Abstract
The Portland cement (PC) production industry is a key contributor of CO2 emission. The demand of cement is mounting day by day due to the rapid infrastructure development in the world. Consequently, CO2 discharge from the construction sector is continuously increasing and accounts for about 8% of the total CO2 emission, which becomes a global concern nowadays. Wide applications of eco-friendly cements can significantly reduce the CO2 release. Therefore, use of magnesium cements (MCs) might be a promising solution to ease such concern. As a rapid hardening cement, MCs can be characterized as low-carbon due to their lower embodied energy and carbon storage ability during the service. This review mainly summarizes the findings of previous studies related to the carbonation performances of PC blended with magnesia and MCs products, and particularly, the influence of Accelerated carbonation curing (ACC) process on the properties of MCs and corresponding CO2 sequestration performance. The effects of ACC on mechanical strength, hydration and mineral carbonation mechanisms, pore structures, pore solution pH and thermal properties are discussed. The limitations of existing research are also discussed, which may provide the directions for future research and development of MC material products.
摘要
波特兰水泥(PC)生产行业是二氧化碳排放的主要贡献 者。由于基础设施的快速发展,水泥的需求日益增加。因此,建筑业的二氧化碳排放量持续增加,约占二氧化碳排放总量的8%,成为当今全球关注的问题。环保水泥的广泛应用显着减少CO2排放。因此,使用镁水泥(MCs)可能是缓 解这种担忧的一个有希望的解决方案。作为一种快硬水泥,MC因其较低的内含能源和使用期间的碳储存能力而具有低 碳特征。本次综述主要总结了PC 与氧化镁和MCs共混后的碳酸化性能,特别是加速碳酸化固化(ACC)过程对MC性 能和相应CO2封存的影响。讨论了 ACC 对机械强度、水合作用和矿物质碳化机制、孔隙结构、孔隙溶液 pH 值和热性 能的影响。还指出了现有研究的局限性,这可能提供MC材料产品未来的研发方向。
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The increasing trend of CO2 gas in atmosphere has threaten the living quality on the earth because the growing rate of CO2 in air is directed to the global climate change as well as environmental imbalance [1]. Most of the reasons for CO2 accumulation in atmosphere are human activities such as production of daily necessary goods, manufacture of raw materials for construction industry. The literature studies reported that cement industry is the second-largest emitter of CO2 among the aforesaid sources, which annually accounts for around 25% of global industrial emissions [2] and about 8% of the global emissions [1, 3,4,5,6] and contributes substantially to global warming [5]. More specifically, PC production is one of the key contributors of CO2 emission [7]. It is noted that PC has been used as an important construction material since ancient era [2]. The demand of PC is still growing due to the rapid infrastructure development worldwide [2]. It is reckoned that about 4.8 billion joule energy is required to yield one ton of PC [8]. In addition, the worldwide annual manufacture of cement was around 3.5 billion metric tons in 2020 [9, 10] and this number is projected to approach 4.83 in 2050 [10]. As a consequence, it may cause the increase of mean temperature of the whole world by 1.4 to 5.8 °C [11]. Furthermore, the PC industries release CO2 mainly from two ways like combustion of fuels and calcination process of CaCO3 (limestone) to form CaO (CaCO3 + heat → CaO + CO2 ↑). It is calculated that nearly 0.5 kg of CO2 is discharged for per kg clinker production [12] and PC contains above 80% of clinker [4], which directs the abundant release of CO2 in air from PC manufacture process. Moreover, a research also stated that about 500 billion cubic yards concrete is produced per year worldwide for infrastructure development [13] and PC is used as a major fraction of this concrete quantity. Therefore, PC concrete production also has a great impact on global warming through emitting the CO2 to atmosphere.
The magnesium cements (MCs) are widely known as ‘CO2-negative’ cements for the absorption of atmospheric CO2 [14,15,16] and form the carbonated magnesium products through reacting with the main component magnesia (MgO) [16]. The applications of MCs have gained increasing popularity as a construction material to reduce the CO2 discharge or capture CO2 from air in context of climate change [17, 18]. Most notably, recent studies have revealed the excellent properties of magnesium cements (MC) over PC, in terms of mechanical strength properties, high-temperature stability [19], CO2 uptake capacity, fire resistance, compatibility with industrial byproducts, stabilization of toxic contaminants in the hydration products [14, 20,21,22]. More importantly, the light-burnt (calcined at 600–1000°C) and hard-burnt (calcined at 1000–1400°C) MgO manufacturing processes causes lower CO2 emission and energy consumption compared to OPC [23]. This indicates that the use of light-burned magnesia (also known as reactive magnesia) can be a possible source of CO2 savings by reducing energy requirements [16]. Another important logic for proving eco-friendly MgO is that the potential of MgO for circular use [24]. Calcined MgO dispersed over the earth surfaces can absorb CO2 to build carbonate mineral [23]. Then the carbonate crystals can be re-gathered and calcined again to obtain the MgO. Such process may lead to the reduction of minimum 2–3 Gt CO2 per year and help suppress global warming [23].
Carbonation denotes the chemical response of cementitious materials to CO2 over the service life or the test protocol period [25]. According to the previous study [26], carbonation curing stimulates the strength gain and CO2 storage of cement materials. Gruyaert et al. [27] report that carbonation leads to the notable changes on the voids, pore size distribution and hydration chemistry of solid cement matrices. Additionally, Soroushian et al. [28] narrated that carbonation curing may improve the durability of plain concrete as a result of the development of carbonate-rich reaction products. Xuan et al. [29] and Zhang et al. [30] explained that carbonation can also improve the performance of fibre-reinforced cementitious composites and recycled aggregate concrete. In the recent years, ACC has been broadly used as an effective CO2 storage for cementitious composites [31]. It facilitates a rapid carbonation system to realize substantial CO2 capture capacity of cement materials through the chemical reactions, which can also mimicry the natural carbonation process by controlling the carbonation factors [31, 32]. The ACC is not only a method to arrest atmospheric CO2, but also an approach to efficiently modify the hydration products, microstructures and enhance the maturity of early age strength properties of mortars and concretes [31, 33]. It is important to note that the ACC process is more economical, environmentally and technically advantageous than natural carbonation [33]. Currently the ACC system has also explored for MC composites for storing and neutralizing the CO2.
This paper presents a review and extensive discussion of the carbon curing/carbonation in comparison with PC counterpart in terms the following issues: 1) impacts of high CO2 emission to environment for high production of PC; 2) carbonation mechanisms and CO2 uptake of PC composites; 3) carbonation mechanisms of MCs; 4) CO2 sequestration enhancement of PC incorporated with MgO; and 5) influence of carbonation curing on the physico-mechanical and microstructural properties of MCs. Finally, conclusions and future perspectives are high-lighted. This review is expected to be able to demonstrate a roadmap for further development of carbonated MCs properties and for widespread applications of carbonated MC products in construction sectors in an eco-friendly way.
2 Carbonation process in PC materials
2.1 Carbon capture method
In the recent years, the global leaders have been raising their voices for saving this planet as a living place through minimizing the environmental pollution occurred by CO2 emission from different sources [34]. So, the scientists and environmentalists have also been attempting to explore the new technology on the reduction of CO2 emission at the sources level of cement production as well as capturing and storage of CO2 [35]. The worldwide total use of captured CO2 is below 0.5% of the total releases for chemical feedstock. Consequently, new solutions are needed to be developed for CO2 capture and utilization [36]. The literature studies [35, 37, 38] stated that carbon capture and utilization (CCU) technique for concrete can be an effective technique to attaining the goal of carbon neutrality, which is capable of storing CO2 expelled from burning fossil fuels. It is a mineral sequestration process, where minerals chemically store CO2 in the form of solid carbonates under the carbonation system [3, 35]. Kamal et al. [3] described the process of CO2 mineral storage in concrete, which can minimize the carbon footprint and provide a cycle of carbon flow to attain a better eco-friendly construction practice. This method not only preserve the CO2 in the inner structure of concrete, but also improves the concrete properties. It is calculated that around 0.1 to 1.4 gigatons of CO2 can be stored in concrete using CCU by the year 2050 [38, 39]. Hanifa et al. [40] and Seifritz [41] illustrated that CO2 mineral storage (i.e. mineral carbonation) is an acceleration system of weathering carbonation or natural carbonation where CO2 is fixed indelibly as a stable crystal structure of carbonate through thermodynamically reactions:
Furthermore, Wang et al. [42] adopted a novel and cost-effective conception where the CO2-rich industrial flowing exhaust gas was utilized to carbonize the RMC and binary cement consisted with 50% RMC and 50% PC. Their investigations revealed that carbonation curing under the CO2-rich industrial exhaust gas improved strength properties of both RMC pastes and binary cement pastes.
