Abstract
In this study, the torrefaction of food waste was carried out using a thermogravimetric analyzer (TGA) to produce biochar and assess its suitability as feedstock for steam gasification. Torrefaction was conducted at temperature from 230 to 290 °C and the heating rate from 10 °C/min to 30 °C/min. Subsequently, the detailed characterization was conducted using proximate, elemental, lignocellulosic, nutrient analysis, and TGA analysis. The proximate and elemental analysis showed that biochar had the highest fixed and elemental carbon at the highest temperature (290 °C) and lowest heating rate (10 °C/min). Similarly, TGA indicated that increase in temperature increased the mass loss, however, increase in heating rate did not yield much change in mass loss. Additionally, lignocellulosic and nutrient analysis showed that lignin fraction increased (up to 80%) with increases in severity of the torrefaction due to significant decomposition of hemicellulose, cellulose, starch, proteins, and lipids. Thereafter, the kinetic parameters (activation energy, pre-exponential factor) of torrefaction of food waste were determined using the two-step decomposition model which showed a good fit with experimental data. Finally, the biochar developed was used for energy production using steam gasification which produced syngas with maximum yield of 3.75 m3/Kg and having hydrogen fraction of around 65% at the optimal conditions (temperature: 290 °C, heating rate: 10 °C/min).
Graphical Abstract

Highlights
• Biochar was developed from food waste using torrefaction.
• Biochar was characterized using various physico-chemical analysis along with kinetic study.
• Steam gasification of biochar generated high quality syngas with 65% H2 fraction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
India is one of the fastest growing economy of the world, and has immense energy requirement which cannot be fulfilled only by the fossil-based energy. It must look for other sources of energy including bio-energy that can be produced in three forms: solid (biochar), liquid (bio-oil), and gases (syngas). Among all the forms, production of biochar is least energy intensive and can further be used as feedstock for many other processes for producing energy including thermochemical processes such as pyrolysis and gasification. Biochar can be derived from various kinds of biomass and wastes including wood, agri-residues, industrial waste, and municipal waste, and in this paper, food waste, a kind of municipal waste, has been used as feedstock for producing biochar. Food waste is generated in large quantities from our households and commercial places like restaurants, canteens, etc. and the major fraction of it ends up in landfills as waste (Singh and Singh 2020; Sinha and Tripathi 2021). Food waste dumped in landfills continuously produces methane, a potent greenhouse gas, due to being buried under other waste and lack of oxygen.
Another common method of food waste management is incineration which also produces another type of greenhouse gas, carbon-di-oxide on combustion, contributing to the total greenhouse emission by 6% globally (Pour and Makkawi 2021), which poses a serious concern, given our current environmental condition. Therefore, to deal with environmental issues, more environment friendly methods need to be explored for food waste management. Biochemical and thermochemical methods are two such methods, however, thermochemical methods are more versatile, faster, and more efficient as compared to biochemical methods (Yadav et al. 2023). Thermochemical methods include processes like pyrolysis, and gasification. Pyrolysis is the heating of the biomass in complete absence of air and gasification is heating of biomass in presence of gasifying agents that include air, oxygen, steam, CO2 or combination of them (Canabarro et al. 2013). Performance of these thermochemical processes strongly depends upon the quality of biomass, however, food waste is not a typical biomass and lacks quality in terms of many physico-chemical properties such as very high moisture content, low mass and energy density, low heating value, storage and transportation issues, heterogeniety, and hygroscopicity, etc. (Poudel et al. 2015).
To address such issues and improve the physical and chemical characteristics of food waste, certain pretreatment techniques need to be employed. Torrefaction is one such technique for enhancing the physico-chemical properties of raw food waste and converting it into biochar. Torrefaction can be carried out between temperature range of 200–300˚C and residence time of 30–120 min (Poudel et al. 2015). Torrefaction increases the mass density, energy density, and heating value of the food waste, thereby improving its quality as feedstock for other energy producing processes (Nam and Capareda 2015). Moreover, the decrease in moisture content leads to a decrease in weight and provides better stability against microbial degradation due to the improved hydrophobic properties. The torrefied biomass or biochar is a moderate density porous product (mass and energy density) with lower mechanical strength, which improves its grindability, which in turn helps in briquetting and its storage and transportation (Chew and Doshi 2011).
Though, there are some studies on the torrefaction of food waste (Poudel et al. 2015; Huang et al. 2020; Pahla et al. 2018), among which only one study, Poudel et al. (2015) presented the kinetic parameters of food waste torrefaction. It was noted in that study that they only considered the torrefaction process up to the temperature of complete moisture release (~ 150 °C), where they justified that their aim was only to measure a rough estimate of activation energy. However, in the present study, the temperature up to 290 °C was used for torrefaction for determining more accurate value of kinetic parameters. The kinetic study presented in this paper is based on a more realistic two-step mechanism proposed by Di-Blasi and Lanzetta (1997). This two-step mechanism has been used for the kinetic study of many other biomasses such as oil palm shell, mesocarp, and empty fruit bunch (Chew et al. 2016) and was found closely matched with the experimental results.
