Abstract
Methane is the second most important greenhouse gas after carbon dioxide, and 8–11% is emitted from paddy fields. Methanogenic microbial processes in water-saturated soils can be alleviated through the oxygenation of soils, which may hamper methane production and emissions in paddies. Here, by mimicking O2 release from rice roots, we report the use of man-made (i.e., silicone tube-based) aerenchymatous tissues (MAT) to continuously release O2 to abate methane emission from paddies. High O2-releasing rates (such as 5 kg O2/ha/d) can be easily achieved by adjusting MAT density (e.g., 0.2 m2 tube/m2 soil) and its inner air pressure (e.g., 25 kPa). Following deployment, MAT significantly increased soil redox potential (from -150 mV to -88.6 mV) and induced active iron redox cycling. This decreased the availability of organic substrates of methanogens and therefore dramatically reduced their abundance (-25.1% active mcrA gene). We quantified the decrease in methane emission both in mesocosms and paddy field trials and found in both setups that ~ 50% of methane emission was reduced. Moreover, we showed that the performance of MAT can be further improved by simply increasing the air pressure in MAT (e.g., -74.2% methane emission at 200 kPa air pressure). This work provides a powerful and sustainable method for mitigating methane emission from rice paddies.
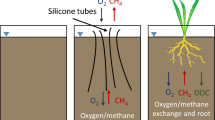
Graphical Abstract

Highlights
➢ A novel method mimicking rice root O2 release is able to significantly reduce methane emission from rice paddies.
➢ Eh in paddies can be considerably promoted by the efficient O2 release from man-made aerenchymatous tissues.
➢ The Eh lift significantly reduces CH4 emission from paddies by reducing methanogens’ activities and substrates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Global warming is a high-level issue facing humankind (Tollefson 2022). Cutting human-caused greenhouse gas emission is believed one of the most effective solutions to mitigate global warming (Coalition, U.N.E.P.a.C.a.C.A. 2021). Methane (CH4) is one of the biggest contributors to global warming and responsible for about 30% of the temperature increase (Kikstra et al. 2022). To limit global warming to 1.5 °C by 2030, a promising way is to reduce CH4 emission from its major sources, since CH4 is 80 times more potent than carbon dioxide as a global warmer over a 20-year time horizon (Nisbet et al. 2009; Pachauri and Meyer 2014). Paddy fields are a major CH4 source and contribute 8–11% of annual anthropogenic CH4 emissions (Ciais et al. 2014; Saunois et al. 2020). According to Climate & Clean Air Coalition (CCAC), cutting CH4 emission from rice paddies is one of the most cost-effective measures to mitigate global warming (Coalition, U.N.E.P.a.C.a.C.A. 2021). As most paddies are distributed in developing countries, a viable method to reduce CH4 emissions must be cost-effective and efficient in paddy fields.
Flooding of paddies tends to deplete active electron acceptors and build up methanogenic conditions (Chen et al. 2019). To mitigate CH4 production, alternate wetting and drying is proposed to supply the strong electron acceptor O2 to paddies (Tyagi et al. 2010; Runkle et al. 2018). However, this approach also facilitates the growth of weeds (Pandey et al. 2020) and creates a new challenge regarding weeds control in paddies. It was also attempted to supply other electron acceptors to paddy soils. For example, iron(III) (Fe(III)) oxides (Van Bodegom et al. 2004; Li et al. 2022) or sulfate salts (Denier van der Gon et al. 2001; Saenjan et al. 2015) are frequently added to suppress CH4 production and emission. However, the added electron acceptors can be quickly consumed by heterotrophic reducers (Li et al. 2022). Although the re-application of electron acceptors could prolong their effect on CH4 emission, it may cause considerable additional costs.
In addition, wetland plants evolve to combat electron acceptor deficiency in flooded soils. Wetland plants, such as rice (Chen et al. 2005), adopt the ability to actively transport O2 from leaves to roots through aerenchymatous tissues (Revsbech et al. 1999). Despite respiration and O2 consumption by rice root cells, the excessive O2 is released into soils and represents additional electron acceptors for microorganisms in the soil. However, the O2 release via the roots leads to Fe(II) oxidation at the rice root surface that then favors Fe(III) oxide cohesion and thus the formation of a compact Fe oxide layer called Fe plaque (Chen et al. 2005). When Fe plaque forms, it serves as a barrier for the continuous O2 release (Møller and Sand-Jensen 2008). As a result, O2 release from rice roots is limited to control CH4 production, especially at the early growth stage when the roots are small and not well developed.
Similar to aerenchymatous tissues, some common materials are water-proof and O2-permeable. Our previous studies demonstrated that most plastic tubes can allow O2 to penetrate and induce Fe(II) oxidation and Fe(III) oxide coating on the tube walls contacting the saturated soil (Yuan et al. 2021c), which is quite similar to Fe plaque formation on rice roots. Similarly to Fe plaque, the Fe oxide coating could greatly inhibit the further O2 diffusion from the tube to reduced soils. Except plastics, silicone tubing was also noticed to allow gas to penetrate (Maier and Schack-Kirchner 2014), but silicone tube surface does not favor Fe oxide cohesion and thus will not form a barrier for O2 release. Therefore, silicone may serve as an ideal tube material to construct man-made aerenchymatous tissues (MAT) to reduce CH4 emission from wetland soils.
