Abstract
Developing highly durable and active Pt-supported catalysts is currently the most prominent and practicable electrocatalyst design strategy for direct methanol fuel cells (DMFC) due to their high electrocatalytic stability and activity. Herein, we fabricated CeO2 nanorods with tiny Pt nanoparticles that possess good electrocatalytic behavior for methanol oxidation reaction (MOR). In addition, the presence of Ce3+ and small Pt nanoparticles (3.46 nm) enhanced strong metal interaction, facilitating the formation of oxygen vacancies on the CeO2 surface to boost the MOR performance. Hence, the Pt-CeO2 catalyst revealed high mass activity (1414.0 mA mg−1) and areal-specific activity (1.615 mA cm−2) towards MOR in an acidic electrolyte, which was three times that of the 20% Pt/C catalyst (545.4 mA mg−1 and 0.856 mA cm−2). Besides, the Pt-CeO2 catalyst exhibited excellent performance of MOR in an alkaline electrolyte and retained 90.44% of its initial mass activity after 5000 cycles due to the unique morphology and synergistic effects between Pt and CeO2. Pt-CeO2 is regarded as a promising MOR electrocatalyst for DMFC applications.
Graphical Abstract

Highlights
• 10% Pt-CeO2 nanorods catalyst has excellent MOR performance in acidic and alkaline electrolytes.
• The high surface area of CeO2 nanorod enhances the stability of Pt metal.
• Redox pair (Ce3+/Ce4+) and Pt-O induced oxygen vacancy may enhance the active surface area.
• Strong metal interaction leads to the synergistic effect of Pt-CeO2 catalyst.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Direct methanol fuel cells (DMFCs) are recognized as potential energy-generating devices for transportation and portable electronic equipment due to their high energy conversion efficiency, power density, and environment-friendly nature (Xia et al. 2019). Effective and durable electrocatalysts are essential in improving the slow kinetics of the methanol oxidation reaction (MOR) (Lou et al. 2022; Yaqoob et al. 2021). Theoretically, platinum-based materials are the most prominent catalyst for MOR performance because of their distinctive electronic structure and high efficiency (Chen et al. 2023; Ojani et al. 2015; Tian et al. 2021). However, the main disadvantages of Pt-based electrocatalysts are their susceptibility to CO intermediate poison on surface active sites and high costs. To overcome the obstacles mentioned above, bi-metallic and tri-metallic platinum-based transition metal catalysts (Fe, Ni, Cu, and Ir) have been employed for MOR in both acidic and alkaline mediums by alloying to achieve electronic structure regulation (Ahmad et al. 2020; Li et al. 2018; Sun et al. 2022).
The electrocatalysts of Pt-embedded non-noble metal oxides (MeOx: CeO2, Fe2O3, SnO2, and TiO2) also have been investigated to boost the stability and activity of Pt (Guo et al. 2020; Murpin Kumar et al. 2022; H. Wang et al. 2021). Despite the regulation of dispersion of Pt nanoclusters in MeOx, the improved electrocatalytic capabilities of Pt-MOx are related to the interaction between Pt and MOx, which modulates the electronic structure and accelerates the CO tolerance (Murpin Kumar et al. 2022; Yu et al. 2023). Among these MeOx, CeO2 is more attentive than other MeOx-based catalysts with favorable physical and chemical characteristics, including oxygen storage capacity, fast electron transfer (Ce3+/Ce4+ redox pair), and high metal dispersion ability (Altamirano-Gutiérrez et al. 2013; Bhuvanendran et al. 2021; Yu et al. 2023). Furthermore, the presence of a Ce3+/Ce4+ redox pair can produce oxygen vacancies on the CeO2 surface, allowing the adsorption of OH−/ CO intermediates and enhancing the CO tolerance of Pt-based catalysts (Su et al. 2023; Xie et al. 2018). Using metal-organic frameworks (MOFs) as substrate, tinny Pt nanoparticles with a size of 3 nm were distributed over CeO2/TiN Nanotubes (TiN NTs) (Zhou et al. 2019). The homogeneously dispersed Pt metal on CeO2 in Pt-CeO2/N-CNTs can enhance the electronic effect and oxygen vacancy between metal-CeO2, which plays a major part in improving the chemical reaction of methanol (Mullins 2015; Murpin Kumar et al. 2022). The catalytic stability and inhibition of bridging CO in a Pt-CeOx catalyst depend on tiny metallic Pt particles (Brummel et al. 2016).
Moreover, the unique morphology of the CeO2 catalyst can influence the MOR performance with various physicochemical aspects such as surface properties and controlled size (An et al. 2013). Most of the electrocatalysts have been synthesized using template-based methods for tuning the morphology size of CeO2 (Tao et al. 2018). Among them, the morphology-controlled Pt-CeO2 mesoporous particle was prepared by seed-mediated chemical reduction method and exhibited good electrocatalytic behavior with an average particle size of 5–10 nm (Murphin Kumar et al. 2017). Porous CeO2-supported Pt catalysts possess high mass activity (229.44 mA mg−1) and areal-specific activity (36.37 mA cm−2) compared to state-of-art Pt/C catalyst owing to the enhanced porous surface area (Murpin Kumar et al. 2022). However, most of these morphology-controlled catalyst preparations are mainly challenging because of their inhomogeneous structure and weak coupling interaction (Zhang et al. 2015). To solve these issues, the effective regulation of green and eco-friendly methods has been employed to enhance the sustainability of catalysts (Wang et al. 2013). Some research works reported that the well-dispersed CeO2 quantum dots (QDs) and Pt/C controlled the electronic configuration of Pt at the Pt-CeO2 design to form catalytically active sites along with many oxygen vacancies for the MOR process by using facile and eco-friendly approach (Su et al. 2023).
