Abstract
Besides carbon sequestration, biochar amendment in soils can regulate the loss of applied fertilizer-nitrogen (N) into the environment. Soil aggregates are essential in controlling soil N stocks' stabilization and supply. However, unraveling the rate-dependent impact of biochar on stable soil aggregates and their associated N in fertilized soils over an extended period is a prerequisite to understanding its implications on soil-N dynamics. We unraveled how high and low biochar application rates combined with inorganic fertilizer (20- and 40-tons ha−1 [FB1 and FB2]) affected soil N fractions, stable soil aggregates, aggregates associated-N, and microbial responses to regulate N supply for Nageia nagi after one year. Results revealed that biochar amendment, especially at higher rate, increased the concentration of inorganic N and some amino acids compared to sole fertilizer (F). Available N increased by 16.5% (p = 0.057), 23.8% (p = 0.033), and 34.8% (p = 0.028) in F, FB1, and FB2, respectively, compared to the control (C). Also, 28.1% and 32.8% significant increases in the availability of NH4+-N were recorded in FB1 and FB2, respectively, compared to F. NO3−-N availability was significantly increased by 15.2%, 21.8%, and 20.8% in FB1, FB2, and C, compared to F. Biochar amendment, irrespective of rate, increased stable microaggregates (< 0.25 mm). However, FB2 significantly increased macro- and intermediate-aggregate-N, and urease activity, and hence higher N supply capacity to meet the N need of N. nagi even after one year. Hence, the N content of N. nagi was 41.3%, 28.8%, and 12.2% higher in FB2, FB1, and F, respectively, compared to the control. Biochar amendment decreased bacterial species diversity but increased the proportion of NH4+-oxidizers (especially the Betaproteobacteria) to maintain the mineralization and slow release of N. Although a low biochar rate was more beneficial than sole fertilization, higher biochar application rate could sustain higher N supply by stabilizing soil microaggregates and increasing macro- and intermediate-aggregates N, its mineralization, and slow-release over longer periods.
Highlights
• Alteration of soil aggregates and aggregates-N to regulate N supply varied with biochar rate
• Enhanced soil macro- to intermediate aggregates-N by high biochar rate improved N supply over time
• High biochar rate increased urease and NH4+-oxidizers' abundance to promote extended N supply
AbstractSection Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
As a key limiting nutrient element required for plant productivity, nitrogen (N) is repeatedly applied to soils to optimize crop production (Cassman et al. 2002). The excessive use of N-based fertilizers in soil results in the loss of reactive N via volatilization, runoff, and leaching, resulting in greenhouse gas emission, eutrophication, and groundwater pollution (Bei et al. 2018; Shi et al. 2020). Besides, excessive N fertilization often results in soil acidification and low N use efficiency (Bei et al. 2018; Dai et al. 2020). While perennial tree crops in plantations often require higher N-doses than arable crops (Ibrahim et al. 2022), the negative impact of this heavy fertilization on N losses to the environment is of grave environmental consequences. Therefore, the need for sustainable management practices to improve N retention and reduce its loss to the environment while meeting the plants' needs remains vital to ensuring global environmental sustainability.
Exploring biochar types for improving soil properties and N retention has gained tremendous research attention in the past decade (Gao et al. 2019; Li et al. 2020; Ibrahim et al. 2020a). However, inconsistent influences on its retention and cycling of N have often been reported (Foster et al. 2016; Haider et al. 2017; Ramlow et al. 2019). These inconsistencies were attributed to the different properties of biochar produced from different feedstock materials, production conditions, and soil types evaluated. Meta-analyses have concluded that biochar's impact on N dynamics is variable, thus preventing its generalization (Gao et al. 2019). While these inconsistencies abound, it is unclear how biochar doses alter soil aggregate properties to influence its interaction with inorganic N fertilization, and thus influence N dynamics.
As the basic unit of soil structure, soil aggregates are essential in controlling and stabilizing soil N stocks (Zhu et al. 2017; Ngaba et al. 2020). The formation of micro and macroaggregates by mineral and organic binders in the soil, the stability of these aggregates, and their constituent N are regulated by soil management (Chen et al. 2017). Moreover, soil organic carbon (C) captured in the fine soil fractions (< 0.053 mm) primarily by adsorption and chemical fixation is considered an important C reservoir (Tong et al. 2014) utilized by nutrient-cycling organisms. Since the C and N cycles are interconnected (Ibrahim et al. 2021), the modifying impact of biochar on interaggregate C could significantly impact inter-aggregate N and overall N cycling. However, it remains unclear how biochar or its doses influence the stability of soil aggregates and their associated N in fertilized soils over an extended period to regulate N supply.
In addition, the negative, positive, or insignificant impacts of biochar on microbial responses are often associated with its varied modifying effect on the properties of different soils, sources of biochar feedstock, and production systems (Cayuela et al. 2014; Song et al. 2018; Palansooriya et al. 2019). These inconsistencies result in varied impacts of biochar on N cycling processes. However, there remains uncertainty on the effect of low and high biochar doses on the abundance and dynamics of soil bacteria populations, particularly those related to the N-cycling processes in the soil over an extended period.
Nageia nagi is a perennial tree crop widely spread across Asia, often maintained in plantations, possessing significant commercial, pharmaceutical, and aesthetic values (Zheng et al. 2018). Like for other plantation crops, heavy doses of inorganic fertilizers, especially N fertilizer, are supplied to promote plant growth and productivity. With the roles of biochar in N retention and slow release, it could be effectively used to manage and regulate N fertilization for crops grown for an extended period (Ibrahim et al. 2022). However, the rate-depended impact of biochar on stable soil aggregates and their constituent N, N mineralization, and microbial dynamics to regulate N availability for plants over a more extended period remains a significant research gap in biochar research.
We hypothesize that, i. compared to low biochar application, a high biochar rate would promote stability of microaggregates, while improving aggregate-associated N retention and supply after one year. Compared to a low rate, a higher biochar rate would promote increased urease activity and soil bacterial abundance, especially those related to N-mineralization, to ensure continuous availability of retained N over an extended period. Therefore, the study aims to evaluate how biochar application rates modify the stability of soil aggregates and their associated N to regulate N retention, cycling, and supply after a year of soil amendment. We also aim to evaluate the biochar rate-dependent modification of soil properties to regulate bacterial mediation of N-cycling in the rhizosphere of N. nagi after an extended period.
2 Materials and methods
2.1 Experimental resources and design
The soil used for the study was a subtropical Alfisol collected from a mixed secondary forest ecosystem site in Southern China (25° 51' N, 116° 49' E). The soil was obtained from the 0–20 cm depth at different points within the forest. The soil was screened to remove non-soil particles, dried under shade, sieved (2 mm), and thoroughly homogenized to form a composite soil sample. A subsample of the composite soil sample was analyzed in triplicates for its basic properties as follows: pH (5.5), total N (0.42 g kg−1), available N (19.21 mg kg−1), available P (7.2 mg kg−1), available K (142. 7 mg kg−1), total C (0.54%), and soil texture ( clayey loam).
Waste mushroom substrate (WMS), an abundant agricultural waste with high nutritional value, was collected and used for biochar production. The WMS was oven-dried at 45 °C for 72 h and crushed to 8 mm before its slow pyrolysis (500 °C and held for 2 h) under anoxic conditions (under N2 gas) (Zhao et al. 2019). The resulting biochar was crushed (0.5 mm) for subsequent characterization. Chemical characterization of the derived biochar was done using the methods outlined by Ibrahim et al. (2020b), and its properties are presented in Table 1. In addition, surface organic groups present on the biochar were detected by Fourier transform infrared spectrometry (Thermo Fisher, MA, USA), while its surface morphology was visualized using scanning electron microscopy (Nova™ NanoSEM 230; Oregon, USA).
