Abstract
The utilization of nuclear energy power and nuclear weapon tests not only releases large amounts of radionuclides into environment, but also needs 235U as nuclear fuel for nuclear energy generation. Covalent organic frameworks (COFs) have the advantages of tunable porous structures, adjustable active sites and enough special functional groups, which assure the high selective preconcentration of target radionuclides from complex solutions. In this perspective, the selective extraction of radionuclides (U(VI) as representative cationic ion, Tc(VII) as representative anionic ion, I2 as gaseous nuclide and other nuclides) by COFs through sorption, and photocatalytic strategies are described, and the results show the high efficiency of COFs in target radionuclides removal. The perspective and challenges for the real applications of COFs in future are discussed in the end.
Graphical Abstract

Highlights
• Selective sorption of uranium, iodine and pertechnetate was described.
• Photoreduction of U(VI) to U(IV) was reviewed and discussed.
• The challenges for the real application of COFs in future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the fast development and utilization of nuclear energy, large amounts of radionuclides are released into the natural environment, which not only have impacts on environmental pollution, but also threaten the human health through food chain. On the other hand, the sustainable development of nuclear power utilization, the extraction of uranium from spent fuel, wastewater or seawater is crucial to supply the nuclear fuel. In the last decade, many methods such as sorption, precipitation, photoreduction, electrochemistry precipitation, crystallization, chemical complexation, piezocatalytic, membrane separation, etc., have been used to separate the target radionuclides from complex systems (Chen et al. 2023a; Hao et al. 2023; Liu et al. 2023; Yang et al. 2021; Wen et al. 2023). However, the highly efficient selective extraction of target radionuclide is not only dependent on the properties of radionuclide itself, but also related to the properties of the materials such as structures, channel pore space, active sites, and special functional groups. Different kinds of materials such as polymers, metal-organic frameworks (MOFs), covalent-organic frameworks (COFs), graphene, etc., have been investigated and the results showed that the active sites, inner pore sizes, tunable structures and special functional groups are critical parameters to improve the high selective sorption of radionuclides (Gu et al. 2022; Leng et al. 2023; Sheng et al. 2017; Sun et al. 2018; Yang et al. 2023a; Yin et al. 2017). To improve the photocatalytic reduction of high valent radionuclide to low valence such as U(VI) to form U(IV) precipitate, the strategies to enhance visible light absorption and separation of photogenerated e-/h+ pairs are utilized. For the electrochemical enrichment of radionuclide, the active sites as electron transfer platform in the electrode, high conductivity and high stability of the electrode are most important parameters for separation of radionuclides (Chen et al. 2023b; Niu et al. 2023; Xie et al. 2023). Besides experiments, machine leaning can be firstly carried out to understand the interaction of radionuclides with nanomaterials, which is helpful to design and construct the nanomaterials with high performance (Wei et al. 2024a, b). Although many reviews have summarized the recent works about the sorption of radionuclides from solutions (Abney et al. 2017; Di et al. 2022; Mei et al. 2023; Wang et al. 2019; Xie et al. 2022a), the separation of cationic, anionic and gaseous radionuclides from complex systems using COFs by sorption, photocatalytic and electrocatalytic strategies are not systematically described. In this perspective, we mainly described the selective extraction of representative cationic radionuclide U(VI), anionic radionuclide Tc(VII) and gaseous radionuclide I2 by COFs through the abovementioned techniques, and challenges for real applications are summarized in the end.