Furthermore, the manufactured metal-oxide (CeO2) nanorods have outstanding benefits with high surface area free energy that stabilizes and disperses metal nanoparticles, boosting mass transfer and active chemical availability to electrode surfaces (Tao et al. 2018). The morphology of Pt-CeO2-P nanorod was successfully synthesized by plasma irradiation, which is an alternative method to the conventional approach for architecting physical and electronic interaction between metal oxides and Pt nanoparticles to enhance MOR activity (Su et al. 2023). In the present study, we synthesized a 10% Pt/CeO2 catalyst with small Pt nanoparticles on CeO2 nanorods using a multi-step synthesis approach for enhancing the MOR process in both acidic and alkaline electrolytes. The CeO2 nanorods were fabricated using hydrothermal methods, followed by embedding the Pt NPs using simple chemical reduction methods. Due to the distinctive morphology and strong metallic interaction, the as-synthesized 10% Pt/CeO2 catalyst exhibits better catalytic activity and durability than a 20% Pt/C catalyst. Furthermore, the obtained tinny Pt nanoparticles (3.46 nm) and pyrolyzed CeO2 nanorods enable better availability of active surface area with higher catalytic conductivity to improve MOR efficiency.
2 Experimental section
2.1 Chemicals
Cerium (III) chloride (CeCl3), hexachloroplatinic acid (H2PtCl6.6H2O), commercial Pt/C (20%), and Polyvinylpyrrolidone (PVP) were purchased from Sigma-Aldrich. Urea, sodium hydroxide (NaOH), potassium hydroxide (KOH), sodium hydroxide (NaOH), ethylene glycol, and ethanol were purchased from Guangzhou Chemical Reagent Co., Ltd.
2.2 Preparation of CeO2 nanorods
A unique morphology of CeO2 nanorods was prepared by one-pot hydrothermal as follows (Fig. 1). 2.7 g CeCl3 was dissolved in 50 mL distilled water, followed by the addition of 0.2 g PVP dispersed through magnetic stirring for 30 minutes. Then, 1 g of urea was added to the above solution and stirred for 6 h to obtain the homogeneous mixture. The final suspension was transferred to a 100 mL hydrothermal autoclave and heated at 180 °C for 24 h. The brown residue was rinsed multiple times with water and ethanol using centrifugation at 10000 rpm and then dried at 60 °C for 12 h. The final material was pyrolyzed in a N2 atmosphere at 900 °C for 2 h.
2.3 Preparation of 10% Pt-CeO2 electrocatalyst
An impregnation method was used to synthesize a 10% Pt-CeO2 nanorod using a standard chemical reduction procedure. 100 mg CeO2 nanorod was dissolved into 24 mL ethylene/glycol mixture, followed by the addition of 11.5 mg H2PtCl6.6H2O precursor (0.01931 M) under the magnetic stirring for 30 minutes. Then, the above mixture solution was adjusted to pH 8 by adding 0.1 M KOH with constant stirring for another 30 minutes. The above solution was poured into a 50 mL three-necked flask, and the temperature was maintained at 110 °C for 6 h using an oil bath with magnetic stirring. The final product was centrifuged numerous times to remove inorganic impurities and dried in a vacuum atmosphere.
2.4 Physical and electrochemical measurements
The crystalline structure of 10% Pt-CeO2 and Pt/C was investigated using X-ray diffraction (XRD, Bruker D8 Advance, Germany) with Cu Kα radiation, and the surface electron structure of the catalyst was examined by X-ray Photoelectron Spectroscopy (XPS- Escalab 250Xi, Thermo fisher, Japan). The catalyst morphology was investigated employing a field emission scanning electron microscope (SEM, SU8220, Hitachi, Japan) and a high-resolution transmission electron microscope (HRTEM, Talos F200S, FEI, USA). The Raman spectra were carried out utilizing a 532 nm laser on a Senterra-Bruker Raman spectrometer, and pore volume and specific surface area were estimated with the Brunauer-Emmett-Teller (BET) technique (ASAP-2460, Micrometrics, USA).
All electrochemical measurements were conducted in a three-electrode cell linked by an electrochemical workstation (CHI 760E, ChenHua Instrument, China), in which an Ag/AgCl (saturated KCl) and platinum wire served as the reference and counter electrodes, and a glassy carbon rotating disc electrode (GC-RDE) was performed as the working electrode, respectively. All the potentials were measured using a reversible hydrogen electrode (RHE) scale. Before drop casting the active material on the working electrode, the GC electrode was polished with paste and rinsed with ultra-pure water. Then, 10 μL of catalyst ink was coated on the electrode (10.12 μgPt cm−2), which was prepared by ultrasonicating 5 mg of catalyst mixture (2.5 mL of ethanol and 20 μL of 5% Nafion) for 30 minutes. The cyclic voltammogram (CV) tests were performed in an N2 purged aqueous solutions of 1 M KOH + 1 M CH3OH and 0.5 M H2SO4 + 0.5 M CH3OH at a scan rate of 50 mV s−1 for MOR measurements. The stability of the catalyst was studied utilizing an accelerated durability test (ADT) that involved continuous cycling between 0.55 V and 0.95 V vs. RHE in N2-purged mixed solution of 1 M KOH + 1 M CH3OH and 0.5 M H2SO4 + 0.5 M CH3OH at 50 mV s−1 for 5000 cycles. The chronoamperometry (CA) curves were recorded at 0.85 V under similar conditions.