The experiment was carried out as a pot experiment in a glasshouse using plastic pots with a dimension of 25 × 30 cm (diameter x height). For each pot, a soil mass of 7 kg was uniformly mixed with biochar, depending on the biochar rate to be evaluated. Based on the treatments, the biochar was applied at 1 and 2% of the total soil mass, corresponding to 20- and 40-tons biochar ha−1, respectively. These rates were chosen based on the reasonable rates of biochar commonly evaluated in reported studies (including greenhouse and field trials). Although higher rates (> 40 tons ha−1) have been utilized in previous studies, we evaluated economically viable rates for field adoption. A total of four treatments with five replicates per treatment were evaluated. These included a control soil (C) without any amendment, inorganic Fertilizer (F), Fertilizer + Biochar at 20 tons ha−1 (FB1), and Fertilizer + Biochar at 40 tons ha−1 (FB2). Inorganic compound nitrogen, phosphorus, and potassium (NPK) fertilizer comprising 15:15:15 (N:P2O5:K2O) was applied at 500 kg ha−1 twice a year (at 6 months intervals).
The pots were randomized in the greenhouse, and each pot was supplied with sufficient water to reach its field capacity using deionized water. The pots were left to equilibrate for 7 days before transplanting seedlings of Nageia nagi at one plant per pot. Each seedling had an average height of 52 cm and a stem diameter of 10 mm. The soil's moisture level was monitored using a digital hand-held moisture meter (FieldScout, Spectrum Tech, USA). Soil water was supplemented at 5 days intervals to maintain optimum moisture content (55–60%). Being a glass-house experiment, photoperiod, temperature, and relative humidity in the glasshouse varied during the one-year growth period of the plants. This was because the plants were exposed to direct sunlight through the transparent roof of the structure. Hand weeding within each pot was carried out during the growth period when necessary.
2.2 Plant and soil sampling
The potted N. nagi plants were maintained for 12 months before terminating the experiment. The middle leaves were carefully detached from several points on each plant per treatment and replicate. The leaves were oven-dried at 60 °C for 48 h, ground, and sieved to a 0.15 mm size fraction for chemical analysis.
Several soil samples were carefully obtained from around the root zone of the plants and bulked before and after plant removal. Sampling for soil microbial analysis was carried out before plant removal to avoid disruptions in soil microbial composition owing to plant removal. The samples were collected from around the root zone (rhizosphere region) per replicate using a mini soil auger of 1 cm radius and bulked. A 5 g subsample was put into sample bags and immediately stored at − 80 °C before microbial analysis. After carefully pulling out the plants, additional soil sampling was carried out and homogenized per replicate for chemical analysis. Part of the fresh soils was subsampled and refrigerated at 4 °C to analyze for NH4+, NO3−, and urease activity, while the rest were air-dried and sieved (0.5 and 1 mm) for chemical analyses. For soil aggregate size analysis, minimally disturbed soil samples were taken out from the pots into steel boxes and oven-dried at 45 °C for 48 h and subsequently sieved to 8 mm size. Visible plant materials and stones were screened out before analysis.
2.3 Samples analyses
The distribution of the soil aggregate sizes was carried out using the wet-sieving procedure outlined by Kemper and Rosenau (1986). Eighty grams of the 8 mm sized soil fraction were introduced into the uppermost of a set of six nested sieves of 2, 1, 0.5, and 0.25 mm to obtain aggregate sizes of > 2, 2–1, 1–0.5, 0.5–0.25, and < 0.25 mm fractions. The sieves were submerged in water for 5 min and gently shaken using slow vertical movements for 30 min at 35 strokes min−1. The retained soil on each sieve was carefully washed into aluminous containers (pre-weighed) and placed in the oven for 24 h at a temperature of 105 °C. The final weight of the soil was obtained by the difference using the initial weight of the containers.
The pH of the soil in water was obtained using a soil: deionized water (1:2.5 (w/v)). Extractable phosphorus (P) was assayed by soil extraction using a solution of 0.5 M NaHCO3, followed by color development using molybdenum-antimony and read spectrophotometrically (Olsen and Sommers 1982). Available potassium (K) was estimated using a flame photometer after soil extraction with 1N NH4OAc solution (pH 7). The NH4 + and NO3 − in the pre-refrigerated fresh soil samples were determined after 100 mL 0.2 M KCl was mixed with 15 g soil and shaken for 1 h. The mixture was centrifuged and filtered using Whatman filter paper#1 and read on a continuous flow Auto Analyzer (Skalar, Netherlands). Individual amino acids were detected in the soil extracts after extraction using hot water using an amino acid analyzer (Hitachi, L8900, Japan). The elemental carbon and N composition of the soil, soil aggregates, and plant were measured by an elemental analyzer (VarioMax, LB, Germany). The activity of the N-cycling enzyme (urease) was assayed as proposed by Tabatabai and Bremner (1972). The soil and plant P and K concentrations were measured after digesting 0.1 g of samples with 5 mL each of H2O2 and H2SO4 at 120 °C (Enders and Lehmann 2012).
2.4 Statistical analyses
The obtained data from plant and soil chemical analyses were statistically processed using a one-way analysis of variance (ANOVA). The means of the measured variables per treatment were separated using the Least Significant Difference (LSD) at p < 0.05. Data analyses and processing was done on SPSS 25.0 (IBM Corp., NY, USA) and OriginPro 2021.
2.4.1 Microbial analysis and bioinformatics
Soil DNA extraction was conducted from 0.5 g of the homogenized fresh soil using the PowerSoil DNA kit (MoBio OH, USA), and its concentration was determined using 2% agarose gel. The v3-v4 regions of the 16S rRNA genes were amplified using the PCR with the primers; 338F and 806R with barcodes, as outlined by Ibrahim et al. (2021). The PCR products were purified using the E.Z.N.A® Gel Extraction Kit (Omega Bio-Tek, USA), and the obtained amplicons were quantified by fluorometry (Thermo Fisher Scientific, USA). The equimolar concentration of the recovered PCR amplicons (2 × 300 base pairs) was used for high-throughput sequencing on an Illumina MiSeq® platform (San Diego, CA, USA).
The elimination of chimeric sequences from the derived raw pair-end reads was done using the UCHIME algorithm. The obtained clean tags were processed using the UPARSE algorithm to generate operational taxonomic units (OTU) (Edgar 2013). The OTUs were allocated at a 97% similarity level using the QIIME2 toolkit (Bolyen et al. 2019) with reference to the Silva database (Release 138, https://www.arb-silva.de). The alpha diversity indices were estimated using the Chao1 and Shannon indexes, while the beta diversity was obtained using the principal component analysis (PCA). The differences in the distribution of the bacterial phyla and class members among the treatments were estimated using the relative abundance (> 1%). The relationships between the treatments, soil properties, and bacterial structure were estimated using the Redundancy analysis (RDA).
3 Results
3.1 Properties of biochar and its rate-dependent impacts on soil properties and N dynamics
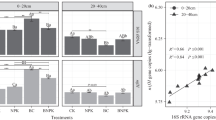
The SEM image of the biochar reveals its porous nature (Fig. 1a), which has implications for the retention of nutrients and for creating a conducive environment for microbial colonization. Also, the FTIR spectra of biochar (Fig. 1b) show the different peaks of functional groups, particularly O-bound groups, which were negatively charged. Compared to the control (C) and sole inorganic fertilization (F), the combined application of fertilizer and biochar at 40 tons ha−1 (FB2) significantly improved all the soil chemical parameters evaluated (Table 1). However, the values for FB2 were statistically similar to those obtained at 20 tons ha−1 (FB1) for pH, TN, and the CN ratio. Notably, available N (AN) was 16.5% (p = 0.057), 23.8% (p = 0.033), and 34.8% (p = 0.028) higher in F, FB1, and FB2, respectively, compared to C.