2 Removal of radioactive uranium
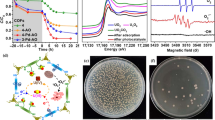
235U(VI) is the most important element for nuclear fuel. The extraction of 235U(VI) from wastewater or seawater is thereby crucial for nuclear energy utilization. The commonly used methods for uranium extraction currently include adsorption, ion exchange, electrochemistry, solid-phase extraction, membrane filtration, biological treatment, photocatalysis, etc. (Wu et al. 2023; Zhang et al. 2023). In recent years, adsorption photocatalytic synergy has become a research hotspot. In addition, the adsorption sites of photocatalysts for U (VI) photoreduction reactions are the decisive factor in photocatalytic reduction of uranium. When U (VI) is adsorbed by the U (VI) adsorption site of the photocatalyst, the adsorbed U (VI) becomes the site for electron capture. As a result, the adsorbed U (VI) can easily obtain electrons and then be reduced. Therefore, constructing efficient U (VI) adsorption sites on photocatalysts is a necessary condition for developing advanced photocatalytic uranium reduction catalysts (Chen et al. 2022). COFs with special functional groups such as amidoxime group could form strong surface complexes with 235U(VI), to achieve the high selective sorption of 235U(VI) from solutions. Through tuning the local structure to enhance the visible light absorption, to generate/separate the e-/h+ pairs and to facilitate the charge transfer to adsorbed 235U(VI), the photoreduction of adsorbed U(VI) to U(IV) species could be enhanced and continuously formed precipitates on COFs. In this sorption-photocatalytic reduction strategy, the first step is the selective sorption of U(VI) by COFs, and then the second step is the reduction of U(VI) to form U(IV) precipitates (Chen et al. 2023b; Yang et al. 2023b). Through tuning the donor-acceptor (D-A) sites (Fig. 1a), the distribution of local charge and separation of charge carrier resulted in the enhancement of photocatalytic ability. The EPR analysis showed that the free active radicals of ·O2-, ·OH and 1O2 were generated under visible light irradiation. These oxygen-containing free radicals inhibit the growth of marine microorganisms, enabling COFs to exhibit excellent anti-biological pollution activity, thus ensuring efficient uranium extraction photocatalysis in seawater. The XAFS characterization showed the photocatalytic reduction of U(VI) to form UO2 precipitate. Through the advanced spectroscopy analysis, the reduction mechanism of U(VI) is illustrated in Fig. 1b. Furthermore, Feng et al. (2022) constructed hydrogen-bonded COFs using Cl- ions to connect the organic ligands. The electron-rich of COFs strengthened H-bonds and photogenerated electrons under visible light conditions could efficiently reduce adsorbed U(VI) photocatalytically to form U(IV) precipitate, with the capacity of 1.7 g U/g COFs. The results showed that the soluble U(VI) could be immobilized by the COFs, thereby reduced the dangerous of U(VI) in the environment.
a The strategy for the construction of D-A system in multivariate COFs. b Photocatalytic mechanism of U(VI) reduction to U(IV) by COFs under visible light irradiation (Yang et al. 2023b). c Adsorption kinetics of ReO4- by TFPM-PZ-Cl. d Removal efficiency of ReO4- by TFPM-PZ-Cl in the presence of competitive anions (Zhang et al. 2022). e Structure diagram and crystal structure (large spheres indicate pore size) (Guo et al. 2020). f Mixed-component (150 ppm I2 and 50 ppm CH3I) breakthrough experiments performed at 25 °C (Xie et al. 2022b). g Survey of Xe/Kr separation performance in top-performing MOFs, COFs, and porous organic cages (Jia et al. 2021)
Up to now, most of the methods for uranium removal have revolved around the adsorption of the radionuclide uranium through the design of rational COFs structural sites. In addition, with the development of solar energy utilization technology, the intramolecular D-A COFs exhibit excellent U(VI) photocatalytic reduction properties. However, the COFs materials are usually in powder state, which is unfavorable for recovery and recycling, and can be considered to composite with other adsorption materials or made into electrode state. The electrocatalytic strategy for U(VI) extraction from seawater is also a very promising method for continuously deposition of U(VI) on the electrode with high selectivity and reusability. The combination of adsorption with photocatalytic and electrocatalytic methods for the removal of uranium deserves to be studied.