The electrochemically active surface area was estimated by integrating the hydrogen adsorption/desorption peaks at 0.03–0.2 V vs. RHE using the following eq. (1) (Ravichandran et al. 2021b).
Wherein QHdes is the amount of charge transferred on the catalyst surface during the hydrogen desorption process (μC cm−2). m represents the Pt loading (10.12 μgPt cm−2).
3 Results and discussion
3.1 Physical characterization
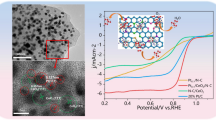
Figure 2 displays the SEM, TEM, and HRTEM images utilized to characterize the structure features and particle size distribution of 10% Pt-CeO2 electrocatalysts as synthesized. The low-magnification SEM and TEM images (Fig. 2a, b) reveal the unique morphology of CeO2 nanorods with tiny Pt nanoparticles forming sphere-like nanoclusters on the CeO2 surface. Nevertheless, the TEM images (Fig. 2b, c) illustrate some tightly packed Pt nanoparticle assemblies generating a sphere-like Pt nanocluster, highlighted as a yellow circle. The average diameter of the Pt nanocluster is about 23.52 nm ± 4.71 nm. As shown in Fig. S1, the average size of Pt nanoparticles is about 3.46 nm. Furthermore, the HRTEM image indicates that the lattice fringes of interplanar spacing (d-spacing) values were 0.22 and 0.31 nm, corresponding to the crystalline planes of Pt (111) and CeO2 (111), respectively, which are compatible with the literature (Murphin Kumar et al. 2017; Su et al. 2023). The polycrystalline nature of the 10% Pt-CeO2 catalyst was confirmed from selected area electron diffraction (SAED) patterns with well-defined rings (Fig. 2d), which is strongly associated with the XRD results. The HAADF-STEM images coupled with EDS mapping reveal the elemental distribution of 10% Pt-CeO2 catalyst, while Pt nanoparticles are found to be well interconnected with CeO2 nanorods and form a cluster-like morphology, especially at the nanostructure edges (Fig. 2e-g).
In addition, the Barrett-Joyner-Halenda (BJH) technique and the adsorption isotherm were used to calculate the pore volume and specific surface area (SSA) of CeO2 through a N2 absorption/desorption isotherm profile (Yu et al. 2018). As illustrated in Fig. S2a, b, the estimated SSA was about 73.62 m2/g, and the average pore volume was found to be 0.1440 cm3 g−1 for CeO2. This result reveals that the CeO2 nanorods exhibit a mesoporous nature and large surface area, which enhances the mass transfer in methanol electro-oxidation (Chen et al. 2020; Su et al. 2023). The crystalline nature and structural information of 10% Pt-CeO2, CeO2, and 20% Pt/C catalysts were analyzed by using XRD (Fig. 3a). The diffraction characteristic peaks of CeO2 located at 28.54 °, 33.06 °, 47.51 °, 56.31 °, 59.10 °, 69.38 °, 76.65 °, 79.30 ° and 88.44 ° corresponding to (111), (200), (220), (311), (222), (400), (331), (420), and (422) crystalline planes, indicating the fluorite lattice structure of CeO2 (JCPDS: 34–0.394) (Bhuvanendran et al. 2021; Chen et al. 2020). Based on the distinct and intense peak of the CeO2 (111) crystalline plane, the Scherrer equation was utilized to calculate the average crystallite size of 16 nm. Furthermore, the typical peaks of 10% Pt-CeO2 and 20% Pt/C catalysts were observed at 39.61 ° (111), 47.06 ° (200), 67.34 ° (220), and 81.34 ° (311) that are ascribed to face-cantered cubic (fcc) structure of metallic Pt (JCPDS: 04–0802) (Ravichandran et al. 2021a, 2021b). In addition, the 10% Pt-CeO2 catalyst shows less intense diffraction peaks for all the Pt crystalline planes than 20% Pt/C, which might be due to CeO2 overlap (Murpin Kumar et al. 2022).
Figure 3b shows the Raman spectra of 10% Pt-CeO2 and 20% Pt/C catalysts that are used to confirm the chemical state and structure information. 10% Pt-CeO2 and 20% Pt/C catalysts exhibit two prominent peaks with D and G bands at around 1332.83 cm−1 and 1592.86 cm−1, corresponding to typical amorphous spectra (Ravichandran et al. 2021a, 2021b). Furthermore, a less intense peak (453.89 cm−1) was found beside the D and G band, associated with the F2g mode of the fluorite structure of CeO2 (Bhuvanendran et al. 2021). This finding suggests that CeO2 nanorods and Pt coexist in 10% Pt-CeO2, which correlates well with the XRD results. In addition, a wide peak between 2500 and 3000 cm−1 was ascribed to the resonance of the D band and also recognized as the ‘2D’ band, as shown in Fig. 3b.
