Abstract
Structures and functions were varied among roots with different diameters, and responded differently to nitrogen (N) addition, a phenomenon called hierarchical trait. However, the presence of this trait in carbon (C) metabolite of root exudate and rhizosphere microbial community remains unclear. We studied the patterns of C metabolites and microbial communities of rhizosphere soil among different diameter roots of Pinus tabulaeformis and their responses to N addition using metabolomics and high-throughput sequencing methods. Root C metabolites and rhizosphere soil microbial communities had hierarchical traits, in which exudates of R1 roots (0–0.5 mm) were rich in fatty acids and organic acids, while exudates of R3 roots (1–2 mm) were rich in carbohydrates and alcohols. With the increasing root diameters, numbers of bacteria and fungi of rhizosphere soil decreased and increased, respectively, while diversities of bacteria and fungi increased. N addition had a promotion effect on abundance of root C metabolites. Numbers of bacteria and fungi of rhizosphere soil were enhanced and inhibited, respectively, while diversities of bacteria and fungi were facilitated by N addition. N addition promoted microbial populations of rhizosphere soil by reducing exudates of R1 roots, and increasing exudates of R2 (0.5–1 mm) and R3 roots. Hippuric acid and cytidine-5´-monophosphate of R1 roots, lactic acid of R2 roots, glucose 1 and spermidine 2 of R3 roots were the functional C metabolites that affected microbial populations of rhizosphere soil, such as Actinobacteria, Acidobacteria, Proteobacteria, Basidiomycota, and Ascomycota. Hierarchical traits were extended from root to rhizosphere soil in this study.
Graphical Abstract

Highlights
(1) Metabolomics and high-throughput sequencing were used to study root exudate and soil microbial community in forest.
(2) There were hierarchical traits in C metabolite of root and soil microbial community among root diameters.
(3) Changing mechanisms of rhizosphere soil microbial community after N addition were varied among root diameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Root exudation is an important way for plants to “communicate” with external environment and build beneficial microbial community, subsequently affecting plant growth and soil matter cycling (Badri and Vivanco 2009). It is generally believed that fine roots are the organs that secrete carbon (C) metabolites, while thick roots do not secrete C metabolites, so root exudates changing soil microbes only occurred around fine roots (Czarnota et al. 2001; Badri and Vivanco 2009). However, some studies have indicated that thick roots secrete C metabolites as well, and C metabolites were varied among different plant species (Mcdougall 1968; Rovira 1969; Li et al. 2018). Therefore, exudates of different diameter roots were quite different, which called hierarchical trait (Freschet et al. 2021). Nevertheless, the specific C metabolites and patterns among different diameter roots need to be clarified. On the one hand, when plants are in nutrient deficiency, root C metabolite secretion is enhanced to promote soil biochemical reactions, and increase soil nutrient contents and plant resistances (Bloom et al. 1985). While plants are in nutrient sufficiency, root C metabolite secretion is inhibited, and there is limited change in soil microbial activity. On the other hand, some studies have found that when soil nutrients are sufficient, root C metabolite secretion does not decrease, but tends to increase (Zhang et al. 2007; Xu et al. 2014). This result may be related to the rich root nutrients and the passive transport of exudates. Nitrogen (N) addition has the ability to directly improve soil fertility and alleviate plant nutrient limitation, but with the acceleration of N addition, the soil is gradually acidified, and plant nutrient limitation transfers to saturation. The environmental transformation from favorable to adverse will lead to an apparent change in plant survival strategies, while the C metabolites and tendency of root exudate respond to N addition are still unclear.
Rhizospheric microbes are affected by soil properties and root exudates, but their effects are varied. For example, root secreted strigolactone to promote reproduction of arbuscular mycorrhizal fungi, which further alleviated plant N limitation (Yoneyama et al. 2012). While some studies suggest that the role of root C metabolites may be overestimated. Dennis et al. (2010) confirmed that the direct effect of root C metabolites on the bacterial community of rhizosphere soil was limited to the vicinity of root tip. Thus, the effects of root C metabolite and soil property on rhizosphere soil microbes are still controversial. Recently, microbial communities have been found differed between rhizosphere soils of young and old roots (Wang et al. 2017b), and root C metabolites have hierarchical traits according to the above statement, so the relationships between root C metabolites and microbial communities need to be verified. Increasing N addition generally inhibits soil microbial reproduction, and the community significantly changes (Wang et al. 2018). While microbial community of the rhizosphere soil has a more complex change after increasing N addition, which is related to the roots with different diameters. Fine roots have stronger physiological activity, and their mycorrhizal growth is more sensitive to environmental change than thick roots (Kou et al. 2015). It can be speculated that fine roots are more likely to be affected by N addition when uptaking nutrients and secreting C metabolites. These root indicators are closely related to soil microbes, which indicates that the changes in microbial community in the rhizosphere soil of fine roots may be greater than those of thick roots after N addition.
P. tabulaeformis forest was N limited on the Loess Plateau of China (Wang and Zheng 2018), and its root morphology had hierarchical traits (Wang et al. 2013), which was suitable for testing the following hypotheses: (1) there are hierarchical traits in root C metabolites, and N addition has varied effects on C metabolite of different diameter roots; (2) the effect of root C metabolite on the microbe is more important than that of soil property. Due to this effect, rhizosphere soil with increasing root diameters has different microbial communities, and the microbial communities in rhizosphere soil of fine roots were more responsive to N addition than those of thick roots.
2 Materials and methods
2.1 Study area and N addition treatment
The experimental field of P. tabulaeformis forest was located in the Songyugou watershed, Shaanxi Province, China (35°58′34″N, 110°05′38.1″E), with an altitude of 1000–1200 m, a slope of 20°-25° and it is east. This region had a typical continental monsoon climate, with an average annual precipitation of 574.4 mm and an annual average temperature of 9.8 °C. P. tabulaeformis was planted in 1960s, and the stand characteristics in recent years are as follows: a stand density of 1400–1800 ha−1, an average diameter breast height of 10.0 cm, an average tree height of 11.2 m, a forest volume of 75.5 m3 hm−2, and a leaf area index of 6.34. Soil in P. tabulaeformis forest belongs to the gray forest soil (according to FAO soil classification), the basic chemical properties before N addition are shown in Table 1, and a detailed site description has been explained by Jing et al. (2021).