Data in Fig. 2a shows that NH4+-N in FB1 and FB2 increased by 28.1% (p = 0.012) and 32.8% (p = 0.009), respectively, compared to F. On the other hand, NO3−-N availability was 15.2% (p = 0.041), 21.8% (p = 0.032), and 20.8% (p = 0.027) higher in FB1, FB2, and C, compared to F after one year of soil amendment (Fig. 2b). Urease activity was significantly stimulated under high biochar amendment (FB2) compared to the other treatments (Fig. 2c). The impact of biochar rates on amino acids in fertilized soils revealed the predominance of sarcosine across the treatments, while its composition was significantly reduced in F (Fig. 2d). Leucine was significantly stimulated in FB2, while cystine was only detected in FB2. However, alanine and serine were not detected in FB2. Also, alanine and phosphoserine were in high proportion in F (Fig. 2d).
Impacts of biochar rates on soil nitrogen dynamics. Impacts of experimental treatments on soil (a) ammonium (NH4+) concentration, (b) nitrate (NO3 −) concentration, (c) urease activity, and (d) the proportions of each amino acid in total amino acid concentration. Experimental treatments include control (C, no application of fertilizer or biochar), fertilizer only (F), fertilizer + 20 tons biochar ha−1 (FB1) and fertilizer + 40 tons biochar ha−1 (FB2). Each bar represents the mean of five replicates, and error bars indicate standard errors. Different letters on bars indicate statistically significant differences at p < 0.05 among the treatments
3.2 Rate-dependent impact of biochar on aggregates size distribution and aggregate-N composition in the soil after one year
Irrespective of the rate, biochar amendment significantly enhanced the distribution of < 0.25 mm aggregates as compared with the F and C treatments. However, after one year of application, biochar did not significantly impact the 0.5–0.25 mm aggregates fraction (Table 2). The > 2 mm aggregate fractions significantly increased in F compared to the control and biochar-amended treatments. Compared to FB1, FB2 reduced the > 2 mm, 2–1 mm, and 1–0.5 mm aggregate fractions. However, the reduction of the 1–0.5 mm fractions in FB2 was associated with a significant increase in its associated N (Fig. 3). Besides, the FB2 had a significantly higher N content across all the aggregate sizes except in the 0.5–0.25 mm fraction.
Impact of biochar rates on stable aggregates associated N distribution in fertilized soil after one year. Experimental treatments include control (C, no application of fertilizer or biochar), fertilizer only (F), fertilizer + 20 tons biochar ha−1 (FB1) and fertilizer + 40 tons biochar ha−1 (FB2). Each bar represents the mean of five replicates, and error bars indicate standard errors. Different letters on bars indicate statistically significant differences at p < 0.05 among the treatments
3.3 Effect of biochar rates on the elemental composition of N. nagi
The N concentration of the plant (leaves) significantly increased in F, FB1, and FB2 treatments than in the control (C). FB2 had a significantly higher plant N content (41.3%), followed by FB1 (28.8%) and F (12.2%) when compared to the control (Table 3). An insignificant difference was obtained in the C content of the plant across all the treatments. Plant P concentration was the least in C and had no significant difference across F, FB, and FB2. Plant K concentration was highest in F (11.60 g kg−1) and least in FB1 (5.10 g kg−1) and FB2 (6.15 g kg−1).
3.4 Impacts of biochar rates on the distribution of soil bacterial distribution and composition
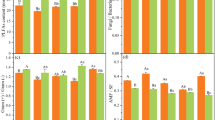
The alpha diversity of the soil bacteria (Chao1 index) revealed a significant reduction in bacterial species diversity in fertilized soil amended with biochar (FB1 and FB2) after one year compared to C or F treatments (Fig. 4a). No significant difference was obtained between the high and low biochar rates (FB1 and FB2) on the Chao1 index. On the other hand, the bacteria community diversity represented by the Shannon index indicated an insignificant impact of sole fertilization or its combination with high and low biochar rates after one year (Fig. 4b).
Alpha diversity indices of soil bacterial population after one year (a) Chao1 index (b) Shannon index. Each boxplot represents the mean of five replicates (p < 0.05). Control (C, no application of fertilizer or biochar), fertilizer only (F), fertilizer + 20 tons biochar ha−1 (FB1) and fertilizer + 40 tons biochar ha.−1 (FB2)
The result of the relative abundance of soil bacteria across the different treatments showed that the Proteobacteria phyla were significantly stimulated in FB1 (41.8%) and FB2 (40.8%) as compared with the control (31.4%) and F (29.5%) treatments after one year (Fig. 5a). The Alphaproteobacteria and Betaproteobacteria were the most dominant Proteobacteria, particularly in FB1 (24.4%) and FB2 (25.0%) (Fig. 5b). However, a reduction was recorded in the proportion of the Chloroflexi and its related class members, JG37-AG-4, in the FB1 (8.1%) and FB2 (1.8%) compared to F (18.7%) and C (19.3%). The abundance of the Gemmatimonadetes was promoted by biochar amendment (Fig. 5a). Its class members had the highest relative abundance in FB2 (24.6%), followed by FB1 (11.3%) when compared to C (3.0%) and F (2.9%) treatments (Fig. 5b). However, an insignificant difference was recorded in the distribution of Actinobacteria across the treatments. Acidobacteria and its class members were significantly less in FB2 (which had the highest pH (Table 1)). The abundance of Firmicutes phyla was significantly increased in F compared to C, FB1, and FB2.
Rate-dependent impact of biochar on the relative abundance of soil bacterial population in fertilized soils after one year. a bacterial Phyla distribution, and (b) bacterial Class distribution. Experimental treatments include control (C, no application of fertilizer or biochar), fertilizer only (F), fertilizer + 20 tons biochar ha−1 (FB1) and fertilizer + 40 tons biochar ha−1 (FB2). Each bar represents the mean of five replicates, and error bars indicate standard errors. Different letters on bars indicate statistically significant differences at p < 0.05 among the treatments. Only the phyla with relative abundances ≥ 1% are presented in this figure
Beta diversity analysis indicated by the principal component analysis (PCA) showed no significant difference in the clustering of bacterial communities in the C and F treatments after one year (Fig. 6a). However, it revealed a distinct clustering pattern in microbial communities under high and low biochar application rates in fertilized soils. Also, the redundancy analysis revealed that all the measured soil properties had a positive and significant association with key bacterial communities involved in N cycling in FB1 and FB2. A significant positive association was observed between C and F, and bacteria groups not actively involved in N cycling.
a Principal component analysis of soil bacterial population and (b) redundancy analysis showing the relationship between soil bacteria and soil properties after one year. Experimental treatments include control (C, no application of fertilizer or biochar), fertilizer only (F), fertilizer + 20 tons biochar ha−1 (FB1) and fertilizer + 40 tons biochar ha.−1 (FB2)
4 Discussion
4.1 Rate-dependent influence of biochar on soil properties and N dynamics in the soil after one year
The existence of several organic groups on the biochar's surface could significantly alter the electrostatic pull amongst ions and modify its adsorption capacity (Chen et al. 2011; Jung and Ahn 2016). The statistical similarity of the increase in soil NH4+ among FB1 and FB2 even after one year of biochar amendment compared to sole fertilizer application (F) indicates the efficiency of biochar for its sorption/retention and slow release (Wang et al. 2015; Zhang et al. 2019), irrespective of its application rate. Also, the functional groups present on the surfaces of biochar surface play a significant role in the sorption of inorganic N (Lehmann and Joseph 2015). Hence, the presence of negatively charged organic groups, as shown on the FTIR image in Fig. 1 could significantly enhance the retention and slow release of NH4+ (Ibrahim et al. 2023a). In addition, the continuous mineralization of the organic N contained in biochar could increase in NH4+ concentration of the amended soils (Haider et al. 2017). The increase in NH4+ concentration in the biochar-amended soils was associated with the stimulation of urease activity, which was crucial for releasing NH4+ after urea hydrolysis (Zhang et al. 2014; Miao et al. 2019; Dotaniya et al. 2019). Despite the high rate of the hydrolysis of urea to NH4+ in FB2 compared that in FB1, the similar NH4+ concentration recorded in these treatments indicates that biochar is effective in equally retaining NH4+ irrespective of its application rate. The high capacity of biochar for NH4 + has been well documented (Chen et al. 2011; Bai et al. 2017). The net negative charges on biochar's surface result in its limited potential for NO3− rather than NH4+ retention. The weak adsorption of NO3− compared to NH4+ by biochar has often been attributed to the net negative charges on biochar relative to positive surface charges (Kameyama et al. 2012). Thus, the poor retention of NO3− in the soil and its increased leaching after biochar application have been previously reported (Laird et al. 2010; Singh et al. 2010). On the other hand, the low NO3− concentration in the soil could be due to its increased uptake, being the fraction of N often taken up by plants, aside from its potential leaching (Reverchon et al. 2014; Ibrahim et al. 2023a). This could explain the high total N concentration of N. nagi in the biochar-amended soils. Also, the pore-filling capacity of biochar, high CEC, and large surface area are important mechanisms that can also improve the sorption and slow release of inorganic N in biochar-applied soils. The large surface area of biochar has been reported to increase the unconventional H-bonding between N ions and the biochar surface (Kammann et al. 2015). Similarly, the physical attraction of inorganic N via physisorption in the pores and on the inner surface of biochar has also been reported (Clough et al. 2013; Ibrahim et al. 2020a, b). Thus, biochar amendment can be helpful in long-term N management, especially for perennial crops.
