods (Saning et al. 2019; Liu et al. 2021a) for overcoming the drawbacks of hydrochar such as low carbonization degree, low aromatization degree, limited specific surface area (SSA) and limited pore volume. However, most modification methods involved extra chemical reagents and high energy consumption (Jalilian et al. 2024). Hence, a more environment-friendly, facile, and low-cost modification method to strengthen the adsorption performance of water hyacinth hydrochar should be deeply investigated. For this, ball-milling modification was considered because it is an energy-saving, simple, flexible method that is easily employed in large-scale production (Lyu et al. 2017). As a product similar to hydrochar but with higher energy consumption, the information on biochar modified by ball-milling is comprehensive (Peterson et al. 2012; Naghdi et al. 2017). Ball-milling usually improves the adsorption capacity of biochar by improving its SSA and pore structure (Lyu et al. 2018). For example, after ball-milling, the SSA and pore volume of corn stalk biochar could increase from ~ 133.0 m2 g−1 and 0.16 m3 g−1, to 255.4 m2 g−1 and 0.27 m3 g−1, respectively, and the adsorption of NOR could be enhanced from 3.92 to 7.29 mg g−1 (Wu et al. 2022). However, it cannot be ignored that the physicochemical properties of hydrochar are significantly different from those of biochar, including elemental composition, pore structure, surface functional groups, and content of dissolved organic matter. (Liu et al. 2019). Ball-milling may not be suitable for improving the pore structure of hydrochar, because hydrochar retains a substantial amount of original carbon, which results in limited pore structure development and a low SSA. The effect of ball-milling on the physicochemical properties of hydrochar is not clear. It has been recognized that pore structure and functional groups of hydrochar play the main roles in adsorbing NOR through mechanisms such as pore-filling, H-bonds, π-π electron-donor–acceptor (EDA), hydrophobic distribution, and electrostatic interactions (Guan et al. 2021; Cheng et al. 2021; Lan et al. 2024). Interestingly, ball-milling could also increase the functional groups of biochar. For instance, the total functional groups of corn stalk biochar could increase from 0.173 mmol g−1 to 0.598 mmol g−1, and the adsorption of methylene blue could be enhanced from 11.0 to 90.2 mg g−1 (Yan et al. 2023). It was hypothesized in the present study that ball-milling could improve other properties (e.g., surface functional groups, hydrophobicity) to enhance the adsorption capacity of hydrochar even if the SSA and pore structure would not be improved. Thus, the impact of ball-milling on the physiochemical properties and adsorption performance of hydrochar should be revealed.
In order to improve the adsorption performance of hydrochar in a green and energy-saving way, ball-milling technology was firstly attempted to modify water hyacinth hydrochar, and the adsorption performance was systematically evaluated by removing norfloxacin from water. Moreover, the adsorption mechanisms were revealed through the combination of experimental data and multiple characterizations. Accordingly, the objectives of this study were to 1) investigate the influence of ball-milling on the physiochemical properties of water hyacinth hydrochar; 2) evaluate the adsorption of NOR on ball-milled water hyacinth hydrochar; and 3) elucidate the adsorption enhancement mechanism of NOR adsorption.
2 Materials and methods
2.1 Materials
Water hyacinth (from Yongzhou, Hunan Province, China) was collected, washed, cut into pieces, and stored in a freezer before use. The water content of water hyacinth (90 ± 0.3%) was determined according to a reported method (Yang et al. 2022). Norfloxacin (NOR, ≥ 98%) was purchased from ANPEL Laboratory Technologies. Sodium hydroxide (NaOH, ≥ 96%), nitric acid (HNO3, ≥ 95%), and sodium nitrate (NaNO3, ≥ 99%) were obtained by Sinopharm Group Chemical Reagent. Humic acid (HA) was bought from Sigma-Aldrich. Deionized water (18.3 MΩ cm) was used in all experiments.
2.2 Hydrochar production
Hydrochar was prepared according to the previous method (Liu et al. 2021b). In brief, 8.5 g (dry weight) of water hyacinth biomass was mixed with 85 mL of water at a feedstock-to-water ratio of 1:10 in a 200 mL autoclave with a p-polyphenylene inner liner. The water content of the water hyacinth was taken into consideration while calculating the additional required water. After heating the autoclave to the required HTC temperature of 180, 200, or 220 °C, it was maintained at peak temperature for 120 min. The solids were washed with deionized water several times after the autoclave cooled naturally to room temperature and then dried at 80 °C. The crushed dry hydrochar was sieved through 0.5–1 mm, collected, stored in a desiccator, and labeled as XHC, where X referred to the HTC temperature.
Ball-milled water hyacinth hydrochar was prepared by mixing 1.80 g hydrochar with 180 g agate balls (10:8:5 mm = 1:1:1) in four 100 mL agate jars and processed in a planetary ball mill machine (DECO-PBM-V-0.4 L, Changsha, China). The operating conditions of rotation speed and direction change time were set at 600 rpm and 0.5 h, respectively, and operated for 3, 6, 9, and 12 h (Lyu et al. 2018). The product was labeled as XBMHC, where X indicated the HTC temperature.
2.3 Hydrochar characterization
The microphotographs were studied by a scanning electron microscope (SEM, Zeiss Supra 55-VP, Germany) with energy dispersive spectroscopy (EDS) at a high resolution of 1 μm. The particle size distribution was measured by a laser diffraction particle size analyzer (Beckman Coulter LS 13 320, USA). The specific surface area was revealed through the Brunauer–Emmett–Teller method by the N2 adsorption–desorption isotherm at 77 K (QuadraSorb Station 1 analyzer, USA). The contact angle was identified by a drop shape analyzer (DSA100, Germany). The elemental contents (C, H, N, and S) were detected by an elemental analyzer (Euro Vector EA3000, Italy). The aromatization degree was analyzed by Raman spectra (inVia Reflex, UK). The functional groups were characterized by Fourier transform infrared spectra (FT-IR, Nicolet iS10 spectrometer, USA) and X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, USA) and quantified by the Boehm titration method (Boehm 1994). The dissolved organic matter (DOM) was characterized by a total organic carbon analyzer (TOC-L, Japan) and a UV–vis spectrometer (1750S, Shimadzu, Japan). The zeta potential under different pH values was tested by a zeta potential analyzer (NanoBrook Omni, USA). The details of the Boehm titration, DOM extraction and analysis were presented in Text S1–2.
