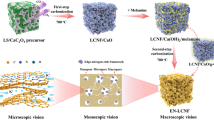
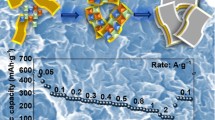
Abstract
Lithium-ion batteries (LIBs) are widely used in portable energy storage. The capacity of commercial graphite is difficult to improve due to the stoichiometry limit of LiC6 of graphite, thus new anodes need to be developed to meet the demand of high-energy–density LIB. The growing interest in graphitized carbon nitride (g-C3N4) stems from its structural resemblance to graphite and its capacity to offer abundant adsorption and intercalation sites. However, g-C3N4, as a semiconductor, has a low lithium transfer rate due to its poor conductivity and high diffusion resistance. Improving the electron transport rate of g-C3N4 and reducing the adsorption energy barrier of Li+ in g-C3N4 are the keys to improving the electrochemical performances of g-C3N4. In this study, lignin and melamine were homogeneously mixed using the spray drying method, followed by the preparation of covalently bonded C3N4/LC material through a one-step carbonization process. The uniform dispersion of g-C3N4 in amorphous carbon can improve the conductivity and reduce the diffusion energy barrier of Li+. As a result, the C3N4/LC-x anode has better electrochemical behavior, including higher reversible capacity, better rate performance, and cycle stability.
Highlights
• The covalently bonded C3N4/LC-x material was prepared through a one-step carbonization method.
• The uniform dispersion of g-C3N4 in amorphous carbon could improve the electronic conductivity and reduce the diffusion energy barrier of Li+ ions.
• C3N4/LC-2 showed high reversible capacity, ideal rate performance, and cycle life.
AbstractSection Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Lithium-ion battery (LIBs) is widely used in portable electronic products, new energy electrical vehicles, aerospace, and other fields. It is necessary to improve the properties of batteries, including energy density, power density, cycle stability, etc., to meet the growing demands of new applications (Dunn et al. 2011; Huang et al. 2023). Graphite, as a commercial anode of LIBs, has a theoretical capacity of 372 mAh g−1 that is difficult to improve due to the stoichiometry limit of LiC6 of graphite (Kubota et al. 2020). Therefore, a new anode material needs to be developed to improve the capacity and rate performance of lithium-ion storage.
Graphitized carbon nitride (g-C3N4) is a typical layered two-dimensional (2D) material. Because of its structural similarities to graphite, layered g-C3N4 can be regarded as graphene with a high nitrogen content and rich planar pores, capable of supplying rich adsorption and intercalation sites (Dong et al. 2017; Xu et al. 2017a), which is garnering the interest of battery researchers. Previous studies have shown that g-C3N4 has a high lithium storage capacity of up to 524 mAh g−1 (vs. 372 mAh g−1 of graphite) (Hankel et al. 2015; Jorge et al. 2014; Veith et al. 2013). The low conductivity of g-C3N4 as a semiconductor, however, restricts the electron transfer rate, resulting in a low real capacity (Shi et al. 2016). Furthermore, g-C3N4 has high adsorption energy for Li+, as shown by density functional theory (DFT) calculations. This means that Li+ faces a significant diffusion barrier of around 2.4 eV between each adsorption site in the g-C3N4 layer (Wu et al. 2013). In addition, significant structural distortion after Li intercalation results in a rapid capacity decline in cycling. Therefore, improving the electron transport rate of g-C3N4, reducing the adsorption energy barrier of Li+ in g-C3N4, and improving the structural stability of g-C3N4 are the keys to improving the electrochemical performance of g-C3N4.
Coupling the conductive amorphous carbon with g-C3N4 to form a composite can effectively alleviate the above issues, making g-C3N4 a feasible strategy for high-performance LIBs anode (Zhu et al. 2019). However, excellent dispersion of g-C3N4 in carbon materials is difficult to achieve. Previous studies have used ball milling or chemical synthesis methods to achieve the combination of conductive carbon skeleton and g-C3N4. However, it is easy to destroy the structure of g-C3N4 or consume too many chemicals in the composite process (Weng et al. 2019). Our previous studies have shown that the chemicals bond between supramolecules (g-C3N4 precursors) and biomass molecules could be used to achieve uniform dispersion of carbon precursors and C3N4. As a result, the amorphous carbon and g-C3N4 can form covalent bonds during carbonization process. This will facilitate the formation of a new g-C3N4-based carbon material (Subramaniyam et al. 2017). As a result, the key is figuring out how to distribute the two precursors evenly.