Dissolved organic N is present as proteins that are degraded by enzymes into bio-available free amino acids, which plants and soil microorganisms competitively utilize (Jones et al. 2005). The amino acid sarcosine is a predominant soil metabolite that can be used as the only source of C and energy by many microorganisms (Zhao and Jorns 2006). This could explain its dominance in treatments containing biochar (FB1 and FB2) with a high proportion of organic N and C. Also, biochar comprises several organic N-functional groups, including amino-acid N that can undergo direct cyclization, dimerization, dehydration, dehydrogenation, and deamination, and can convert into multiple heterocyclic rings utilized and assimilated by plants or other organisms (Chen et al. 2018; Leng et al. 2019). The high proportion of leucine and the presence of cysteine only in FB2 suggests that increasing biochar rates would increase the availability of these amino acids from the biochar matrix (Leng et al. 2019). Biochar could also be vital for releasing these amino acids from their adsorbed state on the soil surface. The adsorption of amino acids in soil matrix could lower their bioavailability to plants and soil microorganisms (Jones and Keilland 2002). Soil amino acid N is competitively taken up with mineral N and can, therefore, be a potential and important factor in the overall ecosystem function and vegetation succession (Henry and Jefferies 2003; Warren 2006). The ability of alanine and glycine to resist microbial decomposition (Gotoh et al. 1986) increased their abundance in the unamended soil with higher microbial diversity (Fig. 4a). Thus, the improved availability of critical amino acids through biochar amendment could, in part, be a useful mechanism for the increased N recovered in plants grown in biochar-applied soils.
4.2 Rate-dependent impact of biochar on stable soil aggregates size and aggregate-N distribution after one year
The aggregate fractions of soils are the basic soil units vital for regulating soil N stocks and their stability over time (Chen et al. 2004; Ngaba et al. 2020). Different soil amendments and management regimes can alter the responses of the soil's stable aggregates and their constituent N in different-size aggregates by altering the binding of soil particles by organic agents (Chen et al. 2017). Biochar application could alter the physicochemical and structural properties of the soil (Blanco-Canqui 2017). The higher < 0.25 mm aggregates (microaggregates) with biochar amendment, irrespective of rate, compared to unamended treatments indicates the increase in stability of microaggregates in fine soil particles rather than larger soil aggregates after extended periods. It was suggested that the capacity of the organic particles of biochar to improve the particle bonding of large particles and promote soil aggregation was more in coarse-textured than in fine-textured soils (Blanco-Canqui 2017). However, aside from the > 2 mm aggregate size, the application of biochar increased the stability of 0.25 – 2 mm fractions. Such a positive impact of biochar on soil aggregate stability has been reported for grassland soils (Herath et al. 2013) and volcanic soils (Curaqueo et al. 2014). Generally, it is estimated that biochar application can increase wet aggregate stability regardless of the differences in soil textural class and initial soil organic C concentration (Blanco-Canqui 2017). However, the insignificant or poor capacity of biochar to improve wet aggregate stability (macroaggregates), similar to some reported studies, suggests that biochar's effects can depend on site-specific characteristics. Interactions among texture, soil slope, organic C, biochar properties, climate, and others could dictate the extent to which biochar application can change soil aggregate stability (Peng et al. 2016). Evidence has shown that biochar can enhance the stability of soil aggregates by providing a loosening effect and introducing micropores, hence reducing bulk density (Guo 2015). This could explain that the increase in microaggregates in FB1 and FB2 was associated with higher soil N contents. This observation indicates that biochar increases N availability by promoting its retention in micro aggregates, which can be easily mineralized to available forms. Such increases in the microaggregates support the increase of soil aggregation under higher SOC (Zhu et al. 2017; Lin et al. 2018).
In addition, the organic complexes provided by biochar were likely to be beneficial for forming and stabilizing macroaggregates-associated N compared to the macroaggregates. A high biochar rate was more effective in improving aggregate N content across the macro- and intermediate aggregates, especially in FB2. The higher N composition in 0.5—> 2 mm aggregate fractions, especially in FB2 could be attributed to the higher water retention potential of higher biochar rates that reduced the leaching of soil N. Studies have found that biochar increased water retention when large amounts of are utilized (Paneque et al. 2016; Xiao et al. 2016). Thus, the potential reduction in N leaching could be a key factor that increased the N concentration in FB2. Biochar amendment effectively reduces nitrification, hence reducing nitrate leaching and improving overall N retention (Borchard et al. 2019; Chen et al. 2019; Ibrahim et al. 2023b). In addition, previous studies have reported the stabilization of macroaggregates due to the binding effect of organic C from litter and exudate from roots (Pohl et al. 2012; Le Bissonnais et al. 2018). Thus, protecting these labile organic C from rapid mineralization by biochar (Ibrahim et al. 2021) could have aided in increasing the stability of the aggregate-associated N. Research has shown that the formation of macroaggregates is a key process in the stabilization of soil organic carbon (Gelaw et al. 2015; Zhu et al. 2017). The stabilization of organic C would ultimately lead to a reduction in N mineralization and hence its protection and increased concentration in soils. Thus, the increased retention of N in the macro- and intermediate aggregates was essential for the slow and continuous mineralization of the retained N. This slow-release process is an important mechanism that ensures the availability of N for use over an extended period.
4.3 Rate-dependent impact of biochar rates on the nutritional composition of N. nagi
The increased concentration of N in the leaves of the plants grown in the biochar-amended soil, particularly in FB2, was is not unconnected with the higher proportion of soil N in this treatment. High N retention by biochar has been associated with an increase in N uptake and concentration in plants (Ibrahim et al. 2023a). Many reports have shown that plants can take up N retained by biochar over time (Krounbi et al. 2021; Rasse et al. 2022). Besides, biochar contains a significant proportion of organic N that can be mineralized and utilized by plants and soil microbes (Gao et al. 2019). Hence, the likelihood of N uptake as amino acids (organic N) (Cao et al. 2016), particularly in the biochar-applied treatments, may have increased the total N contents of the plants. While biochar addition did not significantly influence the carbon concentration of the plants, it increased plant P concentration, similar to sole fertilization (F). Biochar could enhance the solubilization of P for plant and microbial needs by adjusting the soil's pH (Sarfraz et al. 2020). Also, the positive effect of biochar on soil physicochemical properties could create a suitable habitat for diverse microorganisms involved in P mineralization (Sarfraz et al. 2020; Hossain et al. 2020). The observed retention of K in FB1 could be due to its sorption on the abundant negative charges on the surfaces of biochar (Chen et al. 2011; Ibrahim et al. 2023a). However, while the low biochar addition rate reduced K availability and uptake, the high available K in the soils of FB2 did not result in a corresponding K uptake. This could be attributed to the preferable uptake of non-exchangeable or slowly available K, which comprised the bulk of available K detected in the soil. It has been shown that for most soils, the more a plant depends on non-exchangeable K, the lower the K uptake (Hasanuzzaman et al. 2018; Sardans and Peñuelas 2015).