This study aimed i) to investigate whether and to what extent silicone tube-made MAT can modify saturated soil redox and ii) to examine the efficiency of the MAT approach to reduce CH4 emission in rice paddies. We investigated the influence of MAT on saturated soil chemistry, and factors that can easily modulate the O2 emission rate from MAT. MAT can significantly reduce the CH4 emission by modifying the methanogenic condition in saturated soils. Several abiotic or biotic processes could be induced by MAT: i) increase soil Eh, which inhibits the activities of CH4 producers; ii) stimulate Fe(II) oxidation and enhance the adsorption of organic matters by Fe(III) (oxyhydr)oxides, which reduce the availability of organic substrates for CH4 producers; iii) promote the activity of dissimilatory Fe(III)-reducers, which would compete organic substrates with CH4 producers. Here we present the results of using MAT to reduce CH4 emission both in mesocosms and paddy fields.
2 Materials and methods
2.1 Experimental preparation
The paddy soil used in this study was collected from a paddy field at Shangyu, Zhejiang Province, China (29.997°N, 120.788°E). The top layer soil (0–20 cm) was sampled and transported to the lab instantly. The soil was air-dried and stored under room temperature before further use. Sub-soil samples (~ 500 g) were taken from the sampled soil, and then grounded and sieved through a sieve (1.0 mm diameter). The sieved soil was used for soil physical–chemical analyses. To mimic the field condition, the sampled soil without sieving was directly utilized in microcosm and mesocosm incubations. The soil belongs to a loam type soil (40.1% sand, 49.3% silt, and 7.65% clay), with total organic carbon and total Fe concentration of 9.75 g kg−1 and 20.8 g kg−1, respectively.
Rice (O. sativa L.) hybrid, Yongyou-15, was sterilized and germinated following the method described in a previous report (Chen et al. 2012). The seedlings were grown to the four-leaf stage, and then transplanted into flooded soils both in the mesocosm and the paddy field.
2.2 The influence of MAT on soil chemistry
In lab, 1.0 kg paddy soil was put into each pot (diameter*height = 10 cm*15 cm); the soil depth was about 10 cm, and a 3 cm overlying water was maintained during the microcosm incubation (Fig. S1). A silicone tube (inner diameter*outer diameter = 3.0 mm*5.0 mm), purchased from Alibaba Taobao (Hangzhou, China), served as the aerenchymatous tissues of MAT, which was set at 8 cm soil depth. A 3.5 W portable air pump, provided by Mulongju Co., Ltd (China), was employed to pump air through MAT (Fig. S2). During the 30-d incubation, ~ 4.0 mL soil porewater around MAT was sampled by a Rhizon sampler (2.5 mm × 5 cm, MOM, Rhizon, Netherlands), in every 1–10 d; such a sampling volume could represent 0–2 cm soil zones around MAT (Seeberg-Elverfeldt et al. 2005). Soil porewater pH and redox potential (Eh) were instantly measured by a PHB-4 portable pH/OPR meter (Leici, Shanghai, China), then the sample was acidified with 6 M HCl to prevent Fe mineral precipitation (Yuan et al. 2021b). The porewater Fe speciation was determined by the 1, 10-phenanthroline method (λ = 510 nm) (Tamura et al. 1974).
Dissolved organic matter (DOM) in porewater was quantified by a TOC analyzer (Shimadzu TOC-VCPH, Japan). The DOM composition was characterized by a 3D-EEM fluorescence spectrometry (F7000, Hitachi, Japan), following the procedure described by our previous report (Gustave et al. 2019). The DOM composition was further detected by FTICR (Bruker Daltonics Inc, Billerica, MA, USA). The van Krevelen space in this study was divided into six discrete regions, modified from the diagram proposed previously (Hockaday et al. 2009; Ohno et al. 2010): lipids (H:C = 1.5–2.0, OC = 0–0.3); proteins (H:C = 1.5–2.2, OC = 0.3–0.67); lignins (H:C = 0.70–1.5, OC = 0.10–0.67); carbohydrates (H:C = 1.5–2.4, OC = 0.67–1.2); unsaturated hydrocarbons (H:C = 0.70–1.5, OC = 0–0.10); condensed aromatic hydrocarbons (H:C = 0.20–0.70, OC = 0–0.67).
2.3 O2 permeability in MAT
MAT density serves as an important parameter to control O2 release, yet a continuous increase of the density is not economically affordable and feasible in the field. Our preliminary test showed that air pressure in MAT had a big potential to regulate its O2 permeability. Here we tested whether varying air pressures in MAT could serve as an efficient and feasible alternative to control O2 release from MAT. The O2 permeability, under 0, 2, 5, 10, 20, and 50 kPa air pressures (25 °C), was thus measured according to ISO-17455 (ISO-17455 2005). The correlation between the O2 release with air pressure was assessed. Moreover, O2 release rates by MAT under different MAT densities (0–0.5 m2 tube/m2 soil) and air pressures (0–200 kPa) were simulated accordingly during a rice production period (i.e., 120 d).
The O2 permeability, under 25 kPa air pressure (25 °C), was further verified in saturated soils. After a 30 d soil incubation, distance profiles (0–6 cm) of Eh across the soil–water interface and MAT-soil interface were measured by an Eh microelectrode sensor (Unisense Ref-RM and RD-N, 1.1 mm resolution; Science Park, Aarhus, Denmark).