2.2 Carbonation mechanisms
Possan et al. [1] and Wang et al. [43] narrated that calcium hydroxide (Ca(OH)2) is formed as a saturated liquid state under the physical-chemical neutralization process of cement, which finally precipitates as CaCO3 by interacting with CO2. In this reaction, CO2 is consumed from air. When the cement paste is exposed to CO2 in concrete, CO2 dissolves in pore solution through dissociation and form H2CO3 (hydrogen carbonate). A series of reactions occur to develop calcite (CaCO3), shown below [1, 43]:
Figure 1 illustrates the basic carbonation mechanisms of PC, where black arrows designate the dissolving process of cement clinker particles and precipitation of hydration products. In addition, small red arrow symbols characterize the softening of CO2 toward the pore solution, while the big red arrow signs direct the dispersion of CO2 toward the pores of the PC paste [44]. These process results the development of calcium carbonate, which contribute to decrease the pore spaces and increase the strength properties of PC composites. It should be mentioned that the carbonation degree and mechanisms of cement clinker primarily rely on the proper dispersion of CO2 into the cement material and the associated carbonization reactions [45].
Basic carbonation process in PC materials [45]
Moreover, Possan et al. [1] also explains the carbonation gradually transfers from surfaces into the inner structure of concrete as shown in Fig. 2, forming a carbonate. Development of such layer is directly associated to the ingress of CO2 into concrete.
Progress of carbonation in concrete over the service life: a a typical section of a reinforced concrete and b formation of carbonate for ingress of CO2 into concrete (Adapted from [1])
2.3 CO2 uptake measurement
The CO2 uptake denotes the carbonation degree and carbon storage capacity of the cementitious materials [25]. Generally, this term is used to ensure a better CO2 releases balance through measuring the CO2 uptake (%) [46]. As CO2 uptake is an important parameter of carbonation process, global researchers have been calculated in different ways. He et al. [25] estimated the CO2 uptake from TG-DTG analysis using the following equation:
Where, (X)Mass loss at 500–1000 °C indicates the mass loss of carbonated specimens at 500–1000°C and (NA) Mass loss at 500–1000 °C specifies the noncarbonated reference specimens 500–1000°C.
Fang and Chang [47] calculated the CO2 uptake (%) considering the mass curve method using the relation presented here:
Here, CO2 absorbed was measured from the total mass variation in the carbonation method and deducting the mass of CO2 which amount was not participated in the carbonation reaction at conducted chamber.
Xian et al. [48] proposed a mass gain method for estimating the CO2 uptake considering the expression as below:
Here, m1 indicates the mass of sample prior carbonation, m2 denotes the mass of sample after carbonation, mwater designates the mass of water recorded from carbonation curing chamber, and mcement refers the mass of dry cement.
2.4 Applications of carbonation in PC composites
Hanifa et al. [40] summarized the CO2 uptake performances from the literature studies which were conducted on different cement materials as summarized Table 1. It has been well confirmed from the recorded information in Table 1 that CO2 uptake can significantly rise in cement materials through increasing the CO2 concentration, ingestion pressure and curing period. It is interesting to mention that the chemical composition and microstructural characteristics of cement pastes are changed in carbonation weather because of the precipitation of CaCO3. As a consequence, precipitated CaCO3 play a filling role, decrease the cavities of the concrete structure, and enhance the mechanical as well as durability properties [49].
3 Properties of MCs and their carbonation reactions
3.1 Reactive magnesia cement
Reactive magnesia cement (RMC) is a well-known cement and utilized alone as a binder or partial replacement in other cements like PC. RMC can be mixed by 2–3% in PC production for denser microstructures, increased strength, better corrosion and fire resistance, stabilization of the contaminants [42, 54, 55]. Additionally, RMC can chemically capture CO2 and improve the cementing properties under the ACC. The literature studies exemplify that during the carbonation period, CO2 is dissolved and ionized in the cement matrix, and then precipitates as anhydrous and hydrated carbonates in the pore spaces of the matrix, boosting the microstructural solidity and structural integrity [42, 56, 57]. Moreover, RMC contains about 85% of active MgO and CO2 uptake up to 92%, where PC shows around 50.4% [42]. The following reactions occur under the ACC process and hydrated magnesium carbonates are formed as follows [58,59,60,61,62,63]:
Brucite is the key hydration product of RMC. Its strength is enhanced during the carbonation process and MgCO3 is formed, which is a thermodynamically stable mineral. A literature study [64] stated that hydromagnesite and Nesquehonite crystals are developed with the increases of carbonation age through the transition of artinite. Zarandi et al. [65] noted that Nesquehonite is also converted into dypingite for more carbonation intensity. Overall, these carbonation products improve the compactness and interlocked network structures of the carbonated RMC. As a consequence, the strength is well developed into the carbonated RMC [56, 65, 66].
RMC is produced with low energy consumption and possesses good CO2 capture performance and its diverse applications are expanding day by day in the construction sectors. The Fig. 3 illustrates schematically the carbonation reaction steps of magnesia towards the carbonated products.
Carbonation mechanisms of magnesia (Adapted from [11])
3.2 Magnesium phosphate/potassium cement
The MPC/MKPC is broadly known as a rapid hardening cement and mostly used for quick repairing of roads and concrete structures [67, 68]. MPC is shaped by the interactions of dead-burned magnesia (alkaline in nature) and ammonium phosphate (or potassium phosphate) salts (acidic in nature), which is commonly recognized as acid-based reactions. It is to be noted that MPC gains popularity because of its excellent properties such as fast setting, high early strength, superior interfacial bonding to convention concrete, resistant to fire and low drying shrinkage [69,70,71,72]. The main hydration products of MPC is struvite, which is developed as follows [72, 73]:
Due to the rapid hydration of MPC/MKPC, considerable quantities of dead-burned MgO particles may remain at unreacted. Consequently, when the CO2 curing is employed in solid MPC/MKPC specimens, CO2 is reacted with free MgO particles and brucite (Mg(OH)2), forming similar carbonated minerals as of RMC such as MgCO3, MgCO3.3H2O, ((Mg)5(CO3)4(OH)2.5H2O), and ((Mg)5(CO3)4(OH)2.4H2O) [35]. The porosity and structural stiffness of MPC/MKPC are enhanced after developing these carbonated crystals [74].
3.3 Magnesium oxychloride cement (MOC)
The MOC is comprised of the interactions of MgO and MgCl2 (magnesium chloride) solution [75]. The hydration phases, physico-mechanical, and microstructural characteristics of MOC are principally varied by the mix combinations of MgO and MgCl2 in aqueous solution. At room temperature, the hydration phases development of MOC can be illustrated by the below chemical reactions [76]:
The MOC products are usually inclined to CO2 uptake, where hydrated magnesium carbonate like chlorartinite is formed (from Phase 5) by absorbing CO2 [66]:
Phase 3 mineral also uptakes CO2 and forms the new hydration product as below [77,78,79,80]:
It is noted that strength properties of MOC composites are decreased in water environment [77] due to the decomposition of Phase 5 and Phase 3 minerals in contact of water, while both are key contributors of structural rigidness of MOC materials [78]. The crystal Phase 5 may be transformed into Phase 3 structure in the carbonation environment. The researchers observed that the new phase (Mg(OH)2.MgCl2. 2MgCO3 0.6H2O) formed due to carbonation degrades less in water than the Phase 5 and Phase 3 minerals. In other words, the water-resistance performance of MOC is upgraded due to CO2 uptake. Similarly, CO2 sequestration of MOC enhances the strength and toughness properties due to formation of compact microstructures [80, 81]. Figure 4 illustrates the carbonation reaction stages of MOC with CO2, where magnesium carbonate minerals are precipitated like chlorartinite.