According to the atmospheric N deposition rate in the study field and the experimental design in previous studies (Yang et al. 2010; Wang and Zheng 2018), we have conducted N addition treatments (0 (CN), 3 (LN), 6 (MN), and 9 (HN) g N m−2 y−1) in P. tabulaeformis forest since 2014. Each N addition treatment had 4 replicate plots, and the area of each plot was 100 m2 (10 m × 10 m). Ammonium nitrate (NH4NO3) was dissolved in distilled water and sprayed evenly on the soil surface before the light rain in April, June, August, and October every year.
2.2 Soil and root exudate sampling
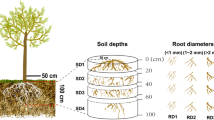
Soil samples include rhizosphere soils with three root diameter classes (R1, 0–0.5 mm; R2, 0.5–1 mm; R3, 1–2 mm) of P. tabulaeformis and non-rhizosphere soil. In October 2019, non-rhizosphere soil was sampled in canopy gaps, where there were no trees and understory vegetation was sparse in each plot. Soil drill was used (5 cm in diameter and 20 cm in length) to obtain the surface (0–20 cm) non-rhizosphere soil and debris were removed at the same time. The operation processes of rhizosphere soil sampling were briefly described as follows. Soil profiles (30 cm in depth) were excavated near the P. tabulaeformis, and there were many root cut surfaces distributed on it. A small spoon was used to collect the soil sample within 0–1 cm around the root with specific diameter class. Meanwhile, multiple collections were operated to meet the fresh mass requirement of 200 g. All soil samples were divided into two parts after removal of foreign matter and passing through a 2 mm sieve. One part was used for chemical analysis after air-drying for several days to a constant weight, and the other one was stored at -80 ℃ for microbial community and quantity analyses.
Root exudate of P. tabulaeformis was collected based on the modified static collection method proposed by Phillips et al. (2008), and the reliability of this method has been confirmed in previous studies (Proctor et al. 2017; Sun et al. 2020). Firstly, intact roots were dug out and washed using deionized water. Then, these roots were placed in a moist fabric tissue and surrounded by the soil and covered with litter for 48 h to recover the potential injury during excavation. Secondly, roots were washed again, and put into a 50 ml centrifuge tube with 750 μm glass beads and C-free nutrient solution (0.5 mM NH4NO3, 0.1 mM KH2PO4, 0.2 mM K2SO4, 0.2 mM MgSO4, 0.3 mM CaCl2). In this case, glass beads were used to facilitate the soil mechanical impedance and porosity. The surface of centrifuge tube was then covered with aluminum foil and litter to prevent disturbances. Thirdly, three days later, the solution in the centrifuge tube was withdrawn with a syringe, and the centrifuge tube was rinsed twice with C-free nutrient solution. All solutions were mixed as root exudate profiling. Root exudate was kept at 4 ℃ and sterilized with ultraviolet light in forest, while filtered through sterile filters and frozen at -20 °C in laboratory for metabolomics analysis. Exudates were collected from roots with three diameter classes (R1, R2 and R3), and there were 6 replicates per treatment.
2.3 Soil chemical analysis
Soil organic carbon (SOC) content was determined using the potassium dichromate and sulfuric acid heating method (Mebius 1960); total nitrogen (TN) content was determined using the Kjeldahl method (Page et al. 1982); total phosphorus (TP) and available phosphorus (AP) contents were determined using the molybdenum antimony colorimetric method (Lu 2000); ammonium (NH4+) and nitrate (NO3−) contents were determined using the continuous flow analyzer method; pH value was determined using the automatic titrator method. The present study focus on metabolites of root and rhizosphere microbial communities, and soil physicochemical properties could be found in our previous studies (Jing et al. 2021; Zhang et al. 2022).
2.4 Soil microbial quantity and community analyses
Soil bacterial and fungal quantities were determined by real time PCR, and detailed soil DNA extraction and PCR analysis were described in the Supplementary material. Soil bacterial and fungal community were evaluated based on the 16S and ITS rRNA genes, and the operations included DNA extraction, Illumina HiSeq2500 high-throughput sequencing, and data analysis. DNA was extracted from 0.4 g fresh soil samples using the soil DNA kit (Omega Bio-Tech, USA), following the manufactural instructions. Then the extracted DNA sample was qualitatively checked using 1% agarose gel electrophoresis, and the concentration was checked using a NanoDrop spectrophotometer (Thermo Fisher Scientific Inc, USA). High-throughput sequencing was performed after PCR amplification, and the operating procedures and instrument parameter of sequencing were described in the Supplementary material. The preliminary microbial community composition of bulk soil of P. tabulaeformis forest can be found in Zhang et al. (2022). High-throughput sequencing data of the present study is provided at the NCBI (SRA) database under the study accession code PRJNA1014789.
2.5 Metabolomics analysis of root exudate
The composition and relative abundances of C metabolites of P. tabulaeformis roots were analyzed by non-targeted metabolomics based on gas chromatography-time-of-flight mass spectrometry (GC–MS). The basic procedures include: (1) extraction and purification of exudates; (2) sample detection using GC–MS; (3) matching of secondary C metabolites to reference data; and (4) standardization of data. Detailed operating procedures were described in the Supplementary material.
2.6 Statistical analyses
The alpha diversity of microbial community was evaluated by Chao1, Simpson, and Shannon indices, and the differences among treatments were analyzed by two-way ANOVA. Multiple comparative analyses (Duncan) were performed to test the differences of microbial copy numbers among N additions and root diameters. Beta diversity was analyzed using the rarefied operational taxonomic unit (OTU) table to investigate the structural variation of microbial communities among treatments based on the nonmetric multidimensional scaling analysis (NMDS) and global and pairwise permutational multivariate analysis of variance (PERMANOVA). Linear discriminate analysis effect size (LEfSe) was carried out and visualized on the platform of Galaxy/Hutlab (http://huttenhower.org/galaxy/) to select differential microbial populations among treatments, depending on the threshold of LDA > 2.0.
The relative abundances of primary C metabolites were calculated by summing the peak area of each metabolite by the sum of the peak area of all metabolites. Meanwhile, the relative abundances of secondary C metabolites were calculated by comparing analytes with standard sample, and the compositions were evaluated using NMDS. The differential root C metabolites were selected depending on the statistically significant threshold of variable influence on projection (VIP) values acquired from the partial least squares discriminant analysis (PLS-DA), and p values acquired from one-way ANOVA. The differential root C metabolites were visualized as the heatmap when the VIP value was greater than 1 and p value was less than 0.05, and the fitting of the PLS-DA model was checked by permutation test. PLS-DA and permutation test were carried out by SIMCA-P 14.0 software (Umetrics Umea, Sweden), and other figures were produced by “ggplot2” package in R v3.5.2 software.