4.4 Impacts of biochar rates on soil bacterial distribution and composition
Applying biochar over extended periods reduced bacterial species diversity, irrespective of its rate. Such reductions in the diversity of bacteria resulting from biochar application have been observed (Li et al. 2020). However, previous reports have indicated enrichment in bacterial diversity due to the combined application of biochar and inorganic fertilizers (Zhang et al. 2019; Zhou et al. 2019). Also, an insignificant impact of biochar amendment on the richness and evenness of the soil bacterial community has been previously reported (Elzobair et al. 2016). The observed reduction in species diversity over extended periods has been linked to the shift of soil bacteria to a more specialized functional group over time while outcompeting nonspecialized bacterial taxa (Ibrahim et al. 2022). Thus, the clustering patterns of soil bacterial groups in the PCA analysis (Fig. 6a) support the specialized nature of soil bacteria and their specific roles in the different treatments, despite the insignificant changes in the community diversity (Fig. 4b).
The proportion of individual bacterial taxa revealed the abundance of Proteobacteria in FB1 and FB2, and their associated class members, the Alpha and Betaproteobacteria. The Proteobacteria are dominant in soils and particularly higher under improved fertility status (Dai et al. 2016; Zhang et al. 2019b; Ibrahim et al. 2023b). This supports the positive relationship of its class members with soil properties, as depicted in the RDA (Fig. 6b). As observed, the Alphaproteobacteria are the most dominant class of Proteobacteria and are non-nitrifying (Spain et al. 2009). However, taxonomic members of the Betaproteobacteria are regarded as ammonium-oxidizing (Voytek and Ward 1995). Biochar application can significantly accelerate ammonia oxidation (Pan et al. 2017). Hence, the high proportion of Betaproteobacteria after biochar amendment was linked to the observed increase in N availability in FB1 and FB2. Besides, the RDA analysis revealed a positive relationship between the Betaproteobacteria and inorganic N in the soils (Fig. 6b). Thus, their high proportion in the biochar-amended soils (FB1 and FB2) compared to the control and F treatments, had a resultant increase in N cycling and supply. Notably, Betaproteobacteria are also known to thrive under increased soil C content (such as in biochar-amended soils) (Cesarano et al. 2017; Yang et al. 2019).
Although the Chlorofexi are phototrophs involved in nitrification (Bryant and Frigaard 2006), their abundance in the control and F treatments rather than in FB1 and FB2 suggests their inability to compete with the Proteobacteria for N substrates. Thus, they might have contributed more to N cycling and supply in the control and F treatments. As observed in our study, the abundance of Actinobacteria under biochar amendment is attributed to their active participation in organic matter decomposition and denitrification (Shen et al. 2013; Xu et al. 2017). These activities could enable them to contribute to the higher inorganic N recorded in the biochar-amended soils. This was also confirmed by their positive relationships with soil inorganic N in the RDA (Fig. 6b). Even though the Gemmatimonadetes thrives under limited soil organic matter, their higher affinity for organic substrates (Fierer et al. 2007) and preference for more favorable pH (Mitchell et al. 2015) were the key factors that promoted their higher relative abundance, in the biochar-amended soils, particularly in FB2. The low relative abundance of the Firmicutes in the biochar amended treatments, unlike that in the F and control treatments, was attributed to the fact that, as gram-positive bacteria, they are characterized by good growth in a stable environment but with a weak capability to adapt to changing environments over time (Mickan et al. 2017; Ibrahim et al. 2022). Gram-negative bacteria are more nutrient-efficient than gram-positive ones (Mickan et al. 2017). The gram-negative bacteria such as Proteobacteria, Actinobacteria, and Gemmatimonadetes (which were in high proportion) in FB1 and FB2 are highly competitive. They could utilize resources faster than gram-positive bacteria in a competitive environment. Evidence has previously shown that members of Firmicutes (e.g., Bacilli) are directly and indirectly involved in the N cycle through the production of hydrolytic enzymes, organic matter decomposition, and mineralization (Park et al. 2017; Azeem et al. 2021). Therefore, they may have contributed to N supply in the control and soil fertilizer treatments where there was a limited abundance of several N cycling bacteria. Besides, the increase in soil pH due to biochar amendment reduced the relative abundance of the Acidobacteria, unlike in the C and F treatments. Thus, the improved soil pH in FB1 and FB2 may have favoured the stimulation of N-cycling bacteria more than in the control and F treatments. This may have, in part, contributed to the higher N cycling and supply due to biochar amendment.
The abundance of the N cycling organisms in the biochar-amended soils and the high aggregate-N recorded in these treatments, particularly in FB2, suggest the colonization of macropores by these organisms to support their active role in N mineralization and supply. The improved porosity of soil after biochar amendment has been associated with higher microbial colonization (Yang et al. 2022). As such, the increased colonization of bacteria in the macro and intermediate aggregates containing higher N concentration contributed to the increased N supply in the biochar-amended soils.
5 Conclusion
The key mechanism regulating the supply of nitrogen (N) after one year under combined biochar and inorganic fertilizer was mainly through the role of biochar in increasing stable microaggregates, while increasing the macro- and intermediate aggregates-associated N, especially at a higher application rate. Thus, there were higher urease activity and continuous mineralization, and slow release of the aggregates-retained N for plant uptake. The functional groups and porous nature of biochar were also critical for its N retention potentials. Despite the reduction in bacteria species diversity due to biochar amendment, the abundance of a more specialized community of NH4+-oxidizers (Betaproteobateria) induced the mineralization and slow release of the retained N to meet the plant's need. Even though a low biochar rate was more beneficial than sole fertilization, higher biochar application rate could sustain more N supply through stabilizing microaggregates, while increasing macro- and intermediate-aggregates-N, its mineralization, and slow-release for plant uptake over one year. Thus, a higher biochar rate could potentially be used for soil N management for crops grown over more extended periods, bearing in mind the cost implication of such an application. However, it is important to note that the pot experiment utilized in this study might not fully capture the complexity and variability of natural soil systems over more extended time frames. This will necessitate the validation of our findings through additional on-field trials. Nonetheless, the insights gained from this study can inform the development of a more accurate N management regime and can guide future research that aims to improve N retention in plantation soil-plant systems.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DNA:
-
Deoxyribonucleic acid
- H2O2 :
-
Hydrogen peroxide
- H2SO4 :
-
Sulfuric acid
- KCl:
-
Potassium chloride
- NaHCO3 :
-
Sodium Bicarbonate
- NH4OAc:
-
Ammonium acetate
- PCR:
-
Polymerase chain reaction
- QIIME:
-
Quantitative Insights Into Microbial Ecology
- RNA:
-
Ribonucleic acid
References
Azeem M, Hassan TU, Tahir MI, Ali A, Jeyasundar PGSA, Hussain Q, Bashir S, Mehmood S, Zhang Z (2021) Tea leaves biochar as a carrier of Bacillus cereus improves the soil function and crop productivity. Appl Soil Ecol 157:103732. https://doi.org/10.1016/j.apsoil.2020.103732
Bai SH, Dempsey R, Reverchon F, Blumfield TJ, Ryan S, Cernusak LA (2017) Effects of forest thinning on soil-plant carbon and nitrogen dynamics. Plant Soil 411:437–449. https://doi.org/10.1007/s11104-016-3052-5
Bei S, Zhang Y, Li T, Christie P, Li X, Zhang J (2018) Response of the soil microbial community to different fertilizer inputs in a wheat-maize rotation on a calcareous soil. Agric Ecosyst Environ 260:58–69. https://doi.org/10.1016/j.agee.2018.03.014
Blanco-Canqui H (2017) Biochar and Soil Physical Properties. Soil Sc Soc Amer J 81(4):687–711. https://doi.org/10.2136/sssaj2017.01.0017
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, et al (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:1091. https://doi.org/10.1038/s41587-019-0209-9.