3 Removal of radioactive 99TcO4 - and ReO4 -
Anionic radionuclides such as 99TcO4- are most toxic radionuclides because of their high mobility, low sorption ability, high water solubility and low complexation property. 99TcO4- can easily diffuse into the natural environment, resulting in the radiotoxicity to human health through the accumulation in the food chain. Because of the high radiotoxicity, 99TcO4- is not allowed in most laboratories for conducting research as the license of 99TcO4-/ReO4- is normally used to understand the behavior of 99TcO4- because of their very similar physicochemical properties. Chen et al. (2023c) synthesized the ionic COFs and applied for the removal of 99TcO4- and ReO4- from solutions. The hydrophobic skeleton and high charge density of the ionic COFs lead to the high selective removal of 99TcO4-/ReO4-. Additionally, unlike 2D COFs, Zhang et al. (2022) constructed 3D COFs through Aldol condensation with TAMP-PZI or TFPM-PZI as cationic tetrahedral building block. The COFs exhibited high irradiation, strong acid and base stability, and batch adsorption experimental results have shown that TFPM-PZ-Cl can quantitatively remove ReO4- with a concentration of 28 ppm from the solution in almost 30 seconds, exhibiting high adsorption kinetics (Fig. 1c). Despite the presence of high concentrations of competing anions, TFPM-PZ-Cl remained good selectivity towards ReO4- (Fig. 1d). The positive ionic sites and the high porosity of the 3D COFs assured the selective adsorption of 99TcO4-/ReO4- with high efficiency. The DFT calculation further indicated the electrostatic repulsion between Cl- and 99TcO4-/ReO4- promoted the adsorption ability of 99TcO4-/ReO4-. In order to improve the stability of the adsorbent under extreme conditions, He et al. (2019) reported the separation of 99TcO4- under extreme conditions by 2D cationic COFs, and the results showed that the cationic COFs exhibited the sorption capacity of 702 mg/g ReO4-, with fast sorption kinetics, high sorption capacity and excellent selectivity through anion exchange of Cl- with 99TcO4-.
At present, various methods such as ion exchange, extraction, and precipitation have been used to capture 99TcO4-. Among them, the removal of 99TcO4- through ion exchange is the most widely used due to its ease of implementation and efficient recovery rate. COFs benefit from their ordered pore structure and large specific surface area, and their adsorption performance can exceed that of polymer materials without crystallinity. However, it is a great challenge to synthesize cationic COFs, which can be used stably for 99TcO4- removal. Therefore, it is crucial for practical applications to design the construction units of COFs reasonably and ensure their chemical stability under strong acid, strong alkali, and high radiation conditions.
4 Removal of radioactive iodine
Radioiodine (131I, 129I) is one most important radionuclide. In nuclear power plants, the iodine (I2) or iodomethane (CH3I) is forms and released from off-gas steam, which is easily volatilized and thereby diffuses to the environment, causing harm to ecological systems and human health (Xie et al. 2024). Liu et al. (2022a) constructed phthalocyanine-based Cu-COFs for the separation of I2 and CH3I, and achieved the sorption capacities of 3.0 g/g for I2 (T = 353 K, ambient pressure, contact time is 48 h) and 492 mg/g for CH3I (T = 298 K, contact time is 24 h). The outstanding ability was attributed to charge transfer from N-rich phthalocyanine and e--rich π-conjugated structures, which formed strong interaction with I2 molecule. I2 obtained charge to form polyiodide (Ix-), which further formed strong complexes with Cu(II) centres through electrostatic interaction, also resulting in the high sorption of I2. Besides the active sites and functional groups to transfer charges from COFs to I2 molecule to form strong electrostatic complexes, the micro-/macro-pores of COFs are also crucial for the separation of I2 or CH3I from solutions through the pore space effect. Hao et al. (2024) synthesized COFs using symmetric building blocks to generate tetragonal or hexagonal pores in COFs using imine-based linkers, and then partitioned the COFs with 2,5-diaminobenzonitrile (C2) or 5''-(4'-amino[1,1'-biphenyl]-4-yl) (C3) to divide the mesopore/micropore into two or three micropores. After partition, the COFs have more N-containing groups which are favorable to improve I2 adsorption performance. The batch experimental results showed that the capture of I2 and CH3I at 75 °C by the C3 divided COF (COF 3-2P) was much higher than that by the undivided COF (COF 3). The Raman, XPS and FTIR characterization illustrated that I2 was adsorbed in the forms of I5- and I3- through the interaction with the N-rich sites. After I2 adsorption, the electrons were transferred from N atoms to I2 