2.4 Adsorption experiments
The batch experiments were conducted on the NOR adsorption performance of hydrochar and carried out in brown bottles with 0.5 g L−1 of hydrochar and 10 mg L−1 of NOR, shaken in a dark environment at 25 °C and 180 rpm. To maintain electron strength, 10 mM NaNO3 was added as a background solution. The adsorption kinetics were evaluated by varying the sampling time (0–2160 min). The adsorption isotherm was obtained by altering the initial concentration of NOR (10–80 mg L−1). The sample 220BMHC was chosen as the adsorbent to study the NOR adsorption thermodynamics at different temperatures (25, 35, and 45 °C) and to evaluate reusability according to the previous method (Salawu et al. 2022). The chemistry of the aqueous phase was also tuned to determine the effect of pH (3–11), humic acid (HA, 1–10 mg L−1), and ionic strength (Na+, Ca2+, Cl−, NO3−, and SO42− = 1–100 mM) on the adsorption of NOR. Experiments were carried out at neutral pH (except when pH was being studied, adjusted from 3 to 11 by HNO3 and NaOH). All the experiments were carried out in triplicate, and the results were reported as a mean of three values for each sampling point. To test the remaining NOR concentration, 1 mL of supernate was withdrawn and filtered for HPLC analysis (Shimadzu LC-20 A, Japan), as specified in earlier research (Wu et al. 2022). The calculation of adsorption capacity, fitted by kinetic models and isotherm models, was described in Text S3.
2.5 Statistic analysis
The statistical significance of the results was evaluated with a one-way analysis of variance (ANOVA) by post-hoc Tukey’s test at a significance level of p ≤ 0.05 using SPSS statistics 19.
3 Results and discussion
3.1 Optimal ball-milling conditions
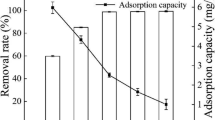
The influence of ball-milling time on the adsorption performance of hydrochar was investigated. NOR adsorption capacity increased with ball-milling time (Fig. S1). For example, it increased from 6.82 ± 0.05 mg g−1 to 14.79 ± 0.27 mg g−1 as the ball-milling time was set at 3 h. With a further increase in the ball-milling time to 6 h, the NOR adsorption capacity increased to 15.96 ± 0.14 mg g−1. However, it was 15.79 ± 0.22 mg g−1 and 16.41 ± 0.11 mg g−1 after ball-milling for 9 h and 12 h, respectively. This may be caused by the agglomeration of the powder with the extension time of ball-milling, which increased the temperature of the agate jars (Qu et al. 2024). Based on these results and taking into account energy consumption, the optimal ball-milling time was chosen as 6 h.
3.2 Hydrochar characterization
As a physical modification technology, ball-milling could introduce a great influence on the morphology, structure, and particle size of hydrochar. SEM images were taken to clarify the impact of ball-milling on the surface morphology of hydrochar (Fig. 1). The form of HCs (180HC, 200HC, and 220HC) was lamellar and cylindrical. A porous structure could be seen on 220HC due to the degradation of cellulose at a higher HTC temperature (Cavali et al. 2023). Besides, spheres around 1 μm were also observed in 220HC, which formed due to the polymerization reaction. Under the potent mechanochemical force of ball-milling, the form of BMHCs (180BMHC, 200BMHC, and 220BMHC) turned into blocky, and the component smaller than 1 μm was increased. It seemed that agglomeration was formed in BMHCs, which were held together by van der Waals forces (Peterson et al. 2012). These results were in accordance with the particle size distribution (Fig. 3a), which showed that 50% of BMHCs had a size smaller than 24.19 μm, and the formation of two peaks might be caused by agglomeration that led to a larger particle size (Gao et al. 2023). These findings showed that ball-milling decreases the particle size of hydrochar, and particle aggregation might occur. The smaller size of hydrochar would have better dispersibility and more contact with contaminants in the solution.
Generally, hydrochar possessed a limited pore structure (Cavali et al. 2023). With a large pore volume and SSA, hydrochar could provide abundant adsorption sites for contaminants. The N2 adsorption-desorption curve was tested to figure out the effect of ball-milling on the pore structure of hydrochar and the results are shown in Fig. 2 and Table 1. With the HTC temperature increasing from 180 to 220 °C, the SSA of HCs increased from 8.70 to 20.43 m2 g−1. Ball-milling induced the SSA of hydrochar to decrease, except for 180BMHC, in which the SSA slightly increased from 8.70 to 12.62 m2 g−1. The reason for the decrease in SSA could be resulted from that the fiber network and pores were damaged through the ball-milling, and the newly created porous planar structure lacked sufficient pores or a high SSA (Xiao et al. 2020). Both HCs and BMHCs possessed a mesoporous texture with an average pore diameter in the range of 22.01–29.03 nm (Table 1), which was beneficial for NOR to enter the pore channel, whereas the three-dimensional size of the NOR molecule was 1.49 × 0.86 × 0.63 nm (Table S1). Particularly, the microporous surface area and microporous pore volume of hydrochar through ball-milling were still ~ 0 m2 g−1 and ~ 0 cm3 g−1, indicating that ball-milling could not improve the micropore structure of hydrochar due to its undeveloped pore structure.
The elemental composition in the values of H/C, O/C, and (O + N)/C could reflect the aromaticity and polarity of hydrochar (Liu et al. 2019). As displayed in Table 2, as the HTC temperature increased from 180 to 220 °C, the atomic ratio (H/C, O/C, and [O + N]/C) decreased gradually from 1.44, 0.67, and 0.70 to 1.25, 0.51, and 0.54, respectively, indicating that higher HTC temperatures induce hydrochar to possess improved aromaticity and declining polarity. There was no obvious change in the elemental composition of hydrochar by ball-milling, possibly caused by ball-milling having more influence on hydrochar surface properties. Raman spectra (Fig. 3b) were applied to further investigate the aromaticity of hydrochar. The ID/IG ratio between the disorder-induced (D) and graphite (G) bands was adopted to reflect the extent of aromaticity in HCs and BMHCs. The ID/IG of HCs and BMHCs were in the range of 0.42–0.58, showing that HCs and BMHCs were carbonaceous materials with amorphous carbon structure and in-plane disordered graphite (Liu et al. 2021a, b). The ID/IG was decreased with higher HTC temperature, suggesting that the aromaticity of hydrochar was increased, which was consistent with the elemental analysis (Table 2). Ball-milling could enhance the aromaticity level, as evidenced by the lower ID/IG. To investigate the hydrophobicity/hydrophilicity of hydrochar, the wettability of the hydrochar surface was studied by observing the contact angle of water droplets (Fig. 3c). The water droplets fully diffused in a short time after contact with 180HC in 20 s, showing a high hydrophilicity of 180HC. The contact angles of 200HC and 220HC were 70.7° and 80.4°, respectively, indicating that the hydrophobicity enhanced with HTC temperature increase. The contact angles of 180BMHC, 200BMHC, and 220BMHC were 78.2°, 89.5°, and 93.6°, respectively, which were both larger than corresponding pristine hydrochar, suggesting that ball-milling could increase the hydrophobicity of hydrochar. The contact angle was influenced by both the surface roughness of sample discs, surface chemistry (He et al. 2013), and the particle size of hydrochar (Edeh and Mašek 2021). Through ball-milling, the hydrochar was more uniform and homogeneous, and the compactability was obviously increased, making ball-milled hydrochar more hydrophobic than pristine hydrochar.
