Abstract
An innovative strategy for synthesizing novel dual-reaction-center (DRC) catalysts from chicken manure (CM) biochar to purify wastewater that contained emerging contaminants (ECs) is proposed to synchronously address the release of ECs and improper disposal of livestock manure. A series of characterization techniques reveal the formation of a special C-O-Ca bond bridge (cation-π) structure on resourcelized CM nanosheets (RCM NSs). RCM NSs exhibit distinct selectivity and anti-interference capability for various ECs removal in complex matrices, and the water purification system remains stable after 1735 hours (equivalent to 3470 cycles) of operation. Density Functional Tomography (DFT) calculations reveal that trace of peroxymonosulfate as an inducer initiates the continuous donation of electrons from electron-rich ECs and the C-O-Ca bond bridges provide a favorable pathway for electron transfer, which facilitates the electron capture effect of dissolved oxygen in the system. This study provides a novel strategy to convert livestock manure into DRC-catalysts for developing energy-saving and high-efficiency environmental remediation technologies.
Graphical Abstract

Highlights
• The chicken manure biochar was converted into a water purification catalyst through a graphitization process.
• Resourcelized catalyst RCM NSs had superior removal capability for various ECs triggering by trace of PMS.
• RCM NSs could quickly eliminate ECs and simultaneously drive NOM removal, even with NOM interference.
• The key role of dissolved oxygen for water purification was illustrated in RCM NSs system.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Emerging contaminants (ECs) such as endocrine disruptors, pharmaceuticals, personal care products and perfluorinated compounds have been found in earth’s surface water, groundwater, industrial wastewater and even drinking water, which pose serious threats to the ecological environment and human health even at trace levels (Chen et al. 2022; de Vidales et al. 2022; McCance et al. 2018). Due to their resistance to biological degradation (Tong et al. 2021), it is difficult to remove them with conventional water treatment technologies. The elimination of ECs is also susceptible to interference from dissolved organic matter (DOM), inorganic anions, etc., in aqueous environments (Sillanpaa et al. 2018).
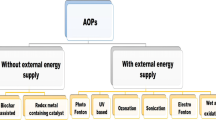
Advanced oxidation process (AOP) is considered a promising water treatment technology to remove ECs due to the relatively strong oxidation capacity and fast reaction rate (Menacherry et al. 2022). Peroxymonosulfate (PMS) activation, one of the widely concerned AOPs, relies on the generation of sulfate radicals (SO4•-), hydroxyl radicals (•OH) and singlet oxygen (1O2) to attack pollutants, showing better performance for water purification. The defects of homogeneous PMS activation process include the large dosage of reagents (metal salts and PMS), the generation of metal sludge, the slow reaction rate due to the limitation of reduction of high-valent metal species, and the difficulty of recycling of active ingredients, which limit the application of homogeneous process (Ghanbari and Moradi 2017; Zhang et al. 2020; Zhu et al. 2020). The development of heterogeneous technologies have solved the above problems to some extent, but the basic principle of PMS activation has not substantially changed, and other bottlenecks still exist, especially the extremely slow interface electron transfer and circulation, which inhibits the reaction activity for water purification and increases the PMS consumption. External energies, such as light, electricity and ultrasound, are even used to speed up interficial electron transfer (Wang et al. 2019), which significantly increases the energy consumption of water purification. In addition, the preparation of heterogeneous catalysts is also an energy-intensive industry. Various agents, such as metal compounds, carriers, templating agents, are inevitably used as precursors for the catalyst synthesis (Wu et al. 2022; Zhang et al. 2020), which causes excessive consumption of resources and energy. It is urgent to develop high-efficiency and low-consumption water purification technologies for ECs removal.
Compared to metal-based catalysts, carbonaceous materials are considered more sustainable catalysts due to the avoidance of potential secondary contamination from metal leaching. Biochar is therefore an ideal candidate for the preparation of water purification catalysts due to its low cost and excellent electronic properties (Liu et al. 2023; Shao et al. 2022). Among biochar, the global manure production rate of major livestock is approximately 12.71 million ton/d and annually increases with the number of farmed livestock (Chávez-Fuentes et al. 2017). Unfortunately, pollutants and pathogens in livestock manure often leach into soil and rivers, which profoundly affects the rural living environment and life health, and has become a hot topic of global concern. Untreated livestock manure may pose a sanitary hazard, contribute to excessive greenhouse gas emissions and eutrophication, and contain numerous zoonotic pathogens that cause disease in organisms (Lim and Kim 2018). Anaerobic digestion, solid–liquid separation and composting are common livestock manure treatment techniques (Palese et al. 2020; Tan et al. 2021). However, the high energy consumption, large land occupied, and long composting times become the evident disadvantages. Conventional livestock manure disposal methods often cannot effectively protect water resources from contamination by excess nutrients, microbial pathogens and pharmaceuticals in the waste (Yang et al. 2020). Hence, an eco-friendly and innovative resourcelized approach to treat livestock manure must be developed.
Actually, livestock manure is rich in organic carbon, complexes and metals, which can serve as the components of environmental functional materials. For example, chicken manure (CM) contains the elements of C, O, N, S, Mg, P, Ca, and Si. The coupling of these elements can be used to well modulate the electronic nonequilibrium state of the catalyst and construct dual-reaction-center (DRC) Fenton-like catalysts for wastewater treatment according to our previous studies (Lu et al. 2020). The construction of the cation-π structure with strong polarities can largely modulate the surface electron distribution and form surface electron-poor/rich microregions (Deng et al. 2021b; Lyu et al. 2020; Lyu et al. 2018; Zhang et al. 2021a). Pollutants were adsorbed on the catalyst surface and acted as electron-donar for the system through cation-π interactions, which dramatically reduced the consumption of additional oxidants and reductants. It is promising to utilize these substances in CM, especially organic carbon, to construct an environmental functional catalyst that contains surface carbon (π) rings for water purification.