2.4 Influence range of MAT
Moreover, under air pressure of 25 kPa, the distance profiles (0–4.4 cm) of soluble Fe in every 2 mm interval were measured by IPI samplers, following our previous report (Yuan et al. 2019). In addition, the depth profiles of Fe in the solid phase were also measured, through manual slicing in every 0.5–1.0 cm interval. 0.5 g of sliced wet soil was immediately weighed and extracted by 6 mL 1 M HCl for 24 h. The extract was filtered through a 0.22 μm cellulose membrane. Iron speciation and organic carbon in the extract were measured by the 1, 10-phenanthroline method and TOC analyzer, respectively.
2.5 CH4 emission measurement
In the mesocosm, three pots (length*width*height = 15.5 cm*15.5 cm*17.3 cm) with or without MAT deployment were prepared, and 4.5 kg paddy soil was put into each pot. MAT was deployed at 8 cm soil depth in the middle of each pot, and a 3 cm overlying water depth was maintained during the incubation. Two seedlings were transplanted into each pot, and grown at room temperature and at a glass greenhouse under natural light conditions for 30 d (elongation stage of rice). After the incubation, the overlying water in each pot was carefully removed by a 50 mL plastic syringe. Every pot was placed into a custom-made PVC chamber (inner dimensions: 80 cm height, 25 cm diameter) with a rubber septum at the top and incubated to 48 h. About 50 mL headspace samples from each pot were withdrawn with a 50 mL plastic syringe, and stored in a 50 mL gas sampling bag.
In the field, two 5 m × 6 m plots were prepared, and the hill space of 0.25 m × 0.15 m was utilized. One plot was set as the control, and another plot was settled with MAT. MAT was deployed at ~ 8 cm soil depth in the middle of every rice row. Two seedlings were transplanted into each hill, and ~ 5 cm overlying water was maintained during the growth. Three gas samples were taken every week (8:00 to 10:00 am) from three locations in the field of each treatment by custom-made PVC chambers (inner dimensions: 80 cm height, 25 cm diameter), during the active CH4 emission period in rice paddies (i.e., vegetative growth period of rice) (Ma et al. 2008; Wang et al. 2022). About 50 mL headspace samples from each PVC chamber were withdrawn with a 50 mL plastic syringe, and stored in a 50 mL gas sampling bag.
The gas sample was immediately transported to the lab, and then withdrawn by a 500 µL syringe and injected into an Agilent 6890N gas chromatograph (Agilent Technologies, Santa Clara, CA), equipped with a DB-624 capillary column (30 m*0.32 mm, 1.8 μm) and a flame ionization detector.
2.6 Microbial analysis
Total RNA was extracted from soils around MAT (0–5 cm) in every 1 cm using the PowerSoil RNA Isolation Kit (MO BIO Laboratories, Inc. Carlsbad, USA) following the manufacturer’s protocols. cDNA was prepared by reverse transcription of the extracted RNA using the PrimeScript RT reagent kit with gDNA Eraser (TAKARA, China).
The generated cDNA was subjected to barcode amplification of the V3-V4 hypervariable region of the 16S rRNA gene at Hangzhou KaiTai Biotechnology Co., Ltd. (Hangzhou, China). The sequencing process is detained in the supporting information. Two genes, including mcrA (α-subunit of methyl-coenzyme M reductase, the key enzyme for CH4 production) and Geo (ferric Fe reduction gene), were quantified by real-time qPCR. The oligonucleotide primers used for the noted genes are detailed in Table S1. The 16S rRNA gene sequence data have been deposited in the NCBI database with accession number PRJNA891874.
2.7 Statistical analysis
Results are displayed as mean ± standard errors (n = 3), unless stated elsewise. Graphs were plotted by R4.1.0 or Origin 2019 software (Origin Lab, Northampton, USA). The statistical difference was analyzed by SPSS22.0 software (IBM SPSS, Armonk, NY, USA), using the unpaired two-tailed Student’s t-test with the significance level of 0.05 (variables include pH, Eh, DOM, Fe speciation).
3 Results
3.1 Soil chemistry changes
Man-made aerenchymatous tissues (MAT) can efficiently release air when blowing air through the tissue, and the released air bubbles in water can be observed by the naked eye after a 5 min air blowing (SI, video1). We further investigated whether MAT could significantly modify saturated soil chemistry. Our results strongly support that MAT can dramatically influence soil chemistry like porewater Eh, dissolved Fe concentration, and dissolved organic matter (DOM) concentration (Fig. 1).