Finally, the biochar produced from torrefaction was used as feedstock for energy production via steam gasification. The high carbon content of biochar produced after torrefaction of food waste would help produce high quality syngas in terms of high hydrogen fraction.
2 Materials and methods
2.1 Sample preparation and characterization
Food waste was collected from the university dining halls over a week and then sun dried and crushed in a ball mill crusher to produce particle size greater than British Standard Sieve (BSS) 12 or 1.4 mm. Food waste samples were characterized by proximate, elemental, ligno-cellulosic, and nutrient analysis. The proximate analysis was conducted following standard methods of ASTM D-3173–11, ASTM D 3175–11, ASTM D-3174–11 to determine moisture, volatile matter, and ash content, respectively. The fixed carbon is calculated using the following Eq.
Elemental analysis was done using Thermo Finnigan Flash 1112 Series Elemental analyzer. The lignocellulosic analysis was conducted using Tappi T222 om-02 standard method for lignin content, TM1 – A11 2001 standard method for hemicellulose, and TM1 – A9 2001 method for holocellulose content. Cellulose content was determined by subtracting hemicellulose from hollocellulose content.
Nutrient analysis was conducted for finding the starch, protein, and lipids content in the food waste sample. For quantitative estimation of crude protein, AOAC (2001.11) Official Method was used which is also known as Kjeldahl method. The starch content was determined by two methods: (i) anthrone method and ii) phenol–sulphuric acid method. The lipids were quantified using (AOAC 2003.05) protocol with the help of organic solvents such as n-hexane in the Soxhlet extraction apparatus.
The HHV of the sample was also calculated using the following Eq. (Parikh et al. 2005):
2.2 Thermogravimetric analysis
Thermogravimetric analysis (TGA) data of food waste samples from Thermogravimetric analyzer (Mettler Toldeo, USA) was obtained by temperature programming with two stages, one is dynamic heating and the other one is isothermal heating. The initial weight of the sample was 5.9 mg and the sample was first heated from 0 to 105 °C and then kept there for 10 min to remove any moisture present in the sample. From 105 °C, the sample was heated to the desired temperature of 230—290 °C. During the second stage of isothermal heating, samples were held at the desired temperature for one hour. The heating rate varied from 10 to 30 °C/min and nitrogen gas was supplied to maintain an inert atmosphere. The change in mass of food waste samples with respect to time and temperature was recorded by the software from TGA. Finally, the TGA and DTG thermos-grams were plotted.
2.3 Decomposition kinetic model
As proposed by Di-Blasi and Lanzetta (1997), the two-stage decomposition was considered for the torrefaction of kitchen food waste. Two sets of parallel reactions took place in which first stage reactions formed the reaction intermediates and second stage reactions converted intermediates in the final product, i.e., char, as given below:

A refers to food waste sample, B refers to the intermediate product, and C refers to the char produced. P1 and P2 are volatile products from torrefaction. Kp1 and KAB are the reaction rate constants for first stage devolatilization and charring whereas Kp2 and KBC are the rate constants for second stage devolatilization and charring.
If we assume first order reaction, the rate of reaction can be written as follows:
[A], [B], and [C] refer to the concentration of A, B, and C as products.
All the rate constants used above are dependent on activation energy and pre-exponential factor as the following relation:
where i = A, B, C, P1, P2.
On solving the Eqs. 2, 3 and 4 by the method proposed by Di Blasi and Lanzetta (1997), the global reaction rate for first and second stage can be obtained as follows:
m is the instantaneous mass, mo is the initial mass, mc is the residual solid mass, and m* is the mass at the start of the second stage, t* is the start time of the second stage and k1 and k2 can be obtained by the slope of Eqs. 5 and 6.
The yield of volatiles and reaction constants can be related as follows:
Kp1and Kp2 can be determined from Eqs. 7 and 8 and KAB and KBC can be obtained from Eq. 9. The activation energy and frequency factor can be obtained from Arrhenius plot by taking the log of both side and then plot ln (k) vs I/T in an excel sheet.