The elemental composition and surface chemical states of the 10% Pt-CeO2 catalyst were investigated by using XPS. As illustrated in Fig. 4a and Table S1, the XPS survey spectrum confirms the chemical composition of all the elements, Pt (9.8%), C (69.89%), N (2.58%), O (14.45%) and Ce (3.28%), with binding energies of 70.22 eV, 281.80 eV, 401.50 eV, 528.72 eV, and 896.71 eV. The deconvoluted Pt 4f emission of the Pt-CeO2 catalyst can be split into two doublet bands of Pt 4f5/2 and Pt 4f7/2 (Fig. 4b; Xu et al. 2016; Zhao et al. 2023). The doublet peaks observed at 73.66 eV (4f5/2) and 70.23 eV (4f7/2) were ascribed to metallic Pt0, while another doublet peaks at 74.45 eV (4f5/2) and 70.78 eV (4f7/2) can be ascribed to the Pt2+ oxidation state, indicating the existence of Pt-O or Pt-OH species (Ravichandran et al. 2021a, 2021b).
In addition, Ce 3d spectra (Fig. 4c) show two doublet peaks of 3d5/2 and 3d3/2 corresponding to the Ce4+ and Ce3+ oxidation states. The prominent peaks obtained at binding energies of 898.93 eV and 917.12 eV for 3d5/2 and 3d3/2 are ascribed to Ce4+, while another satellite peaks were found at 88.99 eV (3d5/2), and 901.51 eV (3d3/2) associated with Ce3+, respectively (Bhuvanendran et al. 2021; Murpin Kumar et al. 2022). Furthermore, two satellite peaks were found at 887.44 and 906.54 eV and denoted as SU1 and SU2, is consistent well with published literature (Pandit et al. 2019). The presence of Ce3+ and Ce4+ confirms the redox pair (Ce3+/Ce4+) on the CeO2 surface, demonstrating efficient oxygen release and storage capabilities (Yu et al. 2023), which improves the electrochemical methanol oxidation process for 10% Pt-CeO2 catalyst.
Figure 4d illustrates the N 1 s high-resolution XPS spectrum with three different peaks assigned to the pyridinic N (398.39 eV), pyrrolic N (399.43 eV), and graphitic N (400.78 eV), which are suggested to play major functions in MOR behavior (Ravichandran et al. 2023; Xu et al. 2016). The XPS spectra of C 1 s were deconvoluted (Fig. 4e) into four peaks. Two peaks at binding energies of 283.51 eV (C-C) and 283.99 eV (C-OH) were ascribed to sp2 carbon and carbon-hydroxyl bond. Two comparatively less intense and broad peaks were obtained at 285.53 eV and 287.30 eV, representing the carbonyl and carboxyl groups (C=O and [O=C-OH]) (Dai et al. 2018; Ravichandran et al. 2023). In Fig. 4f, the O 1 s spectra at 532.15 eV are ascribed to chemisorbed oxygen species over the metal oxide support (OC: OH* radical, adsorbed oxygen and carbonyl group [C=O]), and the high intense peak at 528.66 eV indicates that lattice oxygen is present in CeO2 (OL: O2− around Ce4+) (Dao et al. 2019). In contrast, the broad peak was obtained at 530.86 eV, corresponding to oxygen vacancy on the catalyst surface (OV: C-OH) (Bhuvanendran et al. 2021; Su et al. 2023). The increasing oxygen vacancy and Ce3+ content in Pt/CeO2 catalysts may lead to the formation of Pt-O-Ce linkage at their surface, resulting in more active sites in MOR (Murpin Kumar et al. 2022).
3.2 Electrochemical characterizations
The MOR performance of 10% Pt-CeO2 and 20% Pt/C catalyst were analyzed by cyclic voltammetry in N2-saturated 0.5 M H2SO4 electrolyte at a scan rate of 50 mV s−1. As illustrated in Fig. 5a, the ECSA was estimated from CV curves based on the integrated hydrogen desorption charge process using Eq. (1) (Ravichandran et al. 2021a, 2021b). The calculated ECSA was found to be 87.57 and 63.70 m2 g−1 for 10% Pt-CeO2 and 20% Pt/C catalysts, respectively, indicating a smaller Pt particle size and good distribution on CeO2 surface area (Yu et al. 2023).
Figure 5b shows the MOR electrochemical behavior of 10% Pt-CeO2 and 20% Pt/C catalysts, which was evaluated in N2 saturation 0.5 M methanol containing 0.5 M H2SO4 solution. As can be seen from MOR CV curves, the 10% Pt-CeO2 catalyst has a high forward current density of 14.31 mA cm−2 at 0.87 V with an onset potential of about 0.41 V by the direct electro-oxidation of methanol, which is 3 times higher than the forward current density of 20% Pt/C (5.52 mA cm−2 at 0.86 V), and an onset potential of about 0.49 V. The maximum current density in the backward scan was 13.29 mA cm−2 at 0.65 V, indicating that carbonaceous intermediates were further oxidized (Ravichandran et al. 2023).
Furthermore, a catalyst with a low onset potential and a high mass activity (MA) performs better, suggesting enhanced MOR kinetics of the 10% Pt-CeO2. As illustrated in Fig. 5d and Table 1, the 10% Pt-CeO2 catalyst has high specific activity (1.615 mA cm−2) and mass activity (1414.0 mA mg−1) compared to the commercial 20% Pt/C catalyst (0.856 mA cm−2 and 545.4 mA mg−1). The major electrochemical performance of all catalysts is compared to the published literature, as shown in Table S2. It clearly reveals that the Pt-CeO2 nanorods stabilize the platinum nanoparticles on its surface with sphere-like nanoclusters, which exposes most of them to the MOR reaction (Li et al. 2021). Similarly, oxygen vacancy on the surface of CeO2 will boost additional electron transfer and electrostatic adsorption process, resulting in a synergistic interaction between Pt and CeO2 species, which could improve the kinetics of MOR (Brummel et al. 2016; Sedighi et al. 2017).