Theoretical illustration of MOC carbonation mechanisms [75]
3.4 Magnesium oxysulfate cement
The MOS is fabricated by the chemical responses of light-burned MgO and MgSO4· 7H2O (magnesium sulfate) solution, which concept in parallel with that of MOC [31]. As MOS is formed with lower energy consumption [82] and lower calcination-based magnesia [83], it is also known as eco-friendly cement. Previous research detected that the dominating hydration reaction product is 5Mg(OH)2· MgSO4· 7H2O (5·1·7 phase) [84]. Another research stated that hydration phases of MOS can be defined as xMg(OH)2· yMgSO4·zH2O, which can be designated as different phases. Formation reactions of these phases can be expressed as follows [85]:
The durability properties of MOS matrices can be enhanced by employing carbonation curing. The CO2 curing causes the strength improvement up to a limit at the early CO2 sequestration and afterwards leads to a negative influence on the structural integrity [31]. Ba et al. [86] explained that MgCO3 structure can be developed under carbonation curing of MOS through neutralizing the MgSO4 and Mg(OH)2 phases, which refine the microstructures, capillary porosity and improve the mechanical strength characteristics to a certain extent [31, 84]. The carbonation reaction of MOS can be written as follows [86]:
4 Properties changes and mechanisms of carbonated PC incorporating RMC
4.1 Improvement of physico-mechanical properties
As the RMC is very sensitive to CO2 absorption, researchers have been exploring the CO2 sequestration performance over a decade on the binary cement that combines PC and RMC. Chemical compositions of used PC and RMC are presented in Table 2. In the binary compositions, RMC is used as the partial substitution of PC, entitling the binary cement and exhibits some good facilities such as noteworthy carbonation efficiency and, comparatively rigid microstructures and durability [42, 87]. It is worth noting here that the carbonation performance of cement materials depends on the mix proportion of binders, water to cement proportion, pressurized concentration level of CO2, exposed surface area, curing temperature and relative humidity. Figures 5, 6 and 7 represent the analyzed properties of carbonated binary cement reported by the literature studies. Figure 5 shows the enrichment of CS, apparent density, pore volume and porosity of the binary cement as compared with the carbonated control specimens (i.e., PC mixture). As is observed, the aforesaid properties were significantly improved. In addition, Fig. 6 illustrates the CO2 uptake percentage of the binary and control PC samples, where it is quite clearly seen that CO2 uptake (%) performance of the former was considerably increased and ranged from 21 to 35% at 7d to 56d carbonation ages. Moreover, Fig. 7 demonstrates the carbonation degree of examined samples. Pu and Unluer [88] observed the higher carbonation degree at the peripheral surfaces of binary cement matrices as compared to the inner portions of the carbonated samples.
4.2 CO2 sequestration mechanisms of PC and MgO binary system
The CO2 sequestration is a process where CO2 is chemically stabilized in the cement materials through converting into different crystals and entrapped forever [3]. This technique can play a crucial role in enhancing the cement materials properties and decreasing the carbon footprint in the world [3, 91]. Previous studies stated that free calcium ions (Ca2+) are available in PC matrices because of the dissolution of hydrated lime (Ca(OH)2), and decalcification of CSH and ettringite [90]. In addition, Ca2+ ions may leach from calcium silicate anhydrous [92].
Similarly, the pore solution of RMC products contains Mg2+ ions. Consequently, the pore solution of binary cement having PC and MgO mixes comprise of both Ca2+ and Mg2+ ions, where Mg2+ ions interact with CaCO3 and crystalize to magnesian calcite (CaxMg1-xCO3) as expressed below [90]:
Pokrovsky [93] specified that the amount of magnesian calcite (e.g., CaMg(CO3)2) in the carbonated hydration environment of binary cement is varied depending on the existing quantities of Ca2+ and Mg2+ ions in the pore solution. Furthermore, the magnesium carbonate crystal can develop through the reaction between Mg2+ ions and \({{\text{CO}}}_{3}^{2-}\) as follows [90, 94]:
The key factors influencing the CO2 sequestration degree and capacity in the binary cement compounds can be classified into two types: (1) the dissolution amount and diffusion of Ca2+ and Mg2+ into the pore solution, and (2) the degree of dissolved CO2 in pore water and its movement in the capillary pore networks of solid cement materials [90].
5 Influence of carbonation curing on MCs properties
5.1 Mechanical strength
Tables 3 and 4 summarize the existing findings on how ACC influences the strength properties of magnesium cements. Pu and Unluer [88] investigated that ACC led to 83% higher CS than the standard control specimens (i.e., without ACC). Jang et al. [95] reported that the CS values of the RMC samples increased proportionally with the CO2 sequestration level. They also detected that the CS increased because of the formation of carbonates of hydromagnesite (Mg5(CO3)4(OH)2 0.4H2O) mineral that filled the internal cavities of porous microstructures. Table 2 also shows the better strength results of carbonated MPC matrices in relation to the control curing process. For instance, Jeon et al. [35] observed that CO2 cured MPC specimens offered the higher CS and strain at the peak stress in comparison with the specimens cured in standard air. They demonstrated that the structural integrity was improved of carbonated matrices due to the densification of hydration environment through the reaction between the CO2 and hydration products of dead-durned magnesia. Gao et al. [96] also recorded the similar findings on MPC materials and they explained an interesting thing is that fly ash-based MPC mixes exhibited the significant improved strength under ACC as compared to non-carbonated MPC samples. Table 3 illustrates the comparison of strengths of the carbonated and non-carbonated specimens for MOC and MOS composites. He et al. [77] stated that MOC paste comprising PFA (pulverized fuel ash) and cured in CO2 showed the better strength and water-resistant performance than the control paste. They interpreted that this good performance was possibly due to the development of new carbonated phases. Moreover, Ba et al. [86] specified that carbonation curing improves the structural strength properties and toughness of MOS samples to some extent, as a result of lower water to cement ratio and refinement of meso-pores stimulated by the carbonation process. Similar results were reported by Zhang et al. [31], who described that hydration products of MOS may react with CO2 to form new crystals, which contribute to the strength enhancement.
5.2 Pore structure
The influence of carbonation curing on the pore structures of magnesium cements are discussed in this section. It is observed that ACC changes the pores properties in the microstructures. Mo and Panesar [90] analyzed the pore size distribution (PSD) of PC incorporating RMC and GGBFS (ground granulated blast furnace slag) slag, which were cured at 99.9% concentration of CO2 as displayed in Fig. 8. Their study revealed that lower pore volumes were found in carbonated specimens in relation to non-carbonated samples. Slag led to a coarser PSD, where RMC showed the opposite effect. Introduction of 10–20% RMC to PC paste reduced the pore sizes and total porosity of the blends in comparison to control matrices under the ACC system.
Pore size distribution of carbonated and non-carbonated PC paste containing RMC and GGBFS at 56d [90]
Gao et al. [96] reported the MIP (Mercury intrusion porosimetry) analysis results of MPC paste blended with fly ash considering the ACC and standard air curing systems as exhibited in Fig. 9. It is seen from the cumulative pore size (CPS) and PSD curves that the porosity of pure MPC was increased to some extent after carbonization, where MPC having fly ash exhibited the porosity reduction and strength improvement in the ACC system. Fly ash promoted the formations of mineral and amorphous phases, which instigated the reduction of pore sizes and pore volumes.
Pore size distribution analysis of MPC samples containing fly ash under carbonation and non-carbonation system (Adapted from [96])
Ba et al. [86] examined the pore structures of MOS cement specimens under the standard air and carbonation curing methods and the findings are presented in Fig. 10. It is quite clear that MOS sample prepared with the W/C of 0.5 showed the lower volume porosity (%) and significantly reduced pore sizes when cured in ACC system as compared to the standard air curing samples (Fig. 10). Furthermore, it is well evident from X-CT images (resolution of 38 µm) as shown in Fig. 11 that the meso-porous volume of hardened MOS paste having the W/C of 0.5 decreased in carbonation, specifically in the nearer regions of carbonized layer (in reconstructed images). Most notably, it was reckoned that the mean pore volume was about 12.74% in standard curing, where carbonization specimen presented refined microstructures and average pore volume of around 10.6% at 28d. For this reason, strength properties of MOS cement paste were enhanced to some extent under the ACC environment.
Influence of ACC and non-carbonation system on porosity of MOS cement prepared with different W/C [86]
Meso-porous structures of MOS cement sample: a transverse and longitudinal sections without carbonation and b transverse and longitudinal sections with carbonization [86]
Zhang et al. [31] also analyzed the micro-pore structure properties of MOS cement specimens employing the standard air curing and ACC systems. The obtained results are shown in Table 5. As it is seen, the MOS cement samples cured in ACC atmosphere exhibited the lower average pore sizes and considerably decreased gel pore (< 10 nm) and capillary pore sizes (10–100 nm) than the standard air cured matrices, indicating the compactness of MOS pastes obtained in the ACC system.