Change of saturated soil pH (A), redox potential (Eh, vs. Ag/AgCl reference electrode; B), porewater iron (Fe; C), dissolved organic matter (DOM; D-F) with man-made aerenchymatous tissues (MAT) during the microcosm incubation. The error bars are standard errors (n = 3). Porewater was extracted from 0–2 cm soil zones around MAT by Rhizon samplers. Panels E and F refer to EEM fluorescence spectra of DOM and FTICR mass-spectrum of DOM, respectively
MAT significantly increased Eh and also influenced other processes sensitive to Eh shifts in saturated soils. During a 30 d incubation, MAT had no significant influence on soil pH (p = 0.103; Fig. 1A, Table S2). The soil pH was maintained around neutral conditions (6.89 ± 0.122), regardless of deploying MAT or not. In contrast, MAT dramatically increased soil Eh (p < 0.001; Fig. 1B). Compared to the strongly reducing condition (as low as -150 mV vs. Ag/AgCl reference electrode) in the control, MAT turned the soil redox state from strongly to mildly reducing conditions (-88.6 ± 23.7 mV). At the same time, MAT dramatically decreased porewater Fe concentration (p < 0. 001; -66.3%, Fig. 1C). This indicates that Fe(II) oxidation in the soil is strongly enhanced by MAT. In addition, MAT also significantly decreased DOM concentration (p < 0.001; as low as -39.1%, Fig. 1D), which is most likely due to the strong adsorption of DOM by the newly formed Fe(III) (oxyhydr)oxides in the soil. The fluorescence analysis additionally indicated that MAT not only decreased DOM but also influenced the DOM composition (Fig. 1E). The FTICR analysis further revealed that MAT promoted the oxidation state of the DOM (Fig. S3), and preferentially removed unsaturated and condensed aromatic hydrocarbons (Fig. 1F).
3.2 O2 releasing control
Our results revealed a linear relationship between the released O2 and the air pressure (Fig. 2A). This strongly supports the idea that increasing air pressure in MAT, together with a suitable density, can efficiently control O2 release from MAT. As shown in Fig. 2B, the targeted O2 releasing rate can be easily obtained by tuning the density and air pressure of MAT. For example, a considerable O2 releasing rate (~ 600 kg O2/(ha·season)) was achieved under the density and air pressure of 0.2 m2 tube/m2 soil and 25 kPa, respectively. The released O2 can additionally host more than 1.1 × 1028 electrons/(ha·season) stemming from DOM oxidation in saturated soils, which is comparable to applying a large amount of other electron acceptors such as 1332 kg Na2SO4/(ha·season) or 5998 kg Fe2O3/(ha·season).
Control of O2 releasing from MAT. The x-axis indicates the air pressure in MAT. A rice production season was set as 120 d. According to the linear regression obtained from panel A, O2 release rates by MAT under different MAT densities (0–0.5 m2 tube/m2 soil) and air pressures (0–200 kPa) were calculated and mapped in panel B
3.3 Soil chemical and microbial profiles
During MAT application, a commercial 3.5 W air pump can maintain an air pressure of 25 kPa in MAT. The influence range of MAT in saturated soil was first tested under the noted air pressure. Figure 3 shows that MAT can influence soil Eh on a centimeter-wide scale in the soil.
Soil chemical and microbial changes around MAT under 25 kPa air pressure. A redox potential (Eh; vs. standard hydrogen electrode) across soil–water interface and MAT-soil interface (n = 4); B porewater Fe across MAT-soil interface; C-D Fe speciation and organic matter in the soil solid phase; E the difference in the operational taxonomic units (OTUs) between two groups; F active methyl-coenzyme M reductase (mcrA) gene and Fe-reducing gene (Geo) distribution across MAT-soil interface and in the control. The dashed horizontal line in A means the soil–water interface, while the solid horizontal line indicates the soil depth where MAT was horizontally deployed. MAT-1.0 cm in E means soil samples at ~ 1.0 cm from MAT surface
MAT significantly increased soil Eh (on a cm-scale) and also posed huge impacts on other processes sensitive to Eh shifts in saturated soils. In the absence of MAT, Eh rapidly decreased from + 447 to + 87 mV across the soil–water interface from overlying water to subsurface soils (5.5 cm depth). By contrast, Eh decreased relatively mild from + 448 to + 110 mV and then increased to + 189 mV when approaching MAT deployed at 5.5 cm soil depth (Fig. 3A). The relative change between MAT and the control more clearly showed that MAT dramatically increased soil Eh (on a cm-scale), and might also facilitate O2 diffusion from overlying water into top-layers of the soil (Fig. S4A). Additionally, in the absence of MAT, porewater Fe was extremely high (100 ± 11.2 mg/L) in the subsurface soil at 5.5 cm depth. By contrast, porewater Fe was dramatically decreased within 4.4 cm distance around MAT (-55% to -90%, Fig. 3B). It should be noted that fresh Fe(III) (oxyhydr)oxides were formed in centimeter-vicinity of the soils around MAT, which was observed via a transparent observation window. This indicated that, with MAT, Fe(II) oxidation was strongly promoted in saturated soils. This was also supported by the Fe speciation analysis in the soil solid (Fig. 3C & Fig. S5A-B). As shown in Fig. 3C, Fe(III) was 3.50 times higher than that in the control (p < 0.001), while the opposite was true for Fe(II) in the solid phase (-40.9%, p < 0.001). Moreover, the organic carbon in the solid phase showed a similar trend as Fe(III) (25%, p < 0.001; Fig. 3D & Fig. S5C), indicating DOM was strongly fixed by the newly formed Fe (oxyhydr)oxides.