2.4 Experimental setup and procedure for torrefaction and steam gasification
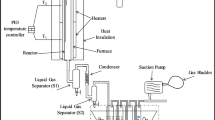
The torrefaction of food waste and steam gasification of biochar developed in this study was conducted in a downdraft fixed bed gasifier setup as shown in Fig. 1. The setup has a feeding, reaction, separation, and cleaning system. The feed material (food waste for torrefaction and biochar for gasification) was fed from feeding system via hopper placed at the top of the reactor and the gasifying agent (steam) (only for gasification) and carrier gas (N2) (for both torrefaction and gasification) was fed through sideways using a valve. The reactor was heated to a desired temperature (torrefaction temperature range was from 230 ˚C to 290 ˚C and gasification temperature was 800 ˚C) by a furnace where torrefaction was carried out for 30 min residence time and steam gasification was carried out till the end of the production of hydrogen. The biochar was removed from the reactor after 30 min residence time. For steam gasification, the gases exiting the reactor passed through condenser where all the condensable gases got separated by gas liquid separator and non-condensable gases passed through three impinger bottles placed in ice filled bucket where tar and suspended particulate matters (SPM) got removed. Thereafter, gases passed through a ceramic filter where the remaining SPM got separated out before going through a wet gas flow meter where the gas flow rate was measured. Finally, the gas samples were taken just before the conduct of the gas flow meter for gas composition analysis which was performed using a gas chromatograph (GC).
3 Results and discussion
3.1 Characterization of raw food waste
Table 1 shows the results of proximate analysis in terms of moisture content, volatile matter content, ash content, and fixed carbon content in the food waste sample. It also shows HHV value of raw food waste. The raw food waste had ~ 75% volatile matter that was close to volatile matters in typical biomass and ~ 15% fixed carbon that was less than its content in typical biomass (> 20%) (Yadav et al. 2023). Less fixed carbon will generate less biochar on thermochemical treatment, and thus provide less carbon for gasification reactions. The value of fixed carbon content increases with torrefaction as torrefaction removes moisture and releases many volatile compounds.
The elemental analysis presented in Table 1 shows a very comparable fraction of carbon element (Chew et al. 2016), however it also had sizable fraction of oxygen which is not much desired in any biomass as it leads to higher combustion of carbonaceous compounds (Adamovics et al. 2018). Furthermore, there were few traces of nitrogen in the MFW sample, which could result in the production of harmful NOx during thermochemical treatment (Mansor et al. 2018). The HHV of the food waste sample was found to be ~ 17.02 MJ/Kg, very close to the lower limit of the HHV range (18–21 MJ/Kg) of typical biomass (Yadav et al. 2023). However, the hydrogen and oxygen fractions reduced significantly on torrefaction, thus increasing the food waste quality as biomass.
Table 2 shows the percent composition of cellulose, hemicellulose, and lignin along with the composition of various nutrients in the food waste sample. The combined percent composition of lignocellulosic compounds was found to be around 49%, and the remaining 51% consisted of moisture (7.14%), ash (2.55%), and various nutrients (41.3%). The individual proportion of lignocellulosic compounds in the food waste sample was found to be 5.03% for hemicellulose, 26.20% for cellulose, and 13.04% for lignin. The percent composition of hemicellulose in the food waste was quite less because it got degraded significantly during the cooking of the food as it was an easily degradable compound at even low temperatures (≤ 200 °C). Consecutively, its composition fell below the recommended range of hemicellulose percent composition in any biomass (20%-35%) (Abraham 2017). However, the cellulose and lignin were present in significant quantity in the food waste sample as in case of other biomasses (Adamovics et al. 2018).
Table 2 also shows that food waste contained additional compounds such as 12.15% protein, 4.13% starch, and 25.02% lipids. Proteins in a food waste sample mainly came from legumes, cereals, meats, and dairy products. The starch content was 4.13%. The detailed analysis of it was carried out using two revised methods (phenol-sulphuric acid method and anthrone method) (Masuko et al. 2005; Zhang et al. 2020), and both of these methods determined the starch percentage in the same range of values, i.e., (4.13 ± 3.93) % and (4.11 ± 4.4) %, respectively.
Furthermore, Table 2 shows the presence of lipids that include fats, oils, waxes, and vitamins in a significant quantity (25%). These lipids can be extracted from food waste via torrefaction and then can be converted into biodiesel through trans-esterification or hydro-treated esters and fatty acids (HEFA) (Chiaramonti et al. 2015).