to form I5- and I3-. The DFT calculation revealed that the adsorption of I2 or CH3I was mainly attributed to the interaction with the triazine N sites. The column experiments showed that I2 and CH3I were efficiently separated by COF 3-2P under different conditions. The breakthrough curves of I2 and CH3I in the COF 3-2P column were affected by the temperature obviously, and the adsorption of I2 and CH3I were affected by the temperature. The pore space of COFs could be partitioned into uniformly micropore size, which not only enhance the adsorption of I2 or CH3I molecules by adjusting the pore space of the COFs, but also separate them through controlling the pore size using suitable symmetric agents. This work firstly reported the partition of COFs using symmetric building blocks to change the inner-pore space and to introduce more N-rich sites, which were suitable to adsorb I2 molecules in the inner-pore spaces with the help of N-rich groups to improve I2 adsorption. Similarly, the adsorption of I2 by cage-derived COFs also indicated that the binding interaction of I5- and I3- with N-rich cage contributed the high adsorption of I2 (T = 348 K, contact time is 50 h) (Liu et al. 2022b). In order to further improve performance and structural stability, Guo et al. (2020) synthesized colyliform 2D COFs with quasi-3D topologies, large pore spaces and flexible constitution units (Fig. 1e). The COFs exhibited high adsorption capacity of I2 (6.29 g/g) at 75 °C and 1 bar with high irradiation stability and reusability. The V-shaped monomer in the COFs not only increased the adaptive ability and flexibility of COFs, but also reduced the π-π interaction of COF interlayers, thereby enhanced I2 adsorption. Adsorbents that can effectively remove I2/CH3I are being increasingly studied, but few adsorbents can effectively adsorb low concentrations of I2 and CH3I simultaneously. Xie et al. (2022b) reported the simultaneous adsorption of I2 and CH3I by COF-TAPT. In the dynamic mixed-gas adsorption with 150 ppm of I2 and 50 ppm of CH3I, COF-TAPT presents an excellent total iodine capture capacity (1.51 g·g-1 at 25 °C), surpassing various benchmark adsorbents (Fig. 1f). The simultaneous capture of I2 and CH3I was attributed to the intermolecular interaction with I2 through the phenyl rings, triazine and imine moieties, and the N-methylation reactions at the nucleophilic N sites to promote CH3I capture.
Currently, the adsorption of radioactive iodine (volatile iodine and organic iodine) by COFs is limited to the laboratory stage. It is possible to simulate the real environment where radioactive iodine is located under the premise of guaranteeing the safety, to determine the selectivity and adsorption performance of COFs under real and complex conditions, and to evaluate their stability. Thereby, it is necessary to provide more persuasive research data for the practical application of radioactive iodine treatment.
5 Removal of other radioactive nuclides
Thorium is also one of the naturally occurring radioactive elements. In nature, the content of thorium is four times that of uranium. Thorium is considered the ‘nuclear fuel of the future’ because of its physical and chemical properties that are superior to those of uranium, its higher energy density and the fact that 232Th can be converted to 233U by neutron bombardment in nuclear reactors (Jyothi et al. 2023). A three-dimensional COF material named COF-DL229 has been successfully synthesized and applied to effectively selective entrapment of Th (IV) (Liu et al. 2022c). Due to the exposure of nitrogen-containing imine bonds, the maximum saturated adsorption capacity of this COF for Th (IV) can reach 513 mg g-1, with a fast capture rate and reaching equilibrium within 1 minute. The adsorption mechanism was further explored through DFT calculations. The DOS results indicates that the addition of Th (IV) can reduce the band gap of COF-DL229, improve both conductivity and adsorption energy. Therefore, this work provides scientific research value for using three-dimensional COF materials in the field of Th (IV) adsorption. Compared to the solid and liquid fission products generated by nuclear reactors, the safe disposal of gaseous fission products is more difficult. Jia et al. (2021) prepared two novel sub-nanoporous covalent organic frameworks (COFs) using a multiple-site alkylation strategy and applied them for the separation of xenon/krypton for the first time. Due to their excellent stability, designability of structure, and slightly larger pore size (~7 Å) than the dynamic diameter of krypton/xenon, the COFs realize the effective adsorption and sieving of krypton/xenon. In comparison with previously reported metal organic frameworks (MOFs) and porous organic cages, TFP-TAPA-Bu exhibits outstanding adsorption and separation performance for Xe/Kr (Fig. 1g).