The high-value utilization of industrial lignin is low at present (Jian et al. 2022; Zhu et al. 2023), and the high carbon content and rich functional phenolic structure give lignin the prerequisite for the carbon electrode precursor and lignin could be easily regulated microstructure (Kizzire et al. 2021; Wen et al. 2022; Zhang et al. 2024). Lignin-derived carbon is expected to be a sustainable and low-cost electrode material (Shen et al. 2019; Zhang et al. 2022). In this work, the homogeneous mixing of lignin and melamine was achieved by a spray drying method, and then the covalently bonded C3N4/LC-x material was prepared by a one-step carbonization strategy. The uniform dispersion of g-C3N4 in amorphous carbon could improve the electrical conductivity and reduce the diffusion energy barrier of lithium ions. A high proportion of edge nitrogen and a low proportion of graphite nitrogen could regulate the nitrogen-containing groups in g-C3N4 and improve the structural stability of g-C3N4. Therefore, compared with the g-C3N4 anode, the C3N4/LC-x electrode has better electrochemical behavior, including higher reversible capacity, better rate performance, and cycling stability.
2 Experimental section
First, the melamine was dissolved in 1 L of deionized water, heated at 90 ℃ and stirred until completely dissolved. Then 6.5 g corncob lignin and 2 mL ammonia were added and continued to stir for 6 h. The mass ratio of lignin to melamine was 1:1, 1:2, 1:3, and 1:10, respectively. The precursor was obtained by spray drying the obtained solution. The precursor was recorded as MA/AL-x, and x was 1, 2, 3, and 10 corresponding to the mass ratio of lignin to melamine 1:1, 1:2, 1:3, and 1:10, respectively. Then the precursor was carbonized in a tube furnace at 550 ℃ for 2 h under N2 atmosphere to obtain C3N4/LC-x. x was 1, 2, 3, and 10 corresponding to the mass ratio of lignin to melamine 1:1, 1:2, 1:3, and 1:10, respectively (Fig. 1).
3 Results and discussion
3.1 Characterization of C3N4/LC-2
According to the surface morphology of the precursor (Fig. S1), the texture of MA/AL-2 was the most unconsolidated, reflecting that the ratio of precursor lignin to melamine was the most evenly dispersed. According to the SEM of Fig. 2a, b, and c, C3N4 was unconsolidated in texture, but the composite C3N4/LC-2 shows a larger particle size than LC. LC, the lignin-carbon concave sphere was filled after the composite of C3N4. Preferable composite changes in the material surface morphology. During the carbonization process, amorphous carbon could destroy the structure of the bulk C3N4. The formed small C3N4 fragments could adhere to amorphous carbon, forming C3N4/LC materials with large interlayer spacing. The combination of amorphous carbon and C3N4 can improve the diffusion kinetics of lithium ions. Comparing LC with other composite proportions of C3N4/LC-1, 2, 3, and 10, the larger the proportions of C3N4, the smaller the particle size (Fig. S2), indicating that preferable composite may generate larger intermolecular forces (π-π bond, etc.), making the structure of C3N4/LC-2 denser. In Fig. 2d, e, f, TEM images, the 2D geometry of LC was irregular, C3N4 appears as a regular spherical shape, and the composite C3N4/LC-2 indicated the carbon layer connected with the sphere, providing ion transport channels. HRTEM (Fig. 2g, h, i) indicated the difference between the layered structure after composite and the unconsolidated structure before composite, indicating that the layers of the composite form through intermolecular forces (π-π bond, etc.) (Fu et al. 2017). The SEM elemental mapping images in Fig. 2j show that a homogeneous distribution of N, O, and C elements indicates a uniform dispersion of C3N4/LC-2, suggesting that lignin and melamine achieved uniform dispersion.