Moreover, MAT had important implications on soil microbial community. As shown in Fig. 3E, MAT significantly changed 969 operational taxonomic units (OTUs) in soils adjacent to its surface. The effect slightly decreased from the near to the far side of MAT (e.g., 471 and 292 OUTs were significantly changed at ~ 3.0 and ~ 5.0 cm distance from MAT; Fig. S4B&C). For example, we observed that MAT significantly promoted Proteobacteria but inhibited Hydrogenedentes (Fig. S6). The former phylum contains the popular Fe(III)-reducer Geobacter, while the latter one is often associated with methanogenic environments (Nobu et al. 2015). Meanwhile, compared to the control, we detected that the key gene of methanogenesis (i.e., mcrA) decreased from the far to the near side of MAT surface (-25.1%), indicating that MAT may negatively influence the abundance and activity of methanogens (Fig. 3F). At the same time, the Fe reduction gene (i.e., Geo) was significantly more abundant at the far compared to that at the near side of the MAT surface (33.5%). The decrease of active Fe(III)-reducers in the soil zone adjacent to MAT was possibly due to those microorganisms inhibited by the relatively high Eh at MAT surface.
3.4 CH4 emission
CH4 emission rates from mesocosms and fields with MAT were measured (Fig. 4), under 25 kPa air pressure and a MAT density of 0.2 m2 tube/m2 soil. Similar to the soil microcosm incubation, MAT significantly reduced porewater Eh both in mesocosms (-57.7%) and field trials (-34.2%) (Fig. S7). The CH4 emission from the mesocosm with MAT was -54.3% lower than that in the control (0.211 vs. 0.463 mM/pot/h; Fig. 4A), at the elongation stage of rice. And, MAT also reduced 45.4% CH4 emission in the field (1.44 vs. 2.65 mM/m2/h, p = 0.043; Fig. 4B, Table S2). Yet, the CH4 emission rate from the control and MAT treatment was relatively the same at 42 days of rice transplanting. This might be due to two causes: i) CH4 emission in paddy soils decreased to a relatively low rate after ~ 40 days of rice transplanting; ii) the relatively high variation of gas sampling and measurement.
CH4 emission in the mesocosm and paddy field. A CH4 emission rate normalized to each pot as a function of time in the mesocosm; B CH4 emission rate normalized to the soil surface area as a function of days after transplanting in the paddy field. MAT was set as 25 kPa air pressure and 0.2 m2 tube/m2 soil density both in the mesocosm and paddy field. Silicone tubes of MAT were buried ~ 8 cm below the soil–water interface
4 Discussion
In this study, we proposed using MAT for mitigating CH4 emissions from saturated soils. Within our experimental scale, MAT can supply abundant O2 to saturated soils from the atmosphere and increase the soil Eh. This strongly inhibits CH4 production while causing no negative influence on the soil quality. This process does not require the addition of chemicals and only consumes little energy that can be powered by solar panels when applied in the field. Thus, we propose that MAT may serve as a powerful and sustainable tool to abate CH4 emission from wetlands.
MAT-mediated ~ 50% of CH4 emission reduction is one of the highest reported reduction efficiencies compared to studies where addition of oxidizing chemicals (e.g., Fe2O3 (Van Bodegom et al. 2004; Li et al. 2022), sulfate salts (Denier van der Gon et al. 2001; Saenjan et al. 2015)) or application of alternate wetting and drying (Tyagi et al. 2010; Runkle et al. 2018) was performed. In contrast to the noted approaches, MAT is only applied once and is much more sustainable. This is because MAT can continuously input O2 to the saturated soil, while other methods only input electron acceptors once during their short-term application (Saenjan et al. 2015; Li et al. 2022). Moreover, we found that the main factor that can efficiently and conveniently modulate the O2 release is the air pressure in MAT (Fig. 2). Adjusting this factor is much superior to that of MAT density, due to two reasons: i) the cost is much cheaper since no additional materials are needed to be consumed; ii) the operation is much more flexible since most of the MAT systems do not need to be changed despite the changing air pressure in MAT. It is reasonable to expect that the performance of MAT could be further enhanced by simply increasing the air pressure in MAT. For example, through elevating the air pressure to 200 kPa in MAT, the performance of MAT was further improved in the mesocosm. Compared to 25 kPa air pressure (-54.3%) (Fig. 4A), much more CH4 emission can be cut (-74.2%) under 200 kPa air pressure (Fig. S8). Yet, it should be noted that continuously increasing air pressure may facilitate ebullition of O2 and other gases in the soil, which may reduce the residence time of O2 and negatively influence the performance of MAT. Hence, further studies are essentially required to optimize the setup of MAT in paddy fields (parameters may include air pressure, the extent and quantity of silicone tube deployment, etc.). And, further work is also essentially required to validate its efficiency under different field conditions.
When deployed in saturated soils, MAT induced several abiotic or biotic processes and reduced CH4 production (Fig. 5). First, MAT increases soil Eh by releasing O2 to the soil (Fig. 1B), which would restrict the living niches of methanogens (Fig. 3F). Methanogens favor strongly reducing conditions where soil oxidants are scarce (Neue 1993), and tend to become inactive when soil Eh is increased by supplying easily used electron acceptors such as O2 (Runkle et al. 2018), nitrate (Roy and Conrad 1999), sulfate (Saenjan et al. 2015), or Fe(III) (oxyhydr)oxides (Li et al. 2022). Second, Fe(II) oxidation induced by MAT O2 release generates Fe(III) (oxyhydr)oxides, which provide additional adsorption sites for DOM in saturated soils (Conrad 2002; Song et al. 2022; Wei et al. 2022). This will directly reduce the availability of organic substrates for methanogens. Due to this cause, previous studies tried to supply Fe(III) (oxyhydr)oxide minerals to reduce CH4 production in saturated soils (Jäckel and Schnell 2000; Li et al. 2022). However, the supplied Fe minerals only have a short-term effect (e.g., < = 6 d) (Li et al. 2022), since they can be quickly reduced and dissolved by anaerobic Fe(III)-reducers (Achtnich et al. 1995; Hori et al. 2010). By contrast, MAT can maintain a centimeter-wide pool of Fe(III) (oxyhydr)oxides in soils through continuously releasing O2, which is most likely to steadily hold much DOM in the solid phase (Fig. 3D-E & S5). Third, the Fe (oxyhydr)oxides induced by MAT provide Fe(III) substrates as the electron acceptor for anaerobic Fe(III)-reducers, and hence can activate and stimulate dissimilatory Fe(III) reduction. Microbial Fe(III) reduction is superior to methanogenesis since the former process has a lower Gibbs energy than the latter (-41.5 vs. -22.0 kJ/mol) (He and Sanford 2004; Dolfing 2014). Therefore, the induced microbial Fe reduction would outcompete methanogens for organic substrates and further lower CH4 production (Achtnich et al. 1995; Hori et al. 2010).