In summary, existing work reports have demonstrated the enormous potential of COFs in spent fuel reprocessing and nuclear environment remediation, providing important theoretical and experimental basis for their practical applications.
6 Perspective
From the abovementioned representative works about the synthesis and application of different types of COFs for the separation or extraction of radionuclides uranium, iodine and technetium from complex systems, one can conclude that COFs exhibited high selectivity and sorption capacity to adsorb the target radionuclides from complex solutions under different conditions, even from strong acidic or basic solutions. The modification of active sites and stable active centres, layer stacking mode, inner pore size and space configuration, adaptive and flexibility of structures, functional groups, and electron transfer pathway could provide special properties of COFs, which are favorable for the binding of target radionuclide with high selectivity, ability, stability, and reusability through ion exchange, surface complexation, photocatalytic reduction and electrocatalytic precipitation strategies. However, for the real application of COFs in the extraction of radionuclides from wastewater, there are still several challenges. For example, 1) the synthesis of COFs is generally carried out under relative complicated conditions. The mild condition for the synthesis of COFs should be developed; 2) the construction of COFs is generally dependent on the experimental experiences. The accurate design of COFs is really a little difficult without abundant background knowledge. Machine learning could help us to understand, to predict and to select the most suitable precursors for COFs construction; 3) the stability of COFs is usually under very extreme conditions, especially in nuclear spent fuel conditions such as strong irradiation, strong acid and presence of different homologue radionuclides. The very similar physicochemical properties of the homologues make them difficult to be separated efficiently; 4) the synthesis of COFs in large scale at low price is the main challenge for real application. With the development of technology and the experience of COFs synthesis, COFs could be synthesized easily at low cost in large scale. The grafting of special groups to bind the target radionuclides or the post-modification of COFs with tunable pore space and structure could guarantee the special separation of radionuclides under extreme conditions. Thereby, we could conclude that COFs could be the promising materials for the radioactive wastewater treatment in future.
Availability of data and materials
Authors can confirm that all relevant data are included in the article.
References
Abney CW, Mayes RT, Saito T, Dai S (2017) Materials for the recovery of uranium from seawater. Chem Rev 117:11133–11140. https://doi.org/10.1021/acs.chemrev.7b00355
Chen T, Yu KF, Dong CX, Yuan X, Gong X, Lian J, Cao X, Li MZ, Zhou L, Hu BW, He R, Zhu WK, Wang XK (2022) Advanced photocatalysts for uranium extraction: elaborate design and future perspectives. Coord Chem Rev 467:214615. https://doi.org/10.1016/j.ccr.2022.214615
Chen ZS, Li Y, Cai YW, Wang SH, Hu BW, Li BF, Ding XD, Zhuang L, Wang X (2023a) Application of covalent organic frameworks and metal-organic frameworks nanomaterials in organic/inorganic pollutants removal from solutions through sorption-catalysis strategies. Carbon Res 2:8. https://doi.org/10.1007/s44246-023-00041-9