3.2 Characterization of torrefied food waste
Table 3 shows data on characterization of torrefied food waste that includes the variation of moisture content, volatile matter content, ash content, and fixed carbon content with torrefaction temperature and heating rates. It can be noted from Table 3 that there was a significant change in volatile matter content, ash content, and fixed carbon content as the torrefaction temperature changed, however, their content did not change much with the change in heating rate. The volatile matter content in torrefied food waste reduced to ~ 37% at the highest torrefaction temperature (290 °C) and highest heating rate (30 °C/min) whereas the ash content and the fixed carbon content increased to 13.5% and 47.2%, respectively, at the same torrefaction conditions. Volatile matter content reduced in torrefied food waste or biochar because of devolatilization through the decomposition of hemicellulose, cellulose, lipids, protein, and starch. Presence of less volatile matter in a biochar sample will produce less bio-oil from its pyrolysis because there will be less amount of condensable present in the gas phase (Yadav et al. 2023). Higher ash content facilitates the production of more biochar and syngas because some components in ash promote condensation reaction of bio-oil components and some components of ash such as Na + , K + , Ca + + facilitate thermal cracking of larger and complex molecules in bio-oil to produce smaller molecules which become part of gas phase, thus producing more syngas (Dhyani and Bhaskar 2018).
Furthermore, higher fixed carbon content in biochar facilitates the production of more syngas from steam gasification of biochar because of the more occurrence of water gas reaction between more biochar and steam that produces more hydrogen and carbon mono oxide (Singh and Yadav 2021). The residence time of gas phase in the reactor as well as reactor temperature is an important factor to decide the relative production of bio-oil and syngas from pyrolysis and gasification of biochar. Heating rate is another factor and changing the heating rate to 20 °C/min at a constant temperature influenced the decomposition of food waste compounds to some extent, however, when it further increased to 30 °C/min, it did not change the analysis data much.
Table 4 shows the variation of elemental carbon, hydrogen, oxygen and nitrogen with different torrefaction conditions. It can be noted that the elemental carbon content did not change much when changing the torrefaction conditions as opposed to fixed carbon content that changed significantly as seen in the previous table. Additionally, it can be noticed that the fixed carbon content was less than elemental carbon at all the torrefaction conditions. Both results can be attributed to the way ultimate analysis is conducted where the carbon associated with volatile matters was included in the ultimate analysis. Furthermore, the change in elemental hydrogen was significant when increasing the temperature to 290 °C at the same heating rates. However, when the heating rate was changed at the same temperature, not much change was noticed in elemental hydrogen content in food waste. The opposite was observed in case of elemental oxygen whose content did not change much in response to variations in the temperature ae well as heating rates.
Table 5 shows the variation in lignocellulosic composition with various torrefaction conditions. It can be noticed that hemicellulose was present in a very small quantity even at the lowest torrefaction temperature because most of it got further decomposed (some of it got decomposed during cooking) after thermal treatment during torrefaction. It had been reduced to an almost negligible quantity as the temperature further increased. Similarly, cellulose fraction in biochar reduced significantly (to 8%) after torrefaction at the lowest torrefaction temperature (230 °C) which did not change much as the heating rate changed at the same temperature. Generally, cellulose degradation started only after 270 °C and peaked around 340 °C. However, in this case, it started below 270 °C because cellulosic bonds got weakened during cooking and got easily broken at even the lowest of the torrefaction temperature. By increasing the torrefaction temperature and heating rates, the cellulose composition was further reduced to 3.5%.
Furthermore, the lignin percentage continuously increased with the increasing severity of torrefaction and reached up to 80%, also mainly due to significant degradation of cellulose and hemicellulose and negligible degradation of lignin, thus increasing the overall lignin percentage in torrefied food waste. The presence of nutrients in the food waste sample was also impacted by the torrefaction condition as evident in the nutrient analysis presented in Table 5. It shows that crude proteins and lipids got degraded completely and starch was also degraded significantly (~ 50%) when treated at the highest torrefaction temperature.
3.3 Thermogravimetric analysis
Figures 2 and 3 illustrate TGA and derivative thermogravimetry DTG curve for the food waste decomposition at three temperatures (230, 260, 290 °C) and three different heating rates (10 °C/min, 20 °C/min, and 30 °C/min). It can be noted that total mass loss curve for 10 °C/min was different from that of the other two heating rates. Mass loss curve or TGA curve at a higher heating rate of 20 °C/min was steeper than that at 10 °C/min, indicating faster mass loss at higher heating rates. However, there was not much change in mass loss curve between 20 °C/min and 30 °C/min indicating not much mass loss as the heating rate increased. Higher heating rates would not give sufficient time for particular decomposition reaction to take place, which was able to take place at slower heating rates. Generally, 10 °C/min is considered to be an optimal heating rate for biomass TGA as recommended by international confederation of thermal analysis and calorimetry (ICTAC) (Vyazovkin et al. 2011).