The relationships between differential microbial populations and C metabolites were analyzed by co-occurrence network of SparCC, which was carried out on the platform of M2IA (http://m2ia.met-bioinformatics.cn/), and the operations include correlation coefficient calculation and the p value was proposed by permutation test (1000 bootstraps and iteration of default-i 20). The co-occurrence networks were constructed using only significant correlations. Differential microbial populations and C metabolites were selected by LEfSe and PLS-DA. The mechanisms of N addition changing microbial community were analyzed by PLS-PM with the R package “plspm”. N addition implied the four rates of N addition, and root diameter implied the maximum values of the three root diameter classes, 0.5, 1, and 2. Soil property included the chemical indices that significant changed under N addition (ANOVA, p < 0.05). Microbial community and root exudate indicated the first principal coordinate (PCo1) of differential microbial populations and C metabolites among N additions by Bray–Curtis distance matrices. Path coefficients were showed in PLS-PM figure after 1000 bootstraps, and goodness of fit (GOF) indicated the fitting of the model, which should be greater than 0.6 (Bi et al. 2021; Cui et al. 2021). The applicability of PLS-PM model was improved by adjusting the causal relationship between observed and potential variables, and detailed processes were described by Rodrigues et al. (2019).
3 Results
3.1 Soil microbial quantity, richness, and structure
There were significant variations in bacterial and fungal copy numbers among rhizosphere soil of different diameter roots (Table 2; p < 0.05). The highest microbial copy number (72.16 × 107 g soil−1) and bacterial copy number (68.70 × 107 g soil−1) were found in rhizosphere soil of R1 root, followed by those of R2 and R3 roots. Fungal copy number in rhizosphere soil of R3 root (59.75 × 106 g soil−1) was higher than that of R2 and R1 roots. N addition significantly changed the bacterial and fungal copy numbers of rhizosphere soil (Table 2; p < 0.05). The microbial and bacterial copy numbers showed parabolic responses to N addition, and rhizosphere soil of R1 root had the maximum value in MN treatment, while rhizosphere soils of R2 and R3 roots had the maximum values in LN treatment. Higher N addition significantly decreased fungal copy number, and rhizosphere soils of R1, R2 and R3 roots had the maximum values in CN or LN treatments.
Chao1 (2742.6 (bacteria), 294.1 (fungi)), simpson (0.9983 (bacteria), 0.9765 (fungi)) and shannon (10.23 (bacteria), 6.75 (fungi)) indices of non-rhizosphere soil were significantly higher than those of rhizosphere soils (Fig. 1; p < 0.05). Microbial diversity indices of rhizosphere soil increased with root diameters, while Chao1 index of fungi decreased with root diameters. N addition significantly increased the bacterial diversity indices in rhizosphere soil of R1 root, with the maximum values observed in HN treatment. Bacterial diversity indices of other rhizosphere soils increased first and then decreased with N additions, and reached the maximum values in MN treatment. Similarly, fungal diversity indices of all rhizosphere soils increased first and then decreased with N additions, and reached the maximum values in MN treatment.
Richness and diversity measures of microbial community in rhizosphere soil of different root diameters and non-rhizosphere soil among N addition treatments (n = 4). Lines inside the boxes represent medians, dots represent means, and the upper and lower edges of the boxes represent the 25th percentile and 75th percentile, respectively. The top bars show the maximum values, and the bottom bars show the minimum values. R1, rhizosphere soil of 0–0.5 mm root; R2, rhizosphere soil of 0.5–1 mm root; R3, rhizosphere soil of 1–2 mm root; Nor, non-rhizosphere soil. CN, LN, MN, and HN are 0, 3, 6, and 9 g N m−2 y−1, respectively
There were significant differences in microbial community among rhizosphere soils and non-rhizosphere soil (Fig. 2e and S1e; Table 3; p < 0.05). A total of 17 differential microbial populations were observed among rhizosphere soils and non-rhizosphere soil at phylum and genus levels (Fig. 3a). Specifically, 10 differential microbial populations were dominant in non-rhizosphere soil, mainly Ascomycota, Tetracladium, Fusarium, etc.; differential populations of Chloroflexi and Sebacina were dominant in rhizosphere soil of R1 root; differential populations of Basidiomycota and Acidimicrobiaceae were dominant in rhizosphere soil of R2 root; differential populations of Actinobacteria and Solirubrobacteraceae were dominant in rhizosphere soil of R3 root. N addition significantly changed the microbial community (Table 3; p < 0.05). HN and MN had the greatest effects on fungal and bacterial communities, respectively. There was no significant interaction effect between root diameter and N addition on soil microbial community, which means that microbial community of different soils had the similar response to N addition.
NMDS analysis based on Bray–Curtis distances depicting the compositions of bacterial community in rhizosphere soils of R1 (a), R2 (b), R3 (c), and non-rhizosphere soil (d) among N addition treatments and root diameters at CN (e) treatment. CN, LN, MN, and HN are 0, 3, 6, and 9 g N m−2 y−1, respectively. R1, rhizosphere soil of 0–0.5 mm root; R2, rhizosphere soil of 0.5–1 mm root; R3, rhizosphere soil of 1–2 mm root; Nor, non-rhizosphere soil
LEfSe of rhizosphere soil microbial populations among root diameters at CN treatment and among N addition treatments at different root diameters (n = 4). _g, genus; _p, phylum. a, microbial populations among root diameters at CN treatment; b, microbial populations among N addition treatments at rhizosphere soil of R1 root; c, microbial populations among N addition treatments at rhizosphere soil of R3 root; d, microbial populations among N addition treatments at non-rhizosphere soil.; e, microbial populations among N addition treatments at rhizosphere soil of R2 root. CN, LN, MN, and HN are 0, 3, 6, and 9 g N m−2 y−1, respectively. R1, R2, and R3 are < 0.5 mm, 0.5–1.0 mm, and 1.0–2.0 mm, respectively; Nor, non-rhizosphere soil
3.2 Composition and relative abundance of C metabolites
Metabolomics revealed 523 secondary C metabolites of P. tabulaeformis roots, which were classified into 17 primary C metabolites (Fig. 4). Exudates were released by all three diameter roots, while the compositions were significantly different among three diameter roots (Fig. 5a and S2). In general, there were 91 differential C metabolites among root diameters (Fig. 6a and S3a), in which 47 differential C metabolites had the highest relative abundances in R1 roots; 11 differential C metabolites had the highest relative abundances in R2 roots; 33 differential C metabolites had the highest relative abundances in R3 roots. Specifically, the relative abundances of 10 organic acids, 9 fatty acids, 4 fats, 3 phenols and 3 nucleotides of R1 roots were higher than those of R2 and R3 roots; the relative abundances of 3 nucleotides and 2 amino acids of R2 roots were higher than those of R1 and R3 roots; the relative abundances of 4 carbohydrates, 3 alkylamines and 3 alcohols of R3 roots were higher than those of R1 and R2 roots.