From the respective CV curves (Fig. 5b), the ratio of If/Ib values was utilized to calculate the CO anti-poisoning capability during the MOR process. The 10% Pt-CeO2 catalyst has the highest If/Ib ratio (1.05) than the 20% Pt/C catalyst (1.07), indicating that CeO2 has a prominent advantage on CO resistance in methanol electro-oxidation (Ravichandran et al. 2023; Ravichandran et al. 2021a, 2021b). As shown in Fig. 5c, the catalytic stability and activity of electrocatalysts can be assessed using chronoamperometry (CA), which was measured for 7200 sec in 0.5 M H2SO4 + 0.5 M CH3OH solution at 0.85 V in an inert environment. In contrast to 20% Pt/C, 10% Pt-CeO2 exhibited a slower current drop and better stable current, indicating that CeO2 plays an important role in boosting the electrocatalytic performance by anti-poisoning capabilities towards CO intermediates (Fig. 5c) (Murphin Kumar et al. 2017). Furthermore, research proposed that the existence of 4f electrons in the CeO2 ground state as a result of f-p hybridization makes the electron transition to the 3d orbital of Pt easier, which may assist CO removal (Zheng et al. 2018).
An accelerated durability test (ADT) was carried out to determine the stability of 10% Pt-CeO2 and 20% Pt/C catalyst. Figure 6a-d shows the ADT-CV carried out in N2-saturated 0.5 M H2SO4 at 50 mV s−1 for all the electrocatalysts. The ADT-CV was tested with continuous potential cycles between 0.55 and 0.95 V vs. RHE for up to 5000 cycles. The 10% Pt-CeO2 catalyst had 29.80% ECSA losses of its initial value even after 5000 cycles, as shown in Fig. S3a, which is comparatively lower than the commercial 20% Pt/C (30.59%). Figure S3b depicts the catalyst stability after 5000 cycles investigated employing the MA. It was discovered that the 10% Pt-CeO2 catalyst retained 30.12% of its initial value, showing that it is more durable than commercial 20% Pt/C catalyst (30.98%). This may be due to the unique structural morphology, large specific surface area, and utilization of Pt. Notably, the improved MOR activity of as-synthesized 10% Pt-CeO2 catalyst might correspond to the synergistic effect between the supported Pt nanoparticles and CeO2 nanorods through Pt-O-Ce bonds, thereby enhancing Ce3+ species at the catalyst surface as demonstrated by XPS results (Murpin Kumar et al. 2022; Xu et al. 2016).
Furthermore, the MOR behavior of 10% Pt-CeO2 and 20% Pt/C catalysts were analyzed in N2 saturation 1 M methanol + 1 KOH using cyclic voltammetry. In a pure alkaline electrolyte (Fig. 7a), all the catalysts exhibited different peaks corresponding to H adsorption and desorption in the potential range from 0.03 to 0.2 V vs. RHE. Based on the peaks, the ECSA was found to be 72.41 and 60.71 m2 g−1 for 10% Pt-CeO2 and 20% Pt/C catalysts, respectively (An et al. 2018). Figure 7b shows the MOR onset potential of 10% Pt-CeO2, which was lower (0.47 V) than that of Pt/C (0.53 V), indicating that OH adsorption species (OH ads) on catalyst sites influenced reaction kinetics towards MOR. Besides, the low onset potential and high mass activity aim to reduce adsorption strength and enhance the kinetics of further methanol oxidation (Shi et al. 2023; Zhang et al. 2019). The term of MA was calculated using the peak current density of forward oxidation peaks (34.56 mA cm−2 and 3409 mA mg−1), and SA was about 4.593 mA cm−2 for 10% Pt-CeO2 catalyst, which was twice the SA for commercial 20% Pt/C catalyst as demonstrated in Fig. 7d and Table 2. The Pt nanoclusters with generated oxygen vacancies on the surface-active sites of CeO2 could significantly boost the electrocatalytic performance of MOR.
The long-term stability and durability of the catalyst were performed using CA and ADT for all the catalysts (Figs. 7c and 8a-d). The chronoamperometry profile was recorded at 0.77 V for 7200 sec in a mixture of 1 M methanol + 1 M CH3OH solution, as shown in Fig. 7c. The initial current density of the 20% Pt/C catalyst was gradually decreased (17.12 mA cm−2) compared to the 10% Pt-CeO2 catalyst (32.94 mA cm−2). As a result, the current decay is caused by two factors: (1) double-layer charge and many active sites of Pt nanoparticles on the catalytic surface caused the difference in behavior (Zhang et al. 2019). (2) Pt oxides and persistent intermediate of CO adsorbed species might cause poisoning during MOR (Zheng et al. 2018; Zhu et al. 2022). After 7200 sec, the 10% Pt-CeO2 was about 3.55 mA cm−2, which was 4–5 times greater than that of the 20% Pt/C (0.87 mA cm−2) catalyst. This result shows that the 10% Pt-CeO2 catalyst has higher stability and activity than the 20% Pt/C catalyst owing to the high oxygen storage capacity of CeO2. To further explore the stability test, ADT was analyzed using cyclic voltammetry recorded in N2-purged 1 M KOH at 50 mV s−1 for all the electrocatalysts, as shown in Fig. 8a-d. After 5000 cycles, 10% Pt-CeO2 catalyst retained 90.68% of ECSA initial value compared to 20% Pt/C (86.27%) (Fig. S4a), respectively. Moreover, the MOR current density substantially dropped after 5000 cycles, indicating an MA loss of 9.56% for the 10% Pt-CeO2 catalyst, significantly higher than other catalysts, as shown in Fig. S4b. In addition, TEM analysis was used to examine the morphological changes of the 10% Pt-CeO2 catalyst following 5000 stability cycles. As shown in Fig. S5, the TEM images exhibited considerable morphological alterations from extended durability tests. However, the Pt nanoparticles were integrated with CeO2 nanorods, which could be a significant factor in enhancing the catalytic activity and stability. The oxygen vacancies and surface defects also significantly impact the crystalline plane and determine the catalyst performance (Bhuvanendran et al. 2021).