5.3 pH of pore solution
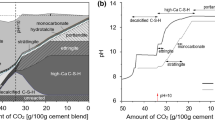
The pH variations of carbonated pore solution of the RMC and MOS compounds at different carbonation ages are presented in Fig. 12. It is seen that the pH of pore solutions ranges from 10.0 to 10.5 for non-carbonated (i.e. at air curing) RMC and MOS specimens, while reduced to the range of 10.0–10.3 because of carbonation curing under the 10% to 20% CO2 environment. The reduction seems to be marginal. More importantly, the pore solution pH was not noticeably changed with the prolonged carbonation ages, possibly due to the pH buffering mechanism supplied by the brucite (Mg(OH)2) mineral in the hydration products [99]. Hay and Celik [97] stated the similar finding that uncarbonated Mg(OH)2 assists to balance the pH buffering issue in the carbonated MC samples.
5.4 Hydration properties
5.4.1 Mineralogical formations
The influence of carbonation curing on the hydration reactions and mineralogical changes in contact to CO2 are discussed in this section. Wang et al. [42] analyzed the crystal formation changes of RMC applying different concentration of CO2 and the experimental results are displayed in Fig. 13. Figure 13a shows the gradually increasing contents of amorphous phase about 31.2% for 70% and 100% CO2 curing at 1d duration, whereas the unreacted MgO content was decreased proportionally. Moreover, MgCO3 and other secondary hydration products of RMC paste were increased with ages noticeably in 10% CO2 environment (Fig. 13b), which indicates that low intensity of CO2 curing needs adequate time to stimulate the hydration reactions for forming carbonation of RMC products.
X-ray diffractions and mineral formation quantities analysis of RMC pastes with varying the CO2 intensity and observation ages (Adapted from [42])
Jeo et al. [35] conducted XRD tests on carbonated and non-carbonated samples of MPC at 3d and 28d ages (Fig. 14). It was found that K-struvite (MgKPO4 0.6H2O) developed as a main hydration product of MKPC pastes, whereas the high peaks of unreacted MgO and MgCO3 were overlapped at 42.82° for standard air and carbonated samples. Additionally, it is quite evident from the results that few small peaks of Nesquehonite were detected in carbonated specimens at 3d and 28d matrices and such peaks were not noticed in air cured pastes, which signifies the chemical interactions of CO2 and Mg (OH)2 to form Nesquehonite.
X-ray diffractions results MKPC pastes at a 3d and b 28d cured at standard air (4N) and carbonation conditions (4C) (Adapted from [35])
He et al. [77] performed the X-ray diffraction experiments on MOC paste containing 30% fly ash and pastes cured in > 99% CO2. It is noted that carbonated MOC samples blended with fly ash showed better water-resistant capacity, due to the huge amorphous contents formed in the hydration region through the interaction between fly ash and MOC. Such formation was greatly enhanced by the ACC system. Consequently, the microstructural rigidity was improved and the intensity of Phase 5 (mineral) remained unchanged in the water media. Similarly, it is seen that the peak intensities of brucite (Mg (OH)2), periclase and alumina were detected almost same even after 14d of water curing.
Li et al. [83] prepared the MOS comprised with fly ash (FA) and GGBFS and cured in standard air and CO2. Then the samples were also cured in water up to 28 days and performed the XRD tests. The analytical results are exhibited in Fig. 15a, where high peaks of Mg (OH)2 (brucite), Phase 5·1·7, magnesite (MgCO3), and free MgO particles were noticed. It is also clearly seen that peak intensities of MgCO3 were increased for CO2 curing, because of the reaction of CO2 and free MgO particles to produce MgCO3 products (Fig. 15a). Noticeable modification of peaks intensities of Phase 5 was not observed for CO2 curing. Additionally, intensities of brucite were noticed clearly after 28d in water region, which attributed to the interactions of water and MgO particles (Fig. 15b). Furthermore, the peaks of brucite were hardly marked after 28d of water immersion due to the solidification of CO2 curing. It designates the sequestration of CO2 that decreased the unreacted magnesia particles in the hardened MOS pastes.
X-ray diffraction results of MOS blended with fly ash and slag cured at air and CO2: a before water immersion and b after water immersion (Adapted from [83])
5.4.2 Microscopic observations
Wang et al. [42] inspected the microscopic pictures of air cured RMC and carbonated RMC pastes with 10% CO2 as shown in Fig. 16. As detected, the standard cured specimen showed the rough surface and existence of free MgO particles in abundant quantities, whereas dense structure and regular sized hydration products were noted in the carbonated RMC samples. Similar characteristics were explored by Jang et al. [95] for light RMC pastes cured in 10% and 20% CO2 as depicted in Fig. 17.
SEM observations of RMC pastes at 1-d 0% CO2 and 10% CO2 system [42]
SEM image of carbonated RMC paste: a RMC paste cured in 10% CO2 and b RMC paste cured in 20% CO2 [95]
It was observed in Fig. 17 that the number and sizes of hydromagnesite crystal was augmented in 20% CO2 cured RMC paste (Fig. 17b) as compared to the 10% CO2 cured sample (Fig. 17a). As a result, RMC specimen cured by 20% CO2 presented the higher structural strength properties due to the filling of internal pores by hydromagnesite development [95].
Jeon et al. [35] analyzed the microscopic properties like micro-pores and micro-cracks of MKPC pastes considering the standard air and ACC systems. Their captured BSE images are shown in Fig. 18. It was specified that comparatively higher micro-cracks and pores were detected in the interfacial transition zone (ITZ) regions of air cured samples (i.e. MKPC-4N) than carbonated specimens (i.e. MKPC-4C). It is fairly clear that carbonation curing stimulated to form the solid microstructure through forming the carbonates minerals that reduces of cavities in the carbonated microstructures [35].
BSE images of MPC pastes at 28d: a MKPC sample with air curing and b MKPC sample with CO2 curing [35]
Li et al. [83] observed the SEM images of control MOS (Fig. 19) and MOS blended with FA 30% or GGBFS 30% pastes (Fig. 20) cured at air for 13d and 1d in CO2. Then the samples were kept in water for 28d. The narrow-like minerals namely 5·1·7 phase having the diameter around 0.25 μm and height about 4 μm were noticed in the samples cured in air (Fig. 19a). After the 1-d carbonation, compactness of needle-like crystals was increased (Fig. 19b). In addition, micro-cracks were revealed in the microstructure of water cured matrices, which implies the degradation of MOS hydration products in contact with water.
Microscopic observations of MOS paste cured in water for 28d considering air and CO2 sequestration treatment: a control with air and b control with CO2 [83]
Microscopic observations of MOS paste cured in water for 28d considering air and CO2 sequestration treatment: a MOS+ fly ash 30% with CO2 curing, and b MOS+ slag 30% with CO2 curing [83]
On the other hand, solid MOS combinations containing supplementary materials like FA or GGBFS and treated with CO2 (Fig. 20), unveiled the denser structure in comparison to standard air cured samples. It was possibly due to the development of the amorphous phases, nanocrystalline C-S-H, M-S-H gel and HCMs in the hydration regions for including FA and GGBFS [100,101,102]. For this reasons, the metrics of carbonated MOS incorporating FA and GGBFS attained the better durability properties [83].
5.4.3 Thermal properties
Wang et al. [42] conducted thermogravimetry analysis (TGA) tests on RMC pastes cured in air and different CO2 concentrations at various hydration ages. As illustrated in Fig. 21, 1-d carbonated RMC samples lost more mass than the ambient air cured specimen. No significant transformation was seen of brucite (Mg(OH)2) into HMCs phases in the specimens cured at low CO2 concentration such as 10% and 40%, whereas notable changes were detected at high CO2 intensities (i.e. 70% and 100% concentration). Additionally, in comparison to 1-d carbonated RMC paste, mass loss peak of 3-d CO2 cured paste shifted from 370 °C to 390 °C. Furthermore, it was reported that the total mass loss dropped around 22.8% for 7d of 10% CO2 specimens. Similar result was observed for 1d aged specimens cured in 100% CO2 [42].
TGA test results of RMC pastes (Adapted from [42]): a mass loss curves cured at 1-d in different CO2 intensities and DTG curves corresponding to them, b mass loss curves for 10% CO2 curing at different periods and DTG curves corresponding to them
Jeon et al. [35] performed the thermogravimetric analysis on the different aged carbonated MKPC pastes and the attained data are presented in Fig. 22. It was revealed that more mass loss occurred for carbonated samples than the air cured counterparts at the same ages. Existence of more water in the K-struvite minerals might cause the decrease of mass below 200 °C [103]. Decomposition of HMCs and MgCO3 was detected at around 560 °C [97]. Finally, it was stated that carbonated MKPC samples exhibited the higher peaks of dehydration with increasing the curing ages, while the air cured matrices showed no change.