To confirm the successful composite of lignin carbon with C3N4 and its chemical bond properties, a variety of analytical techniques were used. In the XRD pattern of the precursor (Fig. S3a), the characteristic peaks of the composite precursor MA/AL-x were shown. In the FT-IR spectra of the precursor (Fig. S3b), the characteristic peaks of AL after composite MA/AL-x were significantly changed in comparison, the shrinkage of OH and NH at around 3500 to 3000 cm−1 and the shrinkage of C-N at 1050 cm−1 became stronger. The larger x is, the stronger the stretching vibration containing the nitrogen bond is. The benzene peak of MA/AL-2 was the strongest(Yang et al. 2007), which indicates that the combination of this ratio enhances the π-π bond and provides conditions for the strong intermolecular force of C3N4/LC-2. The result shows that the π-π bond can be strengthened by the composite of this ratio, which provides conditions for the strong intermolecular force of C3N4/LC-2. In the XRD pattern of Fig. 3a, the (100) peak of C3N4 at 12.73° was caused by the trigonal nitrogen linkage of tri-s-triazine units (Niu et al. 2012; Yang et al. 2013), (002) peak contrast LC was located at 26.96° at a larger angle, C3N4/LC-2 after composite, (002) peak shifted to a larger angle, and (101) peak did not change. Compared with C3N4, C3N4/LC-2 did not have the same high crystalline phase content, but retained the characteristics of amorphous carbon. It could indicate that C3N4 interacted with the LC, which was attributed to the fact that C-N–C bond formed between the lone pair of pyridine nitrogen in the C3N4 void and the carbon in the LC. The XRD pattern of C3N4/LC-1, 2, 3, and 10 (Fig. S4a) also shows that as the ratio of melamine in precursor became larger, the (002) peak shifted to a larger angle, which was closer to the characteristic peak of C3N4 (Fina et al. 2015; Guo et al. 2016). In the Raman spectrum of Fig. 3b, C3N4 has no Raman scattering peak, and the ID/IG value of C3N4/LC-2 was 1.15, much higher than the ID/IG value of LC 0.76, indicating more defects and disordered phases in C3N4/LC-2. Although the formation of C3N4 results in the decrease of the graphitization degree, it will enhance the surface wettability as well as provide extra defects to enhance Li+ storage (Li et al. 2016). The comprehensive results confirm its positive influence on the resultant materials. However, the Raman spectra of C3N4/LC-3, 10 (Fig. S4c) did not have obvious D and G peaks. With the proportion of melamine increased in the precursor, the carbonized sample could generate more crystalline C3N4. Strong fluorescence coverage of crystalline C3N4 could cover the D and G bands in the Raman spectrum. Excessive crystalline C3N4 was detrimental to conductivity and diffusion kinetics of lithium ions. The FT-IR spectrum (Fig. 3c) suggests that C3N4/LC-2, compared with LC, has decreased C-H shrinkage at 800 cm−1, shrinkage of benzene carbon at 1400 cm−1, stretching vibrations of C-N and C = C at 800 cm−1, and slightly stretching vibrations at about 3300 cm−1 (Zhang et al. 2018). It indicates the presence of C3N4 fragment in C3N4/LC-2. The delocalized electrons of C-N and the electron cloud of π bond on C = C were conducive to increasing the electron transfer rate and reducing the adsorption energy of lithium ions (Wang et al. 2018). In contrast, the FT-IR spectrum of C3N4/LC-3, 10 (Fig. S4b) showed the characteristic stretching vibration of crystalline C3N4, which was not conducive to improving the electron transfer rate. In (Fig. 3d) the XPS full spectrum, it can be seen that when compared C3N4/LC-2 with LC, the N content significantly increased, but C3N4/LC-3 had higher nitrogen content (Fig. S4d). The XPS C 1 s spectrum in Fig. 3e shows the chemical bonds change of C. Compared with LC, C-N and N–C = N appear in C3N4/LC-2, which was completely different from LC. The XPS C 1 s spectra of C3N4/LC-1, 3, 10 were compared with C3N4/LC-2 (Fig. S4e), and the C–C/C = C ratio of C3N4/LC-3, 10 was too low, which was not conducive to the intercalation of lithium. The N–C = N ratio of C3N4/LC-1 was too low, the increase of delocalized electrons may be less than that of C3N4/LC-2, and the increase of electron transfer rate may be insufficient (Li et al. 2015; Tahir et al. 2013). Figure 3f shows the XPS N 1 s spectrum. Due to the low nitrogen content of LC, the analysis of chemical bonds could not be fitted. C3N4/LC-2 had higher content of N-(C)3 and lower content of C-NHx. This may be because when the precursor was mixed, the dispersion of melamine in the lignin was better, so the amino group was relatively dispersed, resulting in low thermal stability of the amino group and decomposition in the pyrolysis process (Zhao et al. 2023). Comparing XPS N 1 s spectrum of C3N4/LC-1, 3, 10 with that of C3N4/LC-2 (Fig. S4f), the proportion of N-(C)3 in C3N4/LC-3, 10 was too low, and the proportion of N–C = N in C3N4/LC-1 was too low, which was not beneficial for the improvement of conductivity (He et al. 2015; Tahir et al. 2014; Yin et al. 2023).