Hydrochar possessed abundant functional groups (Liu et al. 2019). The variations in functional groups of hydrochar through ball-milling were reflected in FT-IR (Fig. 4), and the assignments of differentiated FT-IR peaks are summarized in Table S2. The C=C stretching vibration of hydrochar slightly shifted from 1617 to 1624 cm−1 after ball-milling, which was probably caused by the π cloud density increase (Harindintwali et al. 2023). Besides, the peaks of -OH (3341–3435 cm−1) and C-O (1059 cm−1) for ball-milled hydrochar were dramatically stronger than those of pristine hydrochar, which was consistent with the Boehm titration results (Fig. 3d). It should be found that the concentrations of acidic functional groups in 180HC, 200HC, and 220HC were 2.02, 3.18, and 4.10 mmol g−1, respectively. After ball-milling, the concentrations of acidic functional groups were increased to 2.93, 3.71, and 4.80 mmol g−1 for 180BMHC, 200BMHC, and 220BMHC, respectively. These results confirmed that ball-milling introduced more acidic functional groups on the hydrochar surface, which was due to the oxidation atmosphere during ball-milling in air conditions (Xu et al. 2021).
Hydrochar was produced at a relatively low temperature and was not completely carbonized, containing both noncarbonized organic matter and carbonized organic matter, and dissolved organic matter (DOM) is an important part (Guan et al. 2021). DOM extracted from hydrochar was characterized. As shown in Fig. 3e, the DOM contents of 180HC, 200HC, and 220HC were 5.64, 7.46, and 10.38 mg g−1, respectively. After ball-milling, DOM release of 180BMHC (15.88 mg g−1) and 200BMHC (14.58 mg g−1) was significantly increased by 2.82 and 1.85 times compared to 180HC and 200HC, respectively, while DOM release from 220BMHC (9.11 mg g−1) had no evident change (p < 0.05). The significantly increased DOM concentration of 180BMHC and 200BMHC might be caused by ball-milling, which broke the low degree carbonization hydrochar (180HC and 200HC) into small pieces and induced the inherent noncarbonized organic matter release. To predict the DOM properties of ball-milled hydrochar, three UV indices (\({a}_{m}\) [m−1], \({S}_{275-295}\), and \({SUVA}_{254}\) [L mg−1 m−1]) were measured as provided in Fig. S2. DOM aromaticity was favorably linked with \({SUVA}_{254}\) (Helms et al. 2008). In this study, the aromaticity (\({SUVA}_{254}\)) increased in both HCs and BMHCs with the HTC temperature elevated from 180 to 220 °C. This was due to the fact that macromolecules in DOM were difficult to degrade, thus increasing the aromatization degree (Song et al. 2020; Yu et al. 2023). The \({SUVA}_{254}\) was reduced following ball-milling, particularly in low-temperature hydrochar. This could be explained by low-temperature hydrochar releasing more noncarbonized organic materials, which would have reduced aromaticity of DOM. A greater value of \({S}_{275-295}\) indicated a smaller molecular size. The present study showed that the value of \({S}_{275-295}\) rose with the HTC temperature of HCs increasing from 180 to 220 °C. This was due to that when the HTC temperature increased, the macromolecules in the biomass broke down into smaller molecules, resulting in a decrease in the molecular size of DOM. The \({S}_{275-295}\) value of BMHCs was raised after ball-milling, regardless of the HTC temperature. It was suggested that ball-milling also decreased the molecular size of DOM as the particle size of hydrochar decreased.
3.3 NOR adsorption on hydrochar
Adsorption kinetics investigated the adsorption equilibrium of NOR on hydrochar (Fig. 5a, b). For HCs, NOR adsorption increased gradually in the first 360 min and reached equilibrium in 1440 min. For BMHCs, NOR adsorption increased rapidly before 120 min and became stable after 600 min. The results showed that BMHCs possessed more adsorption sites than HCs, thus leading to higher and faster adsorption performance. The data were fitted with the typically used models, including the pseudo-first-order kinetic model, the pseudo-second-order kinetic model, and the Elovich model (Eqs. S5–S7). The fitting parameters were presented in Table S3. The results showed that the Elovich model (0.986 – 0.994) could fit the experiment data of HCs, and the pseudo-second-order kinetic model (R2 = 0.976 – 0.997) and the Elovich model (R2 = 0.980 – 0.995) both fit for BMHCs well. This means that the adsorbent surface was energetically heterogeneous, and chemisorption was the adsorption rate-limiting step for BMHCs (Wu et al. 2022; Kundu et al. 2024).
To investigate the NOR maximum saturated adsorption capacity of hydrochar, the data of adsorption isotherms were fitted with the typical Langmuir, Freundlich, and Sips models (Eqs. S8–S10) and shown in Fig. 5c, d, and the parameters were also displayed in Table S4. For both HCs and BMHCs, the Sips model (R2 = 0.995 – 0.999) was better fitted than the Langmuir model (R2 = 0.963 – 0.983) and the Freundlich model (R2 = 0.987 – 0.998), suggesting that the adsorption was an inhomogeneous process (Wang et al. 2023; Hu et al. 2024). The \(n\) of the Freundlich model represented the heterogeneity of the sorbent surface. The closer \(n\) was to 1, the more adsorption was controlled by hydrophobic distribution (Jing et al. 2022). The \(n\) of this study was in the range of 0.38 – 0.48, which belonged to medium nonlinearity. According to the results of Langmuir model fitting, the theoretical maximum saturated adsorption capacity (\({q}_{m}\)) of HCs was decreased with the HTC temperature increase, i.e., 180HC (36.86 ± 2.21 mg g−1) > 200HC (25.83 ± 1.11 mg g−1) > 220HC (24.29 ± 1.34 mg g−1). Notably, the \({q}_{m}\) of 180BMHC, 200BMHC, and 220BMHC were 51.35 ± 3.22, 69.68 ± 3.28, and 68.53 ± 3.15 mg g−1, respectively, which were 1.39, 2.70, and 2.82 times greater than those corresponding to the original hydrochar. These results showed that ball-milling was a feasible way to modify water hyacinth hydrochar to enhance the effectiveness of remediating antibiotics in the water environment. 200BMHC (69.68 ± 3.28 mg g−1) and 220BMHC (68.53 ± 3.15 mg g−1) possessed similar NOR adsorption capacity. Considering 220BMHC released less DOM than 200BMHC (Fig. 3e), 220BMHC was chosen for follow-up experiments. Compared with other biochar produced for NOR adsorption in the literature (Table S5), such as phosphoric acid-assisted cornstalk hydrochar (69.12 mg g−1) (Lan et al. 2024) and Fe2O3 modified poplar wood chip biochar (38.77 mg g−1) (Liang et al. 2022), it was suggested that 220BMHC (68.53 mg g−1) possessed a comparatively great adsorption capability, and its preparation method was green, sustainable, and scalable.