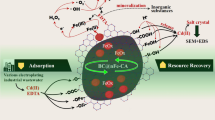
Herein, to address the two issues of efficient removal of ECs from water and effective utilization of livestock manure resources synchronously, a new strategy for the resourcelized conversion of CM into water purification catalyst is proposed. In this work, we successfully convert the disordered structure of CM into an ordered structure to form resourcelized CM nanosheets (RCM NSs) through a innovative graphitization process. Astonishingly, RCM NSs exhibit excellent performance for water purification through eliminating ECs, including ciprofloxacin (CIP), bisphenol A (BPA) and acid orange 7 (AO7), under trace of PMS trigger, which is still very stable after 1735 hours (equivalent to 3470 cycles) of operation. Even actual industrial wastewater can be quickly purified in this system. The structure and typical C-O-Ca (cation-π) configuration of the catalyst are characterized and revealed by a series of technologies. An innovative water purification mechanism for the efficient use of pollutants and dissolved oxygen through the surface excitation of PMS is proposed for the first time.
2 Materials and methodology
2.1 Resourcelized conversion of CM to RCM NSs
No additional chemicals were added to the process of resourcelized conversion of chicken manure to RCM NSs. The obtained wet chicken manure was naturally dried and dehydrated. After proper selection, the required dry chicken manure raw material was used for subsequent catalyst synthesis (the organic functional groups contained in chosen chicken manure are shown in Fig. S1). Before pyrolysis, the dried chicken manure was chopped into small pieces. Then, the RCM NSs catalyst was synthesized by temperature programmed-pyrolysis in the following processes. First, dried chicken manure pieces were placed in ceramic crucibles and subsequently in a muffle furnace for pyrolysis to form preliminary biochar. The temperature was initially fixed at 100 °C for 2 h. A slow heating rate within a certain pyrolysis time will prolong the retention time of pyrolysis materials in the low-temperature zone and promote the charring reaction of substances, which increases the carbonization yield. Afterwards, the temperature was increased to 600 °C in 10 °C min−1 increments and maintained for 1 h in a muffle furnace to fully carbonize into biochar. Finally, the crucibles were cooled to room temperature; then, the prepared material was ground into powder to obtain the RCM NSs catalyst.
2.2 Procedures and analysis
Acid orange 7 (AO7), 2-chlorophenol (2-CP), rhodamine B (RhB), ciprofloxacin (CIP) and bisphenol A (BPA) were selected as representative ECs to evaluate the catalytic performance of the RCM NSs. In general, 0.025 g of the catalyst powder was first mixed with 50 mL of the pollutant solution (10 mg L−1, natural pH, 35 °C) in an appropriate volume glass beaker. Then, the reaction was triggered by adding PMS (2 mM) under magnetic stirring. The system was used in all experiments unless otherwise specified. At certain intervals, 1 mL reaction suspension and 3 mL printing and dyeing wastewater reaction suspension were collected with a syringe, filtered with a filter (0.22 μm) for follow-up analysis, and quenched with a sufficient amount of sodium thiosulfate solution. The pollutant concentrations were immediately measured through Agilent 1200 series high-performance liquid chromatography (HPLC) with an XDB-C18 column and fluorescence spectrophotometer. The RhB and AO7 concentrations were measured using a spectrophotometer (752 N) at 554 nm and 485 nm, respectively. The total organic carbon (TOC) concentrations before and after the reaction were measured with a TOC-VCPH analyzer (Shimadzu, Japan) based on the high-temperature combustion method. The pH values involving in the experiment were adjusted with 1 M solutions of H2SO4 and NaOH. To test the reusability and stability, the catalyst after the reaction was recovered by filtering the reaction, washing several times with distilled water, and drying in a vacuum oven at 60 °C. To investigate the long-term stability of the catalyst, a fixed-bed reactor was established to continuously remove AO7 dye wastewater, and the PMS concentration was maintained at 2 mM, which was synchronously pumped into the reactor with the raw water. Other characterization methods and experimental procedures in this paper are shown in Supplementary material.
3 Results and discussions
3.1 Efficient removal of ECs in a complicated water environment medium
The catalytic performance of RCM NSs was evaluated by the degradation of ECs such as endocrine disruptors, pharmaceuticals and dyes under natural conditions. Surprisingly, BPA was completely degraded at 15 minutes in the RCM NSs/PMS system, and approximately 48% of BPA was eliminated in only 5 min, which indicates the high efficiency of resourcelized RCM NSs for the ECs removal. Furthermore, the removal rate of BPA by RCM NSs/PMS suspension was 213 and 520 times higher than that of RCM NSs suspension and PMS suspension, respectively, which demonstrates the excellent catalytic performance of RCM NSs (Fig. 1a). To explore the optimal reaction conditions of the RCM NSs/PMS system, the activity evaluation experiments for BPA degradation under different conditions, including the different catalyst dosage and PMS concentration, were performed. Obviously, when the concentration of PMS was 2 mM, the rate of BPA removal by RCM NSs was positively correlated with the dosage of the catalyst (Fig. S2a). It is worth noting that when the catalyst dosage was raised to 1.0 g L−1, the degradation rate of BPA was negatively affected. The reaction rate constants (k) showed that 0.5 g L−1 RCM NSs was the optimal dosage for further investigation (Fig. 1b). Apparently, when the concentration of PMS was increased from 0.5 mM to 2.0 mM, the BPA removal efficiency was largly enhanced. The catalyst performance was slightly inhibited when the PMS concentration was elevated to 5 mM (Fig. S2b). This result suggests that high concentrations of PMS do not necessarily lead to increased activity, which is mainly attributed to the quenching of free radicals by excess PMS. From the perspective of degradation efficiency and economic cost, [catalyst] = 0.5 g/L and [PMS] = 2.0 mM were used as the optimal catalytic conditions for all subsequent experiments.