3.2 Electrochemical performances
Figure 4a shows the first three cycles of the C3N4 anode CV plot, with a severe irreversible reaction. The first three cycles of C3N4/LC-1, 3, 10 anode CV also had different degrees of irreversible reaction, and the first three cycles of LC anode CV were irregular in shape and not close to capacitive reaction (Fig. S5). As demonstrated in Fig. 4b, the first three cycles of the C3N4/LC-2 anode CV had a lower degree of irreversibility and a more trapezoidal shape, suggesting that the electrochemical reaction was closer to the capacitive reaction. This suggests that the uniform dispersion of C3N4 aids in enhancing the rate of electron transfer and lowering the adsorption energy of lithium ions. The first cycle of GCD curves in Fig. 4c shows a higher capacity and ICE of C3N4/LC-2, reaching 749.2 mAh g−1 and 59.9%, respectively. The reason for the low initial coulombic efficiency of C3N4/LC-2 was caused by excessive irreversible defects from C3N4. The rate and cycling performance in Fig. 4d, e also demonstrate the high-rate performance and cycling stability of C3N4/LC-2. Compared with other composite ratios of C3N4/LC-x, C3N4/LC-2 also had the best rate performance and cycling stability (Fig. S6). It was due to the electronic structure of C3N4 being properly regulated and large interlayer spacing could improve the stability of the carbon layer.
Figure 5a shows the b value of the half-cell of C3N4/LC-2 anode. The b value of the anode was as high as 0.917, close to 1, indicating that the electrochemical reaction of the anode of C3N4/LC-2 was mainly due to capacitance contribution and adsorption (Xu et al. 2017b; Zhang et al. 2021). Figure 5b shows the capacitance contribution of the anode of C3N4/LC-2 at different scan rates, and the proportion of capacitance contribution was higher with the increase of scan rate, indicating that the electrochemical reaction of the anode of C3N4/LC-2 was mainly adsorption (Yin et al. 2021, 2020). In order to elucidate the electrochemical behavior of C3N4/LC-2 anode, the impedance of C3N4/LC-2 anode was measured, and the impedance data were analyzed after preparation and cycling. The Nyquist plot (Fig. 5c) consists of a semicircle at high frequency and a straight line at low frequency which were attributed to the electrolyte or solution resistance (Rs) offered at the electrode–electrolyte interface and the charge transfer resistance (Rct) in the case of the semicircle, and the Warburg lithium diffusion (Wdiff) in the case of the straight line (Zhong et al. 2022). By comparing the EIS curves of C3N4/LC-2, C3N4 and LC negative electrodes, the Rs and Wdiff (Rs = 1.6 Ω, Wdiff = 90.7 Ω) of C3N4/LC-2 negative electrodes were the lowest (Table S1), indicating that the preferable composite of C3N4/LC effectively promoted ion diffusion and reduced the adsorption energy of lithium ions. The diffusion impedance and interface impedance were reduced. The Rct of the anode of C3N4/LC-2 was also lower than that of C3N4, indicating that the uniform dispersion of C3N4 was conducive to electron transfer. The Warburg coefficient (σ, its unit is Ω/\(\sqrt{s}\)) can be obtained from the slope of the Warburg plot (Fig. 5d), and the diffusion coefficient of lithium-ions DLi+ at the interfacial regions can be estimated by the equation σ = \(\frac{RT}{{An}^{2}{F}^{2}C\sqrt{2{D}_{{Li}^{+}}}}\) (S4) (Yi et al. 2017). The relationship between the actual resistance (Z') of the three anodes and the square root of the frequency (ω−0.5) was obtained (Ding et al. 2018). The diffusion coefficient DLi+ of C3N4/LC-2 anode was the highest, 2.7*10−13, which was much higher than 1.2*10−13 of LC anode and 1.2*10−14 of C3N4 negative electrode, indicating that the preferable composite of C3N4/LC-2 and the uniform dispersion of C3N4 effectively promoted lithium-ion diffusion.