Relative abundance of the root primary C metabolites among N addition treatments and root diameters. The relative abundance is calculated by the summation of the peak area of each C metabolite by the summation of the peak area of all C metabolites. CN, LN, MN, and HN are 0, 3, 6, and 9 g N m−2 y−1, respectively. R1, R2, and R3 are < 0.5 mm, 0.5–1.0 mm, and 1.0–2.0 mm, respectively
Score scatter plot of PLS-DA for identified differential C metabolites among root diameters at CN treatment and among N addition treatments at different roots (n = 6). a, root C metabolite profiles among root diameters at CN treatment; b, root C metabolite profiles among N addition treatments in R1 root; c, root C metabolite profiles among N addition treatments in R2 root; d, root C metabolite profiles among N addition treatments in R3 root. CN, LN, MN, and HN are 0, 3, 6, and 9 g N m−2 y−1, respectively. R1, R2, and R3 are < 0.5 mm, 0.5–1.0 mm, and 1.0–2.0 mm, respectively
Clustered heatmap of the top 40 differential secondary C metabolites in VIP values and their associated primary C metabolites. a, root secondary C metabolites among root diameters at CN treatment; b, root secondary C metabolites among N addition treatments in R2 root; c, root secondary C metabolites among N addition treatments in R1 root; d, root secondary C metabolites among N addition treatments in R3 root. CN, LN, MN, and HN are 0, 3, 6, and 9 g N m−2 y−1, respectively. R1, R2, and R3 are < 0.5 mm, 0.5–1.0 mm, and 1.0–2.0 mm, respectively
The significant difference in the composition of C metabolites of R1 roots was the largest among N addition treatments, followed by the significant difference in composition of C metabolites of R1 and R2 roots. (Fig. 5b, c, and d; p < 0.05). Specifically, there were 87 differential C metabolites in R1 roots among N addition treatments (Fig. 6 and S3), and relative abundances of C metabolite were the highest in MN treatment; R2 roots had 110 differential C metabolites, which were abundant in MN and HN treatments; R3 roots had 91 differential C metabolites, which were abundant in HN treatment. The differential C metabolites affected by treatments were mainly organic acids, amino acids, carbohydrates, and fatty acids.
3.3 Effects of N addition on rhizosphere microbial community by changing root exudate
N addition had significant positive effects on microbial populations of rhizosphere soil by reducing the C metabolite abundance of R1 roots (Fig. 7c and 8). N addition increased the C metabolite abundance of R2 roots, but did not promote the microbial populations of rhizosphere soil. As for R3 roots, N addition significantly increased the C metabolite abundance and further promoted the microbial populations of rhizosphere soil. In non-rhizosphere soil, N addition had a direct positive effect on microbial populations.
Network of correlation analysis between differential secondary C metabolites and bacterial populations. Only significant correlations are shown (p < 0.05). The green lines show significant positive correlations, the red lines show significant negative correlations, and the dashed lines show the relationships between secondary C metabolites and primary C metabolites, and the relationships between microbial genus and phylum. a, correlation analysis between C metabolites and bacterial populations in rhizosphere soil of R2 root; b, correlation analysis between C metabolites and bacterial populations in rhizosphere soil of R3 root; c, correlation analysis between C metabolites and bacterial populations in rhizosphere soil of R1 root
Path analysis of the effects of N addition on root exudate, soil chemical property, and microbial populations of rhizosphere soil with different root diameters and non-rhizosphere soil. The width of the arrow is proportional to the absolute value of path coefficient. Red indicates a negative effect, and blue indicates a positive effect. Coefficient is calculated after 1000 bootstraps. Solid lines indicate a significant effect between variables, while dashed lines indicate and insignificant effect between variables. GOF, goodness of fit. * p < 0.05; ** p < 0.01
The relationships between C metabolites and microbial populations varied greatly among different diameter roots (Fig. 7). 2-hydroxypyridine, cytidine-5´-monophosphate and hippuric acid secreted by R1 roots were significantly correlated with 17 bacterial genera, with 15 negative correlations and 12 positive correlations. Lactic acid, conduritol b epoxide 2, and ethanolamine secreted by R2 roots had significant relationships with 11 bacterial genera, and there were 12 negative correlations and 7 positive correlations. Glucose 1, spermidine 2, and lactic acid secreted by R3 roots had significant relationships with 5 bacterial genera, and there were 5 negative correlations and 8 positive correlations. Bacterial genera affected by root C metabolites mainly belonged to Actinobacteria, Acidobacteria and Proteobacteria. Basidiomycota and Ascomycota were the fungal phyla affected by root C metabolites (Fig. S4). 2-hydroxypyridine, conduritol b epoxide 2, cytidine-5´-monophosphate secreted by R1 roots promoted 4 fungal genera. Lactic acid and spermidine 2 secreted by R3 roots promoted 4 fungal genera, while urea and glucose1 inhibited 2 fungal genera.
4 Discussion
4.1 C metabolites of root exudates
Root system with different diameters were varied in chemical composition, physiological function, and response to environmental changes, referred to as hierarchical traits (Freschet et al. 2021). Most C metabolites had the highest abundances of exudate of R1 roots. This result is consistent with our hypothesis and supports previous studies (Rovira 1969; Badri and Vivanco 2009). The relative abundances of 47 C metabolites such as organic acids, fatty acids, and fats of finer roots were higher than those of other diameter roots. Organic acids have the function of improving the availability of soil nutrients, regulating soil pH, and inhibiting the reproduction of harmful microbes (Dakora and Phillips 2002). Fatty acids, fats, and phenols have the ability to strengthen the resistance of roots and shape the microbial community of rhizosphere soil. The high abundances of these C metabolites represent vigorous physiological activity and diverse functions of finer roots. In addition, there was C metabolite secretion of thick roots as well, the relative abundances of carbohydrates, alcohols and alkylamines were higher than those of other diameter roots. Likewise, the exudation of carbohydrate of cucumber occurred not only at root tips, but also throughout the whole root system (Li et al. 2018). Our results support previous studies due to the similar traits of root system, and indicate that thick roots produce higher abundances of alcohols and alkylamines. Alcohols can be used as energy substances by specific microbes (Zhu et al. 2016), while alkylamines act as the common organic amine, which has the ability to enhance plant resistance (Mench and Martin 1991). The higher abundances of these C metabolites implied that thick roots not only provide energy for microbes, but also have functions of affecting soil microbial community and plant adaptability.