Performance evaluation of RCM NSs. a Removal of BPA in diverse systems (the inset shows the pseudo-first-order kinetic rate plots of the different systems); b k comparison of the BPA degradation in different catalyst dosages and different PMS concentrations; c effect of the initial pH on BPA degradation in RCM NSs/PMS (the inset shows the pseudo-first-order kinetic rate plots of the different systems); d effect of common inorganic anions on the BPA degradation in RCM NSs/PMS; e TOC residual rate during reaction in RCM NSs/PMS suspension. f Degradation of different pollutants in RCM NSs/PMS (the inset shows the pseudo-first-order kinetic rate plots of the different systems). Reaction conditions: [pollutants] = 10 mg L−1, [catalyst] = 0.5 g L−1, [PMS] = 2 mM, natural pH, [Temp] = 35 °C, [inorganic anions] = 0.1 mol L−1
To assess the adaptability of resourcelized conversion catalyst RCM NSs to a complicated water environment medium, influence experiments of initial pH and different salts (anions) were performed. As depicted in Fig. 1c, the RCM NSs still exhibited excellent activity for BPA removal over a wide pH range. Even in neutral or alkaline conditions, BPA could be removed within 1 hour in the RCM NSs/PMS system and the main reactive species did not change. In addition, non-target natural water constituents such as inorganic anions and dissolved organic matter usually have a detrimental effect on the performance of AOPs (Ghanbari and Moradi 2017). However, in the RCM NSs/PMS system, the removal ability of BPA was not evidently inhibited by various salts (Fig. 1d). Abnormally, the existence of Cl− or PO42− had a significant promotion effect on the degradation of BPA, and BPA was completely removed within only 5 min, which attributed to the possible role of anions in facilitating electron donation from pollutants. These results embody the superior catalytic activity of RCM NSs even in complex aqueous environments (high doses of anions or extremely acidic and alkaline). Furthermore, the total organic carbon (TOC) removal rate reflects the mineralization rate of pollutants in water. BPA was completely removed from the system within 15 min, with 63% of TOC eliminated (Fig. 1e), which is even better than that of the previous SACo-NGs/PMS system (TOC removal rate ~ 50%; Zhang et al. 2021a). Similarly, the RCM NSs/PMS system was used to remove other ECs under natural conditions. The achievement of complete oxidation of various pollutants was fulfilled within 15–30 min, which suggests the admirable catalytic adaptability and pollutant degradation ability of RCM NSs (Fig. 1f). Based on these results, resourcelized conversion catalyst RCM NSs have superior pollutant removal capability and great potential in actual wastewater treatment.
A fixed-bed reactor was built to further investigate the long-term stability and practical application capabilities of RCM NSs (Figs. 2a and S3). The reactor first operated without RCM NSs to eliminate the effect of quartz sand on the pollutant removal, and the removal of AO7 by PMS/quartz sand was only maintained at approximately 40% after 30 days (Fig. S4). Then, 1.0 g of catalyst and quartz sand were alternately loaded into a new fixed-bed reactor (hydrodynamic residence time of 30 min). Astonishingly, the reactor could stably remove over 90% of 10 ppm AO7 (containing 2 mM PMS) after 72 consecutive days without any additional operations (include washing catalyst, adding new or washed catalyst), which is equivalent to 3470 cycles (Fig. 2b). The elimination of pollutants far exceeded that of the device with only quartz sand, revealing that RCM NSs play a primary role in the long-term stable removal of pollutants. Furthermore, actual kitchen wastewater (the satellite imagery of the sampling site was shown in Fig. 2e) was used to test whether RCM NSs/PMS had high degradation activity in a complex environment that contained multiple organic compounds, and the results are shown in Fig. 2c and d. In general, regions I and II are related to simple aromatic proteins such as tyrosine; region IV is considered microbial byproduct-like compounds; regions III and V are associated with fulvic acid-like and humic acid-like organics, respectively (Chen et al. 2003). Two main peaks associated with simple aromatic proteins and microbial byproduct-like compounds were identified, which indicates that the raw water was poorly biochemically degradable and heavily contaminated (Liu et al. 2016; Tang et al. 2018). After the reaction, the two main peaks almost disappeared. Overall, dissolved organic matter was almost removed after treatment, which significantly improved the biodegradability of the water.
Schematic illustration of (a) fixed-bed reactor; b stability test of RCM NSs: run continuously for 1735 hours in a fixed-bed reactor (1.0 g of catalyst and quartz sand, hydrodynamic residence time of 30 min); 3D-EEM fluorescence spectroscopy of the kitchen wastewater sample by RCM NSs/PMS after (c) 0 min and (d) 30 min of reaction; e satellite imagery of the kitchen wastewater sampling site; f BPA degradation in UP water, tap water and river water; g satellite imagery of river water sampling site; h 3D-EEM fluorescence spectroscopy of the different samples: BPA solution (UP water); i Pearl River water sample; j Pearl River water/BPA sample; k Pearl River water/BPA sample by RCM NSs/PMS after 5 min of reaction. Reaction conditions: [pollutants] = 10 mg L−1, [catalyst] = 0.5 g L−1, [PMS] = 2 mM, natural pH, [Temp] = 35 °C
The adaptability of the RCM NSs system was further evaluated in different aquatic systems (ultrapure water, tap water, and Pearl River water; Fig. 2f). Amazingly, the removal rate of BPA by RCM NSs/PMS system in tap water and Pearl River water (containing DOM and inorganic anions; the satellite imagery of the sampling site was shown in Fig. 2g) was accelerated instead of inhibited, which is consistent with previous studies (Wang et al. 2022a; Wang et al. 2022b). BPA could be completely degraded in just 1 minute, with significantly improved degradation efficiency, which illustrates the potential value of catalysts in actual applications. Meanwhile, an interesting phenomenon was discovered. DOM was analyzed in a Pearl River water system containing BPA during a reaction using 3D fluorescence technology. Figures 2h and i show the 3D-EEM fluorescence patterns of BPA solution and Pearl River raw water, respectively. The Pearl River water contains a large amount of DOM such as simple aromatic proteins (regions II), fulvic acid-like (regions III) and humic acid-like (regions V) organics. After 5 minutes of reaction, the corresponding peaks of ECs were completely removed even with a large amount of NOM interference (Fig. 2j), surprisingly, the associated peaks of NOM also disappeared, which illustrates the simultaneous promotion of DOM removal (Fig. 2k). These results suggest that the RCM NSs/PMS system has high activity, strong adaptability, and long-term stability in water treatment.