(a) Fitting slope b value of C3N4/LC-2 CV; (b) relative contributions of the capacitive and diffusion-controlled charge storage processes at different scan rates of C3N4/LC-2; (c) Nyquist plots for LC, C3N4/LC-2, and C3N4; (d) corresponding relationship between Z’ and ω.−0.5 (where ω = 2πf) of LC, C3N4/LC-2, and C3N4
4 Conclusion
In this study, the C3N4/LC-2 composite was crafted and synthesized through a straightforward and convenient method. This synthesized material enables uniform dispersion of C3N4 in an amorphous carbon matrix, facilitates the generation of numerous nitrogen active sites, promotes electron transfer, and reduces the energy barrier for lithium-ion adsorption. C3N4/LC-2 exhibits outstanding properties, such as high reversible capacity, favorable rate performance, and extended cycle life. Moreover, it introduces a novel approach to the structural design of modified carbon-based anodes for lithium-ion batteries.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ding R, Zhang J, Qi J, Li Z, Wang C, Chen M (2018) N-doped dual carbon-confined 3d architecture rgo/fe(3)o(4)/ac nanocomposite for high-performance lithium-ion batteries. ACS Appl Mater Interfaces 10:13470–13478. https://doi.org/10.1021/acsami.8b00353
Dong J, Xue Y, Zhang C, Weng Q, Dai P, Yang Y, Zhou M, Li C, Cui Q, Kang X, Tang C, Bando Y, Golberg D, Wang X (2017) Improved Li(+) storage through homogeneous n-doping within highly branched tubular graphitic foam. Adv Mater 29:1603692. https://doi.org/10.1002/adma.201603692
Dunn B, Kamath H, Tarascon JM (2011) Electrical energy storage for the grid: a battery of choices. Science 334:928–935. https://doi.org/10.1126/science.1212741
Fina F, Callear SK, Carins GM, Irvine JTS (2015) Structural investigation of graphitic carbon nitride via xrd and neutron diffraction. Chem Mater 27:2612–2618. https://doi.org/10.1021/acs.chemmater.5b00411
Fu J, Yu J, Jiang C, Cheng B (2017) g-C3N4-Based heterostructured photocatalysts. Adv Energy Mater 8:1701503. https://doi.org/10.1002/aenm.201701503
Guo W, Li X, Xu J, Liu HK, Ma J, Dou SX (2016) Growth of highly nitrogen-doped amorphous carbon for lithium-ion battery anode. Electrochim Acta 188:414–420. https://doi.org/10.1016/j.electacta.2015.12.045
Hankel M, Ye D, Wang L, Searles DJ (2015) Lithium and sodium storage on graphitic carbon nitride. J Phys Chem C 119:21921–21927. https://doi.org/10.1021/acs.jpcc.5b07572
He B, Li W-C, Lu A-H (2015) High nitrogen-content carbon nanosheets formed using the Schiff-base reaction in a molten salt medium as efficient anode materials for lithium-ion batteries. Journal of Materials Chemistry A 3:579–585. https://doi.org/10.1039/c4ta05056h
Huang S, Qiu X-Q, Wang C-W, Zhong L, Zhang Z-H, Yang S-S, Sun S-R, Yang D-J, Zhang W-L (2023) Biomass-derived carbon anodes for sodium-ion batteries. New Carbon Mater 38:40–66. https://doi.org/10.1016/s1872-5805(23)60718-8
Jian W, Zhang W, Wu B, Wei X, Liang W, Zhang X, Wen F, Zhao L, Yin J, Lu K, Qiu X (2022) Enzymatic hydrolysis lignin-derived porous carbons through ammonia activation: activation mechanism and charge storage mechanism. acs appl mater interfaces 14:5425–5438. https://doi.org/10.1021/acsami.1c22576
Jorge AB, Corà F, Sella A, McMillan PF, Brett DJL (2014) Electrochemical properties of graphitic carbon nitrides. Int J Nanotechnol 11:737. https://doi.org/10.1504/ijnt.2014.063784