Thermogravimetric analytical curves of carbonated (MKP-4C) and air (MKP-4N) cured MKPC specimens at different ages [35]
Figure 23 represents the DSC/TG results on MOS specimens employing air and carbonation curing process directed by Li et al. [83]. FA and GGBFS were also added in MOS pastes. It was reported from DSC curves that the control sample showed mass loss ranging from 7.48% to 8.73% in the heat range of 50 °C to 180 °C. In the other side, carbonated MOS sample revealed a higher mass loss for heat about 180 °C than the reference sample. It was also seen that carbonated samples showed first decomposition at higher temperature as compared to air cured specimens, which was possibly due to the effects of forming Mg (OH)2 and HMCs crystal-like structure [97, 104]. Consequently, CO2 treatment could improve the water-resistance performance of MOS matrix. It is clearly seen from the DSC curves of MOS comprising FA30 and GGBFS30 that carbonation curing influenced their way of dehydration and decomposition. An exothermic peak was spotted in FA30 and GGBFS30 blended samples at about 850 °C due to crystallization of the M-S-H gel (i.e., MgSiO3 compound). It is recognized that M-S-H gel has an insoluble structure and can improve the water proofing properties of MOS cement [105].
DSC-TG curves of air and carbonation cured MOS samples: a before immersion into water and b after 28d of water immersion [83]
6 CO2 sequestration in RMC
Up to now, a large number of existing studies are found for measuring the CO2 sequestration in RMC composites, whereas very few studies are available for MOC matrices in the literature. Figure 24 summarizes the CO2 sequestration (%) information from published papers on RMC. All the specimens were cured in the ACC system under different CO2 concentration levels, temperatures and RH (%). Then the sequestration percentages were measured through the thermogravimetry analysis. It is seen that the sequestration (%) ranged from about 4% to 46% at early ages to 28d for RMC solid specimens. It signifies that for 1 ton of RMC materials, ∼40–460 kg CO2 could be the effectiveness of offsetting CO2 absorption via the use of RMC as an alternate binder, in spite of the variation of CO2 sequestration quantity may vary due to the different mix designs of raw materials proportions, testing ages, curing conditions etc. For example, Hay and Celik [106] applied the supercritical CO2 pressure under 9.5 MPa at 35 ± 2 ◦C condition and noticed that around 8.8% CO2 sequestration was achieved at 4 h of experiment, which was very high amount considering the short carbonation age. It seems that supercritical CO2 pressure suppressing the primary nucleation seeding and development of carbonates in the higher intensity of carbonation degree [95, 107].
Power et al. [75] examined the CO2 sequestration of MOC-based particle boards for long periods. They reported that 1 kg CO2/m2 was sequestrated within 15 years, where the passive carbonation degree was about 0.07 kg CO2/m2/year. Furthermore, Jankovský et al. [80] put forward an estimation that Phase 3 (3Mg(OH)2.MgCl2 0.8H2O) mineral of MOC can uptake CO2 around 211 g/kg while 331 g/kg for Phase 5 (5Mg(OH)2.MgCl2 0.8H2O) crystal.
7 Comparative sustainability assessment of MgO and PC composites
The LCA of construction materials prepared with PC and RMC amalgams was conducted to analyze the sustainability issue of MCs composites [66, 108, 109]. McQueen et al. [23] demonstrated that MgO along with high-purity CO2 is produced from the burning process of magnesite (MgCO3). Then this MgO reacts with atmospheric CO2 through spreading over the earth surface to form carbonate minerals again, which is recollected and re-calcined [110]. Therefore, a magnesia loop can be formed to facilitate a sustainable way to keep the environment balance. Table 6 illustrates the comparative analysis of sustainability of MgO and PC products considering different factors. It is noticeable that RMC and hard-burned magnesia (HBM) are manufactured at lower temperature than OPC, which leads to lower energy cost [23]. Although the incineration process of MgO releases little higher CO2 as compared to PC, the overall MgO production shows around 73% lower net CO2 emission than PC in the context of carbonation capability. In addition, Ruan and Unluer [108] studied the LCA considering the environmental impacts of RMC and PC manufacturing processes. They concluded that MgO presents fairly lesser impact on the whole ecological quality and capitals, when compared to PC. On account of the strength ratio modification (i.e. strength performance), previous research reported MgO manufacture results in about ∼13% and ∼32% smaller CO2 intensity and impact to human health in relation to PC, correspondingly. Finally, it can be concluded that the use of MgO composites generates a lower ecological impact in terms of the total service life performance [14].
8 Discussion and limitation of existing research
Magnesium cements have been known as low-carbon construction materials and drawn increasing attention globally from researchers and industrial investors for their lucrative properties over the PC composites. It is well-known that MCs respond well to CO2 curing and show significant carbonation degree to uptake CO2. Therefore, a lot of research has been conducted broadly over the last few years on the carbonation performance of MCs composites to demonstrate their environmental sustainability.
The current review primarily focuses on the changes of engineering properties and CO2 sequestration performances of the MCs composites employing the ACC process. According to the information conveyed in the previous sections, noteworthy reduction was observed of porosities, improvement of density and strength properties, and CO2 sequestration in PC materials comprising with RMC due to the carbonation curing. Carbonated products are formed through stimulating the hydration reactions of RMC and formation of hybrid Ca-Mg products. These carbonated crystals refine the pore structures and improve the structural integrity of PC composites. It is quite obvious that the inclusion of RMC into the PC mixtures enhances the carbonation degree and CO2 uptake capacity. As a consequence, the pore volume and porosity of the binary cement compositions are reduced, leading to the improved engineering properties. In addition, ACC method also significantly influence the microstructural as well as hydration reaction properties of MCs compounds, due to the dispersion of CO2 within the micropores system and thus dense carbonate network. Consequently, better engineering performances (including better water resistance) are achieved in carbonated MCs than the non-carbonated. Furthermore, low carbon emission-based construction materials can be manufactured with MCs. Net CO2 emissions can be considerably minimized through the use of low-calcined MgO and optimizing the; mix design, water to cement proportion, binder to aggregate ratio, carbonation degree and carbonation curing process [66].
However, further research is needed for better understanding the engineering properties of MCs. Although numerous papers have been published on the properties of carbonated RMC, relatively few research articles are found on the characteristics of carbonated MPC/MKPC, MOC, and MOS composites as compared in Table 7.
It is shown in Table 6 that the properties like pore solution pH, CO2 sequestration (%), water proofing performance, and pore structures of carbonated MPC/MKPC, MOC and MOS compounds with and without supplementary cementitious materials need further exploration. It is commonly recognized that MC binders show good strength properties in comparison to their PC counterparts, for example CS ranges in RMC 20-70 MPa, > 40 MPa for MPC (at 3 h), 60–150 MPa for MOC and 25-45 MPa for MOS [66]. Therefore, it should be a good step forward to more explore and improve the properties of MCs through carbonation process for eco-friendly practical applications.
9 Conclusions
This paper has presented a comprehensive state-of-the-art review on the previous studies related to the carbonation mechanisms of MCs, the properties change under the carbonation curing and CO2 sequestrations. Additionally, the influence of carbonation on the binary cement (PC and MgO) properties is also discussed here. The review can lead to the following concluding remarks:
-
(1)
Significant efforts have been spent over the past two decades on the storage of CO2 in PC materials through applying the mineral storage process. It is widely accepted that CaCO3 is precipitated as a carbonation product in PC and formed by the interaction between CaO and CO2. About 0.1 to 1.4 gigatons of CO2 can be stored in PC concrete using CCU by the year 2050.
-
(2)
Inclusion of different dosages of RMC into PC mixtures enhances its density and strength properties, and reduces its porosity and pore sizes. Also, presents a good viability to improve the CO2 uptake performances of the PC and RMC blended composites in ACC system. Carbonated products are formed through stimulating the hydration reactions of RMC and form the hybrid Ca-Mg products. These carbonated crystals refine the pore structures and improve the structural integrity of PC composites. It was noted that addition of 10–50% RMC into PC composition increases the CO2 uptake (%) performances by around 20% –35% and carbonation degree about 10%-60%. For these advantages, the applications of MC have been considerably increased over the last few years and gained increasing popularity as a construction material to reduce the CO2 discharge or capture CO2 from air in the context of climate change.