3.2 Morphology and structure characterization of RCM NSs
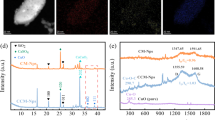
The morphology and structure characterization were investigated to comprehend the structure-activity relationship of resourcelized conversion RCM NSs. The adsorption/desorption isotherm of RCM NSs exhibits a hysteresis loop (Fig. 3a), which belongs to a special IV type. The surface area exceeds 19 m2/g, and the average pore diameter is 18.1 nm, indicating that RCM NSs is a classical mesoporous material. Transmission electron microscopy (TEM) images show that the RCM NSs present a distinct layered nanostructure that resembles graphene-like nanosheets (Fig. S5a). Meanwhile, regular fine fringes in the high-resolution TEM (HR-TEM) image explicitly demonstrate the presence of a graphite structure. Adjacent lattice stripes with a spacing of 0.309 nm immediately adjacent to the lamellar region (grphite C) were observed, which are attributed to the interplanar distances of the CaCO3 (104) crystal planes (Fig. S5b). In addition, the element mapping diagram (Fig. S5c) shows the predominant existence of Ca, C, and O elements and the three elements were evenly distributed in the RCM NSs framework. These structures were also corroborated by X-ray diffraction (XRD) and Raman analyses. As shown in Fig. 3b, the XRD pattern of RCM NSs exhibited definite diffraction peak characteristics. RCM NSs displayed a characteristic peak at 29.35° which mainly corresponded to the (104) plane of CaCO3 (PDF card no. 01–0837). Another peak at 26.43° was largely attributed to the (002) plane of the graphitic structure (Li et al. 2016). The ordered catalyst structure was clearly obtained by graphitization. The evident D and G bands were observed at 1354 cm−1 and 1596 cm−1 in the Raman spectra, which represent defects and graphitic carbon, respectively, and validate the graphene-like feature of RCM NSs. A broad but relatively weak peak was located at 2753 cm−1, which attributed to the laminar structure of the RCM NSs (Fig. 3c; Gao et al. 2020b). Additionally, XRD was applied to analyze the structural stability of the RCM NSs before and after the reaction (Fig. S6). The structure was stable and remained with graphene-like and CaCO3 as the main structure after the reaction. Stable structure of RCM NSs is an important reason for maintaining stable activity for such a long time. Based on these results, it was tentatively concluded that the CM was converted from a disordered into a stable and ordered structure through a graphitization process.
3.3 Chemical bond analysis and identification of the reaction site of RCM NSs
To explore the bonding between graphene-like and CaCO3, X-ray photoelectron spectroscopy (XPS) was conducted to investigate the chemical bonding and valence state of RCM NSs (Fig. S7). In the C1s XPS spectra (Fig. 4a), the peak at 284.78 eV was largely attributed to the graphite-like carbon (sp2 C-C/C=C bonds; Zhang et al. 2014). In addition, the peak at 286.08 eV confirmed the formation of the C-O-M bond, which is composed of C atoms of the graphene-like carbon ring bonding to the deprotonated hydroxyl groups (Lyu et al. 2018). This peak was generated by the interaction between Ca and graphene-like (Yang et al. 2018). In combination with the Ca XPS spectra analysis of RCM NSs (Fig. 4b), the two peaks of Ca 2p3/2 and Ca 2p1/2 were slightly shifted compared with CaCO3 (Qi et al. 2023), which indicates that the graphene-like carbon substrate interacted with CaCO3, and the C-O-M bond occurred at the Ca sites (Qian et al. 2021). For O1s XPS spectra, two corresponding peaks at 531.48 eV and 532.88 eV were attributed to Olatt and Oads, respectively (Fig. 4c; Liang et al. 2022). Based on the above results, the graphite-like substrate and CaCO3 are linked by the C-O-Ca bond bridge. In addition, electronic structure information of RCM NSs was detected by electron paramagnetic resonance (EPR) spectroscopy (Fig. 4d). The RCM NSs had a significant symmetrical EPR signal of free electrons relative to the original sample. The graphene-like skeleton structure and cross-linking Ca and C rings through C-O-Ca bridges to form cation-π structures, so the electrons were activated and detected (Deng et al. 2021b).
Simultaneously, more visual information on the catalyst structure and reaction sites was presented in the FTIR spectra (Fig. 4e). Compared to the fresh RCM NSs catalyst, the three peaks of C=C, and C-O-C related to the carbon (π) rings (Li et al. 2020) showed a weak blueshift after adsorption of BPA, which was attributed to π-π stacking between the aromatic ring of BPA and the graphene-like carbon (π) ring. The pollutant molecules were adsorbed on the surface of the catalyst, which shifted the characteristic peak of the carbon (π) ring. Additionally, Raman spectroscopy was used to reveal the role of defects in RCM NSs (Zhu et al. 2021). As illustrated in Fig. 4f, after the adsorption of BPA, the ratio of the D band to the G band (ID/IG) was 0.99, which was significantly lower than that of fresh RCM NSs (1.03) because the π-π interaction occupied a certain defect position, which reduced the defect degree of RCM NSs. After the reaction, ID/IG (0.96) was further weakened, which indicates that the defects provided adequate active sites of electron transfer for PMS (Ouyang et al. 2019).