Kizzire DG, Richter AM, Harper DP, Keffer DJ (2021) Lithium and sodium ion binding mechanisms and diffusion rates in lignin-based hard carbon models. ACS Omega 6:19883–19892. https://doi.org/10.1021/acsomega.1c02787
Kubota K, Shimadzu S, Yabuuchi N, Tominaka S, Shiraishi S, Abreu-Sepulveda M, Manivannan A, Gotoh K, Fukunishi M, Dahbi M, Komaba S (2020) Structural analysis of sucrose-derived hard carbon and correlation with the electrochemical properties for lithium, sodium, and potassium insertion. Chem Mater 32:2961–2977. https://doi.org/10.1021/acs.chemmater.9b05235
Li H, Gan S, Wang H, Han D, Niu L (2015) Intercorrelated superhybrid of agbr supported on graphitic-c3n4-decorated nitrogen-doped graphene: high engineering photocatalytic activities for water purification and co2 reduction. Adv Mater 27:6906–6913. https://doi.org/10.1002/adma.201502755
Li D, Zhang L, Chen H, Wang J, Ding L-X, Wang S, Ashman PJ, Wang H (2016) Graphene-based nitrogen-doped carbon sandwich nanosheets: a new capacitive process controlled anode material for high-performance sodium-ion batteries. Journal of Materials Chemistry A 4:8630–8635. https://doi.org/10.1039/c6ta02139e
Niu P, Zhang L, Liu G, Cheng HM (2012) Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv Func Mater 22:4763–4770. https://doi.org/10.1002/adfm.201200922
Shen Y, Peng F, Cao Y, Zuo J, Wang H, Yu H (2019) Preparation of nitrogen and sulfur co-doped ultrathin graphitic carbon via annealing bagasse lignin as potential electrocatalyst towards oxygen reduction reaction in alkaline and acid media. J Energy Chem 34:33–42. https://doi.org/10.1016/j.jechem.2018.09.021
Shi M, Wu T, Song X, Liu J, Zhao L, Zhang P, Gao L (2016) Active Fe2O3 nanoparticles encapsulated in porous g-C3N4/graphene sandwich-type nanosheets as a superior anode for high-performance lithium-ion batteries. Journal of Materials Chemistry A 4:10666–10672. https://doi.org/10.1039/c6ta03533g
Subramaniyam M, C., Deshmukh K.A., Tai Z., Mahmood N., Deshmukh A.D., Goodenough J.B., Dou S.X., Liu H.K. (2017) 2D Layered graphitic carbon nitride sandwiched with reduced graphene oxide as nanoarchitectured anode for highly stable lithium-ion battery. Electrochim Acta 237:69–77. https://doi.org/10.1016/j.electacta.2017.03.194
Tahir M, Cao C, Butt FK, Idrees F, Mahmood N, Ali Z, Aslam I, Tanveer M, Rizwan M, Mahmood T (2013) Tubular graphitic-C3N4: a prospective material for energy storage and green photocatalysis. Journal of Materials Chemistry A 1:13949. https://doi.org/10.1039/c3ta13291a
Tahir M, Cao C, Mahmood N, Butt FK, Mahmood A, Idrees F, Hussain S, Tanveer M, Ali Z, Aslam I (2014) Multifunctional g-C(3)N(4) nanofibers: a template-free fabrication and enhanced optical, electrochemical, and photocatalyst properties. ACS Appl Mater Interfaces 6:1258–1265. https://doi.org/10.1021/am405076b
Veith GM, Baggetto L, Adamczyk LA, Guo B, Brown SS, Sun X-G, Albert AA, Humble JR, Barnes CE, Bojdys MJ, Dai S, Dudney NJ (2013) Electrochemical and solid-state lithiation of graphitic C3N4. Chem Mater 25:503–508. https://doi.org/10.1021/cm303870x
Wang S, Shi Y, Fan C, Liu J, Li Y, Wu XL, Xie H, Zhang J, Sun H (2018) Layered g-C(3)N(4)@reduced graphene oxide composites as anodes with improved rate performance for lithium-ion batteries. ACS Appl Mater Interfaces 10:30330–30336. https://doi.org/10.1021/acsami.8b09219
Wen F, Zhang W, Jian W, He X, Yin J, Shi J, Lin H, Lu K, Qin Y, Qiu X (2022) Sustainable production of lignin-derived porous carbons for high-voltage electrochemical capacitors. Chem Eng Sci 255:117672. https://doi.org/10.1016/j.ces.2022.117672