Chen Z, Wang J, Hao M, Xie Y, Liu X, Yang H, Waterhouse GIN, Wang X, Ma S (2023b) Tuning excited electronic structure and charge transport in covalent organic frameworks for enhanced photocatalytic performance. Nat Commun 14:1106. https://doi.org/10.1038/s41467-023-36710-x
Chen XR, Zhang CR, Liu X, Liang RP, Qiu JD (2023c) Ionic covalent organic framework for selective detection and adsorption of TcO4-/ReO4-. Chem Commun 59:9521–9524. https://doi.org/10.1039/d3cc02429f
Di ZY, Mao YN, Yuan H, Zhou Y, Jin J, Li CP (2022) Covalent organic frameworks (COFs) for sequestration of 99TcO4-. Chem Res Chinese University 38:290–295. https://doi.org/10.1007/s40242-022-1447-9
Feng LJ, Yuan YH, Yan BJ, Feng TT, Jian YP, Zhang JC, Sun WY, Lin K, Luo GS, Wang N (2022) Halogen hydrogen-bonded organic framework (XHOF) constructed by singlet open-shell diradical for efficient photoreduction of U(VI). Nat Commun 13:1389. https://doi.org/10.1038/s41467-022-29107-9
Gu H, Liu X, Wang S, Chen Z, Yang H, Hu B, Shen C, Wang X (2022) COF-based composites: extraordinary removal performance for heavy metals and radionuclides from aqueous solutions. Rev Environ Contam Toxicol 260:23. https://doi.org/10.1007/s44169-022-00018-6
Guo XH, Li Y, Zhang MC, Cao KC, Tian Y, Qi Y, Li SJ, Li K, Yu XQ, Ma LJ (2020) Colyliform crystalline 2D covalent organic frameworks (COFs) with quasi-3D topologies for rapid I2 adsorption. Angew Chem Int Ed 59:22697–22705. https://doi.org/10.1002/anie.202010829
Hao MJ, Liu YF, Wu WJ, Wang SY, Yang XY, Chen ZS, Tang ZW, Huang QF, Wang SH, Yang H, Wang XK (2023) Advanced porous adsorbents for radionuclides elimination. Energy Chem 5:100101. https://doi.org/10.1016/j.enchem.2023.100101
Hao M, Xie Y, Lei M, Liu X, Chen Z, Yang H, Waterhouse GIN, Ma S, Wang X (2024) Pore space partition synthetic strategy in imine-linked multivariate covalent organic frameworks. J Am Chem Soc 146:1904–1913. https://doi.org/10.1021/jacs.3c08160
He L, Liu S, Chen L, Dai X, Li J, Zhang M, Ma F, Zhang C, Yang Z, Zhou R, Chai Z, Wang S (2019) Mechanism unravelling for ultrafast and selective 99TcO4- uptake by a radiation-resistant cationic covalent organic frameworks: a combined radiological experiment and molecular dynamics simulation study. Chem Sci 10:4293–4305. https://doi.org/10.1039/C9SC00172G
Jia ZM, Yan ZT, Zhang J, Zou YD, Qi Y, Li XF, Li Y, Guo XH, Yang CT, Ma LJ (2021) Pore Size Control via Multiple-Site Alkylation to Homogenize Sub-Nanoporous Covalent Organic Frameworks for Efficient Sieving of Xenon/Krypton. ACS Appl Mater Interfaces 13:1127–1134. https://doi.org/10.1021/acsami.0c14610
Jyothi RK, De Melo LGTC, Santos RM, Yoon HS (2023) An overview of thorium as a prospective natural resource for future energy. Energy Res 11:1132611. https://doi.org/10.3389/fenrg.2023.1132611
Leng R, Sun YC, Feng R, Zhao GX, Qu Z, Wang CZ, Han B, Wang JJ, Ji ZY, Wang XK (2023) Design and Fabrication of Hypercrosslinked Covalent Organic Adsorbents for Selective Uranium Extraction. Environ Sci Technol 57:9615–9626. https://doi.org/10.1021/acs.est.3c02916
Liu XW, Zhang AR, Ma R, Wu B, Wen T, Ai YJ, Sun MT, Jin J, Wang SH, Wang XK (2022a) Experimental and theoretical insights into copper phthalocyanine-based covalent organic frameworks for highly efficient radioactive iodine capture. Chinese Chem Lett 33:3549–3555. https://doi.org/10.1016/j.cclet.2022.03.001