3.4 Interfacial electron transfer mechanism of the RCM NSs/PMS process for ECs removal
In PMS-involved water treatment process, PMS is usually reduced to •OH/SO4•- as an electron acceptor or oxidized to 1O2 as electron donors (Gao et al. 2021; Wang et al. 2020). These reactive oxygen species (ROS) can oxidize organic pollutants in water. To generate more ROS and increase the efficiency of degrading organic pollutants, it is inevitable to continuously consume PMS (Gu et al. 2021). However, in the RCM NSs/PMS system, the decomposition of PMS was only 12.1% after 60 min with the rapid removal of BPA (Fig. 5a). The results showed that PMS was only consumed at the beginning of the chain reaction, and the consumption of PMS was almost negligible as the reaction progressed (Fig. 5a). On the contrary, BPA was still removed at a high reaction rate, indicating that a rapid electron transfer process between the interfaces remained. This surprising result indicated that PMS was not activated into ROS in the system, so trace amounts of PMS only just plays a role in the reaction system as an inducer to initiate the continuous supply of electrons from electron-donor pollutants. BMPO and TEMP were used to capture the •OH/SO4•-, /O2•- and 1O2 generated by the system to further explore the function of PMS in the system and the electron transfer pathway of the RCM NSs/PMS system to degrade BPA. As shown in Fig. S8, there were no visible BMPO-•OH/SO4•- signal peaks in the system even in the presence of PMS (Gao et al. 2020a). The result indicates that PMS is not activated to •OH/SO4•- in the reaction system, which precisely explains the result of the very low decomposition rate of PMS. A particularly strong signal consisting of three characteristic peaks of TEMP-1O2 was observed in the RCM NSs/PMS system (Fig. 5b; Gao et al. 2020b), which demonstrated that PMS interacted with defects as a critical factor in breaking the electronic equilibrium of the system and enabled the system to generate 1O2. After adding BPA, the signal intensity of TEMP-1O2 of the RCM NSs/PMS system was nearly twice as strong, which confirms that pollutant molecules enhance the electron/energy transfer in the system through π-π interactions with the carbon (π) ring, proving the contribution of organic pollutants to the generation of active species (Zhang et al. 2021a). Meanwhile, a weak O2•- signal was generated in the RCM NSs/PMS system (Fig. 5c). The signal intensity did not diminish in the presence of pollutants, which indicates a constant source of electrons/energy to enable the system to produce O2•-. Quenching tests also obtained consistent results (Fig. 5d) with no significant change in degradation rate of BPA after the addition of the •OH/SO4•- quencher ethanol, which indicates that no •OH/SO4•- was produced in the system (Wu et al. 2021). However, the degradation of BPA was significantly inhibited when quenched against O2•- and 1O2 (Huang et al. 2022). The degradation efficiency decreased from 100% to 71.4% and 55.6%, respectively. This result illustrates that 1O2 and O2•- played a predominant role in the system.
a PMS concentration and removal of BPA in RCM NSs/PMS (inset shows the consumption rate of PMS and the removal rate of BPA); b 1O2 in the RCM NSs suspensions in the absence/presence of PMS with/without BPA; BMPO spin-trapping EPR spectra for (c) O2•- in the RCM NSs suspensions in the absence/presence of PMS with/without BPA; d effect of quenching different active species on the degradation of BPA; e effect of N2, air, and O2 in the RCM NSs/PMS system; f EIS spectra of the RCM NSs electrode in different reaction systems. Reaction conditions: [pollutants] = 10 mg L−1, [catalyst] = 0.5 g L−1, [PMS] = 2 mM, natural pH, [Temp] = 35 °C, [Ethanol (•OH/SO4•-)] = [p-benzoquinone (HO2•/O2•-)] = [furfuryl alcohol (1O2)] = 1 mM
In addition to PMS, dissolved oxygen (DO) is one of the possible origins of O2•- and 1O2 (Sun et al. 2021). To investigate the formation of ROS in the presence of such trace PMS consumption, the degradation experiments of BPA were performed in nitrogen (N2) atmosphere, air atmosphere, and oxygen (O2) atmosphere to analyze the effect of DO on the degradation of BPA (Fig. 5e). The degradation efficiency of BPA under N2 was significantly inhibited compared to the reaction under air conditions. Surprisingly, the removal of BPA was dramatically accelerated under O2 conditions; the removal rate of BPA was as high as 50% at 1 minute (Fig. S9), and BPA was completely removed within only 5 minutes. Reaction rate constants (k) were obtained from the quasi-level kinetics (Fig. 5e), and comparing k gave k(O2) > k(Air) > k(N2), which indicates that DO makes a significant contribution to the degradation of BPA by the resourcelized conversion catalyst RCM NSs. Figure 5f shows the electrochemical impedance spectroscopy (EIS) of RCM NSs in different systems. Evidently, the arc radius decreased in the presence of BPA, which confirms that strong interface interaction occurs between the pollutant and catalyst and facilitates the electron transfer of the system. This phenomenon was even more noticeable after the addition of PMS. The maximum electron transfer rate was reached, which signifies that the addition of PMS plays a crucial role in the enhancement of the electron transfer rate of the system and further corroborates the reaction triggering effect of PMS.
To further reveal the triggering effect of PMS on the surface of RCM NSs for pollutant degradation, the adsorption models of pollutants at the key local sites of RCM NSs were investigated through DFT computation. The optimal model of Ca-O coordination graphene-like structure was constructed based on structural characterization and experimental phenomenon (Fig. S10). Low electrostatic potential regions (red Ca sites, which tend to obtain electrons) and high electrostatic potential regions (blue graphene-like sites, which tend to lose electrons) exist on the catalyst surface, which constitute the electron poor/rich microregions on the surface established through Ca-O-C bond bridge (Fig. S11). Phenol was used as the target pollutant to construct the adsorption model. As shown in Fig. 6a, b, the adsorption energy (Ead) of PMS at Ca site was − 3.93 eV and that of phenol at Ca site was − 0.60 eV, both of which were thermodynamically spontaneous. For simultaneous adsorption of phenol and PMS at the Ca site (Fig. 6c), the adsorption energy (Ead) was elevated to − 1.38 eV compared to phenol adsorption alone, which indicates that the presence of PMS significantly enhances the molecular forces between the contaminant and the catalyst surface. Figures 6d-f demonstrate the electrostatic potential of the adsorption process. The electrostatic potential of the phenol surface in Fig. 6f was lower compared to that in Fig. 6e, indicating that phenol was more inclined to lose electrons in the presence of PMS. This further suggests that PMS enhances the electron-donating effect of pollutants on the catalyst surface, which promotes faster electron transfer between interfaces, resulting in an efficient PMS- triggered water purification process.