Additionally, mass loss curve changed significantly with the respect to changes in temperature. TGA curves shown in Fig. 2 (a)-(c) have three distinct zones: the first zone corresponds to the loss of the moisture from the food waste sample as well as the removal of some lipids, whereas the second zone corresponds to degradation of hemicellulose and cellulose and the third zone corresponds to the steady stage that came after mass loss up to the end of experiments. First zone represents the first stage of model proposed by Di-Blasi and Lanzetta (1997) and second and third zones represent the second stage of Di-Blasi and Lanzetta's (1997) model when most of the chemical decomposition of food waste took place. The co-ordinates for defining these zones were different for different temperatures and heating rates. For example, if we used 10 °C/min heating rate and 290 °C as the heating rate and the temperature, the first zone ended at around 26 minutes with 85% remaining mass and the second zone ended around 38 minutes with ~58% remaining mass followed by the third stage. Additionally, Fig. 2 shows that there was a shift of these zones towards the left on a time scale on increasing the heating rate from 10 °C/min to 30 °C/min suggesting that higher heating rates resulted in earlier mass loss. Furthermore, there was an increase in mass loss when increasing the torrefaction temperature as indicated by the larger slop of decomposition curve corresponding to both zones. For the heating rate of 10 °C/min, the mass loss at an increasing temperature from 230 °C to 290 °C was approximately between 12-15% after the first zone and between 25-55% after the second zone. Therefore, it can be concluded that increasing the heating rate beyond 10 °C/min would not help in increasing the decomposition of food waste, however, increasing the temperature would increase the decomposition of food waste. This conclusion is in coherence with the recommendation of ICTAC.
Figure 3 shows the DTG curve of decomposition of food waste indicating the rate of mass loss with time. The curves shown for food waste torrefied at different temperatures were quite different from each other for a particular heating rate. The peak areas in DTG curve for torrefaction at the low temperature of 230 °C with a low heating rate (Fig. 3a) were broad and small indicating a low rate of conversion or decomposition of food waste sample. The early peaks were due to the removal of some vegetable oils present in food waste. However, no peaks corresponding to the decomposition of lignocellulosic compounds were observed at this temperature, which was understandable as not much hemicellulose was present in food waste (due to cooking) and cellulose decomposed only when the temperature was higher than 250 °C (Luangkiattikhun et al. 2008). Therefore, as the final temperature increased, the decomposition of cellulose became very obvious by a peak shown at around 30 min. The same was observed even for a higher temperature of 290 °C. However, the peak area was larger at 290 °C suggesting a higher rate of decomposition of cellulose at that temperature.
Additionally, Fig. 3(b) and (c) show the rate of decomposition at a higher heating rate of 20 °C/min and 30 °C/min, respectively. It can be noted that the peaks corresponding to cellulose decomposition shifted to the left-hand side for a higher heating rate indicating the early arrival of the torrefaction temperature. Additionally, the area of the peaks was larger at the higher heating rate indicating more decomposition of cellulose and nutrients (starch, protein, and lipids) at a higher heating rate, though the difference was slim for cellulose decomposition rate at a higher heating rate and 290 °C. Furthermore, three zones can also be noticed in the DTG curve corresponding to three zones in TGA. When the heating rate was 10 °C/min, heating rate was low in the first 26 minutes (first zone) and then in the first to a high peak value (4.3) followed by a decrease (second zone) to a similar rate as in the first zone. Thereafter, the mass loss rate stabilized after 38 minutes (third zone).
Figure 4 shows the change in mass yield with change in torrefaction conditions as obtained from TGA data. The highest mass yield (75%) was found for the mildest torrefaction conditions and the lowest mass yield (40%) was found at the highest torrefaction temperature of 290 °C and medium heating rate of 20 °C/min. The highest mass yield results can be attributed to the minimum devolatilization of food waste at mildest torrefaction, due to which, it would not lose much mass, hence would result in high mass yield. The lowest mass yield can be attributed to the maximum devolatilization at the corresponding conditions. The 20 °C/min heating rate had a stronger effect on devolatilization than that of 30 °C/min as discussed in paragraph 3 of section 3.3).
3.4 Kinetic study parameters
The kinetic parameters for torrefaction of food waste determined from this study are shown in Table 4. It can be noticed that the activation energy for the torrefaction of food waste was higher for the first stage than that for the second stage which is understandable because a more packed structure would have caused low reactivity of food waste. After the first stage, the packing of the structure loosened after the release of moisture and some volatiles and lowered the activation energy for further release of volatiles and formation of torrefied bio-char during the second stage. The overall activation energy for the first stage was 65.1 kJ/mol and for the second stage, it was 48.3 kJ/mole for 10 °C/min heating rate and these values are close to the values reported by Chew et al. (2016). Additionally, both stages had two components of activation energy, the activation energy for devolatilization and activation energy for the formation of char. It can be noticed that the activation energy for devolatilization in the second stage was quite low as compared to its value in the first stage because of the loosening of the chemical structure as explained above. The activation energy for the first stage and second stages at 10 °C/min heating rate were 98.2 kJ/mol and 10.1 kJ/mol, respectively.