-
(3)
It was noticed that carbonation curing advances the structural integrity and toughness of MCs samples. Consequently, carbonated MC composites presented the better strength and water-resistance properties than the non-carbonated ones.
-
(4)
Carbonated MC specimens revealed the lower pore volumes in relation to non-carbonated samples. The ACC promoted the formations of minerals and amorphous phases, which instigated the reduction of pore sizes and pore volumes. It was also reported that the MCs comprising with supplementary materials such as fly ash exhibited the lower gel pore (< 10 nm) and capillary pore sizes (10–100 nm) than the standard air cured matrices, where fly ash promoted the amorphous phases under the CO2 curing.
-
(5)
X-ray diffraction analysis showed that ACC greatly influences the hydration reactions of MC materials. Carbonation curing stimulates to form the minerals like Nesquehonite, MgCO3, secondary hydration products and more amorphous contents, which refines the meso-pores and increase the densification of microstructures. Most notably, the peak intensity of MgCO3 is increased by CO2 curing because of the reaction of CO2 and free MgO particles. Brucite was also found disappeared gradually from MCs due to the solidification in the carbonation environment. It specifies the CO2 sequestration hardened MCs composites.
-
(6)
Relatively few studies were found in the literature on the estimation of CO2 sequestration percentage of MC materials employing the ACC system. It was observed that about 4% to 46% CO2 was sequestered in the RMC mixtures at 28d. The magnitude of CO2 sequestration mainly depends on the mix compositions of raw materials in MC matrices, quantities of supplementary materials, carbonation intensities and curing ages.
10 Recommendations for further studies
In spite of the many studies available on the carbonation of RMC materials, they are still a relatively new topic in comparison to the carbonation of PC. Research on the engineering, microstructural and CO2 uptake properties of carbonated MPC/MKPC, MOC, and MOS are still very limited. Hence, there is a great opportunity to explore the properties and CO2 sequestration performances of MC mortars and concretes employing the ACC system. The future studies on the carbonation of MCs may be conducted from the following aspects:
-
1)
Accelerated carbonation studies can be conducted on MCs blended with supplementary materials (SMs) such as silica fume, red mud, bauxite, calcine clay and limestone. As these SMs play a great role in improving the properties of MCs.
-
2)
The effects of carbonation on the pore solution pH and corrosion probability of steel reinforced MC concretes containing SMs should be further clarified.
-
3)
Long term durability study can be performed on carbonated MC specimens under accelerated exposure schemes like high alkaline and acidic environment.
-
4)
Advanced material characterization techniques like Raman spectroscopy, nucleic magnetic resonance, and micro-X-ray computed tomography can be performed in order to gain deep insights into carbonated MCs samples.
-
5)
CO2 sequestration study can be implemented using the wet carbonation as well as carbonation on fresh mixtures of MCs composites.
-
6)
Life cycle assessment on MCs like MPC, MOC and MOSC composites can be studied for sustainable field applications in future.
-
7)
Influence of carbonation on the engineering properties of magnesium cement concrete can be monitored.
11 Nomenclature
ACC Accelerated carbonation curing
CCU Carbon capture and utilization
CSH Calcium silicate hydrate
CO2 Carbon dioxide
CS Compressive strength
FS Flexural strength
HMCs hydrated magnesium carbonates
LCA Life cycle assessment
MCs Magnesium cements
MPC/MKPC Magnesium phosphate/potassium cement
MOC Magnesium oxychloride cement
MOS Magnesium oxysulfate cement
PC Portland cement
RMC Reactive magnesia cement
References
Possan, E., Thomaz, W. A., Aleandri, G. A., Felix, E. F., & dos Santos, A. C. P. (2017). CO2 uptake potential due to concrete carbonation: A case study, Case Stud. Construction Materials, 6, 147–161. https://doi.org/10.1016/j.cscm.2017.01.007
Chen, C., Xu, R., Tong, D., Qin, X., Cheng, J., Liu, J., Zheng, B., Yan, L., & Zhang, Q. (2022). A striking growth of CO2emissions from the global cement industry driven by new facilities in emerging countries. Environmental Research Letters, 17(4), 044007. https://doi.org/10.1088/1748-9326/ac48b5
Kamal, N. L. M., Itam, Z., Sivaganese, Y., & Beddu, S. (2020). Carbon dioxide sequestration in concrete and its effects on concrete compressive strength. Materials Today: Proceedings, 31, A18–A21. https://doi.org/10.1016/j.matpr.2020.11.185
Ali, M. B., Saidur, R., & Hossain, M. S. (2011). A review on emission analysis in cement industries. Renewable and Sustainable Energy Reviews, 15, 2252–2261. https://doi.org/10.1016/j.rser.2011.02.014
He, Z. J., Zhu, X. D., Wang, J. J., Mu, M. L., & Wang, Y. L. (2019). Comparison of CO 2 emissions from OPC and recycled cement production. Construction and Building Materials, 211, 965–973. https://doi.org/10.1016/j.conbuildmat.2019.03.289
Haselbach, L., & Thomas, A. (2014). Carbon sequestration in concrete sidewalk samples. Construction and Building Materials, 54, 47–52. https://doi.org/10.1016/j.conbuildmat.2013.12.055
Alimi, W. O., Adekunle, S. K., Ahmad, S., & Amao, A. O. (2022). Carbon dioxide sequestration characteristics of concrete mixtures incorporating high-volume cement kiln dust. Case Studies in Construction Materials, 17, e01414. https://doi.org/10.1016/j.cscm.2022.e01414
Kene, S. D., Domke, P. V., Deshmukh, S. D., & Deotale, R. S. (2011). Assessment of concrete strength using fly ash and rice husk ash. International Journal of Engineering Research and Applications ISSN, 1, 524–534.
Global cement production from 1990 to 2030 (2022). https://www.statista.com/statistics/373845/global-cement-production-forecast/. Accessed 30 Dec 2022.