Optimized model of pollutant adsorption on RCM NSs surface. a PMS adsorbed at active Ca sites; b phenol adsorbed at active Ca sites; c schematic diagram of phenol purification; d ESP distribution of PMS adsorption model; e ESP distribution of phenol adsorption model; f ESP distribution of phenol/PMS adsorption model
Combined with these results, pollutants were adsorbed on the catalyst surface by π-π/cation-π stacking (Chen et al. 2020; Sun et al. 2022), leading electrons transferred from the electron donor pollutant to the catalyst. PMS as an inducer initiated the continuous supply of electrons from electron-donors pollutants. These electrons were transferred through the C-O-Ca bond bridge and resulted in the activation of DO to O2•-/1O2 in tne Ca site (Deng et al. 2021a), which greatly facilitated the activation of DO and pollutant removal (Fig. 7).
The intermediates produced in the RCM NSs/PMS system were identified through high-performance liquid chromatography-mass spectrometer (LC-MS) techniques to identify the degradation pathway of BPA. The relevant details and mass spectra are shown in Figs. S12 and S13. According to the obtained results, a possible degradation pathway for BPA is proposed (Fig. 8). O2•-/1O2 generated by the system directly attacks BPA and generates oxidation products (You et al. 2021). Moreover, BPA molecule loses electrons through a strong interface interaction on RCM NSs surface and is directly cleaved. Meanwhile, the attack of 1O2 on the C-site between two phenyl groups of BPA produces cleavage products such as p-tert-butylphenol and phenol (Zhu et al. 2021). These intermediates can further react with ROS to obtain low-molecular-weight compounds and finally mineralize to CO2 and H2O (Cheng et al. 2020).
4 Conclusions
In summary, a new strategy to synthesize chicken manure biochar into a special catalyst for water purification is proposed in this work. The resourcelized RCM NSs exhibited excellent pollutant removal in the presence of PMS and DO. Typical emerging pollutants in water are completely removed within 15 min. Notably, the second-order kinetic constant for BPA removal is 92 times higher than that of PMS consumption. The electron-donating effect of the pollutant was induced using π-π/cation-π interactions between the pollutant and graphene-like. The trace amount of PMS triggered a rapid electron transfer on the surface of RCM NSs, and the formation of C-O-Ca bond bridges provided a favorable pathway for electron transfer, which enabled energy in the system to be recycled. These electrons/energy were eventually utilized at the Ca site to activate DO in the water to generate 1O2/O2•-, and further accelerate the degradation of organic pollutants. Moreover, the RCM NSs exhibited potential applicability in the advanced oxidation of actual wastewater and the stable removal of organic pollutants for extended periods of time. This work provides an important engineering strategy to prepare biomass waste chicken manure into advanced oxidation catalysts for environmental remediation. It is an innovative environmental remediation technology to efficiently treat ECs in wastewater through resourcelized conversion of CM into catalysts, which could reduce the energy consumption for livestock manure disposal and wastewater treatment, and achieve zero emissions and zero pollution, simultaneously.
Data availability
Data will be made available on request.
References
Chávez-Fuentes JJ, Capobianco A, Barbušová J et al (2017) Manure from our agricultural animals: a quantitative and qualitative analysis focused on biogas production. Waste Biomass Valorization 8(5):1749–1757. https://doi.org/10.1007/s12649-017-9970-5
Chen J, Li H, Fan C et al (2020) Dual single-atomic Ni-N4 and Fe-N4 sites constructing Janus hollow graphene for selective oxygen Electrocatalysis. Adv Mater 32(30):e2003134. https://doi.org/10.1002/adma.202003134
Chen WWP, Leenheer JA, Booksh K (2003) Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ Sci Technol 37(24):5701–5710. https://doi.org/10.1021/es034354c
Chen Y, Lin M, Zhuang D (2022) Wastewater treatment and emerging contaminants: bibliometric analysis. Chemosphere. 297:133932. https://doi.org/10.1016/j.chemosphere.2022.133932
Cheng F, Zhou P, Huo X et al (2020) Enhancement of bisphenol a degradation by accelerating the Fe(III)/Fe(II) cycle in graphene oxide modified Fe(III)/peroxymonosulfate system under visible light irradiation. J Colloid Interface Sci 580:540–549. https://doi.org/10.1016/j.jcis.2020.07.029
de Vidales MJM, Palomo de la Fuente E, Atanes-Sánchez E et al (2022) New compact multi option photo reactor for the removal of contaminants of emerging concern from wastewater. J Environ Chem Eng 10(3):107700. https://doi.org/10.1016/j.jece.2022.107700
Deng K, Gao T, Fang Q et al (2021b) Vanadium tetrasulfide cross-linking graphene-like carbon driving a sustainable electron supply chain from pollutants through the activation of dissolved oxygen and hydrogen peroxide. Environ Sci: Nano 8(1):86–96. https://doi.org/10.1039/d0en00982b
Deng K, Gu Y, Gao T et al (2021a) Carbonized MOF-coated zero-Valent cu driving an efficient dual-reaction-center Fenton-like water treatment process through utilizing pollutants and natural dissolved oxygen. ACS ES&T Water 2:174–183. https://doi.org/10.1021/acsestwater.1c00331
Gao Y, Chen Z, Zhu Y et al (2020b) New insights into the generation of singlet oxygen in the metal-free peroxymonosulfate activation process: important role of electron-deficient carbon atoms. Environ Sci Technol 54(2):1232–1241. https://doi.org/10.1021/acs.est.9b05856