To clarify the spontaneity of NOR adsorption by ball-milled hydrochar, thermodynamic experiments were conducted, and the data fitted with the Freundlich model (R2 = 0.994 – 0.995) were shown in Fig. 6. The thermodynamic parameters, including Gibbs free energy \({(\Delta G}^{0})\), enthalpy \(({\Delta H}^{0})\), and entropy \(({\Delta S}^{0}\)), were calculated (Eqs. [1]–[3]), and the results are shown in Table 3. The negative values of \({\Delta G}^{0}\) (from -5.566 to -6.115 kJ mol−1) indicated the adsorption of NOR onto 220BMHC was thermodynamically feasible and spontaneous. With the temperature increase, \({\Delta G}^{0}\) was less negative, showing that the spontaneity of the adsorption process was weakened, which was not conducive to the adsorption. Given the negative \({\Delta H}^{0}\) (-14.277 kJ mol−1) obtained, indicating that the adsorption process was exothermic. It was reported that when \({\Delta H}^{0}\) lies in the range of 0–20 kJ mol−1, the adsorption of NOR on 220BMHC was mostly dominated by physical mechanisms (Kundu et al. 2024). The absolute value of \({\Delta H}^{0}\) in this study was in the above range, implying the dominant role of physical adsorption. \({\Delta S}^{0}\) (-27.346 J K−1 mol−1) was negative, indicating the adsorption process reduced the disorder at the solid–liquid two-phase interface in the reaction system. In summary, the adsorption process of NOR on 220BMHC was physically spontaneous and exothermic.
,where \(R\) is the universal gas constant (8.314 J mol−1 K−1); \(T\) is the Kelvins temperature (K); \({K}_{f}\) is the equilibrium constant obtained from the Freundlich model (mg1−n Ln g−1).
Reusability was an important index to evaluate the economic benefit of the adsorbent. The recycle potential of 220BMHC was evaluated in this study, and the results are shown in Fig. 7a. The NOR adsorption increased slightly after the first cycle from 16.47 ± 2.21 mg g−1 to 18.11 ± 0.25 mg g−1 in the second cycle and then decreased slightly to 17.10 ± 0.03 mg g−1 in the third cycle; the slight increase might be attributed to the enhanced adsorption sites by using NaOH as the desorption agent (Wang et al. 2017). It was found that 220BMHC can be reused and regenerated multiple times with very little effect on its adsorption capacity. Electron microscopy and elemental mapping of spent 220BMHC were studied to confirm the results. Compared with virgin 220BMHC (Fig. 1), spent 220BMHC showed no obvious change in surface morphology (Fig. S3), and the uniformly distributed F indicated the obvious adsorption of NOR onto 220BMHC (Hu et al. 2024). Furthermore, the F 1s content increased from 0.98% to 1.47% upon NOR adsorption, as indicated by the XPS data (Fig. 9a), and the FTIR bands of HCs and BMHCs revealed new peaks at 1384 cm−1 (N = O) (Fig. 4) after NOR adsorption (Zhou et al. 2024), both of which further confirmed NOR was adsorbed. The stable structure of 220BMHC after adsorption and the minimal decrease in adsorption capacity of 220BMHC after multiple cycles emphasize its potential for recycling.
3.4 Mechanisms of NOR adsorption enhancement
It was worth noting that 180HC (36.86 ± 2.21 mg g−1) performed the best NOR adsorption capacity among HCs. However, 220BMHC (68.53 ± 3.15 mg g−1) showed the greatest improvement and the highest NOR adsorption capability. The NOR adsorption enhancement mechanism of ball-milled hydrochar was investigated. Commonly, pore-filling, electrostatic interaction, hydrophobic distribution, H-bonds, and π-π EDA were the main mechanisms involved in organic contaminants being removed by hydrochar (Jalilian et al. 2024; Huang et al. 2023).
It was believed that a larger SSA and abundant pore volume were beneficial for pollutant adsorption by pore-filling interaction (Lyu et al. 2018). However, for HCs, the increase of SSA (Table 1) with the HTC temperature increase was not consistent with the decreased NOR adsorption capacity, indicating that pore-filling was not the dominant mechanism of NOR adsorption by hydrochar. The NOR adsorption was normalized with SSA (Table S6) and found that the normalized NOR adsorption increased from 1.51 (200HC) and 1.19 mg m−2 (220HC) to 4.65 (200BMHC) and 5.52 mg m−2 (220BMHC), indicating that other adsorption mechanisms were enhanced. Whereas, 180HC and 180BMHC owned similar normalized NOR adsorption capacities (4.24 and 4.07 mg m−2), indicating that pore-filling might be the NOR adsorption enhancement mechanism of 180HC after ball-milling.
NOR was an ionizable organic matter that had two different pKa values (Table S1 and Fig. S4). When pH < 6.2, NOR was cationic (NOR+), when pH > 8.5, NOR was anionic (NOR−), and when 6.2 < pH < 8.5, NOR was zwitterionic (NOR±). Meanwhile, the hydrochar surface was negatively charged at solution pH values > 3.23 (Fig. 7d). To investigate the contribution of electrostatic interaction, the pH effect on NOR adsorption by hydrochar was compared as shown in Fig. 7b, c. It could be observed that BMHCs had higher NOR adsorption than HCs no matter what pH values, and pH played an important role in NOR adsorption (Jing et al. 2022). In the pH range of 4.97–9.04, the NOR adsorption performances of BMHCs were highly effective. According to the pH change before (pHinitial) and after (pHfinal) adsorption (Fig. 7e), it was found that when pHinitial values were at 4.97, 6.71, and 9.04, the pHfinal values were stable around 6.08–6.58. Considering the pH of hydrochar (5.41–5.59) was weakly acidic (Table 2), the stable pHfinal was attributable to the pH buffering effect of hydrochar (Cavali et al. 2022). If the electrostatic interaction was dominant, the NOR adsorption performance should be enhanced in the acid pH (3.96 or 3.04) condition because hydrochar possessed a negative charge while NOR existed as cations and electrostatic attraction occurred. However, no matter whether the pH value decreased from 4.97 to 3.04 or the pH value increased from 9.04 to 10.97, the NOR adsorption was decreased (Fig. 7b, c), suggesting that the electrostatic interaction was not the main adsorption mechanism. Besides, the surface potential of BMHCs was less negative than that of HCs (Fig. 7d), which was also observed on ball-milled corn stalk biochar (Li et al. 2021), suggesting that the NOR adsorption contribution from electrostatic interaction was decreased after hydrochar modified by ball-milling.