Furthermore, changes in the activation energy with change in heating rate are also presented in Table 6. It can be seen that overall activation energy reduced (from 65.1 to 59.8) with an increase in heating rate from 10 °C/min to 30 °C/min, though the change was very small. The same can be observed for the activation of different stages. The activation energy of the food waste torrefaction was higher than the activation energy of Xylan as reported by Di Blasi and Lanzetta (1997). The xylan is hemicellulose and does not have a tightly packed crystalline structure as cellulose and thus it requires less energy to decompose. The food waste sample used in this study had a high percentage of cellulose (~26%), therefore, the torrefaction of this food waste sample required much higher activation energy than that for torrefaction of Xylan. Table 6 also contains the values of the pre-exponential factor. The pre-exponential values were higher for first stage than that of second stage for all the heating rates and by comparing these values for different heating rates, it can be noticed that pre-exponential factor is has a higher value for less heating rate.
Figure 5 shows the comparison between experimental data and data obtained from the proposed model for a heating rate of 10 °C/min. It can be seen that the proposed model fits well with the experimental data. The coefficient of determination was found to be 0.97 for this fit. This study clearly shows that Di-Blasi and Lanzetta's (1997) model is the best to predict the most accurate behaviour of food waste during torrefaction. Such kinetic study will help better design the reactor after determining process parameters such as heating rate and temperature. Torrefaction is generally as a pre-step for studies on thermochemical process such as pyrolysis and gasification.
3.5 Steam gasification of biochar
3.5.1 Syngas yield
Figure 6 shows the gas yield from the steam gasification of biochar generated from torrefaction of food waste at different torrefaction conditions.
Syngas yield increased with increase in torrefaction temperature which can be attributed to more water gas reaction due to more fixed carbon left in biochar after torrefaction at a higher temperature. The torrefaction at increasing temperature, when temperature increases, causes more devolatilization which in turn leaves more fixed carbon in the biochar sample. However, the more devolatilization also results in release of gases and may result in reducing the syngas yield, however syngas production from steam gasification of fixed carbon left after devolatilization offsets that loss and results in production of more syngas. The highest syngas yield was found to be 3.75 m3/Kg for B 290 (biochar produced at 290 °C), much more than the syngas yield from torrefaction of raw food waste sample.
3.5.2 Syngas composition and H2 fraction
Figure 7 shows the change in syngas composition with change in biochar sample used for steam gasification. The hydrogen is the most desirable component in produce gas as it is a clean fuel. It can be noticed that the lowest hydrogen fraction in syngas was found for raw food waste sample and highest hydrogen fraction was found for biochar sample torrefied at 290 °C (B290). The lowest hydrogen fraction in syngas from steam gasification of raw food waste was mainly due to the lowest fixed carbon content in it. The main gasification reaction, water gas shift reaction has carbon and steam as main reactants \(C\mathit+H_{\mathit2}O\mathit\rightarrow CO\mathit+H_{\mathit2}\) and low fixed carbon content resulted in less occurrence of water gas reaction, thus it produces less amount of hydrogen (Seo et al. 2022). Additionally, devolatilization of raw food waste at gasification temperature generates large amount of non-condensable gases such as CH4, CO, and CO2 and decreases the fraction of hydrogen in produce gas, although CH4 and CO undergo further reaction via steam reforming reaction and water gas shift reaction respectively to produce hydrogen (Valizadeh et al. 2022). The fraction of CH4 in produce gass depends upon relative value of rate, where CH4 is a product and steam reforming reaction rate, where CH4 is a reactant. Similarly, CO fraction in product gas depends upon relative value of water gas reaction rate and devolatilization rate, where CO is a product and water gas shift reaction, where CO is a reactant (Singh et al. 2020).
As the raw food waste sample was torrefied at 230 °C, the biochar generated, B230 had more fixed carbon (25%) than that in raw food waste (15%). The increase in the fixed carbon content enhanced the hydrogen production due to more occurrence of water gas shift between more carbon and steam. Additionally, there’ll be less release of non-condensable gases from B230 that forms part of product gas as it has already gone through significant devolatilization during torrefaction at 230 °C. Therefore, more hydrogen production from water gas reaction and less release of non-condensable gases via devolatilization increased the hydrogen fraction in producing gas. As the torrefaction temperature further increased, the B260 will have more fixed carbon than that in raw food waste sample and B230, thus producing more hydrogen than that from raw sample and B230. Release of non-condensable gases will further be reduced during devolatilization of B260 and contribute less to total gas production and increase the contribution from products from water gas reaction, i.e., H2 and CO. However, the CO further gets consumed by water gas shift reaction, thus its fraction is less than the fraction of H2. Similarly, the hydrogen fraction is more than that in raw sample, B230, and B260.