Liu C, Jin YC, Yu ZH, Gong L, Wang HL, Yu BQ, Zhang W, Jing JZ (2022b) Transformation of porous organic cages and covalent organic frameworks with efficient iodine vapor capture performance. J Am Chem Soc 144:12390–12399. https://doi.org/10.1021/jacs.2c03959
Liu XJ, Xiao ST, Jin TT, Gao F, Wang M, Gao YA, Zhang W, Ouyang YG, Ye GA (2022c) Selective entrapment of thorium using a three-dimensional covalent organic framework and its interaction mechanism study. Sep Purif Technol 296:121413. https://doi.org/10.1016/j.seppur.2022.121413
Liu XL, Li Y, Tan CH, Chen ZS, Yang H, Wang XK (2023) Highly selective extraction of U(VI) from solutions by metal organic framework-based nanomaterials through sorption, photochemistry and electrochemistry strategies. Langmuir 39:18696–18712. https://doi.org/10.1021/acs.langmuir.3c02739
Mei DC, Liu LJ, Yan B (2023) Adsorption of uranium(VI) by metal-organic frameworks and covalent organic frameworks from water. Coord Chem Rev 475:214917. https://doi.org/10.1016/j.ccr.2022.214917
Niu CP, Zhang CR, Liu X, Liang RP, Qiu JD (2023) Synthesis of propanone-linked covalent organic frameworks via Claisen-Schmidt reaction for photocatalytic removal of uranium. Nat Commun 14:4420. https://doi.org/10.1038/s41467-023-40169-1
Sheng D, Zhu L, Xu C, Wang Y, Chen L, Diwu J, Chen J, Chai Z, Albrecht-Schmitt TE, Wang S (2017) Efficient and selective uptake of 99TcO4- by a cationic metal-organic framework material with open Ag+ sites. Environ Sci Technol 51:3471–3479. https://doi.org/10.1021/acs.est.7b00339
Sun Q, Aguila B, Earl LD, Abney CW, Wojtas L, Thallaally PK, Ma SQ (2018) Covalent organic frameworks as a decorating platform for utilization and affinity enhancement of chelating sites for radionuclide sequestration. Adv Mater 30:1705479. https://doi.org/10.1002/adma.201705479
Wang X, Chen L, Wang L, Fan Q, Pan D, Li J, Chi F, Yu S, Xie Y, Xiao C, Luo F, Wang J, Wang X, Chen C, Wu W, Shi W, Wang X (2019) Synthesis of novel nanomaterials and their application in efficient removal of radionuclides. Sci China Chem 62:933–967. https://doi.org/10.1007/s11426-019-9492-4
Wei X, Lu Z, Ai Y, Shen L, Wei M, Wang X (2024a) Implementing and understanding the unsupervised transfer learning in metal organic framework toward methane adsorption from hypothetical to experimental data. Sep Purif Technol 330:125291. https://doi.org/10.1016/j.seppur.2023.125291
Wei X, Liu Y, Shen L, Lu Z, Ai Y, Wang X (2024b) Machine learning insights in predicting heavy metals interaction with biochar. Biochar 6:10. https://doi.org/10.1007/s42773-024-00304-7
Wen CM, Yao YJ, Meng LY, Duan EZ, Wang M, Chen ZS, Wang XX (2023) Photocatalytic and electrocatalytic extraction of uranium by COFs: a review. Ind Eng Chem Res 62:18230–18250. https://doi.org/10.1021/acs.iecr.3c02831
Wu Y, Xie YH, Liu XL, Li Y, Wang JY, Chen ZS, Yang H, Hu BW, Shen C, Tang ZW, Huang QF, Wang XK (2023) Functional nanomaterials for selective uranium recovery from seawater: Material design, extraction properties and mechanisms. Coord Chem Rev 485:215097. https://doi.org/10.1016/j.ccr.2023.215097
Xie Y, Liu ZY, Geng YY, Li H, Wang N, Song YP, Wang XL, Chen J, Wang JC, Ma SQ, Ye G (2022a) Uranium extraction from seawater: material design, emerging technologies and marine engineering. Chem Soc Rev 52:97–162. https://doi.org/10.1039/d2cs00595f