N addition significantly increased the relative abundances of root C metabolites, reaching the maximum values in MN or HN treatments. Root exudates were released to attract microbes to fix more N and relieve N limitation of plants (Jones 1998). Exogenous N input directly provides sufficient nutrients for plants, thus reducing the release of root exudate (Carvalhais et al. 2010; Jauregui et al. 2015). Some others argued that root exudate secretion did not increase under plant N limitation (Haase et al. 2007), while root exudate secretion was improved when plant N limitation was relieved (Yang 2015). This may be due to the relief of N limitation that increased the root nutrient contents, and excess amino acids were released in rhizosphere soil by increasing the output of exudates. These results indicate that plants have different survival strategies in response to environmental changes. In the present study, N addition significantly affected the relative abundances of various C metabolites, including organic acids, amino acids, and carbohydrates. These C metabolites are expected to shape microbial community of rhizosphere soil and improve the plant health (Shepherd and Davies 1994; Jiang et al. 2009; Kachroo and Kachroo 2009).
PLS-DA showed that C metabolite composition of R1 roots had the largest difference among N addition treatments, followed by that of R2 and R3 roots (Fig. 5). The effects of N addition on composition of root C metabolite were weakened with root diameters, which was consistent with the hypothesis. Varied responses of different diameter roots to environmental changes have been demonstrated in many studies, such as root biomass, morphological structure, respiration, turnover, etc. (Guo et al. 2004; Kou et al. 2015; Wang et al. 2017a; Jing et al. 2019). These indicators of root system were closely related to the exudate secretion (Badri and Vivanco 2009), which may be the reasons that exudates of different diameter roots had varied responses to N addition as well.
4.2 Microbial communities of rhizosphere soil
In rhizosphere soil, bacterial and fungal copy numbers decreased and increased with root diameters, respectively. Soil bacteria had abundant species and strong tolerance to environmental changes (Brown and Jumpponen 2014), while soil fungi had fewer species and were sensitive to environmental changes (Chen et al. 2021). Although rhizosphere soil of finer roots had rich nutrient contents, acid exudates inhibited the fungal reproduction (Jones 1998). On the contrary, thick roots released more carbohydrates, which provided a favorable environment for fungal reproduction (Jones and Darrah 1994; Li et al. 2018). As for bacteria, their reproduction was more active in rhizosphere soil of finer roots due to the rich soil nutrient contents. In addition, our study found that bacterial diversity did not significantly change between rhizosphere and non-rhizosphere soils, while fungal diversity of non-rhizosphere soil was significantly higher than that of rhizosphere soil. This result indicated that soil fungal community was more sensitive to environmental changes than bacterial community, and rhizosphere soil was not conducive to fungal diversity (Gqozo et al. 2020; Chen et al. 2021). With the increase of root diameters, bacterial and fungal diversities of rhizosphere soil were increased, which confirmed the hierarchical trait of soil microbial diversity. Finer roots produced a variety of acids, such as organic acids and fatty acids (Shepherd and Davies 1994; Xing and Chin 2000). These C metabolites promoted the reproduction of specific microbes, but inhibited the reproduction of others. Gqozo et al. (2020) suggested that the reduction of microbial diversity was attributed to the pressure exerted by roots. In this study, microbial diversity of rhizosphere soil increased with root diameters, indicating that finer roots have a greater selection effect on soil microbial community than thick roots.
The dominant populations and diversity of microbial community of non-rhizosphere soil were more abundant than those of rhizosphere soil. These microbial indicators have the same pattern, and the changing reasons are similar as well. In addition, it was found that specific populations dominated the rhizosphere soil of different diameter roots. Actinobacteria has a low nutrient requirement and belongs to oligotrophic bacteria (Chen et al. 2021), which was dominant in the rhizosphere soil of thick roots. Basidiomycota is the dominant populations of fungi, including ectomycorrhiza and macromolecular decomposers (Syed et al. 2010; Kjoller and Rosendahl 2014), and its abundance was rich in rhizosphere soil of fine root. Chloroflexi has a strong tolerance to the environmental changes, especially heavy metal and acidity (DeBruyn et al. 2011). Sebacina is the most common fungus of litter in coniferous forests (Obase et al. 2009). As a result, they were dominant in rhizosphere soil of finer roots, which is related to the low soil pH and active biochemical reactions (Obase et al. 2009; Bi et al. 2021). These results are consistent with the pattern of microbial quantity and diversity of rhizosphere soil among root diameters. Therefore, hierarchical traits exist in the microbial populations of rhizosphere soil.
Soil bacterial copy number had parabolic responses, while fungal copy number had decreasing responses to N addition. The responses of fungi and bacteria were quite different, and bacterial reproduction requires more N than fungus. Microbes of fungal community have the capacity of N-fixation (Smith and Read 2008), and N input has a limited promotion effect on their reproduction, while soil acidification might inhibit their reproduction (Han et al. 2020). However, bacteria generally do not have the ability to fix N (Brown and Jumpponen 2014), and N input alleviates the nutrient limitation of bacterial community and promotes the reproduction (Su et al. 2016). Therefore, the quantity and diversity of bacterial community were promoted by N addition, while those of fungal community were inhibited and promoted by N addition, respectively. Microbial quantity and diversity had different responses to environmental changes (Su et al. 2016). The opposite changes of fungal diversity and quantity in our study were due to the significant inhibition of N addition on the reproduction of dominant populations, leading to the decrease of fungal quantity. The reproduction of other fungal populations was promoted, so there was no significant change or increase in diversity.
Microbial copy number in rhizosphere soil of thick roots was more responsive to N addition than that of fine roots, which is opposite to the responses of root exudates. Soil microbial community has a high-level homeostasis when there is sufficient supply of C substances, resulting in minimal variation in microbial quantity. While homeostasis of soil microbial community is unstable when the C substances supply is deficient, and microbial quantity is easily affected by environmental changes (Yanardag et al. 2017). The microbial copy numbers in rhizosphere soil of thick roots were more sensitive than those of fine roots, which may be related to their low C substance supply. Furthermore, the sensitivities of soil microbial diversity to N addition were basically the same as the sensitivities of soil microbial quantity, and the reasons for changes might be the same as well. By screening out differential microbial populations among N additions, it was found that rhizosphere soil of R1 root had more differential microbial populations than non-rhizosphere soil, followed by rhizosphere soil of R3 root. The sensitivities of microbial dominant populations to N addition were quite different from the sensitivities of microbial quantity and diversity. Zhao et al. (2013) confirmed that N addition had no significant effect on the soil microbial community of eucalyptus plantation, but significantly changed the fungal quantity. Microbial dominant populations had different responses to environmental changes due to the species-specific trait, while structure and diversity reflected the overall state of microbial community. Therefore, changes in these microbial indicators may also be different.