4 Conclusion
We proposed a simple and environmentally friendly technique for fabricating a 10% Pt/CeO2 catalyst with small Pt nanoparticles (3.46 nm) on CeO2 nanorods. In the first step, the CeO2 nanorods were prepared using hydrothermal methods, and the Pt NPs were embedded using facile chemical reduction methods. The Pt-CeO2 catalyst showed good MOR electrocatalytic performance in an acidic electrolyte with high mass activity (1414.0 mA mg−1) and specific activity (1.615 mA cm−2) at 0.87 V, compared to the 20% Pt/C catalyst (545.4 mA mg−1 and 0.856 mA cm−2). Furthermore, the Pt-CeO2 catalyst also has excellent stability in an alkaline electrolyte, retaining 90.68% of the ECSA initial value compared to 20% Pt/C (86.27%) due to the unique electronic structure of Pt and the high surface area of CeO2. Moreover, the formation of strong metal interaction between Pt and CeO2 support at their functional interface leads to the generation of Ce3+ and oxygen vacancy. These results indicate that it reduces CO intermediate species and increases the possibility of more active Pt sites for the methanol oxidation process.
Data Availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmad YH, El-Sayed HA, Mohamed AT, Aljaber AS, Al-Qaradawi SY (2020) Rational one-pot synthesis of ternary PtIrCu nanocrystals as robust electrocatalyst for methanol oxidation reaction. Appl Surf Sci 534:147617. https://doi.org/10.1007/s11581-021-04311-7
Altamirano-Gutiérrez A, Fernández A, Varela FR (2013) Preparation and characterization of Pt-CeO2 and Pt-Pd electrocatalysts for the oxygen reduction reaction in the absence and presence of methanol in alkaline medium. Int J Hydrog Energy 38(28):12657–12666. https://doi.org/10.1016/j.ijhydene.2012.12.140
An K, Alayoglu S, Musselwhite N, Plamthottam S, Melaet GRM, Lindeman AE, Somorjai GA (2013) Enhanced CO oxidation rates at the interface of mesoporous oxides and Pt nanoparticles. J Am Chem Soc 135(44):16689–16696. https://doi.org/10.1021/ja4088743
An M, Du L, Du C, Sun Y, Wang Y, Yin G, Gao Y (2018) Pt nanoparticles supported by sulfur and phosphorus co-doped graphene as highly active catalyst for acidic methanol electrooxidation. Electrochim Acta 285:202–213. https://doi.org/10.1016/j.electacta.2018.07.237
Bhuvanendran N, Ravichandran S, Kandasamy S, Zhang W, Xu Q, Khotseng L et al (2021) Spindle-shaped CeO2/biochar carbon with oxygen-vacancy as an effective and highly durable electrocatalyst for oxygen reduction reaction. Int J Hydrog Energy 46(2):2128–2142. https://doi.org/10.1016/j.ijhydene.2020.10.115
Brummel O, Waidhas F, Faisal F, Fiala R, Vorokhta M, Khalakhan I et al (2016) Stabilization of small platinum nanoparticles on Pt–CeO2 thin film electrocatalysts during methanol oxidation. J Phys Chem C 120(35):19723–19736. https://doi.org/10.1021/acs.jpcc.6b05962
Chen G, Ma J, Zhang J, Lin Y, Wang T, Cheng Y et al (2023) Insights into the synergy of platinum and nickel carbonate hydroxide for efficient methanol electro-oxidation. Appl Surf Sci 616:156587. https://doi.org/10.1016/j.apsusc.2023.156587
Chen J, Li Z, Chen Y, Zhang J, Luo Y, Wang G, Wang R (2020) An enhanced activity of Pt/CeO2/CNT triple junction interface catalyst prepared by atomic layer deposition for oxygen reduction reaction. Chem Phys Lett 755:137793. https://doi.org/10.1016/j.cplett.2020.137793
Dai S, Zhang J, Fu Y, Li W (2018) Histidine-assisted synthesis of CeO2 nanoparticles for improving the catalytic performance of Pt-based catalysts in methanol electrooxidation. New J Chem 42(22):18159–18165. https://doi.org/10.1039/C8NJ03972K
Dao DV, Le TD, Adilbish G, Lee I-H, Yu Y-T (2019) Pt-loaded au@CeO2 core–shell nanocatalysts for improving methanol oxidation reaction activity. J Mater Chem A 7(47):26996–27006. https://doi.org/10.1039/C9TA09333H
Guo Y, Tang L, Zhang Y, Wei H, Shu X, Cui J et al (2020) Construction of three-dimensional hierarchical Pt/TiO2@ C nanowires with enhanced methanol oxidation properties. Int J Hydrog Energy 45(58):33440–33447. https://doi.org/10.1016/j.ijhydene.2020.09.052
Li C, Chen X, Zhang L, Yan S, Sharma A, Zhao B et al (2021) Synthesis of Core@Shell cu-Ni@Pt-cu Nano-Octahedra and their improved MOR activity. Angew Chem Int Ed 60(14):7675–7680. https://doi.org/10.1002/anie.202014144
Li J, Rong H, Tong X, Wang P, Chen T, Wang Z (2018) Platinum–silver alloyed octahedral nanocrystals as electrocatalyst for methanol oxidation reaction. J Colloid Interface Sci 513:251–257. https://doi.org/10.1016/j.jcis.2017.11.039