Weng GM, Xie Y, Wang H, Karpovich C, Lipton J, Zhu J, Kong J, Pfefferle LD, Taylor AD (2019) A promising carbon/g-C(3) N(4) composite negative electrode for a long-life sodium-ion battery. Angew Chem Int Ed Engl 58:13727–13733. https://doi.org/10.1002/anie.201905803
Wu M, Wang Q, Sun Q, Jena P (2013) Functionalized graphitic carbon nitride for efficient energy storage. J Phys Chem C 117:6055–6059. https://doi.org/10.1021/jp311972f
Xu J, Xu F, Qian M, Xu F, Hong Z, Huang F (2017a) Conductive carbon nitride for excellent energy storage. Adv Mater 29:1701674. https://doi.org/10.1002/adma.201701674
Xu J, Zhang W, Hou D, Huang W, Lin H (2017b) Hierarchical porous carbon derived from Allium cepa for supercapacitors through direct carbonization method with the assist of calcium acetate. Chin Chem Lett 28:2295–2297. https://doi.org/10.1016/j.cclet.2017.10.041
Yang H, Yan R, Chen H, Lee DH, Zheng C (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86:1781–1788. https://doi.org/10.1016/j.fuel.2006.12.013
Yang S, Gong Y, Zhang J, Zhan L, Ma L, Fang Z, Vajtai R, Wang X, Ajayan PM (2013) Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light. Adv Mater 25:2452–2456. https://doi.org/10.1002/adma.201204453
Yi L, Liu L, Guo G, Chen X, Zhang Y, Yu S, Wang X (2017) Expanded graphite@SnO2@ polyaniline composite with enhanced performance as anode materials for lithium ion batteries. Electrochim Acta 240:63–71. https://doi.org/10.1016/j.electacta.2017.04.012
Yin J, Zhang W, Wang W, Alhebshi NA, Salah N, Alshareef HN (2020) Electrochemical zinc ion capacitors enhanced by redox reactions of porous carbon cathodes. Adv Energy Mater 10:2001705. https://doi.org/10.1002/aenm.202001705
Yin J, Zhang W, Alhebshi NA, Salah N, Alshareef HN (2021) Electrochemical zinc ion capacitors: fundamentals, materials, and systems. Adv Energy Mater 11:2100201. https://doi.org/10.1002/aenm.202100201
Yin J, Jin J, Chen C, Lei Y, Tian Z, Wang Y, Zhao Z, Emwas AH, Zhu Y, Han Y, Schwingenschlogl U, Zhang W, Alshareef HN (2023) preferential pyrolysis construction of carbon anodes with 8400 h lifespan for high-energy-density k-ion batteries. Angew Chem Int Ed Engl 62:e202301396. https://doi.org/10.1002/anie.202301396
Zhang H, Zhang W, Ming H, Pang J, Zhang H, Cao G, Yang Y (2018) Design advanced carbon materials from lignin-based interpenetrating polymer networks for high performance sodium-ion batteries. Chem Eng J 341:280–288. https://doi.org/10.1016/j.cej.2018.02.016
Zhang W, Sun M, Yin J, Wang W, Huang G, Qiu X, Schwingenschlögl U, Alshareef HN (2021) Rational design of carbon anodes by catalytic pyrolysis of graphitic carbon nitride for efficient storage of Na and K mobile ions. Nano Energy 87:106184. https://doi.org/10.1016/j.nanoen.2021.106184
Zhang W, Qiu X, Wang C, Zhong L, Fu F, Zhu J, Zhang Z, Qin Y, Yang D, Xu CC (2022) Lignin derived carbon materials: current status and future trends. Carbon Research 1:14. https://doi.org/10.1007/s44246-022-00009-1
Zhang W, Huang Z, Alshareef HN, Qiu X (2024) Synthesis strategies and obstacles of lignocellulose-derived hard carbon anodes for sodium-ion batteries. Carbon Research 3:28. https://doi.org/10.1007/s44246-024-00122-3