4.3 The mechanisms between microbial communities and root exudates
Based on the PLS-PM, it was found that root exudate was the main driving factor of N addition affecting microbial community of rhizosphere soil, which was consistent with our hypothesis. Rhizosphere is the hotspot of matter cycling due to the rich soil nutrients and the abundant root exudates (Rovira 1969). However, recent studies suggest that the effects of root exudates on microbes have been overestimated (Dennis et al. 2010), because previous results were mostly obtained from indoor hydroponics experiments. There is an urgent need to verify the effect of root exudates on soil microbes under field conditions. In the present study, root exudates had an important effect on microbes of rhizosphere soil in P. tabulaeformis forest, which supports the previous studies. N addition promoted microbial populations of rhizosphere soils by reducing the C metabolite abundance of finer roots, and increasing the C metabolite abundance of thick roots. The reduced C metabolite abundance of finer roots had the promotion effect on microbial populations of rhizosphere soil, which might be related to the fact that exudates of finer roots were rich in organic acids and fatty acids. These C metabolites inhibit the reproduction of acid-intolerant microbes, and the exudate reduction is favorable for the microbial reproduction (Dakora and Phillips 2002). Exudates of thick roots were rich in carbohydrates and alcohols, which are nutrients for the reproduction of most microbes (Jones and Darrah 1994; Li et al. 2018). Therefore, exudates of thick roots were conducive to the microbial reproduction. Additionally, the driving effect of C metabolites of medium fine roots on the influence of N addition affecting microbial populations was insignificant, which might be related to the low abundance of C metabolites of medium fine roots.
R1 roots secreted hippuric acid and cytidine-5´-monophosphate, R2 roots secreted lactic acid and conduritol b epoxide 2, and R3 roots secreted glucose 1 and spermidine 2 were the main functional C metabolites affecting microbes of rhizosphere soils. Different diameter roots had varied functional C metabolites, which were related to the specific composition of root exudates (Mcdougall and Rovira 1970; Li et al. 2018). However, the affected microbial populations of rhizosphere soil were similar among different diameter roots. Actinobacteria, Acidobacteria and Proteobacteria were the affected bacteria, and Basidiomycota and Ascomycota were the affected fungi. These microbes are the dominant populations of soil microbial community in P. tabuliformis forest (Urbanova et al. 2015), and thus microbial community would be altered by root exudates as well. In addition, with the increasing root diameters, bacterial populations of rhizosphere soil affected by exudates were reduced, and the exudates of finer roots had more inhibitory effects, while the exudates of thick roots had more promoting effects. These results support the PLS-PM that N addition affected microbial community by changing the exudates of finer roots and thick roots (Fig. 8). The effects of acid C metabolites on microbes were stronger than those of carbohydrates, which might be the reason that microbial populations in rhizosphere soil of finer roots were more likely to be affected by exudates than those of thick roots.
5 Conclusion
Different diameter roots had varied responses to N addition. Specifically, N addition had a promotive effect on the abundances of root C metabolites, and the changes of C metabolites of finer roots were more apparent than those of thick roots. N addition increased the rhizosphere microbial diversity and bacterial quantity, but decreased fungal quantity. In addition, the microbial quantity and diversity in rhizosphere of thick roots were more sensitive to N addition than those of finer roots. Root exudates were confirmed to be an important driving factor of N addition affecting rhizosphere soil microbes in forest ecosystem. Meanwhile, the functional C metabolites were quite different, but the affected microbial populations of rhizosphere soil were similar among different diameter roots. Future studies should reveal the roles of these functional C metabolites and extend the applicability of hierarchical traits to more plant species.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- N:
-
Nitrogen
- C:
-
Carbon
- NH4NO3 :
-
Ammonium nitrate
- SOC:
-
Soil organic carbon
- TN:
-
Total nitrogen
- TP:
-
Total phosphorus
- AP:
-
Available phosphorus
- NH4 + :
-
Ammonium
- NO3 − :
-
Nitrate
- GC-MS:
-
Gas chromatography-time-of-flight mass spectrometry
- OTU:
-
Operational taxonomic unit
- NMDS:
-
Nonmetric multidimensional scaling analysis
- PERMANOVA:
-
Pairwise permutational multivariate analysis of variance
- LEfSe:
-
Linear discriminate analysis effect size
- VIP:
-
Variable influence on projection
- PLS-DA:
-
Partial least squares discriminant analysis
- GOF:
-
Goodness of fit
References
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant Cell Environ 7(32):666–681
Bi B, Wang K, Zhang H, Wang Y, Fei H, Pan R, Han F (2021) Plants use rhizosphere metabolites to regulate soil microbial diversity. Land Degrad Dev 24(32):5267–5280
Bloom AJ, Chapin FS, Mooney HA (1985) Resource limitation in plants-an economic analogy. Annu Rev Ecol Syst 16:363–392
Brown SP, Jumpponen A (2014) Contrasting primary successional trajectories of fungi and bacteria in retreating glacier soils. Mol Ecol 20(23):481–497
Carvalhais LC, Dennis PG, Fedoseyenko D, Hajirezaei MR, Borriss R, von Wirén N (2010) Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. J Plant Nutr Soil Sci 22(174):3–11
Chen L, He Z, Wu X, Du J, Zhu X, Lin P, Tian Q, Kong J (2021) Linkages between soil respiration and microbial communities following afforestation of alpine grasslands in the northeastern Tibetan Plateau. Appl Soil Ecol 161:103882
Cui Y, Bing H, Fang L, Jiang M, Shen G, Yu J, Wang X, Zhu H, Wu Y, Zhang X (2021) Extracellular enzyme stoichiometry reveals the carbon and phosphorus limitations of microbial metabolisms in the rhizosphere and bulk soils in alpine ecosystems. Plant Soil 458:7–20