For hydrophobic distribution interaction, NOR is a hydrophobic molecule due to its low solubility (280 mg L−1, 25 °C) and high log Kow (0.46) (Table S1), while hydrochar possessed a large amount of noncarbonized organic matter, which could bind NOR through hydrophobic interaction with NOR (Guan et al. 2021). The best adsorption performance happened when NOR was zwitterionic (Fig. 7b, c), where the solubility of NOR was lowest, indicating the importance of hydrophobic interaction. Ionic effect experiments were used to assess the involvement of hydrophobic distribution (Fig. 8a, c). It was possible that Ca2+ was an exchangeable cation that was highly hydrated and might occupy some of the hydrophobic sites on the hydrochar surface and weaken the hydrophobic interaction (Huang et al. 2018). In this study, the effect of Ca2+ on NOR adsorption by 220BMHC was significant (p < 0.05), whereas Na+, Cl−, NO3−, and SO42− had no clear effect on NOR adsorption, indicating that the adsorption process could possess high tolerance to such common coexisting ions (Qu et al. 2023). The inhibition of Ca2+ on NOR adsorption also proved the important role of hydrophobic distribution. 180HC had the lowest degree of carbonization (Table 2) due to its low HTC temperature; this might be the reason that 180HC performed better adsorption performance among pristine hydrochar. In addition, the results of contact angle showed that ball-milling increased the hydrophobicity of hydrochar (Fig. 3c), indicating that ball-milling could enhance the hydrophobic interaction between BMHCs and NOR. However, the \(n\) of the Freundlich model (Table S4) showed middle un-linear (0.38–0.48), indicating that hydrophobic interaction was also not the dominant mechanism.
For H-bonds, NOR owned 7 acceptors and 2 donors of H-bond (Table S1), and hydrochar owned large amounts of oxygen-functional groups such as hydroxyl and carboxyl groups (Fig. 3b), which could form H-bonds with NOR (Kah et al. 2017). The HA effect experiment was used to assess the involvement of H-bonds (Fig. 8d). It was reported that HA primarily formed H-bonds with NOR (Salawu et al. 2022). Thus, in the presence of HA, the NOR bonded with 220BMHC through H-bonds would decrease, leading to a decrease in the removal of NOR by 220BMHC. In this study, the effect of HA on NOR adsorption by 220BMHC was significant (p < 0.05), indicating that H-bonds were involved in the adsorption of NOR on BMHC. Besides, the -OH peaks of HCs and BMHCs obtained from FTIR after NOR adsorption shift from ~ 3424 cm−1 to ~ 3413 cm−1 (Fig. 4), suggesting that H-bonds interaction was involved (Qu et al. 2022). With the HTC temperature increase, the NOR adsorption capacity of HCs was decreased (Fig. 5c), but the concentrations of functional groups were increased (Fig. 3d), indicating that the H-bonds interaction was not the dominant mechanism. After ball-milling, the concentrations of functional groups (Fig. 3d) and the NOR adsorption capacity of BMHCs (Fig. 5d) were both increased compared to corresponding HCs, proving that the H-bonds interaction between NOR and BMHCs might be enhanced.
For π-π EDA interaction, the benzene ring of NOR with a strong electron-absorbing group of fluorine could act as a π-electron acceptor (Lan et al. 2024), and the oxygen-functional groups and the aromatic ring on the hydrochar could be used as electron donors. Thus, π-π interactions between NOR and hydrochar would happen. The C 1s XPS spectra of 220 BMHC before and after NOR adsorption were analyzed to identify the involvement of π-π EDA interaction. The main convolution peaks of 220BMHC were C-Si (282.5 eV), C–C/C-H/C = C (284.7 eV), C–O–C/C–OH (286.69 eV), and C = O (287.88 eV) (Li et al. 2021; Zhou et al. 2024). The C–C/C-H/C = C on 220BMHC could act as π electron donors to react with the π electron acceptors (benzene and six-membered rings) in NOR (Table S1). After NOR adsorption, the relative abundances of C–C/C-H/C = C on 220BMHC reduced by 19.38%, indicating that π-π EDA interaction occurred (Zhou et al. 2024). For HCs, with the increased HTC temperature, the aromaticity of hydrochar increased (Fig. 2b and Table 2), but the NOR adsorption capacity decreased, indicating π-π EDA interaction was not the dominant mechanism. The conclusion could also be proved from the results of the pH (3) effect (Fig. S5). When pH = 3, the hydrophobic distribution and electrostatic interaction contribution of NOR adsorbed to HC were weak (Yang et al. 2012), and the NOR adsorption of HCs was similar (1.03–1.44 mg g−1). It was reported that ball-milling could increase the π cloud density and therefore enhance the π-π EDA interaction (Harindintwali et al. 2023). At pH = 3, the adsorption of NOR on 200BMHC (from 1.14 to 2.33 mg g−1) and 220BMHC (from 1.03 to 2.64 mg g−1) obviously (p < 0.05) increased after ball-milling, while NOR adsorption on 180BMHC (1.45 mg g−1) was similar (p < 0.05) to 180HC (1.44 mg g−1). The results showed that ball-milling slightly enhanced the π-π EDA interaction. The lack of significant change in 180HC and 180BMHC might be due to their low degree of carbonization (Table 2).
Hydrochar had a high content of DOM (Fig. 3e), which could bind with organic pollutants through multiple mechanisms (Fu et al. 2018; Yang et al. 2020). To evaluate the contribution of DOM, the DOM-released hydrochar (freeze-drying) was used in NOR adsorption, and the results were shown in Fig. 8b. It could be observed that after removing DOM from hydrochar, the NOR adsorption was slightly decreased (1.42–10.57%), indicating that DOM released from hydrochar was involved in NOR adsorption. However, the minimal changes in NOR adsorption performance might be attributed to the enhanced SSA and pore volume of hydrochar upon removal of DOM, exposing more adsorption sites (Wang et al. 2017).