Schneider, M., Romer, M., Tschudin, M., & Bolio, H. (2011). Sustainable cement production-present and future. Cement and Concrete Research, 41, 642–650. https://doi.org/10.1016/j.cemconres.2011.03.019
Abdel-Gawwad, H. A., Hassan, H. S., Vásquez-García, S. R., Israde-Alcántara, I., Ding, Y. C., Martinez-Cinco, M. A., Abd El-Aleem, S. A., Khater, H. M., Tawfik, T. A., & El-Kattan, I. M. (2020). Towards a clean environment: The potential application of eco-friendly magnesia-silicate cement in CO2 sequestration. Journal of Cleaner Production, 252, 1–9. https://doi.org/10.1016/j.jclepro.2019.119875
Bosoaga, A., Masek, O., & Oakey, J. E. (2009). CO2 capture technologies for cement industry. Energy Procedia., 1, 133–140. https://doi.org/10.1016/j.egypro.2009.01.020
Ahmad, M. R., Chen, B., Oderji, S. Y., & Mohsan, M. (2018). Development of a new bio-composite for building insulation and structural purpose using corn stalk and magnesium phosphate cement. Energy and Buildings, 173, 719–733. https://doi.org/10.1016/j.enbuild.2018.06.007
Liska, M., & Al-Tabbaa, A. (2009). Ultra-green construction: Reactive MgO masonry products. Proceedings of the Institution of Civil Engineers: Waste Resource Management, 162, 185–196. https://doi.org/10.1680/warm.2009.162.4.185
Liska, M., Al-Tabbaa, A., Carter, K., & Fifield, J. (2012). Scaled-up commercial production of reactive magnesium cement pressed masonry units. Part I: Production. Proceedings of the Institution of Civil Engineers-Construction Materials, 165, 211–223. https://doi.org/10.1680/coma.10.00032
Walling, S. A., & Provis, J. L. (2016). Magnesia-based cements: A journey of 150 years, and cements for the future? Chemical Reviews, 116, 4170–4204. https://doi.org/10.1021/acs.chemrev.5b00463
Scott, A., Oze, C., Shah, V., Yang, N., Shanks, B., Cheeseman, C., Marshall, A., & Watson, M. (2021). Transformation of abundant magnesium silicate minerals for enhanced CO2 sequestration. Communications Earth & Environment, 2, 1–6. https://doi.org/10.1038/s43247-021-00099-6
Shen, W. G., Cao, L., Li, Q., Wen, Z. J., Wang, J., Liu, Y., Dong, R., Tan, Y., & Chen, R. F. (2016). Is magnesia cement low carbon? Life cycle carbon footprint comparing with Portland cement. Journal of Cleaner Production, 131, 20–27. https://doi.org/10.1016/j.jclepro.2016.05.082
Hou, P. K., Cai, Y. M. , Cheng, X., Zhang, X. Z., Zhou, Z. H., Ye, Z. M., Zhang, L. N., Li, W. G., & Shah, S. P. (2017). Effects of the hydration reactivity of ultrafine magnesium oxide on cement-based materials. Magazine of Concrete Research, 69, 1135–1145. https://doi.org/10.1680/jmacr.16.00212
Gonçalves, T., Silva, R. V., De Brito, J., Fernández, J. M., & Esquinas, A. R. (2019). Hydration of reactive MgO as partial cement replacement and its influence on the macroperformance of cementitious mortars. Advances in Materials Science and Engineering, 2019. https://doi.org/10.1155/2019/9271507
Unluer, C., & Al-Tabbaa, A. (2013). Impact of hydrated magnesium carbonate additives on the carbonation of reactive MgO cements. Cement and Concrete Research, 54, 87–97. https://doi.org/10.1016/j.cemconres.2013.08.009
Liska, M., & Al-Tabbaa, A. (2008). Performance of magnesia cements in pressed masonry units with natural aggregates: Production parameters optimisation. Construction and Building Materials, 22, 1789–1797. https://doi.org/10.1016/j.conbuildmat.2007.05.007
McQueen, N., Kelemen, P., Dipple, G., Renforth, P., & Wilcox, J. (2020). Ambient weathering of magnesium oxide for CO2 removal from air. Nature Communications, 11, 1–10. https://doi.org/10.1038/s41467-020-16510-3
Yang, J., Lu, S. W., & Wang, L. Y. (2020). Fused magnesia manufacturing process: A survey. Journal of Intelligent Manufacturing, 31, 327–350. https://doi.org/10.1007/s10845-018-1448-1
He, Z., Li, Z., & Shao, Y. X. (2017). Effect of carbonation mixing on CO2 uptake and strength gain in concrete. Journal of Materials in Civil Engineering, 29, 1–8. https://doi.org/10.1061/(asce)mt.1943-5533.0002031
El-Hassan, H., & Shao, Y. X. (2015). Early carbonation curing of concrete masonry units with Portland limestone cement. Cement and Concrete Composites, 62, 168–177. https://doi.org/10.1016/j.cemconcomp.2015.07.004
Gruyaert, E.,Heede, V. D. P., & Belie, D. N. (2013). Carbonation of slag concrete: Effect of the cement replacement level and curing on the carbonation coefficient - Effect of carbonation on the pore structure. Cement and Concrete Composites, 35, 39–48. https://doi.org/10.1016/j.cemconcomp.2012.08.024
Soroushian, P., Won, J. P., & Hassan, M. (2012). Durability characteristics of CO2-cured cellulose fiber reinforced cement composites. Construction and Building Materials, 34, 44–53. https://doi.org/10.1016/j.conbuildmat.2012.02.016
Xuan, D. X., Zhan, B. J., & Poon, C. S. (2016). Assessment of mechanical properties of concrete incorporating carbonated recycled concrete aggregates. Cement and Concrete Composites, 65, 67–74. https://doi.org/10.1016/j.cemconcomp.2015.10.018
Zhang, J. K., Shi, C. J., Li, Y. K., Pan, X. Y., Poon, C. S., & Xie, Z. B. (2015). Influence of carbonated recycled concrete aggregate on properties of cement mortar. Construction and Building Materials, 98, 1–7. https://doi.org/10.1016/j.conbuildmat.2015.08.087
Zhang, N., Yu, H. F., Ma, H. Y., Ba, M. F., & He, Z. M. (2022). Effects of CO2 curing on properties of magnesium oxysulfate cement. Journal of Materials in Civil Engineering, 34, 1–14. https://doi.org/10.1061/(asce)mt.1943-5533.0004490
Liu, Z., & Meng, W. N. (2021). Fundamental understanding of carbonation curing and durability of carbonation-cured cement-based composites: A review. Journal of CO2 Utilization, 44, 101428. https://doi.org/10.1016/j.jcou.2020.101428
Sharma, D., & Goyal, S. (2018). Accelerated carbonation curing of cement mortars containing cement kiln dust: An effective way of CO2 sequestration and carbon footprint reduction. Journal of Cleaner Production, 192, 844–854. https://doi.org/10.1016/j.jclepro.2018.05.027
Poudyal, L., & Adhikari, K. (2021). Environmental sustainability in cement industry: An integrated approach for green and economical cement production. Resources, Environment and Sustainability, 4, 100024. https://doi.org/10.1016/j.resenv.2021.100024
Jeon, I. K., Qudoos, A., & Kim, H. G. (2021). Influence of carbonation curing on hydration and microstructure of magnesium potassium phosphate cement concrete. Journal of Building Engineering, 38, 102203. https://doi.org/10.1016/j.jobe.2021.102203
Ghiat, I., & Al-Ansari, T. (2021). A review of carbon capture and utilisation as a CO2 abatement opportunity within the EWF nexus. Journal of CO2 Utilization, 45, 101432. https://doi.org/10.1016/j.jcou.2020.101432
Ruan, S. Q., Wang, T., Guo, R. N., & Unluer, C. (2021). Assessment of the properties and environmental impact of carbonated reactive magnesia containing industrial waste. Thermochimica Acta, 706, 179051. https://doi.org/10.1016/j.tca.2021.179051
Ravikumar, D., Zhang, D., Keoleian, G., Miller, S., Sick, V., & Li, V. (2021). Carbon dioxide utilization in concrete curing or mixing might not produce a net climate benefit. Nature Communications, 12, 1–13. https://doi.org/10.1038/s41467-021-21148-w
Hepburn, C., Adlen, E., Beddington, J., Carter, E. A., Fuss, S., Dowell, N. M., Minx, J. C., Smith, P., & Williams, C. K. (2019). The technological and economic prospects for CO2 utilization and removal. Nature, 575, 87–97. https://doi.org/10.1038/s41586-019-1681-6
Hanifa, M., Agarwal, R., Sharma, U., Thapliyal, P. C., & Singh, L. P. (2023). A review on CO2 capture and sequestration in the construction industry: Emerging approaches and commercialised technologies. Journal of CO2 Utilization, 67, 102292. https://doi.org/10.1016/j.jcou.2022.102292
Seifritz, W. (1990). CO2 disposal by means of silicates. Nature, 345, 486. https://doi.org/10.1038/345486b0
Wang, L., Chen, L., Provis, J. L., Tsang, D. C. W., & Poon, C. S. (2020). Accelerated carbonation of reactive MgO and Portland cement blends under flowing CO2 gas. Cement and Concrete Composites, 106, 103489. https://doi.org/10.1016/j.cemconcomp.2019.103489
Wang, Y. X., Li, X. L/, & Liu, R. (2022). The capture and transformation of carbon dioxide in concrete: A review. Symmetry (Basel), 14(12), 2615. https://doi.org/10.3390/sym14122615
Zhang, D., Ghouleh, Z., & Shao, Y. X. (2017). Review on carbonation curing of cement-based materials. Journal of CO2 Utilization, 21, 119–131. https://doi.org/10.1016/j.jcou.2017.07.003