Czarnota MA, Paul RN, Dayan FE, Nimbal CI, Weston LA (2001) Mode of action, localization of production, chemical nature, and activity of sorgoleone: A potent PSII inhibitor in Sorghum spp. root exudates. Weed Technol. 4:813–825
Dakora FD, Phillips DA (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 95(245):35–47
DeBruyn JM, Nixon LT, Fawaz MN, Johnson AM, Radosevich M (2011) Global biogeography and quantitative seasonal dynamics of gemmatimonadetes in soil. Appl Environ Microbiol 77(17):6295–6300
Dennis PG, Miller AJ, Hirsch PR (2010) Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol Ecol 72(3):313–327
Freschet GT, Roumet C, Comas LH, Weemstra M, Bengough AG, Rewald B, Bardgett RD, De Deyn GB, Johnson D, Klimesova J, Lukac M, McCormack ML, Meier IC, Pages L, Poorter H, Prieto I, Wurzburger N, Zadworny M, Bagniewska-Zadworna A, Blancaflor EB, Brunner I, Gessler A, Hobbie SE, Iversen CM, Mommer L, Picon-Cochard C, Postma JA, Rose L, Ryser P, Scherer-Lorenzen M, Soudzilovskaia NA, Sun T, Valverde-Barrantes OJ, Weigelt A, York LM, Stokes A (2021) Root traits as drivers of plant and ecosystem functioning: current understanding, pitfalls and future research needs. New Phytol 232(3):1123–1158
Gqozo MP, Bill M, Siyoum N, Labuschagne N, Korsten L (2020) Fungal diversity and community composition of wheat rhizosphere and non-rhizosphere soils from three different agricultural production regions of South Africa. Appl Soil Ecol 151:103543
Guo D, Mitchell RJ, Hendricks JJ (2004) Fine root branch orders respond differentially to carbon source-sink manipulations in a longleaf pine forest. Oecologia 140:450–457
Haase S, Neumann G, Kania A, Kuzyakov Y, Romheld V, Kandeler E (2007) Elevation of atmospheric CO2 and N-nutritional status modify nodulation, nodule-carbon supply, and root exudation of Phaseolus vulgaris L. Soil Biol Biochem 39(9):2208–2221
Han Y, Feng J, Han M, Zhu B (2020) Responses of arbuscular mycorrhizal fungi to nitrogen addition: A meta-analysis. Glob Change Biol 26(12):7229–7241
Jauregui I, Aparicio-Tejo PM, Avila C, Rueda-Lopez M, Aranjuelo I (2015) Root and shoot performance of Arabidopsis thaliana exposed to elevated CO2: A physiologic, metabolic and transcriptomic response. J Plant Physiol 189:65–76
Jiang C, Shimono M, Maeda S, Inoue H, Mori M, Hasegawa M, Sugano S, Takatsuji H (2009) Suppression of the rice fatty-acid desaturase gene OsSSI2 enhances resistance to blast and leaf blight diseases in rice. Mol Plant Microbe Interact 22(7):820–829
Jing H, Zhang P, Li J, Yao X, Liu G, Wang G (2019) Effect of nitrogen addition on the decomposition and release of compounds from fine roots with different diameters: the importance of initial substrate chemistry. Plant Soil 438:281–296
Jing H, Liu Y, Wang G, Liu G (2021) Contrasting effects of nitrogen addition on rhizosphere soil CO2, N2O, and CH4 emissions of fine roots with different diameters from Pinus tabulaeformis forest using laboratory incubation. Sci Total Environ 780:146298
Jones DL (1998) Organic acids in the rhizosphere – a critical review. Plant Soil 205:25–44
Jones D, Darrah P (1994) Amino-acid influx at the soil-root interface of Zea mays L. and its implications in the rhizosphere. Plant and Soil. 163:1–12
Kachroo A, Kachroo P (2009) Fatty acid-derived signals in plant defense. Annu Rev Phytopathol 47:153–176
Kjoller R, Rosendahl S (2014) Cultivated and fallow fields harbor distinct communities of Basidiomycota. Fungal Ecol 9:43–51
Kou L, Guo D, Yang H, Gao W, Li S (2015) Growth, morphological traits and mycorrhizal colonization of fine roots respond differently to nitrogen addition in a slash pine plantation in subtropical China. Plant Soil 391:207–218
Li X, Dong J, Chu W, Chen Y, Duan Z (2018) The relationship between root exudation properties and root morphological traits of cucumber grown under different nitrogen supplies and atmospheric CO2 concentrations. Plant Soil 425:415–432
Lu R (2000) Methods for soil agrochemistry analysis. Agricultural Science and Technology Press Beijing China. pp 106–310
Mcdougall BM (1968) Exudation of 14C-labelled substances from roots of wheat seedlings. Int Soc Soil Sci Transition 3:647–655
Mcdougall BM, Rovira AD (1970) Sites of exudation of 14C labelled compounds from wheat roots. New Phytol 69(4):999–1003
Mebius LJ (1960) A rapid method for the determination of organic carbon in soil. Anal Chim Acta 22:120–124
Mench M, Martin E (1991) Mobilization of cadmium and other metals from two soils by root exudates of Zea mays L. Nicotiana tabacum L. and Nicotiana rustica L. Plant and Soil. 132:187–196
Obase K, Cha JY, Lee JK, Lee SY, Lee JH, Chun KW (2009) Ectomycorrhizal fungal communities associated with Pinus thunbergii in the eastern coastal pine forests of Korea. Mycorrhiza 20:39–49
Page AL, Miller RH, Dennis RK (1982) Methods of soil analysis. Am Soc Agronomy Soil Sci Soc Am 15:99–100
Phillips RP, Erlitz Y, Bier R, Bernhardt ES (2008) New approach for capturing soluble root exudates in forest soils. Funct Ecol 22(6):990–999
Proctor C, He Y (2017) Quantifying root extracts and exudates of sedge and shrub in relation to root morphology. Soil Biol Biochem 114:168–180
Rodrigues VS, do Valle RF, Fernandes LFS, Pacheco FAL (2019) The assessment of water erosion using Partial Least Squares-Path Modeling: A study in a legally protected area with environmental land use conflicts. Sci Total Environ. 691:1225–1241
Rovira AD (1969) Plant root exudates. Bot Rev 35:35–57
Shepherd T, Davies HV (1994) Patterns of short-term amino acid accumulation and loss in the root-zone of liquid-cultured forage rape (Brassica napus L.). Plant Soil. 158:99–109
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Quart Rev Biol Read 137(3):273–281
Su Y, Huang G, Lin Y, Zhang Y (2016) No synergistic effects of water and nitrogen addition on soil microbial communities and soil respiration in a temperate desert. CATENA 142:126–133
Sun L, Ataka M, Han M, Han Y, Gan D, Xu T, Guo Y, Zhu B (2020) Root exudation as a major competitive fine-root functional trait of 18 coexisting species in a subtropical forest. New Phytol 229(1):259–271