In summary, NOR adsorption by ball-milled hydrochar was physically spontaneous, with multiple adsorption mechanisms involved. SSA and the pore volume of hydrochar were not the most dominant factors in NOR adsorption. As shown in Fig. 10, the enhanced NOR adsorption on ball-milled hydrochar was due to the enhancement of H-bonds, π-π EDA, and hydrophobic interaction, and among them, the hydrophobic interaction played an important role.
3.5 Economic analysis
To estimate the cost of using ball-milled hydrochar (BMHC) to remediate the NOR-polluted water, large-scale application was considered in assessing the cost of the adsorbent. The cost estimation method (Text S4) developed by Chen et al. (2023) was adopted. The total cost of the synthesized BMHC includes material, production, source, labor, and other costs, which is USD$ 41.62/t (Table S7). It was worth noting that hydrothermal water was rich in organic matter; the costs can be further reduced if it can be used effectively rather than treated as wastewater. Compared with the price of other materials used for environmental remediation, which ranged from USD$ 152.8/t to USD$ 9690/t (Table S8), the BMHC is a potential facile, green, and large-scale production adsorbent to remove NOR in contaminated water.
4 Conclusions
Ball-milling was a feasible way to enhance the functional groups and hydrophobicity of water hyacinth hydrochar (BMHC), but not the specific surface area of hydrochar. The norfloxacin adsorption on BMHC was greatly increased compared to pristine HC, and this remarkable enhancement was effective in a wide pH range. The adsorption of norfloxacin on BMHC was physically spontaneous and exothermic, and the enhanced adsorption capacity was owing to boosting H-bonds, π-π EDA, and hydrophobic interaction. These findings from present study enlighten that BMHC is a facile, green, effective, and large-scale production adsorbent to remove quinolones or organic pollutants in contaminated water.
Availability of data and material
Data will be made available on the reasonable request.
References
Boehm HP (1994) Some aspects of the surface-chemistry of carbon-blacks and other carbons Some aspects of the surface-chemistry of carbon-blacks and other carbons. Carbon 32(5):759–769. https://doi.org/10.1016/0008-6223(94)90031-0
Cavali M, Libardi Junior N, Mohedano RdA, Belli Filho P, da Costa RHR, de Castilhos Junior AB (2022) Biochar and hydrochar in the context of anaerobic digestion for a circular approach: An overview. Sci Total Environ 822:153614. https://doi.org/10.1016/j.scitotenv.2022.153614
Cavali M, Libardi Junior N, de Sena JD, Woiciechowski AL, Soccol CR, Belli Filho P, Bayard R, Benbelkacem H, de Castilhos Junior AB (2023) A review on hydrothermal carbonization of potential biomass wastes, characterization and environmental applications of hydrochar, and biorefinery perspectives of the process. Sci Total Environ 857(Pt 3):159627. https://doi.org/10.1016/j.scitotenv.2022.159627
Chen X, Liang S, Tao S, Yu W, Yuan S, Jian S, Wan N, Zhu Y, Bian S, Liu Y, Huang L, Duan H, Awasthi MK, Yang J (2023) Sludge-derived iron-carbon material enhancing the removal of refractory organics in landfill leachate: Characteristics optimization, removal mechanism, and molecular-level investigation. Sci Total Environ 904:166883. https://doi.org/10.1016/j.scitotenv.2023.166883
Cheng L, Ji Y, Shao Q (2021) Facile modification of hydrochar derived from cotton straw with excellent sorption performance for antibiotics: Coupling DFT simulations with experiments. Sci Total Environ 760:144124. https://doi.org/10.1016/j.scitotenv.2020.144124
Edeh IG, Mašek O (2021) The role of biochar particle size and hydrophobicity in improving soil hydraulic properties. Eur J Soil Sci 73(1):13138. https://doi.org/10.1111/ejss.13138
Fick J, Soderstrom H, Lindberg RH, Phan C, Tysklind M, Larsson DG (2009) Contamination of surface, ground, and drinking water from pharmaceutical production. Environ Toxicol Chem 28(12):2522–2527. https://doi.org/10.1897/09-073.1
Fu H, Wei C, Qu X, Li H, Zhu D (2018) Strong binding of apolar hydrophobic organic contaminants by dissolved black carbon released from biochar: A mechanism of pseudomicelle partition and environmental implications. Environ Pollut 232:402–410. https://doi.org/10.1016/j.envpol.2017.09.053
Gao J, Zhao M, Xu Z, Liu K, Zhong H, Tsang DCW (2023) Mechanochemical synthesis of calcium-biochar for decontamination of arsenic-containing acid mine drainage. Bioresour Technol 390:129892. https://doi.org/10.1016/j.biortech.2023.129892
Guan J, Liu Y, Jing F, Ye R, Chen J (2021) Contrasting impacts of chemical and physical ageing on hydrochar properties and sorption of norfloxacin with coexisting Cu2+. Sci Total Environ 772:145502. https://doi.org/10.1016/j.scitotenv.2021.145502
Guy Laurent Zanli BL, Tang W, Chen J (2022) N-doped and activated porous biochar derived from cocoa shell for removing norfloxacin from aqueous solution: Performance assessment and mechanism insight. Environ Res 214(Pt 3):113951. https://doi.org/10.1016/j.envres.2022.113951
Harindintwali JD, He C, Xiang L, Dou Q, Liu Y, Wang M, Wen X, Fu Y, Islam MU, Chang SX, Kueppers S, Shaheen SM, Rinklebe J, Jiang X, Schaeffer A, Wang F (2023) Effects of ball milling on biochar adsorption of contaminants in water: A meta-analysis. Sci Total Environ 882:163643. https://doi.org/10.1016/j.scitotenv.2023.163643
He C, Giannis A, Wang J-Y (2013) Conversion of sewage sludge to clean solid fuel using hydrothermal carbonization: Hydrochar fuel characteristics and combustion behavior. Appl Energy 111:257–266. https://doi.org/10.1016/j.apenergy.2013.04.084
Helms JR, Stubbins A, Ritchie JD, Minor EC, Kieber DJ, Mopper K (2008) Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol Oceanogr 53(3):955–969. https://doi.org/10.4319/lo.2008.53.3.0955