The MAT approach has its advantages and constraints (Table S3). MAT is easy to use, and little maintenance is required. Upon deployment, MAT continuously releases O2 to saturated soils, hence it has a long-term good performance. By contrast, although addition of oxidizing chemicals (e.g., sulfate, Fe2O3, etc.) is easy to apply, each addition of the chemical only has a short-term performance (Saenjan et al. 2015; Li et al. 2022). To maintain a sufficient oxidizing capacity in the long term (e.g., during the rice production period), multiple times’ addition is essentially required. Yet, this will greatly increase the cost and may cause negative effects if too many chemicals were added (e.g., acidify the soil, degrade the soil quality or cause hazards to living organisms in the soil) (Zeng et al. 2017; Yuan et al. 2021a). By comparison, MAT is 19.4 ~ 53.9 times lower than the equivalent application of Na2SO4 or Fe2O3 (see calculation in the SI). And, MAT causes no negative influence on the soil. It should be noted that aerobic decomposition and CO2 emissions may be enhanced by MAT, which should be carefully considered before MAT implementation. Additionally, the MAT approach is flexible, and thus could easily be combined with other CH4-reducing methods to maximize the efficiency of reducing CH4 emission. For example, lowering CH4 emission could be further enhanced by coupling MAT with cable bacteria inoculation, which will additionally provide abundant sulfate to saturated soils via enhancing electrogenic sulfide oxidation (Scholz et al. 2020). Therefore, MAT would serve as a powerful and cost-effective tool for humankind to achieve the goal of reducing human-caused CH4 emissions by 45% and thus limit the global temperature rise to 1.5 °C by 2030 (Coalition, U.N.E.P.a.C.a.C.A. 2021; Tollefson 2022).
The MAT approach is still in its infancy and there are three major concerns regarding its feasibility in field application. First, intensive labor is required when deploying MAT, which is estimated at ca. one day’s work per hectare of field. However, MAT deployment can easily be integrated into rice transplanting, and deployment of MAT and transplanting rice seedlings could be simultaneously completed by rice transplanter. Second, MAT may be damaged by sharp stones or deposited glasses in the field, which is a threat to its good performance. To solve this issue, a natural textile could be covered on MAT surface before deployment, thus the potential damage can be avoided. Moreover, the durability of MAT warrants further study, and how to deal with the damaged and defective MAT also need further research. Third, the present portable air pump is unable to maintain a relatively high air pressure (e.g., > 100 kPa) in MAT, which may restrict the further improvement of MAT performance under certain field conditions (e.g., when the soil is very rich in organic matter which can potentially clog the pores of the tubing). We noticed that the portable tire pump, which can maintain high air pressure (up to 1000 kPa), is very universal. Hence it may be possible for using a portable tire pump to manage air pressure in MAT after certain modifications, then the performance of MAT could be well maintained under different field conditions.
5 Conclusion
This study demonstrates MAT as an effective method to reduce CH4 emission from rice paddies. MAT can significantly promote saturated soil Eh by continuously delivering O2 to the soil from the atmosphere. To date, it has been difficult to control Eh in flooded paddies, but MAT may offer a solution. We identifies that MAT mitigates CH4 emission through three processes: i) decreasing CH4 generation by inhibiting methanogens’ activities; ii) stimulating Fe oxidation and enhancing the adsorption of DOM by Fe(III) (oxyhydr)oxides, which decreases the potential organic substrates for methanogens; iii) stimulating dissimilatory Fe(III) reduction process, which outcompetes organic substrates with methanogens. This work provides a novel method for reducing CH4 emission from rice paddies, although further work is required to validate its efficiency under varying field conditions.
Data availability
Data and codes are available from https://doi.org/10.6084/m9.figshare.21359466.