Lou W, Ali A, Shen PK (2022) Recent development of au arched Pt nanomaterials as promising electrocatalysts for methanol oxidation reaction. Nano Res 15(1):18–37. https://doi.org/10.1007/s12274-021-3461-5
Mullins DR (2015) The surface chemistry of cerium oxide. Surf Sci Rep 70(1):42–85. https://doi.org/10.1016/j.surfrep.2014.12.001
Murphin Kumar PS, Thiripuranthagan S, Imai T, Kumar G, Pugazhendhi A, Vijayan SR et al (2017) Pt nanoparticles supported on mesoporous CeO2 nanostructures obtained through green approach for efficient catalytic performance toward ethanol electro-oxidation. ACS Sustain Chem Eng 5(12):11290–11299. https://doi.org/10.1021/acssuschemeng.7b02019
Murpin Kumar PS, Arulmani S, Kim H-i, Koolath Ramakrishnan D, Ponnusamy VK, Pal U, Krishnan SK (2022). An efficient and durable electrocatalyst based on strongly coupled Pt nanoparticles on CeO2 microspheres for CO-resilient methanol oxidation. J Phys Chem C, 126(44), 18670–18682. https://doi.org/10.1021/acs.jpcc.2c05069
Ojani R, Hasheminejad E, Raoof JB (2015) Direct growth of 3D flower-like Pt nanostructures by a template-free electrochemical route as an efficient electrocatalyst for methanol oxidation reaction. Energy 90:1122–1131. https://doi.org/10.1016/j.energy.2015.06.061
Pandit B, Sankapal BR, Koinkar PM (2019) Novel chemical route for CeO2/MWCNTs composite towards highly bendable solid-state supercapacitor device. Sci Rep 9(1):5892. https://doi.org/10.1038/s41598-019-42301-y
Ravichandran S, Bhuvanendran N, Hao S, Sun S, Maiyalagan T, Su H et al (2023) PANI-SnO2 nanorods decorated with Pdx-Niy nanoparticles for improved electrooxidation of methanol with extended durability. Colloids and Surfaces A. Physicochemical and Engineering Aspects, p 131833. https://doi.org/10.1016/j.colsurfa.2023.131833
Ravichandran S, Bhuvanendran N, Peng K, Zhang W, Xu Q, Su H (2021) Pt nanoparticles decorated on Fe2O3/N, P-doped mesoporous carbon for enhanced oxygen reduction activity and durability. J Electrochem Energy Convers Storage 18(2):021006. https://doi.org/10.1115/1.4048083
Ravichandran S, Bhuvanendran N, Xu Q, Maiyalagan T, Su H (2021) Improved methanol electrooxidation catalyzed by ordered mesoporous Pt-Ru-ir alloy nanostructures with trace ir content. Electrochim Acta 394:139148. https://doi.org/10.1016/j.electacta.2021.139148
Sedighi M, Rostami AA, Alizadeh E (2017) Enhanced electro-oxidation of ethanol using Pt–CeO2 electrocatalyst prepared by electrodeposition technique. Int J Hydrog Energy 42(8):4998–5005. https://doi.org/10.1016/j.ijhydene.2016.12.014
Shi Y, Zhang L, Zhou H, Liu R, Nie S, Ye G et al (2023) Te-induced fabrication of Pt3PdTe0.2 alloy nanocages by the self-diffusion of Pd atoms with unique MOR electrocatalytic performance. Nanoscale Adv 5(10):2804–2812. https://doi.org/10.1039/d2na00576j
Su H, Gao W, Li L, Yuan H, Wen D (2023) Oxygen vacancy-rich CeO2 quantum dots boost the activity and durability of Pt/C for methanol oxidation and oxygen reduction reactions. ACS Sustain Chem Eng 11(33):12317–12325. https://doi.org/10.1021/acssuschemeng.3c02126
Sun H, Rao P, Deng P, Li J, Chen Q, Shen Y, Tian X (2022) Three-dimensional porous PtCu as highly efficient electrocatalysts for methanol oxidation reaction. Int J Hydrog Energy 47(84):35701–35708. https://doi.org/10.1016/j.ijhydene.2022.08.155
Tao L, Shi Y, Huang Y-C, Chen R, Zhang Y, Huo J et al (2018) Interface engineering of Pt and CeO2 nanorods with unique interaction for methanol oxidation. Nano Energy 53:604–612. https://doi.org/10.1016/j.nanoen.2018.09.013
Tian H, Yu Y, Wang Q, Li J, Rao P, Li R et al (2021) Recent advances in two-dimensional Pt based electrocatalysts for methanol oxidation reaction. Int J Hydrog Energy 46(61):31202–31215. https://doi.org/10.1016/j.ijhydene.2021.07.006
Wang H, Zhang K, Qiu J, Wu J, Shao J, Deng Y et al (2021) Photoassisted reduction synthesis of Pt@ SnO2/graphene catalysts with excellent activities toward methanol oxidation. Energy Fuel 35(15):12516–12526. https://doi.org/10.1021/acs.energyfuels.1c01750
Wang X, Liu D, Song S, Zhang H (2013) Pt@CeO2 multicore@Shell self-assembled Nanospheres: clean synthesis, structure optimization, and catalytic applications. J Am Chem Soc 135(42):15864–15872. https://doi.org/10.1021/ja4069134