Additionally, Fig. 8 shows the variation of HHV of syngas produced from steam gasification of food waste torrefied at different conditions. The HHV of syngas generated from steam gasification of food waste at 290 °C and 10 C/min is the highest with heating value of 22.6 MJ/m3. The same conclusion has been mentioned in the previous sections that 290 °C and 10 °C/min are the optimal torrefaction conditions.
4 Conclusions
This study presented the detailed physico-chemical analysis of raw as well as torrefied food waste using proximate analysis, elemental analysis, lignocellulosic analysis and nutritional analysis. The torrefaction was conducted in a TGA by varying the temperature (230–290 °C) and heating rates (10–30 °C/min). It was noticed that volatile matter reduced significantly to 37.4% and fixed carbon increased significantly to 47.2% at the highest torrefaction condition, a most desirable property for steam gasification of biochar. It was also noticed that hemicellulose, proteins, and lipids got almost completely decomposed whereas cellulose and starch decomposed significantly to 3.5% and 2.1%, respectively, as the torrefaction conditions increased to the highest severity. However, lignin did not exhibit much decomposition and its content increased to 80% at the highest torrefaction conditions. TGA and DTG curves clearly indicated two zones of decomposition followed by steady stage which can be considered as the third stage. The increase in temperature resulted in more mass loss thus producing the TGA curve with a higher slope. Additionally, variation in heating rate resulted in shifting of curves toward the left side, thus resulting in an early finish of the total process. Additionally, the kinetic study was conducted using TGA data in Di-Blasi and Lazentta's (1997) model, and kinetic parameters were determined. The data generated following this model showed a good fit with the experimental data with the coefficient of determination of 0.97. Finally, the biochar produced via torrefaction of food waste was used as feedstock for steam gasification to produce syngas with high hydrogen fraction. The highest hydrogen fraction (~ 65%) was found for syngas with yield of 3.5 m3/Kg for biochar B290 generated at the optimal torrefaction conditions.
Availability of data and materials
I have provided the data in the form of excel sheet with this submission.
Abbreviations
- TGA:
-
Therogravimetric Analyser
- BSS:
-
British Standard Sieve
- ASTM:
-
American Society of Testing and Materials
- AOAC:
-
Association of Official Agricultural Chemists
- GC :
-
Gas Chromotography
- HHV:
-
High Heating Value
- NOx:
-
Nitrogen Oxides
- HEFA:
-
Hydro-treated Esters and Fatty Acids
- DTG:
-
Derivative Thermogravimetry
- E:
-
Activation Energy
- A:
-
Pre-exponential factor
- B230:
-
Biochar produced at 230 °C
- B260:
-
Biochar produced at 260 °C
- B290:
-
Biochar produced at 290 °C
References
Abraham M (2017) Encyclopedia of sustainable technologies. Elsevier
Adamovics A, Platace R, Gulbe ZL, Ivanovs S (2018) The content of carbon and hydrogen in grass biomass and its influence on heating value. Eng Rural Dev 17:1277–1281
Canabarro N, Soares JF, Anchieta CG, Kelling CS, Mazutti MA (2013) Thermochemical processes for biofuels production from biomass. Sustain Chem Process 1:1–10. https://doi.org/10.1186/2043-7129-1-22
Chew JJ, Doshi V (2011) Recent advances in biomass pretreatment–Torrefaction fundamentals and technology. Renew Sustain Energy Rev 15:4212–4222. https://doi.org/10.1016/j.rser.2011.09.017
Chew JJ, Doshi V, Yong S, Bhattacharya S (2016) Kinetic study of torrefaction of oil palm shell, mesocarp and empty fruit bunch. J Therm Anal Calorim 126:709–715. https://doi.org/10.1007/s10973-016-5518-3
Chiaramonti M, Prussi M, Buffi M, Casini D, Rizzo AM (2015) Thermochemical conversion of microalgae: challenges and opportunities. Energy Procedia 75:819–826. https://doi.org/10.1016/j.egypro.2015.07.142
Dhyani V, Bhaskar T (2018) A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew Energy 129:695–716. https://doi.org/10.1016/j.renene.2017.04.035
Di-Blasi C, Lanzetta M (1997) Intrinsic kinetics of isothermal xylan degradation in inert atmosphere. J Anal Appl Pyrol 40:287–303. https://doi.org/10.1016/S0165-2370(97)00028-4
Huang J, Qiao Y, Wang Z, Liu H, Wang B, Yu Y (2020) Valorization of food waste via torrefaction: effect of food waste type on the characteristics of torrefaction products. Energy Fuels 34:6041–6051. https://doi.org/10.1021/acs.energyfuels.0c00790?ref=pdf