Hu Y, Liu Y, Fu W, Yang H (2024) Efficiency and mechanism of enhanced norfloxacin removal using amorphous TiO2-modified biochar. Environ Pollut 351:124027. https://doi.org/10.1016/j.envpol.2024.124027
Huang P, Ge C, Feng D, Yu H, Luo J, Li J, Strong PJ, Sarmah AK, Bolan NS, Wang H (2018) Effects of metal ions and pH on ofloxacin sorption to cassava residue-derived biochar. Sci Total Environ 616–617:1384–1391. https://doi.org/10.1016/j.scitotenv.2017.10.177
Huang J, Feng Y, Xie H, Wu P, Wang M, Wang B, Zhang Q, Zhang S, Liu Z (2023) A bibliographic study reviewing the last decade of hydrochar in environmental application: History, status quo, and trending research paths. Biochar 5(1):12. https://doi.org/10.1007/s42773-023-00210-4
Jalilian M, Bissessur R, Ahmed M, Hsiao A, He QS, Hu Y (2024) A review: Hydrochar as potential adsorbents for wastewater treatment and CO2 adsorption. Sci Total Environ 914:169823. https://doi.org/10.1016/j.scitotenv.2023.169823
Jing F, Guan J, Tang W, Chen J (2022) Mechanistic insight into adsorptive removal of ionic NOR and nonionic DEP organic contaminates by clay-biochar composites. Environ Pollut 310:119881. https://doi.org/10.1016/j.envpol.2022.119881
Kah M, Sigmund G, Xiao F, Hofmann T (2017) Sorption of ionizable and ionic organic compounds to biochar, activated carbon and other carbonaceous materials. Water Res 124:673–692. https://doi.org/10.1016/j.watres.2017.07.070
Kundu D, Sharma P, Bhattacharya S, Gupta K, Sengupta S, Shang J (2024) Study of methylene blue dye removal using biochar derived from leaf and stem of Lantana camara L. Carbon Research 3(1):22. https://doi.org/10.1007/s44246-024-00108-1
Lan Y, Luo Y, Yu S, Ye H, Zhang Y, Xue M, Sun Q, Yin Z, Li X, Xie C, Hong Z, Gao B (2024) Cornstalk hydrochar produced by phosphoric acid-assisted hydrothermal carbonization for effective adsorption and photodegradation of norfloxacin. Sep Purif Technol 330:125543. https://doi.org/10.1016/j.seppur.2023.125543
Li H, Cui S, Tan Y, Peng Y, Gao X, Yang X, Ma Y, He X, Fan B, Yang S, Chen Q (2021) Synergistic effects of ball-milled biochar-supported exfoliated LDHs on phosphate adsorption: Insights into role of fine biochar support. Environ Pollut 294:118592. https://doi.org/10.1016/j.envpol.2021.118592
Li C, Tian Q, Zhang Y, Li Y, Yang X, Zheng H, Chen L, Li F (2022) Sequential combination of photocatalysis and microalgae technology for promoting the degradation and detoxification of typical antibiotics. Water Res 210:117985. https://doi.org/10.1016/j.watres.2021.117985
Liang H, Zhu C, Ji S, Kannan P, Chen F (2022) Magnetic Fe2O3/biochar composite prepared in a molten salt medium for antibiotic removal in water. Biochar 4(1):3. https://doi.org/10.1007/s42773-021-00130-1
Liang H, Zhang J, Hu J, Li X, Li B (2023) Fluoroquinolone residues in the environment rapidly induce heritable fluoroquinolone resistance in Escherichia coli. Environ Sci Technol 57(12):4784–4795. https://doi.org/10.1021/acs.est.2c04999
Liu Y, Sohi SP, Jing F, Chen J (2019) Oxidative ageing induces change in the functionality of biochar and hydrochar: Mechanistic insights from sorption of atrazine. Environ Pollut 249:1002–1010. https://doi.org/10.1016/j.envpol.2019.03.035
Liu L, Sim SF, Lin S, Wan J, Zhang W, Li Q, Peng C (2021a) Integrated structural and chemical analyses for HCl-supported hydrochar and their adsorption mechanisms for aqueous sulfachloropyridazine removal. J Hazard Mater 417:126009. https://doi.org/10.1016/j.jhazmat.2021.126009
Liu Y, Sun Y, Wan Z, Jing F, Li Z, Chen J, Tsang DCW (2021b) Tailored design of food waste hydrochar for efficient adsorption and catalytic degradation of refractory organic contaminant. J Clean Prod 310:127482. https://doi.org/10.1016/j.jclepro.2021.127482
Lyu H, Gao B, He F, Ding C, Tang J, Crittenden JC (2017) Ball-milled carbon nanomaterials for energy and environmental applications. ACS Sustain Chem Eng 5(11):9568–9585. https://doi.org/10.1021/acssuschemeng.7b02170
Lyu H, Gao B, He F, Zimmerman AR, Ding C, Huang H, Tang J (2018) Effects of ball milling on the physicochemical and sorptive properties of biochar: Experimental observations and governing mechanisms. Environ Pollut 233:54–63. https://doi.org/10.1016/j.envpol.2017.10.037
Merry M, Mitan N (2019) Water hyacinth: Potential and Threat. Mater Today: Proc 19:1408–1412. https://doi.org/10.1016/j.matpr.2019.11.160
Monisha RS, Mani RL, Sivaprakash B, Rajamohan N, Vo D-VN (2021) Green remediation of pharmaceutical wastes using biochar: A review. Environ Chem Lett 20(1):681–704. https://doi.org/10.1007/s10311-021-01348-y
Naghdi M, Taheran M, Brar SK, Rouissi T, Verma M, Surampalli RY, Valero JR (2017) A green method for production of nanobiochar by ball milling- optimization and characterization. J Clean Prod 164:1394–1405. https://doi.org/10.1016/j.jclepro.2017.07.084
Peterson SC, Jackson MA, Kim S, Palmquist DE (2012) Increasing biochar surface area: Optimization of ball milling parameters. Powder Technol 228:115–120. https://doi.org/10.1016/j.powtec.2012.05.005
Qu J, Dong M, Bi F, Tao Y, Wang L, Jiang Z, Zhang G, Zhang B, Zhang Y (2022) Microwave-assisted one-pot synthesis of β-cyclodextrin modified biochar for stabilization of Cd and Pb in soil. J Clean Prod 346:131165. https://doi.org/10.1016/j.jclepro.2022.131165
Qu J, Li Y, Sun H, Liu R, Han Y, Bi F, Fan H, Zhang G, Zhang Y, Wang Y, Cao W, Zhang Y (2024) Ball-milled sepiolite/phosphate rock for simultaneous remediation of cadmium-contaminated farmland and alleviation of phosphorus deficiency symptoms in pepper. Chem Eng J 488:150925. https://doi.org/10.1016/j.cej.2024.150925
Qu J, Li Z, Bi F, Zhang X, Zhang B, Li K, Wang S, Sun M, Ma J, Zhang Y (2023) A multiple Kirkendall strategy for converting nanosized zero-valent iron to highly active Fenton-like catalyst for organics degradation. Proc Natl Acad Sci U S A. 120(39):e2304552120. https://doi.org/10.1073/pnas.2304552120.