Xia Z, Zhang X, Sun H, Wang S, Sun G (2019) Recent advances in multi-scale design and construction of materials for direct methanol fuel cells. Nano Energy 65:104048. https://doi.org/10.1016/j.nanoen.2019.104048
Xie Y, Wu J, Jing G, Zhang H, Zeng S, Tian X et al (2018) Structural origin of high catalytic activity for preferential CO oxidation over CuO/CeO2 nanocatalysts with different shapes. Appl Catal B Environ 239:665–676. https://doi.org/10.1016/j.apcatb.2018.08.066
Xu H, Wang A-L, Tong Y-X, Li G-R (2016) Enhanced catalytic activity and stability of Pt/CeO2/PANI hybrid hollow nanorod arrays for methanol electro-oxidation. ACS Catal 6(8):5198–5206. https://doi.org/10.1021/cs500107t
Yaqoob L, Noor T, Iqbal N (2021) Recent progress in development of efficient electrocatalyst for methanol oxidation reaction in direct methanol fuel cell. Int J Energy Res 45(5):6550–6583. https://doi.org/10.1002/er.6316
Yu F, Xie Y, Tang H, Yang N, Meng X, Wang X et al (2018) Platinum decorated hierarchical porous structures composed of ultrathin titanium nitride nanoflakes for efficient methanol oxidation reaction. Electrochim Acta 264:216–224. https://doi.org/10.1016/j.electacta.2018.01.137
Yu Y, He J, Wang T, Qiu X, Chen K, Wu Q et al (2023) One-step production of Pt–CeO2/N-CNTs electrocatalysts with high catalytic performance toward methanol oxidation. Int J Hydrog Energy 48. https://doi.org/10.1016/j.ijhydene.2023.04.137
Zhang J-M, He J-J, Wang X-Q, Fan Y-J, Zhang X-J, Zhong J-P et al (2019) One-step synthesis in deep eutectic solvents of PtV alloy nanonetworks on carbon nanotubes with enhanced methanol electrooxidation performance. Int J Hydrog Energy 44(54):28709–28719. https://doi.org/10.1016/j.ijhydene.2019.09.104
Zhang Y, Xu Y, Zhou Y, Xiang S, Sheng X, Wang Q, Zhang C (2015) Hierarchical structures based on gold nanoparticles embedded into hollow ceria spheres and mesoporous silica layers with high catalytic activity and stability. New J Chem 39(12):9372–9379. https://doi.org/10.1039/C5NJ01990G
Zhao Z-G, Guo P, Shen L-X, Liu Y-Y, Zhang Z-Y, Tu F-D et al (2023) Triple-phase interfacial engineering Pt-CeO2-nitrogen-doped carbon electrocatalysts for proton exchange membrane fuel cells. Appl Surf Sci 609:155302. https://doi.org/10.1016/j.apsusc.2022.155302
Zheng Y, Zhang X, Zhang Z, Li Y, Sun Y, Lou Y et al (2018) Preparation and application of ZnO doped Pt-CeO2 nanofibers as electrocatalyst for methanol electro-oxidation. J Rare Earths 36(9):974–980. https://doi.org/10.1016/j.jre.2018.02.007
Zhou Q, Pan Z, Wu D, Hu G, Wu S, Chen C et al (2019) Pt-CeO2/TiN NTs derived from metal organic frameworks as high-performance electrocatalyst for methanol electrooxidation. Int J Hydrog Energy 44(21):10646–10652. https://doi.org/10.1016/j.ijhydene.2019.01.231
Zhu J, Xia L, Yu R, Lu R, Li J, He R et al (2022) Ultrahigh stable methanol oxidation enabled by a high hydroxyl concentration on Pt clusters/MXene interfaces. J Am Chem Soc 144(34):15529–15538. https://doi.org/10.1021/jacs.2c03982
Acknowledgements
We thank the National Natural Science Foundation of China (22108044), the Research and Development Program in Key Fields of Guangdong Province (2020B1111380002), the financial support from the Guangdong Provincial Key Laboratory of Plant Resources Biorefinery (2021GDKLPRB07), the Special Funds for the Cultivation of Guangdong College Students’ Scientific and Technological Innovation, and the Basic Research and Applicable Basic Research in Guangzhou City (202201010290).
Author information
Authors and Affiliations
Contributions
Sabarinathan Ravichandran was responsible for conceptualization, methodology, investigation, formal analysis, writing the original draft, reviewing, and editing. Shuhua Hao conducted investigation and formal analysis. Wenli Zhang acquired funding, supervised the study, and reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
Wenli Zhang is the lead guest editor of the special issue, Functional Carbon Materials for Electrochemical Energy Conversion and Storage, and was not involved in the editorial review or the decision to publish this article. All authors declare that there are no competing interests.
Additional information
Handling editor: Ke Lu
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ravichandran, S., Hao, S. & Zhang, W. CeO2-supported Pt nanoclusters for improved electrochemical oxidation of methanol. Carbon Res. 3, 21 (2024). https://doi.org/10.1007/s44246-023-00091-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44246-023-00091-z