Syed K, Doddapaneni H, Subramanian V, Lam YW, Yadav JS (2010) Genome-to-function characterization of novel fungal P450 monooxygenases oxidizing polycyclic aromatic hydrocarbons (PAHs). Biochem Biophys Res Commun 399(4):492–497
Urbanova M, Šnajdr J, Baldrian P (2015) Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol Biochem 84:53–64
Wang Z, Zheng F (2018) C, N, and P stoichiometric characteristics of Pinus tabulaeformis plantation in the Ziwuling Region of the Loess Plateau. Acta Ecol Sin 38(19):6870–6880
Wang G, Fahey TJ, Xue S, Liu F (2013) Root morphology and architecture respond to N addition in Pinus tabuliformis, west China. Oecologia 171:583–590
Wang G, Xue S, Liu F, Liu G (2017) Nitrogen addition increases the production and turnover of the lower-order roots but not of the higher-order roots of Bothriochloa ischaemum. Plant Soil 415:423–434
Wang Q, Gao M, Liu M, Wang H, Dong Y, Wang Y (2017) Illumina Miseq sequencing-based fungal community of rhizosphere soils along root orders of poplar plantation. J Appl Ecol 28(4):1177–1183
Wang C, Liu W, Bai E (2018) Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol Biochem 120:126–133
Xing J, Chin CK (2000) Modification of fatty acids in eggplant affects its resistance to Verticillium dahliae. Physiol Mol Plant Pathol 56(5):217–225
Xu G, Li S, Zhao Y, Chen M, Li Y (2014) Effects of straw returning and nitrogen fertilizer application on root secretion and nitrogen utilization of rice. Acta Pratacul Sin 23(2):140–146
Yanardag IH, Zornoza R, Bastida F, Buyukkilic-Yanardag A, Garcia C, Faz A, Mermut AR (2017) Native soil organic matter conditions the response of microbial communities to organic inputs with different stability. Geoderma 295:1–9
Yang W, Tong Y, Qiao L, Liu X, Duan M, Li J (2010) Preliminary estimate of the atmospheric nitrogen deposition in different ecological regions of Shaanxi Province. J Agro-Environ Sci 29(4):795–800
Yang J (2015) Influence of nitrogen deposition and rainfall manipulation on cocentration of forest tree root exudates. University of Chinese Academy of Sciences
Yoneyama K, Xie X, Kim HI, Kisugi T, Nomura T, Sekimoto H, Yokota T, Yoneyama K (2012) How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 235:1197–1207
Zhang J, Wang J, Xu Y, Li H (2007) Effect of nitrogen on the species and contents of organic acids in root exudates of different soybean cultivars. Plant Nutr Fertilizer Sci 13(3):398–403
Zhang J, Ai Z, Liu H, Tang D, Yang X, Wang G, Liu Y, Liu G, Morrie E, Xue S (2022) Short-term N addition in a Pinus tabuliformis plantation: Microbial community composition and interactions show different linkages with ecological stoichiometry. Appl Soil Ecol 174:104422
Zhao J, Wan S, Fu S, Wang X, Wang M, Liang C, Chen Y, Zhu X (2013) Effects of understory removal and nitrogen fertilization on soil microbial communities in Eucalyptus plantations. For Ecol Manage 310:80–86
Zhu S, Vivanco JM, Manter DK (2016) Nitrogen fertilizer rate affects root exudation, the rhizosphere microbiome and nitrogen-use-efficiency of maize. Appl Soil Ecol 107:324–333
Acknowledgements
The authors acknowledged the anonymous reviewers for comments to improve the quality of this work.
Funding
This study was supported by the National Natural Science Foundation of China (No. 42307384, 42077456), and the Department of Science and Technology of Government of Jiangsu Province (NO. BK20220371).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Guoliang Wang and Guobin Liu conceived and designed the research. Hang Jing collected data and wrote the initial paper. Yi Cheng and Huiling Wang revised the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Fangbai Li.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary methods.
Additional file 2: Fig. S1.
NMDS analysis based on Bray-Curtis distances depicting the compositions of fungal community in rhizosphere soils of R1 (a), R2 (b), R3 (c), and non-rhizosphere soil (d) among N addition treatments and among root diameters at CN (e) treatment. CN, LN, MN, and HN are 0, 3, 6, and 9 g N m-2 y-1, respectively. R1, rhizosphere soil of 0-0.5 mm root; R2, rhizosphere soil of 0.5-1 mm root; R3, rhizosphere soil of 1-2 mm root; Nor, non-rhizosphere soil.
Additional file 3: Fig. S2.
NMDS based on Bray-Curtis distances depicting the root C metabolite profiles of P. tabuliformis at different N addition treatments and root diameters. CN, LN, MN, and HN are 0, 3, 6, and 9 g N m-2 y-1, respectively. R1, R2, and R3 are < 0.5 mm, 0.5-1.0 mm, and 1.0-2.0 mm, respectively. QC, quality control samples.
Additional file 4: Fig. S3.
Clustered heatmap of the rest differential secondary C metabolites which VIP values greater than 1 and ANOVA p values less than 0.05 among treatments. a, root secondary C metabolites among root diameters at CN treatment; b, root secondary C metabolites among N addition treatments in R1 root; c, root secondary C metabolites among N addition treatments in R3 root; d, root secondary C metabolites among N addition treatments in R2 root. CN, LN, MN, and HN are 0, 3, 6, and 9 g N m-2 y-1, respectively. R1, R2, and R3 are < 0.5 mm, 0.5-1.0 mm, and 1.0-2.0 mm, respectively.
Additional file 5: Fig. S4.
Network of correlation analysis between differential secondary C metabolites and fungal populations. Only significant correlations are shown (p < 0.05). The green lines show significant positive correlations, the red lines show significant negative correlations, and the dashed lines show the relationships between secondary C metabolites and primary C metabolites, as well as the relationships between microbial genus and phylum. a, correlation analysis between C metabolites and fungal populations of R1 root; b, correlation analysis between C metabolites and fungal populations of R3 root.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jing, H., Wang, H., Wang, G. et al. Hierarchical traits of rhizosphere soil microbial community and carbon metabolites of different diameter roots of Pinus tabuliformis under nitrogen addition. Carbon Res. 2, 47 (2023). https://doi.org/10.1007/s44246-023-00081-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44246-023-00081-1