Borchard N, Schirrmann M, Cayuela ML, Kammann C, Wrage-Mönnig N, Estavillo JM, Fuertes-Mendizábal T, Sigua G, Spokas K, Ippolito JA, Novak J (2019) Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: a meta-analysis. Sci Total Environ 651:2354–2364. https://doi.org/10.1016/j.scitotenv.2018.10.060
Bryant DA, Frigaard N-U (2006) Prokaryotic photosynthesis and phototrophy illuminated. Trends Microbiol 14:488–496. https://doi.org/10.1016/j.tim.2006.09.001
Cao X, Ma Q, Zhong C, Yang X, Zhu L, Zhang J, Jin Q, Wu L (2016) Elevational variation in soil amino acid and inorganic nitrogen concentrations in Taibai mountain. China. PLOS One 11(6):e0157979. https://doi.org/10.1371/journal.pone.0157979
Cassman KG, Dobermann A, Walters DT (2002) Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 31:132–140. https://doi.org/10.1579/0044-7447-31.2.132
Cayuela ML, van Zwieten L, Singh BP, Jeffery S, Roig A, Sánchez-Monedero MA (2014) Biochar’s role in mitigating soil nitrous oxide emissions: a review and meta-analysis. Agric Ecosyst Environ 191:5–16. https://doi.org/10.1016/j.agee.2013.10.00
Cesarano G, De Filippis F, La Storia A, Scala F, Bonanomi G (2017) Organic amendment type and application frequency affect crop yields, soil fertility and microbiome composition. Appl Soil Ecol 120:254–264. https://doi.org/10.1016/j.apsoil.2017.08.017
Chen CR, Xu ZH, Mathers NJ (2004) Soil carbon pools in adjacent natural and plantations forests of subtropical Australia. Soil Sci Soc Am J 68:282–291. https://doi.org/10.2136/sssaj2004.2820
Chen B, Chen Z, Lv S (2011) A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour Technol 102(2):716–723. https://doi.org/10.1016/j.biortech.2010.08.067
Chen C, Liu W, Jiang X, Wu J (2017) Effects of rubber-based agroforestry systems on soil aggregation and associated soil organic carbon: implications for land use. Geoderma 299:13–24. https://doi.org/10.1016/j.geoderma.2017.03.021
Chen W, Yang H, Chen Y, Li K, Xia M, Chen H (2018) Influence of biochar addition on nitrogen transformation during copyrolysis of algae and lignocellulosic biomass. Environ Sci Technol 52:9414–9521
Chen W, Meng J, Han X, Lan Y, Zhang W (2019) Past, present, and future of biochar. Biochar 1:75–87. https://doi.org/10.1007/s42773-019-00008-3
Clough T, Condron L, Kammann C, Müller C (2013) A review of biochar and soil nitrogen dynamics. Agronomy 3:275–293. https://doi.org/10.3390/agronomy3020275
Curaqueo G, Meier S, Khan N, Cea M, Navia R (2014) Use of biochar on two volcanic soils: effects on soil properties and barley yield. J Soil Sci Plant Nutr 14:911–924
Dai Z, Hu J, Xu X, Zhang L, Brookes PC, He Y, Xu J (2016) Sensitive responders among bacterial and fungal microbiome to pyrogenic organic matter (biochar) addition differed greatly between rhizosphere and bulk soils. Sci Rep 6:36101. https://doi.org/10.1038/srep36101
Dai Z, Yu M, Chen H, Zhao H, Huang Y, Su W et al (2020) Elevated temperature shifts soil N cycling from microbial immobilization to enhanced mineralization, nitrification and denitrification across global terrestrial ecosystems. Glob Change Biol 26(9):5267–5276. https://doi.org/10.1111/gcb.15211
Dotaniya ML, Aparna K, Dotaniya CK, Singh M, Regar KL (2019) Role of soil enzymes in sustainable crop production. Enzymes Food Biotechnol: 569–589 https://doi.org/10.1016/b978-0-12-813280-7.00033-5
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998
Elzobair KA, Stromberger ME, Ippolito JA, Lentz RD (2016) Contrasting effects of biochar versus manure on soil microbial communities and enzyme activities in an Aridisol. Chemosphere 142:145–152. https://doi.org/10.1016/j.chemosphere.2015.06.044
Enders A, Lehmann J (2012) Comparison of wet-digestion and dry ashing methods for total elemental analysis of biochar. Commun Soil Sci Plant Analy 43:1042–1052. https://doi.org/10.1080/00103624.2012.656167
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364. https://doi.org/10.2307/27651243
Foster EJ, Hansen N, Wallenstein M, Cotrufo MF (2016) Biochar and manure amendments impact soil nutrients and microbial enzymatic activities in a semi-arid irrigated maize cropping system. Agric Ecosyst Environ 233:404–414. https://doi.org/10.1016/j.agee.2016.09.029
Gao S, DeLuca TH, Cleveland CC (2019) Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: a meta-analysis. Sci Total Environ 654:463–472. https://doi.org/10.1016/j.scitotenv.2018.11.124
Gelaw AM, Singh BR, Lal R (2015) Organic carbon and nitrogen associated with soil aggregates and particle sizes under different land uses in Tigray, northern Ethiopia. Land Degrad Dev 26:690–700. https://doi.org/10.1002/ldr.2261
Gotoh S, Araragi M, Koga H, Ono S (1986) Hydrolyzable organic forms of nitrogen in some rice soil profiles as affected by organic matter application. Soil Sci Plant Nutri 32(4):535–550. https://doi.org/10.1080/00380768.1986.10557536
Guo M (2015) Application of biochar for soil physical improvement. In: Agricultural and environmental applications of biochar: Advances and barriers. https://doi.org/10.2136/sssaspecpub63.2014.0039.5
Haider G, Steffens D, Moser G, Müller C, Kammann CI (2017) Biochar reduced nitrate leaching and improved soil moisture content without yield improvements in a four-year field study. Agric Ecosys Environ 237:80–94. https://doi.org/10.1016/j.agee.2016.12.019
Hasanuzzaman M, Bhuyan MHMB, Nahar K, Hossain MS, Mahmud JA, Hossen MS, Masud AAC, Moumita Fujita M (2018) Potassium: a vital regulator of plant responses and tolerance to abiotic stresses. Agron 8:31. https://doi.org/10.20944/preprints201801.0223.v1
Henry HAL, Jefferies RL (2003) Plant amino acid uptake, soluble N turn over and microbial N capture in soils of a grazed Arctic salt marsh. J Ecol 91:627–636. https://doi.org/10.1046/j.1365-2745.2003.00791.x
Herath HMSK, Arbestain MC, Hedley M (2013) Effect of biochar on soil physical properties in two contrasting soils: an alfisol and an andisol. Geoderma 209–210:188–197. https://doi.org/10.1016/j.geoderma.2013.06.016
Hossain MZ, Bahar MM, Sarkar B et al (2020) Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2:379–420. https://doi.org/10.1007/s42773-020-00065-z
Ibrahim MM, Hu K, Tong C, Xing S, Zou S, Mao Y (2020) De-ashed biochar enhances nitrogen retention in manured soil and changes soil microbial dynamics. Geoderma 378:114589. https://doi.org/10.1016/j.geoderma.2020.114589
Ibrahim MM, Tong C, Hu K, Zhou B, Xing S, Mao Y (2020) Biochar-fertilizer interaction modifies N-sorption, enzyme activities and microbial functional abundance regulating nitrogen retention in rhizosphere soil. Sci Total Environ 739:140065. https://doi.org/10.1016/j.scitotenv.2020.140065
Ibrahim MM, Zhang H, Guo L, Chen Y, Heiling M, Zhou B, Mao Y (2021) Biochar interaction with chemical fertilizer regulates soil organic carbon mineralization and the abundance of key C-cycling-related bacteria in rhizosphere soil. Europ J Soil Biol 106:103350. https://doi.org/10.1016/j.ejsobi.2021.103350