Gao Y, Zhu Y, Chen Z et al (2020a) Insights into the difference in metal-free activation of peroxymonosulfate and peroxydisulfate. Chem Eng J 394:123936. https://doi.org/10.1016/j.cej.2019.123936
Gao Y, Zhu Y, Li T et al (2021) Unraveling the high-activity origin of single-atom Iron catalysts for organic pollutant oxidation via Peroxymonosulfate activation. Environ Sci Technol 55(12):8318–8328. https://doi.org/10.1021/acs.est.1c01131
Ghanbari F, Moradi M (2017) Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: review. Chem Eng J 310:41–62. https://doi.org/10.1016/j.cej.2016.10.064
Gu Y, Gao T, Zhang F et al (2021) Surface sulfur vacancies enhanced electron transfer over co-ZnS quantum dots for efficient degradation of plasticizer micropollutants by peroxymonosulfate activation. Chin Chem Lett 33:3829–3834. https://doi.org/10.1016/j.cclet.2021.12.004
Huang P, Zhang P, Wang C et al (2022) Enhancement of persulfate activation by Fe-biochar composites: synergism of Fe and N-doped biochar. Appl Catal B 303:120926. https://doi.org/10.1016/j.apcatb.2021.120926
Li J, Li S, Dong Z et al (2020) Two-dimensional perturbation correlation infrared spectroscopy for probing pyrolysis of biomass: fundamentals, applications, and mechanistic understanding. Energy Fuel 34(8):9154–9174. https://doi.org/10.1021/acs.energyfuels.0c01921
Li Z, Li B, Liu Z et al (2016) One-pot construction of 3-D nitrogen-doped activated graphene-like nanosheets for high-performance supercapacitors. Electrochim Acta 190:378–387. https://doi.org/10.1016/j.electacta.2015.12.210
Liang J, Fu L, Gao K et al (2022) Accelerating radical generation from peroxymonosulfate by confined variable co species toward ciprofloxacin mineralization: ROS quantification and mechanisms elucidation. Appl Catal B 315:121542. https://doi.org/10.1016/j.apcatb.2022.121542
Lim SJ, Kim TH (2018) Advanced treatment of gamma irradiation induced livestock manure using bioelectrochemical ion-exchange reactor. J Environ Manag 218:148–153. https://doi.org/10.1016/j.jenvman.2018.04.054
Liu J, Yang Q, Wang D et al (2016) Enhanced dewaterability of waste activated sludge by Fe(II)-activated peroxymonosulfate oxidation. Bioresour Technol 206:134–140. https://doi.org/10.1016/j.biortech.2016.01.088
Liu Z, He M, Tang L et al (2023) Dual redox cycles of Mn(II)/Mn(III) and Mn(III)/Mn(IV) on porous Mn/N co-doped biochar surfaces for promoting peroxymonosulfate activation and ciprofloxacin degradation. J Colloid Interface Sci 634:255–267. https://doi.org/10.1016/j.jcis.2022.12.008
Lu C, Deng KL, Hu C et al (2020) Dual-reaction-center catalytic process continues Fenton's story. Front Environ Sci Eng 14(5):82. https://doi.org/10.1007/s11783-020-1261-x
Lyu L, Cao W, Yu G et al (2020) Enhanced polarization of electron-poor/rich micro-centers over nZVCu-cu(II)-rGO for pollutant removal with H2O2. J Hazard Mater 383:121182. https://doi.org/10.1016/j.jhazmat.2019.121182
Lyu L, Yan D, Yu G et al (2018) Efficient destruction of pollutants in water by a dual-reaction-center Fenton-like process over carbon nitride compounds-complexed cu(II)-CuAlO2. Environ Sci Technol 52(7):4294–4304. https://doi.org/10.1021/acs.est.7b06545
McCance W, Jones OAH, Edwards M et al (2018) Contaminants of emerging concern as novel groundwater tracers for delineating wastewater impacts in urban and peri-urban areas. Water Res 146:118–133. https://doi.org/10.1016/j.watres.2018.09.013
Menacherry SPM, Aravind UK, Aravindakumar CT (2022) Critical review on the role of mass spectrometry in the AOP based degradation of contaminants of emerging concern (CECs) in water. J Environ Chem Eng 10:108155. https://doi.org/10.1016/j.jece.2022.108155
Ouyang D, Chen Y, Yan J et al (2019) Activation mechanism of peroxymonosulfate by biochar for catalytic degradation of 1,4-dioxane: important role of biochar defect structures. Chem Eng J 370:614–624. https://doi.org/10.1016/j.cej.2019.03.235
Palese AM, Persiani A, D’Adamo C et al (2020) Composting as manure disposal strategy in small/medium-size livestock farms: some demonstrations with operative indications. Sustainability. 12(8):3315. https://doi.org/10.3390/su12083315
Qi C, Chen H, Chen X et al (2023) In-situ-reduced synthesis of cyano group modified g-C3N4/CaCO3 composite with highly enhanced photocatalytic activity for nicotine elimination. J Environ Sci 126:517–530. https://doi.org/10.1016/j.jes.2022.03.019
Qian B, Wang Y, Zhao Q et al (2021) Preparation and luminescence properties of Eu3+ incorporated in CaCO3 nanocrystals with multiple sites. J Lumin 239:118344. https://doi.org/10.1016/j.jlumin.2021.118344
Shao B, Liu Z, Tang L et al (2022) The effects of biochar on antibiotic resistance genes (ARGs) removal during different environmental governance processes: a review. J Hazard Mater 435:129067. https://doi.org/10.1016/j.jhazmat.2022.129067
Sillanpaa M, Ncibi MC, Matilainen A (2018) Advanced oxidation processes for the removal of natural organic matter from drinking water sources: a comprehensive review. J Environ Manag 208:56–76. https://doi.org/10.1016/j.jenvman.2017.12.009
Sun B, Ma W, Wang N et al (2021) Retraction of “polyaniline: a new metal-free catalyst for Peroxymonosulfate activation with highly efficient and durable removal of organic pollutants”. Environ Sci Technol 55(5):3451. https://doi.org/10.1021/acs.est.0c07981
Sun Y, Hu C, Lyu L (2022) New sustainable utilization approach of livestock manure: conversion to dual-reaction-center Fenton-like catalyst for water purification. Npj Clean Water 5(1):53. https://doi.org/10.1038/s41545-022-00200-2