Abbreviations
- CH4 :
-
Methane
- Fe:
-
Iron
- MAT:
-
Man-made aerenchymatous tissues
- Eh :
-
Redox potential
- DOM:
-
Dissolved organic matter
- OTUs:
-
Operational taxonomic units
References
Achtnich C, Bak F, Conrad R (1995) Competition for electron donors among nitrate reducers, ferric iron reducers, sulfate reducers, and methanogens in anoxic paddy soil. Biol Fert Soils 19:65–72
Chen Z, Zhu YG, Liu WJ, Meharg AA (2005) Direct evidence showing the effect of root surface iron plaque on arsenite and arsenate uptake into rice (Oryza sativa) roots. New Phytol 165:91–97
Chen Z, Huang YC, Liang JH, Zhao F, Zhu YG (2012) A novel sediment microbial fuel cell with a biocathode in the rice rhizosphere. Bioresource Technol 108:55–59
Chen C, Li L, Huang K, Zhang J, Xie WY, Lu Y, Dong X, Zhao FJ (2019) Sulfate-reducing bacteria and methanogens are involved in arsenic methylation and demethylation in paddy soils. ISME J 13:2523–2535
Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell J, Chhabra A, DeFries R, Galloway J, Heimann M (2014) Carbon and other biogeochemical cycles, Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. New York, Cambridge University Press, pp. 465–570.
Coalition, U.N.E.P.a.C.a.C.A (2021) Global methane assessment: benefits and costs of mitigating methane emissions. United Nations Environment Programme, Nairobi
Conrad R (2002) Control of microbial methane production in wetland rice fields. Nutr Cycl Agroecosys 64:59–69
Denier van der Gon HA, van Bodegom PM, Wassmann R, Lantin RS, Metra-Corton TM (2001) Sulfate-containing amendments to reduce methane emissions from rice fields: mechanisms, effectiveness and costs. Mitig Adapt Strat Gl 6:71–89
Dolfing J (2014) Thermodynamic constraints on syntrophic acetate oxidation. Appl Environ Microbiol 80:1539–1541
Gustave W, Yuan ZF, Sekar R, Ren YX, Liu JY, Zhang J, Chen Z (2019) Soil organic matter amount determines the behavior of iron and arsenic in paddy soil with microbial fuel cells. Chemosphere 237:124459
He Q, Sanford RA (2004) Acetate threshold concentrations suggest varying energy requirements during anaerobic respiration by Anaeromyxobacter dehalogenans. Appl Environ Microbiol 70:6940–6943
Hockaday WC, Purcell JM, Marshall AG, Baldock JA, Hatcher PG (2009) Electrospray and photoionization mass spectrometry for the characterization of organic matter in natural waters: a qualitative assessment. Limnol Oceanogr-Meth 7:81–95
Hori T, Müller A, Igarashi Y, Conrad R, Friedrich MW (2010) Identification of iron-reducing microorganisms in anoxic rice paddy soil by 13C-acetate probing. ISME J 4:267–278
Jäckel U, Schnell S (2000) Suppression of methane emission from rice paddies by ferric iron fertilization. Soil Biol Biochem 32:1811–1814
Kikstra JS, Nicholls ZRJ, Smith CJ, Lewis J, Lamboll RD, Byers E, Sandstad M, Meinshausen M, Gidden MJ, Rogelj J, Kriegler E, Peters GP, Fuglestvedt JS, Skeie RB, Samset BH, Wienpahl L, van Vuuren DP, van der Wijst KI, Al Khourdajie A, Forster PM, Reisinger A, Schaeffer R, Riahi K (2022) The IPCC sixth assessment report WGIII climate assessment of mitigation pathways: from emissions to global temperatures. Geosci Model Dev 15:9075–9109
Li Y, Zhu Z, Wei X, Kuzyakov Y, Li B, Kim PJ, Wu J, Liu S, Ge T (2022) Sources and intensity of CH4 production in paddy soils depend on iron oxides and microbial biomass. Biol Fertil Soils 58:181–191
Ma J, Xu H, Yagi K, Cai Z (2008) Methane emission from paddy soils as affected by wheat straw returning mode. Plant Soil 313:167–174
Maier M, Schack-Kirchner H (2014) Using the gradient method to determine soil gas flux: a review. Agr Forest Meteorol 192:78–95
Møller CL, Sand-Jensen K (2008) Iron plaques improve the oxygen supply to root meristems of the freshwater plant Lobelia Dortmanna. New Phytol 179:848–856
Neue HU (1993) Methane emission from rice fields. Bioscience 43:466–474
Nisbet EG, Jones SM, Maclennan J, Eagles G, Moed J, Warwick N, Bekki S, Braesicke P, Pyle JA, Fowler CMR (2009) Kick-starting ancient warming. Nat Geosci 2:156–159
Nobu MK, Narihiro T, Rinke C, Kamagata Y, Tringe SG, Woyke T, Liu WT (2015) Microbial dark matter ecogenomics reveals complex synergistic networks in a methanogenic bioreactor. ISME J 9:1710–1722
Ohno T, He Z, Sleighter RL, Honeycutt CW, Hatcher PG (2010) Ultrahigh resolution mass spectrometry and indicator species analysis to identify marker components of soil-and plant biomass-derived organic matter fractions. Environ Sci Technol 44:8594–8600
Pachauri R, Meyer L (2014) Climate change 2014: synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change.
Pandey S, Yadav S, Hellin J, Balié J, Bhandari H, Kumar A, Mondal MK (2020) Why technologies often fail to scale: Policy and market failures behind limited scaling of alternate wetting and drying in rice in Bangladesh. Water 12:1510
ISO-17455 (2005) Plastics piping systems—multilayer pipes—determination of the oxygen permeability of the barrier pipe. S.