Ibrahim MM, Guo L, Zhang H, Wu F, Zou S, Xing S, Mao Y (2022) Comparative impact of Bacillus spp. on long-term N supply and N-cycling bacterial distribution under biochar and manure amendment. J Soil Sci Plant Nut 22:882–895. https://doi.org/10.1007/s42729-021-00698-4746
Ibrahim MM, Liu D, Wu F, Chen Y, He Z, Zhang W, Xing S, Mao Y (2023) Nitrogen retention potentials of magnesium oxide- and sepiolite-modified biochars and their impacts on bacterial distribution under nitrogen fertilization. Sci Total Environ 866:161358. https://doi.org/10.1016/j.scitotenv.2022.161358
Ibrahim MM, Wu F, Chen Y, Liu D, Zhang W, He Z, Hou E, Xing S, Mao Y (2023) Impacts of MgO- and sepiolite-biochar composites on N-partitioning and dynamics of N-cycling bacteria in a soil-maize system: A field-based 15N-urea tracer study. Geoderma 429:116236. https://doi.org/10.1016/j.geoderma.2022.116236
Jones DL, Kielland K (2002) Simple method to enable the high resolution determination of total free amino acids in soil solutions and soil extracts. Soil Biol Biochem 34(12):1893–1902. https://doi.org/10.1016/s0038-0717(02)00203-1
Jones DL, Shannon D, Junvee-Fortune T, Farrarc JF (2005) Plant capture of free amino acids is maximized under high soil amino acid concentrations. Soil Biol Biochem 37(1):179–181. https://doi.org/10.1016/j.soilbio.2004.07.021
Jung K, Ahn K (2016) Fabrication of porosity enhanced MgO/biochar for removal of phosphate from aqueous solution: application of a novel combined electrochemical modification method. Bioresour Technol 20:1029–1032. https://doi.org/10.1016/j.biortech.2015.10.008
Kameyama K, Miyamoto T, Shiono T, Shinogi Y (2012) Influence of sugarcane bagasse-derived biochar application on nitrate leaching in calcaric dark red soil. J Environ Qual 41(4):1131–1137. https://doi.org/10.2134/jeq2010.0453
Kammann CI, Schmidt HP, Messerschmidt N, Linsel S, Steffens D, Müller C, Koyro HW, Conte P, Joseph S (2015) Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci Rep 5(1):11080. https://doi.org/10.1038/srep11080
Kemper WD, Rosenau RC (1986) Aggregate stability and size distribution. In: Klute A (ed) Methods of soil analysis, Part 1: physical and mineralogical methods, 2nd edn. ASA and Soil Science Society of America, Madison, Wisconsin, pp 425–442
Krounbi L, Enders A, Gaunt J et al (2021) Plant uptake of nitrogen adsorbed to biochars made from dairy manure. Sci Rep 11:15001. https://doi.org/10.1038/s41598-021-94337-8
Laird D, Fleming P, Wang B, Horton R, Karlen D (2010) Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 158(3–4):436–442. https://doi.org/10.1016/j.geoderma.2010.05.012
Le Bissonnais Y, Prieto I, Roumet C, Nespoulous J, Metayer J, Huon S, Villatoro M, Stokes A (2018) Soil aggregate stability in Mediterranean and tropical agro-ecosystems: effect of plant roots and soil characteristics. Plant Soil 424(1–2):303–317. https://doi.org/10.1007/s11104-017-3423-6
Lehmann J, Joseph S (2015) Biochar for environmental management: science, technology and implementation. Earthscan, New York
Leng L, Xu S, Liu R, Yu T, Zhuo X, Leng S, Xiong Q, Huang H (2019). Nitrogen containing functional groups of biochar: an overview. Biores Technol 122286. https://doi.org/10.1016/j.biortech.2019.122286
Li S, Wang S, Fan M, Wu Y, Shangguan Z (2020) Interactions between biochar and nitrogen impact soil carbon mineralization and the microbial community. Soil Tillag Res 196:104437. https://doi.org/10.1016/j.still.2019.104437
Lin Z, Li Y, Tang C, Luo Y, Fu W, Cai X, Li Y, Yue T, Jiang P, Hu S, Chang SX (2018) Converting natural evergreen broadleaf forests to intensively managed moso bamboo plantations affects the pool size and stability of soil organic carbon and enzyme activities. Biol Fertil Soils 54(4):467–480. https://doi.org/10.1007/s00374-018-1275-8
Miao F, Li Y, Cui S et al (2019) Soil extracellular enzyme activities under long-term fertilization management in the croplands of China: a meta-analysis. Nutr Cycl Agroecosyst 114:125–138. https://doi.org/10.1007/s10705-019-09991-2
Mickan BS, Abbott LK, Fan JW (2017) Application of compost and clay under water-stressed conditions infuences functional diversity of rhizosphere bacteria. Biol Fertil Soils 54:55–70. https://doi.org/10.1007/s00374-017-1238-5
Mitchell PJ, Simpson AJ, Soong R, Simpson MJ (2015) Shifts in microbial community and water-extractable organic matter composition with biochar amendment in a temperate forest soil. Soil Biol Biochem 81:244–254. https://doi.org/10.1016/j.soilbio.2014.11.017
Ngaba MJY, Bol R, Hu YL (2020) Stable isotopic signatures of carbon and nitrogen in soil aggregates following the conversion of natural forests to managed plantations in eastern China. Plant Soil 459(1–2):371–385. https://doi.org/10.1007/s11104-020-04754-3
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL et al (eds) Methods of Soil Analysis, Part 2, 2nd edn, Agron Monogr 9. ASA and ASSA, Madison WI, pp 403–430
Palansooriya KN, Wong JTF, Hashimoto Y, Huang L, Rinklebe J, Chang SX, Bolan N, Wang H, Ok YS (2019) Response of microbial communities to biochar amended soils: a critical review. Biochar 1:3–22. https://doi.org/10.1007/s42773-019-00009-2
Pan F, Chapman SJ, Li Y, Yao H (2017) Straw amendment to paddy soil stimulates denitrification but biochar amendment promotes anaerobic ammonia oxidation. J Soils Sedim 17(10):2428–2437. https://doi.org/10.1007/s11368-017-1694-4
Paneque M, Rosa JM, Franco-Navarro JD, Colmenero-Flores JM, Knicker H (2016) Effect of biochar amendment on morphology, productivity and water relations of sunflower plants under non-irrigation conditions. CATENA 147:280–287. https://doi.org/10.1016/j.catena.2016.07.037
Park HG, Jeong MH, Ahn YS (2017) Inoculation with Bacillus licheniformis MH48 to improve Camellia japonica seedling development in coastal lands. Turk J Agric for 41:381–388. https://doi.org/10.3906/tar-1703-147
Peng X, Zhu Q, Xie Z, Darboux F, Holden N (2016) The impact of manure, straw and biochar amendments on aggregation and erosion in a hillslope ultisol. CATENA 138:30–37. https://doi.org/10.1016/j.catena.2015.11.008
Pohl M, Graf F, Buttler A, Rixen C (2012) The relationship between plant species richness and soil aggregate stability can depend on disturbance. Plant Soil 355:87–102. https://doi.org/10.1007/s11104-011-1083-5
Ramlow M, Foster EJ, Del Grosso SJ, Cotrufo MF (2019) Broadcast woody biochar provides limited benefits to deficit irrigation maize in Colorado. Agric Ecosyst Environ 269:71–81. https://doi.org/10.1016/j.agee.2018.09.017
Rasse DP, Weldon S, Joner EJ, Joseph S, Kammann CI, Liu X, O’Toole A, Pan G, Kocatürk-Schumacher NP (2022) Enhancing plant N uptake with biochar-based fertilizers: limitation of sorption and prospects. Plant Soil 475: 213–236. https://doi.org/10.1007/s11104-022-05365-w.