Tan M, Hou Y, Zhang L et al (2021) Operational costs and neglect of end-users are the main barriers to improving manure treatment in intensive livestock farms. J Clean Prod 289:125149. https://doi.org/10.1016/j.jclepro.2020.125149
Tang L, Liu Y, Wang J et al (2018) Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: Electron transfer mechanism. Appl Catal B 231:1–10. https://doi.org/10.1016/j.apcatb.2018.02.059
Tong X, You L, Zhang J et al (2021) A comprehensive modelling approach to understanding the fate, transport and potential risks of emerging contaminants in a tropical reservoir. Water Res 200:117298. https://doi.org/10.1016/j.watres.2021.117298
Wang L, Huang X, Han ME et al (2019) Efficient inhibition of photogenerated electron-hole recombination through persulfate activation and dual-pathway degradation of micropollutants over iron molybdate. Appl Catal B 257:117904. https://doi.org/10.1016/j.apcatb.2019.117904
Wang Y, Wu Y, Yu Y et al (2020) Natural polyphenols enhanced the cu(II)/peroxymonosulfate (PMS) oxidation: the contribution of cu(III) and HO(*). Water Res 186:116326. https://doi.org/10.1016/j.watres.2020.116326
Wang Y, Zhang P, Lyu L et al (2022a) Preferential destruction of micropollutants in water through a self-purification process with dissolved organic carbon polar complexation. Environ Sci Technol 56(15):10849–10856. https://doi.org/10.1021/acs.est.2c03354
Wang Y, Zhang P, Lyu L et al (2022b) Efficient destruction of humic acid with a self-purification process in an Fe0-FeyCz/Fex-GZIF-8-rGO aqueous suspension. Chem Eng J 446:136625. https://doi.org/10.1016/j.cej.2022.136625
Wu L, Sun Z, Zhen Y et al (2021) Oxygen vacancy-induced nonradical degradation of organics: critical trigger of oxygen (O2) in the Fe-co LDH/Peroxymonosulfate system. Environ Sci Technol 55(22):15400–15411. https://doi.org/10.1021/acs.est.1c04600
Wu T, Liu Z, Shao B et al (2022) Hydrogen peroxide-impregnated supramolecular precursors synthesize mesoporous-rich ant nest-like filled tubular g-C3N4 for effective photocatalytic removal of pollutants. Chem Eng J 447:137332. https://doi.org/10.1016/j.cej.2022.137332
Yang S, Liu Y, Hao Y et al (2018) Oxygen-vacancy abundant ultrafine Co3O4/graphene composites for high-rate Supercapacitor electrodes. Adv Sci (Weinh) 5(4):1700659. https://doi.org/10.1002/advs.201700659
Yang Y, Kumar Awasthi M, Du W et al (2020) Compost supplementation with nitrogen loss and greenhouse gas emissions during pig manure composting. Bioresour Technol 297:122435. https://doi.org/10.1016/j.biortech.2019.122435
You J, Zhang C, Wu Z et al (2021) N-doped graphite encapsulated metal nanoparticles catalyst for removal of Bisphenol a via activation of peroxymonosulfate: a singlet oxygen-dominated oxidation process. Chem Eng J 415:128890. https://doi.org/10.1016/j.cej.2021.128890
Zhang H, Li C, Lyu L et al (2020) Surface oxygen vacancy inducing peroxymonosulfate activation through electron donation of pollutants over cobalt-zinc ferrite for water purification. Appl Catal B 118874:270. https://doi.org/10.1016/j.apcatb.2020.118874
Zhang H, Lyu L, Fang Q et al (2021a) Cation−π structure inducing efficient peroxymonosulfate activation for pollutant degradation over atomically dispersed cobalt bonding graphene-like nanospheres. Appl Catal B 286:119912. https://doi.org/10.1016/j.apcatb.2021.119912
Zhang J, Zhang M, Yang C et al (2014) Nanospherical carbon nitride frameworks with sharp edges accelerating charge collection and separation at a soft photocatalytic interface. Adv Mater 26(24):4121–4126. https://doi.org/10.1002/adma.201400573
Zhu J, Yu F, Meng J et al (2020) Overlooked role of Fe(IV) and Fe(V) in organic contaminant oxidation by Fe(VI). Environ Sci Technol 54(15):9702–9710. https://doi.org/10.1021/acs.est.0c03212
Zhu M, Kong L, Xie M et al (2021) Carbon aerogel from forestry biomass as a peroxymonosulfate activator for organic contaminants degradation. J Hazard Mater 413:125438. https://doi.org/10.1016/j.jhazmat.2021.125438
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (52122009, 52070046, 52150056), the Introduced Innovative R&D Team Project under the “Pearl River Talent Recruitment Program” of Guangdong Province (2019ZT08L387), and Basic and Applied Basic Research Project of Guangzhou (202201020163).
Funding
This work was financially supported by the National Natural Science Foundation of China (52122009, 52070046, 52150056), the Introduced Innovative R&D Team Project under the “Pearl River Talent Recruitment Program” of Guangdong Province (2019ZT08L387), and Basic and Applied Basic Research Project of Guangzhou (202201020163).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Yingtao Sun and Yuting Gu. Manuscript supervision, conceptualization, writing-reviewing and editing were performed by Chun Hu and Lai Lyu. The first draft of the manuscript was written by Yingtao Sun and Yuting Gu, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling editor: Fengchang Wu
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary data associated with this article can be found in the online version at xxx.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, Y., Gu, Y., Li, M. et al. Fast elimination of emerging contaminates in complicated water environment medium over the resource conversion product of chicken manure biochar triggered by peroxymonosulfate. Carbon Res. 3, 8 (2024). https://doi.org/10.1007/s44246-023-00096-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44246-023-00096-8