Xie YQ, Pan TT, Lei Q, Chen CL, Dong XL, Yuan YY, Maksoud WA, Zhao L, Cavallo Pinnau I, Han Y (2022b) Efficient and simultaneous capture of iodine and methyl iodide achieved by a covalent organic framework. Nat Commun 13:2878. https://doi.org/10.1038/s41467-022-30663-3
Xie Y, Wu Y, Liu X, Hao M, Chen Z, Yang H, Waterhouse GIN, Ma S, Wang X (2023) Design and modulation of “uranium nanotraps” in covalent organic frameworks to optimize uranium extraction performance in seawater. Cell Rep Phys Sci 4:101220. https://doi.org/10.1016/j.xcrp.2022.101220
Xie Y, Rong Q, Mao F, Wang S, Wu Y, Liu X, Hao M, Chen Z, Yang H, Waterhouse GIN, Ma S, Wang X (2024) Engineering the pore environment of antiparallel stacked covalent organic frameworks for dynamic capture of iodine pollutants. Nat Commun 15:2671. https://doi.org/10.1038/s41467-024-46942-0
Yang H, Liu X, Hao M, Xie Y, Wang X, Tian H, Waterhouse GIN, Kruge PE, Telfer SG, Ma S (2021) Functionalized iron−nitrogen−carbon electrocatalyst provides a reversible electron transfer platform for efficient uranium extraction from seawater. Adv Mater 33:2106621. https://doi.org/10.1002/adma.202106621
Yang X, Wu W, Xie Y, Hao M, Liu X, Chen Z, Yang H, Waterhouse GIN, Ma S, Wang X (2023a) Modulating anion nanotraps via halogenation for high efficiency 99TcO4− removal under wide−ranging conditions. Environ Sci Technol 57:10870–10881. https://doi.org/10.1021/acs.est.3c02967
Yang H, Hao M, Xie Y, Liu X, Chen Z, Wang X, Waterhouse GIN, Ma S (2023b) Tuning local charge distribution in multicomponent covalent organic frameworks for dramatically enhanced photocatalytic uranium extraction. Angew Chem Int Ed 62:e202303129. https://doi.org/10.1002/ange.202303129
Yin ZJ, Xu SQ, Zhan TG, Qi YQ, Wu ZQ, Zhao X (2017) Ultrahigh volatile iodine uptake by hollow microspheres formed from a heteropore covalent organic framework. Chem Commun 53:7266–7269. https://doi.org/10.1039/C7CC01045A
Zhang D, Fang L, Liu LJ, Zhao B, Hu BW, Yu SJ, Wang XK (2023) Uranium extraction from seawater by novel materials: a review. Sep Purif Technol 320:124204. https://doi.org/10.1016/j.seppur.2023.124204
Zhang CR, Cui WR, Yi SM, Niu CP, Liang RP, QI JX, Chen XJ, Jiang W, Liu X, Luo QX, Qiu JD, (2022) An ionic vinylene-linked three-dimensional covalent organic framework for selective and efficient trapping of ReO4- or 99TcO4-. Nat Commun 13:7621. https://doi.org/10.1038/s41467-022-35435-7
Acknowledgements
The authors acknowledged the anonymous reviewers for favorable comments to improve the quality of this review.
Funding
National Natural Science Foundation of China (U2067215; U21A20290) and Beijing Outstanding Young Scientist Program.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The manuscript was written by Qiuyu Rong. The review and editing were performed by Jie Jin, Suhua Wang and Xiangke Wang. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No.
Consent for publication
Agree.
Competing interests
The authors declare that they have no known competing interests that could have appeared to influence the work reported in this paper.
Additional information
Handling editor: Shaobin Wang.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rong, Q., Jin, J., Wang, S. et al. Recent progress of covalent organic frameworks in high selective separation of radionuclides. Carbon Res. 3, 52 (2024). https://doi.org/10.1007/s44246-024-00137-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44246-024-00137-w