Reverchon F, Flicker RC, Yang H, Yan G, Xu Z, Chen C, Bai SH, Zhang D (2014) Changes in δ15N in a soil–plant system under different biochar feedstocks and application rates. Biol Fertil Soil 50(2):275–283. https://doi.org/10.1007/s00374-013-0850-2
Sardans J, Peñuelas J (2015) Potassium: a neglected nutrient in global change. Glob Ecol Biogeogr 24:261–275. https://doi.org/10.1111/geb.12259
Sarfraz R, Yang W, Wang S, Zhou B, Xing S (2020) Short term effects of biochar with different particle sizes on phosphorous availability and microbial communities. Chemosphere 256:126862. https://doi.org/10.1016/j.chemosphere.2020.126862
Shen C, Xiong J, Zhang H, Feng Y, Lin X, Li X, Liang W, Chu H (2013) Soil pH drives the spatial distribution of bacterial communities along elevation on changbai mountain. Soil Biol Biochem 57:204–211. https://doi.org/10.1016/j.soilbio.2012.07.013
Shi W, Ju Y, Bian R, Li L, Joseph S, Mitchell DRG, Munroe P, Taherymoosavi S, Pan G (2020) Biochar bound urea boosts plant growth and reduces nitrogen leaching. Sci Total Environ 134424 https://doi.org/10.1016/j.scitotenv.2019.134424
Singh BP, Hatton BJ, Singh B, Cowie AL, Kathuria A (2010) Influence of biochars on nitrous oxide emission and nitrogen leaching from two contrasting soils. J Environ Qual 39(4):1224–1235. https://doi.org/10.2134/jeq2009.0138
Song D, Tang J, Xi X, Zhang S, Liang G, Zhou W, Wang X (2018) Responses of soil nutrients and microbial activities to additions of maize straw biochar and chemical fertilization in a calcareous soil. Europ J Soil Biol 84:1–10. https://doi.org/10.1016/j.ejsobi.2017.11.003
Spain AM, Krumholz LR, Elshahed MS (2009) Abundance, composition, diversity and novelty of soil Proteobacteria. ISME J 3(8):992–1000. https://doi.org/10.1038/ismej.2009.43
Tabatabai MA, Bremner JM (1972) Assay of urease activity in soils. Soil Biol Biochem 4:479–487. https://doi.org/10.1016/0038-0717(72)90064-8
Tong X, Xu M, Wang X, Bhattacharyya R, Zhang W, Cong R (2014) Long-term fertilization effects on organic carbon fractions in a red soil of China. CATENA 113:251–259
Voytek MA, Ward BB (1995) Detection of ammonium-oxidizing bacteria of the betasubclass of the class Proteobacteria in aquatic samples with the PCR. Appl Environ Microbiol 61(4):1444–1450
Wang X, Zhou W, Liang G, Song D, Zhang X (2015) Characteristics of maize biochar with different pyrolysis temperatures and its effects on organic carbon, nitrogen and enzymatic activities after addition to fluvo-aquic soil. Sci Total Environ 538:137–144. https://doi.org/10.1016/j.scitotenv.2015.08.026
Warren CR (2006) Potential organic and inorganic N uptake by six Eucalyptus species. Funct Plant Biol 33(7):653–660. https://doi.org/10.1071/fp06045
Xiao Q, Zhu L, Zhang H, Li X, Shen Y, Li S (2016) Soil amendment with biochar increases maize yields in a semi-arid region by improving soil quality and root growth. Crop past Sci 67:495–507. https://doi.org/10.1071/CP15351
Xu M, Xia H, Wu J, Yang G, Zhang X, Peng H, Yu X, Li L, Xiao H, Qi H (2017) Shifts in the relative abundance of bacteria after winelees-derived biochar intervention in multi metal-contaminated paddy soil. Sci Total Environ 599–600:1297–1307. https://doi.org/10.1016/j.scitotenv.2017.05.086
Yang CD, Liu JJ, Ying HC, Lu SG (2022) Soil pore structure changes induced by biochar affect microbial diversity and community structure in an Ultisol. Soil Till Res. 224:105505. https://doi.org/10.1016/j.still.2022.105505.
Yang W, Wang S, Ni W, Rensing C, Xing S (2019) Enhanced Cd-Zn-Pb-contaminated soil phytoextraction by Sedum alfredii and the rhizosphere bacterial community structure and function by applying organic amendments. Plant Soil 1–18 https://doi.org/10.1007/s11104-019-04256-x
Zhang T, Wan S, Kang Y, Feng H (2014) Urease activity and its relationships to soil physiochemical properties in a highly saline-sodic soil. J Soil Sci Plant Nutr 14:304–315
Zhang M, Muhammad R, Zhang L, Xia H, Cong M, Jiang C (2019) Investigating the effect of biochar and fertilizer on the composition and function of bacteria in red soil. Appl Soil Ecol 139:107–116. https://doi.org/10.1016/j.apsoil.2019.03.021
Zhao G, Jorns MS (2006) Spectral and kinetic characterization of the Michaelis charge transfer complex in monomeric sarcosine oxidase. Biochemistry 45(19):5985–5992. https://doi.org/10.1021/bi0600852
Zhao Z, Ibrahim MM, Wang X, Xing S, Heiling M, Hood-Nowotny R, Tong C, Mao Y (2019) Properties of biochar derived from spent mushroom substrates. BioResources 14:5254–5277. https://doi.org/10.15376/biores.14.3.5254-5277
Zheng YD, Bai G, Tang C, Ke CQ, Yao S, Tong LJ, Feng F, Li Y, Ding J, Xie H, Ye Y (2018) 7 α,8 α -Epoxynagilactones and their glucosides from the twigs of Podocarpus nagi: isolation, structures, and cytotoxic activities. Fitoterapia 125:174–183. https://doi.org/10.1016/j.ftote.2018.01.007
Zhou Z, Gao T, Zhu Q, Yan T, Li D, Xue J, Wu Y (2019) Increases in bacterial community network complexity induced by biochar-based fertilizer amendments to karst calcareous soil. Geoderma 337:691–700. https://doi.org/10.1016/j.geoderma.2018.10.013
Zhu GY, Shangguan ZP, Deng L (2017) Soil aggregate stability and aggregate-associated carbon and nitrogen between natural restoration grassland and Chinese red pine plantation on the loess plateau. CATENA 149:253–260. https://doi.org/10.1016/j.catena.2016.10.004
Acknowledgements
We acknowledge the Key Research Laboratory of Soil Ecosystem Health and Regulation, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, China for providing the required support during the conduct of the experiments, and the Key Laboratory of Vegetation Restoration and Management of Degraded Ecosystems, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou for providing the required support that ensured the completion of the research output.
Funding
This study was supported by the National Natural Science Foundation of China (32271644; 31870464), the Guangdong Basic and Applied Basic Research Foundation (2022B1515020014), the Fujian Forestry Science and Technology Promotion Project (2020TG17), and University-Industry Cooperation Project of Fujian Province (2021N5002).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. Material preparation, data collection, and analysis were performed by [Muhammed Mustapha Ibrahim], [Zhaofeng Chang], [Zhimin Li], [Jerry Joseph], [Ado Adamu Yusuf], and [Xianzhen Luo]. Data curation, investigation, methodology, writing of the original draft, review, and editing were done by [Muhammed Mustapha Ibrahim]. Supervision, review and editing, project administration, and resources were provided by [Enqing Hou]. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Handling editor: Yilai Lou.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahim, M.M., Chang, Z., Li, Z. et al. Biochar rate-dependent regulation of extended nitrogen supply by modifying stable aggregates-N and microbial responses. Carbon Res. 2, 22 (2023). https://doi.org/10.1007/s44246-023-00056-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44246-023-00056-2