Salawu OA, Han Z, Adeleye AS (2022) Shrimp waste-derived porous carbon adsorbent: Performance, mechanism, and application of machine learning. J Hazard Mater 437:129266. https://doi.org/10.1016/j.jhazmat.2022.129266
Saning A, Herou S, Dechtrirat D, Ieosakulrat C, Pakawatpanurut P, Kaowphong S, Thanachayanont C, Titirici MM, Chuenchom L (2019) Green and sustainable zero-waste conversion of water hyacinth (Eichhornia crassipes) into superior magnetic carbon composite adsorbents and supercapacitor electrodes. RSC Adv 9(42):24248–24258. https://doi.org/10.1039/c9ra03873f
Sevilla M, Fuertes AB (2009) Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chem Eur J 15(16):4195–4203. https://doi.org/10.1002/chem.200802097
Song C, Shan S, Yang C, Zhang C, Zhou X, Ma Q, Yrjala K, Zheng H, Cao Y (2020) The comparison of dissolved organic matter in hydrochars and biochars from pig manure. Sci Total Environ 720:137423. https://doi.org/10.1016/j.scitotenv.2020.137423
Van Doorslaer X, Dewulf J, Van Langenhove H, Demeestere K (2014) Fluoroquinolone antibiotics: An emerging class of environmental micropollutants. Sci Total Environ 500–501:250–269. https://doi.org/10.1016/j.scitotenv.2014.08.075
Wang B, Zhang W, Li H, Fu H, Qu X, Zhu D (2017) Micropore clogging by leachable pyrogenic organic carbon: A new perspective on sorption irreversibility and kinetics of hydrophobic organic contaminants to black carbon. Environ Pollut 220:1349–1358. https://doi.org/10.1016/j.envpol.2016.10.100
Wang M, Yan J, Diao Y, Zhou X, Luo T, Wang H, Quan G, Sun X, Wang J (2023) Ball milled Mg/Al hydroxides modified nitrogen-rich biochar for arsenic removal: Performance and governing mechanism. Carbon Research 2(1):30. https://doi.org/10.1007/s44246-023-00063-3
Wu J, Wang T, Liu Y, Tang W, Geng S, Chen J (2022) Norfloxacin adsorption and subsequent degradation on ball-milling tailored N-doped biochar. Chemosphere 303(Pt 3):135264. https://doi.org/10.1016/j.chemosphere.2022.135264
Xiao Y, Lyu H, Tang J, Wang K, Sun H (2020) Effects of ball milling on the photochemistry of biochar: Enrofloxacin degradation and possible mechanisms. Chem Eng J 384:123311. https://doi.org/10.1016/j.cej.2019.123311
Xu X, Xu Z, Huang J, Gao B, Zhao L, Qiu H, Cao X (2021) Sorption of reactive red by biochars ball milled in different atmospheres: Co-effect of surface morphology and functional groups. Chem Eng J 413:127468. https://doi.org/10.1016/j.cej.2020.127468
Yan N, Hu B, Zheng Z, Lu H, Chen J, Zhang X, Jiang X, Wu Y, Dolfing J, Xu L (2023) Twice-milled magnetic biochar: A recyclable material for efficient removal of methylene blue from wastewater. Bioresour Technol 372:128663. https://doi.org/10.1016/j.biortech.2023.128663
Yang W, Lu Y, Zheng F, Xue X, Li N, Liu D (2012) Adsorption behavior and mechanisms of norfloxacin onto porous resins and carbon nanotube. Chem Eng J 179:112–118. https://doi.org/10.1016/j.cej.2011.10.068
Yang F, Zhang Q, Jian H, Wang C, Xing B, Sun H, Hao Y (2020) Effect of biochar-derived dissolved organic matter on adsorption of sulfamethoxazole and chloramphenicol. J Hazard Mater 396:122598. https://doi.org/10.1016/j.jhazmat.2020.122598
Yang L, Shuang E, Liu J, Sheng K, Zhang X (2022) Endogenous calcium enriched hydrochar catalyst derived from water hyacinth for glucose isomerization. Sci Total Environ 807(Pt 2):150660. https://doi.org/10.1016/j.scitotenv.2021.150660
Yu S, Zhang H, Ni J, Xiang Y, Wei R, Qian W, Chen W (2023) Spectral characteristics coupled with self-organizing maps analysis on different molecular size-fractionated water-soluble organic carbon from biochar. Sci Total Environ 857:159424. https://doi.org/10.1016/j.scitotenv.2022.159424
Yu C, Dan J, Liu Z, Wang J, Wang J, Fang H, Lai F, Li D, Li L, Li F, Zhou C, Huang B (2024) A facile, green strategy to synthesize N/P self-doped, biomass-derived, hierarchical porous carbon from water hyacinth for efficient VOCs adsorption. Fuel 358:130136. https://doi.org/10.1016/j.fuel.2023.130136
Zhang W, Zhou J, Liu Q, Xu Z, Peng H, Leng L, Li H (2024) A novel intelligent system based on machine learning for hydrochar multi-target prediction from the hydrothermal carbonization of biomass. Biochar 6(1):19. https://doi.org/10.1007/s42773-024-00303-8
Zhou Y, Wang Z, Hu W, Zhou Q, Chen J (2024) Norfloxacin adsorption by urban green waste biochar: characterization, kinetics, and mechanisms. Environ Sci Pollut Res Int 31(20):29088–29100. https://doi.org/10.1007/s11356-024-33085-4
Funding
This study was supported by the National Natural Science Foundation of China (No: 41731282).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Jingqi Wu, Tongshuai Wang, Shijia Li and Zilong Zhao. Wei Tang and Shuhan Yu revised the manuscript. Jiawei Chen acquired funding, supervised the study, and reviewed and edited the manuscript. The first draft of the manuscript was written by Jingqi Wu and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Handling Editor: Fengchang Wu.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, J., Wang, T., Li, S. et al. A green method to improve adsorption capacity of hydrochar by ball-milling: enhanced norfloxacin adsorption performance and mechanistic insight. Carbon Res. 3, 60 (2024). https://doi.org/10.1007/s44246-024-00145-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44246-024-00145-w