Revsbech NP, Pedersen O, Reichardt W, Briones A (1999) Microsensor analysis of oxygen and pH in the rice rhizosphere under field and laboratory conditions. Biol Fertil Soils 29:379–385
Roy R, Conrad R (1999) Effect of methanogenic precursors (acetate, hydrogen, propionate) on the suppression of methane production by nitrate in anoxic rice field soil. FEMS Microbiol Ecol 28:49–61
Runkle BR, Suvočarev K, Reba ML, Reavis CW, Smith SF, Chiu Y-L, Fong B (2018) Methane emission reductions from the alternate wetting and drying of rice fields detected using the eddy covariance method. Environ Sci Technol 53:671–681
Saenjan P, Ro S, Vityakon P (2015) Methane fluxes and rice yields as a function of sulfate fertilizer with incorporated rice stubble. Asia-Pac J Sci Technol 20:337–345
Saunois M, Stavert AR, Poulter B, Bousquet P, Canadell JG, Jackson RB, Raymond PA, Dlugokencky EJ, Houweling S, Patra PK (2020) The global methane budget 2000–2017. Earth Syst Sci Data 12:1561–1623
Scholz VV, Meckenstock RU, Nielsen LP, Risgaard-Petersen N (2020) Cable bacteria reduce methane emissions from rice-vegetated soils. Nat Commun 11:1878
Seeberg-Elverfeldt J, Schlüter M, Feseker T, Kölling M (2005) Rhizon sampling of porewaters near the sediment-water interface of aquatic systems. Limnol Oceanogr-Meth 3:361–371
Song X, Wang P, Van Zwieten L, Bolan N, Wang H, Li X, Cheng K, Yang Y, Wang M, Liu T (2022) Towards a better understanding of the role of Fe cycling in soil for carbon stabilization and degradation. Carbon Res 1:5
Tamura H, Goto K, Yotsuyanagi T, Nagayama M (1974) Spectrophotometric determination of iron (II) with 1, 10-phenanthroline in the presence of large amounts of iron (III). Talanta 21:314–318
Tollefson, J (2022) Scientists raise alarm over'dangerously fast'growth in atmospheric methane. Nature. https://www.nature.com/articles/d41586-022-00312-2, https://doi.org/10.1038/d41586-022-00312-2.
Tyagi L, Kumari B, Singh SN (2010) Water management - A tool for methane mitigation from irrigated paddy fields. Sci Total Environ 408:1085–1090
Van Bodegom PM, Scholten JC, Stams AJ (2004) Direct inhibition of methanogenesis by ferric iron. FEMS Microbiol Ecol 49:261–268
Wang S, Sun P, Zhang G, Gray N, Dolfing J, Esquivel-Elizondo S, Peñuelas J, Wu Y (2022) Contribution of periphytic biofilm of paddy soils to carbon dioxide fixation and methane emissions. Innovation 3:100192
Wei L, Zhu Z, Razavi BC, Xiao M, Dorodnikov M, Fan L, Yuan H, Yurtaev A, Luo Y, Cheng W, Kuzyakov Y, Wu J, Ge T (2022) Visualization and quantification of carbon “rusty sink” by rice root iron plaque: Mechanisms, functions, and global implications. Glob Change Biol 28:6711–6727
Yuan ZF, Gustave W, Bridge J, Liang Y, Sekar R, Boyle J, Jin CY, Pu TY, Ren YX, Chen Z (2019) Tracing the dynamic changes of element profiles by novel soil porewater samplers with ultralow disturbance to soil–water interface. Environ Sci Technol 53:5124–5132
Yuan C, Na S, Li F, Hu H (2021a) Impact of sulfate and iron oxide on bacterial community dynamics in paddy soil under alternate watering conditions. J Hazard Mater 408:124417
Yuan ZF, Gustave W, Sekar R, Bridge J, Wang JY, Feng WJ, Guo B, Chen Z (2021b) Simultaneous measurement of aqueous redox-sensitive elements and their species across the soil-water interface. J Environ Sci 102:1–10
Yuan ZF, Pu TY, Jin CY, Feng WJ, Wang JY, Gustave W, Bridge J, Cheng YL, Tang X, Zhu YG (2021c) Sustainable removal of soil arsenic by naturally-formed iron oxides on plastic tubes. J Hazard Mater 439:129626
Zeng M, de Vries W, Bonten LT, Zhu Q, Hao T, Liu X, Xu M, Shi X, Zhang F, Shen J (2017) Model-based analysis of the long-term effects of fertilization management on cropland soil acidification. Environ Sci Technol 51:3843–3851
Acknowledgements
We appreciate the excellent laboratory support from Cheng-Lu Lou, and also the field support from local farmers You-Quan Huang and A-Wei Wang.
Funding
National Science Foundation of China (42122048, 42107008) and China Postdoctoral Science Foundation (2022T150572, 2021M700122).
Author information
Authors and Affiliations
Contributions
X.T. and Z.Y. designed the research; Z.Y. performed the research; Z.Y., X.T., Y.Z., Z.C., Y.W., A.K., and J.X. wrote the paper. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
All authors declare that they are consent for publication in the journal of Carbon Research.
Competing interests
All authors declare that there are no competing interests.
Additional information
Handling Editor: Fengchang Wu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuan, ZF., Zhou, Y., Chen, Z. et al. Reduce methane emission from rice paddies by man-made aerenchymatous tissues. Carbon Res. 2, 17 (2023). https://doi.org/10.1007/s44246-023-00049-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44246-023-00049-1