Luangkiattikhun P, Tangsathitkulchai C, Tangsathitkulchai M (2008) Non-isothermal thermogravimetric analysis of oil-palm solid wastes. Biores Technol 99:986–997. https://doi.org/10.1016/j.biortech.2007.03.001
Mansor AM, Lim JS, Ani FN, Hashim H, Ho WS (2018) Ultimate and proximate analysis of Malaysia pineapple biomass from MD2 cultivar for biofuel application. Chem Eng Trans 63:127–132. https://doi.org/10.3303/CET1863022
Masuko T, Minami A, Iwasaki N, Majima T, Nishimura SI, Lee YC (2005) Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal Biochem 339:69–72. https://doi.org/10.1016/j.ab.2004.12.001
Nam H, Capareda S (2015) Experimental investigation of torrefaction of two agricultural wastes of different composition using RSM (response surface methodology). Energy 91:507–516. https://doi.org/10.1016/j.energy.2015.08.064
Pahla G, Ntuli F, Muzenda E (2018) Torrefaction of landfill food waste for possible application in biomass co-firing”. Waste Manage 71:512–520. https://doi.org/10.1016/j.wasman.2017.10.035
Parikh J, Channiwala SA, Ghosal GK (2005) A correlation for calculating HHV from proximate analysis of solid fuels. Fuel 84(5):487–494. https://doi.org/10.1016/j.fuel.2004.10.010
Poudel J, Ohm T, Oh SC (2015) A study on torrefaction of food waste. Fuel 140:275–281. https://doi.org/10.1016/j.fuel.2014.09.120
Pour FH, Makkawi YT (2021) A review of post-consumption food waste management and its potentials for biofuel production. Energy Rep 7:7759–7784. https://doi.org/10.1016/j.egyr.2021.10.119
Seo MW, Lee SH, Nam H, Lee D, Tokmurzin D, Wang S, Pak Y (2022) Recent advances of thermochemical conversion processes for biorefinery. Bioresour Technol 343:126109. https://doi.org/10.1016/j.biortech.2021.126109
Singh D, Yadav S (2021) Evaluation of the physico-chemical development of kitchen food wastes through torrefaction-a biodiversity case study. Biomass Convers Biorefin 11:1353–1362. https://doi.org/10.1007/s13399-019-00526-x
Singh D, Yadav S, Bhardwaj S, Verma S (2020) Low temperature steam gasification to produce hydrogen rich gas from kitchen food waste: Influence of steam flow rate and temperature. Int J Hyrog Energy 45(41):20843–20850. https://doi.org/10.1016/j.ijhydene.2020.05.168
Singh P, Singh D (2020) Review on biomass. LAP Lambert Academic Publishing, London. ISBN: 9783330025936
Sinha S, Tripathi P (2021) Trends and challenges in valorisation of food waste in developing economies: a case study of India. Case Stud Chem Envir Eng 4:100162. https://doi.org/10.1016/j.cscee.2021.100162
Valizadeh S, Hakimian H, Farooq A, Jeon B, Chen W, Lee SH, Jun S, Seo M, Park Y (2022) Valorization of biomass through gasification for green hydrogen generation: a comprehensive review. Bioresour Technol 365:128143. https://doi.org/10.1016/j.biortech.2022.128143
Vyazovkin S, Burnham AK, Criado JM, Perez-Maqueda LA, Propescu C, Svirrazzuoli N (2011) ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520(1–2):1–19. https://doi.org/10.1016/j.tca.2011.03.034
Yadav S, Singh D, Mohanty P, Sarangi PK (2023) Biochemical and thermochemical routes of H2 production from food waste: a comparative review. Chem Eng Tech 46:191–203. https://doi.org/10.1002/ceat.202000526
Zhang WH, Wu J, Weng L, Zhang H, Zhang J, Wu A (2020) An improved phenol-sulfuric acid method for the determination of carbohydrates in the presence of persulfate. Carbohydr Polym 227:115332. https://doi.org/10.1016/j.carbpol.2019.115332
Acknowledgements
The authors acknowledge the help provided by Subham Misra for the work presented in this study.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Dr. Dharminer Singh contributed to material preparation, data collection, analysis, and manuscript preparation. Dr. Sanjeev Yadav contributed to planning the study, data analysis, and manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known conflict of interest for the work reported in this paper.
Additional information
Handling Editor: Hailong Wang.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yadav, S., Singh, D. Assessment of biochar developed via torrefaction of food waste as feedstock for steam gasification to produce hydrogen rich gas. Carbon Res. 2, 34 (2023). https://doi.org/10.1007/s44246-023-00065-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44246-023-00065-1