Zhao L, Sun S, Lin J, Zhong L, Chen L, Guo J, Yin J, Alshareef HN, Qiu X, Zhang W (2023) Defect engineering of disordered carbon anodes with ultra-high heteroatom doping through a supermolecule-mediated strategy for potassium-ion hybrid capacitors. Nanomicro Lett 15:41. https://doi.org/10.1007/s40820-022-01006-0
Zhong L, Zhang W, Sun S, Zhao L, Jian W, He X, Xing Z, Shi Z, Chen Y, Alshareef HN, Qiu X (2022) Engineering of the crystalline lattice of hard carbon anodes toward practical potassium-ion batteries. Adv Func Mater 33:2211872. https://doi.org/10.1002/adfm.202211872
Zhu Y-P, Lei Y, Ming F, Abou-Hamad E, Emwas A-H, Hedhili MN, Alshareef HN (2019) Heterostructured MXene and g-C3N4 for high-rate lithium intercalation. Nano Energy 65:104030. https://doi.org/10.1016/j.nanoen.2019.104030
Zhu D, Yang B, Wang H, Shahnawaz M (2023) Editorial: Lignin valorization: Recent trends and future perspective. Front Bioeng Biotechnol 11:1190128. https://doi.org/10.3389/fbioe.2023.1190128
Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (U23A6005, 22108044), the Research and Development Program in Key Fields of Guangdong Province (2020B1111380002), the Open Foundation of Shanghai Jiao Tong University Shaoxing Research Institute of Renewable Energy and Molecular Engineering (JDSX2023002). The BL19U2 of the Shanghai Synchrotron Radiation Facility (SSRF) for access to the synchrotron-based SAXS is acknowledged (No. 2022-NFPS-PT-500103, 2023-NFPS-PT-500787, 2023-NFPS-JL-02050). This work is also supported by the User Experiment Assist System of Shanghai Synchrotron Radiation Facility. This work is supported by the Guangdong-Hong Kong-Macao Joint Laboratory for Neutron Scattering Science and Technology (P1622112800001). The authors acknowledge the Analysis and Test Center, Guangdong University of Technology for the structural analysis of our specimens.
Funding
National Natural Science Foundation of China (U23A6005, 22108044), Research and Development Program in Key Fields of Guangdong Province (2020B1111380002), the Open Foundation of Shanghai Jiao Tong University Shaoxing Research Institute of Renewable Energy and Molecular Engineering (JDSX2023002), and the user projects of Shanghai Synchrotron Radiation Facility (SSRF) (No. 2022-NFPS-PT-500103, 2023-NFPS-PT-500787, 2023-NFPS-JL-02050).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Xueqing Qiu, and Wenli Zhang performed the supervision, conceptualization, and funding acquisition. Data collections were performed by Shunsheng Yang, Lei Zhong, and Zehua Lin. Material preparation, and analysis were performed by Lei Zhong, and Shunsheng Yang. The first draft of the manuscript was written by Shunsheng Yang. Lei Zhong, Zejie Zhang, Wenli Zhang, and Qiyu Liu commented on previous versions of the manuscript. The submitted version of the manuscript was finalized by Lei Zhong and Wenli Zhang. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
Wenli Zhang is the Lead Guest Editor of Special Issue on “Functional Carbon Materials for Electrochemical Energy Conversion and Storage” and was not involved in the editorial review, or the decision to publish, this article. All authors declare that there are no competing interests.
Additional information
Handling Editor: Minglei Sun.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, S., Zhong, L., Lin, Z. et al. Carbon/C3N4 heterostructures constructed from lignin toward enhanced lithium-ion storage. Carbon Res. 3, 45 (2024). https://doi.org/10.1007/s44246-024-00128-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44246-024-00128-x