Zajac, M., Lechevallier, A., Durdzinski, P., Bullerjahn, F., Skibsted, J., & Ben Haha, M. (2020). CO2 mineralisation of Portland cement: Towards understanding the mechanisms of enforced carbonation. Journal of CO2 Utilization, 38, 398–415. https://doi.org/10.1016/j.jcou.2020.02.015
Sanjuán, M. Á., Andrade, C., Mora, P., & Zaragoza, A. (2020). Carbon dioxide uptake by cement-based materials: A Spanish case study. Applied Sciences, 10(1), 339. https://doi.org/10.3390/app10010339
Fang, Y. F., & Chang, J. (2015). Microstructure changes of waste hydrated cement paste induced by accelerated carbonation. Construction and Building Materials, 76, 360–365. https://doi.org/10.1016/j.conbuildmat.2014.12.017
Xian, X. P., Zhang, D., & Shao, Y. X. (2021). Flue gas carbonation curing of cement paste and concrete at ambient pressure. Journal of Cleaner Production, 313, 127943. https://doi.org/10.1016/j.jclepro.2021.127943
Shi, Y., He, Y. J., & Cao, Y. (2014). Effect of preconditioning on process and microstructure of carbon dioxide cured concrete. Journal of the Chinese Ceramic Society, 42, 999–1004. https://doi.org/10.7521/j.issn.04545648.2014.08.07
El-Hassan, H., Shao, Y. X., & Ghouleh, Z. (2013). Effect of initial curing on carbonation of lightweight concrete masonry units. ACI Materials Journal, 110, 441–450
Jang, J. G., & Lee, H. K. (2016). Microstructural densification and CO2 uptake promoted by the carbonation curing of belite-rich Portland cement. Cement and Concrete Research, 82, 50–57. https://doi.org/10.1016/j.cemconres.2016.01.001
Shao, Y. X., Mirza, M. S., & Wu, X. R. (2006). CO2 sequestration using calcium-silicate concrete. Canadian Journal of Civil Engineering, 33, 776–784. https://doi.org/10.1139/l05-105
Rostami, V., Shao, Y. X., Boyd, A. J., & He, Z. (2012). Microstructure of cement paste subject to early carbonation curing. Cement and Concrete Research, 42, 186–193. https://doi.org/10.1016/j.cemconres.2011.09.010
Mo, L. W., Zhang, F., Deng, M., Jin, F., Al-Tabbaa, A., & Wang, A. G. (2017). Accelerated carbonation and performance of concrete made with steel slag as binding materials and aggregates. Cement and Concrete Composites, 83, 138–145. https://doi.org/10.1016/j.cemconcomp.2017.07.018
Cao, F. Z., Miao, M., & Yan, P. Y. (2018). Hydration characteristics and expansive mechanism of MgO expansive agents. Construction and Building Materials, 183, 234–242. https://doi.org/10.1016/j.conbuildmat.2018.06.164
Dung, N. T., & Unluer, C. (2017). Carbonated MgO concrete with improved performance: The influence of temperature and hydration agent on hydration, carbonation and strength gain. Cement and Concrete Composites, 82, 152–164. https://doi.org/10.1016/j.cemconcomp.2017.06.006
Shi, C. J., Wu, Z. M., Cao, Z. J., Ling, T. C., & Zheng, J. L. (2018). Performance of mortar prepared with recycled concrete aggregate enhanced by CO2 and pozzolan slurry. Cement and Concrete Composites, 86, 130–138. https://doi.org/10.1016/j.cemconcomp.2017.10.013
Soares, E. G., & Castro-Gomes, J. (2021). Carbonation curing influencing factors of Carbonated Reactive Magnesia Cements (CRMC) – A review. Journal of Cleaner Production, 305, 127210. https://doi.org/10.1016/j.jclepro.2021.127210
Dung, N. T., Lesimple, A., Hay, R., Celik, K., & Unluer, C. (2019). Formation of carbonate phases and their effect on the performance of reactive MgO cement formulations. Cement and Concrete Research, 125, 105894. https://doi.org/10.1016/j.cemconres.2019.105894
Sonat, C., Lim, C. H., Liska, M., & Unluer, C. (2017). Recycling and reuse of reactive MgO cements – A feasibility study. Construction and Building Materials, 157, 172–181. https://doi.org/10.1016/j.conbuildmat.2017.09.068
Unluer, C., & Al-Tabbaa, A. (2014). Enhancing the carbonation of MgO cement porous blocks through improved curing conditions. Cement and Concrete Research, 59, 55–65. https://doi.org/10.1016/j.cemconres.2014.02.005
Gardeh, M. G., Kistanov, A. A., Nguyen, H., Manzano, H., Cao, W., & Kinnunen, P. (2022). Exploring mechanisms of hydration and carbonation of MgO and Mg(OH)2in reactive magnesium oxide-based cements. Journal of Physical Chemistry C, 126, 6196–6206. https://doi.org/10.1021/acs.jpcc.1c10590
Unluer, C. (2018). Carbon dioxide sequestration in magnesium-based binders. Elsevier Ltd. https://doi.org/10.1016/B978-0-08-102444-7.00007-1
Rausis, K., Ćwik, A., & Casanova, I. (2020). Phase evolution during accelerated CO2 mineralization of brucite under concentrated CO2 and simulated flue gas conditions. Journal of CO2 Utilization, 37, 122–133. https://doi.org/10.1016/j.jcou.2019.12.007
Zarandi, A. E., Larachi, F., Beaudoin, G., Plante, B., & Sciortino, M. (2017). Nesquehonite as a carbon sink in ambient mineral carbonation of ultramafic mining wastes. Chemical Engineering Journal, 314, 160–168. https://doi.org/10.1016/j.cej.2017.01.003
Meng, D., Unluer, C., Yang, E. H., & Qian, S. Z. (2023). Recent advances in magnesium-based materials: CO2 sequestration and utilization, mechanical properties and environmental impact. Cement and Concrete Composites, 138, 104983. https://doi.org/10.1016/j.cemconcomp.2023.104983
Haque, M. A., Chen, B., & Liu, Y. T. (2020). The role of bauxite and fly-ash on the water stability and microstructural densification of magnesium phosphate cement composites. Construction and Building Materials, 260, 119953. https://doi.org/10.1016/j.conbuildmat.2020.119953
Haque, M. A., Chen, B., & Li, S. J. (2022). Water-resisting performances and mechanisms of magnesium phosphate cement mortars comprising with fly-ash and silica fume. Journal of Cleaner Production, 369, 133347. https://doi.org/10.1016/j.jclepro.2022.133347
Haque, M. A., Chen, B., & Maierdan, Y. (2022). Influence of supplementary materials on the early age hydration reactions and microstructural progress of magnesium phosphate cement matrices. Journal of Cleaner Production, 333, 130086. https://doi.org/10.1016/j.jclepro.2021.130086
Haque, M. A., Chen, B., Liu, Y. T., Farasat Ali Shah, S., & Ahmad, M. R. (2020). Improvement of physico-mechanical and microstructural properties of magnesium phosphate cement composites comprising with Phosphogypsum. Journal of Cleaner Production, 261, 121268. https://doi.org/10.1016/j.jclepro.2020.121268
Haque, M. A., Chen, B., Maierdan, Y., & Wang, J. M. (2022). Hydration products and microstructural properties analysis of magnesium phosphate cement comprising with industrial residues. Construction and Building Materials, 344, 128228. https://doi.org/10.1016/j.conbuildmat.2022.128228
Haque, M. A., Pen, D., & Chen, B. (2020). Effects of aluminum silicate on mechanical strength and microstructural improvement of magnesium phosphate cement mortar. Journal of Materials in Civil Engineering, 32, 04020360. https://doi.org/10.1061/(asce)mt.1943-5533.0003413
Haque, M. A., & Chen, B. (2020). In vitro and in vivo research advancements on the magnesium phosphate cement biomaterials: A review. Materialia, 13, 100852. https://doi.org/10.1016/j.mtla.2020.100852
Vandeperre, L. J., & Al-Tabbaa, A. (2007). Accelerated carbonation of reactive MgO cements. Advances in Cement Research, 19, 67–79. https://doi.org/10.1680/adcr.2007.19.2.67
Power, I. M., Dipple, G. M., & Francis, P. S. (2017). Assessing the carbon sequestration potential of magnesium oxychloride cement building materials. Cement and Concrete Composites, 78, 97–107. https://doi.org/10.1016/j.cemconcomp.2017.01.003
Qu, Z. Y., Wang, F. Z., Liu, P., Yu, Q. L., & Brouwers H. J. H. (2020). Super-hydrophobic magnesium oxychloride cement (MOC): From structural control to self-cleaning property evaluation. Materials and Structures, 53. https://doi.org/10.1617/s11527-020-01462-3
He, P. P., Poon, C. S., & Tsang, D. C. W. (2017). Effect of pulverized fuel ash and CO2 curing on the water resistance of magnesium oxychloride cement (MOC). Cement and Concrete Research, 97, 115–122. https://doi.org/10.1016/j.cemconres.2017.03.005
Maravelaki-Kalaitzaki, P., & Moraitou, G. (1999). Sorel’s cement mortars: Decay susceptibility and effect on Pentelic marble. Cement and Concrete Research, 29, 1929–1935. https://doi.org/10.1016/S0008-8846(99)00197-0
De Castellar, M. D., Lorente, J. C., Traveria, A., & Tura, J. M. (1996). Cracks in Sorel’s cement polishing bricks as a result of magnesium oxychloride carbonatation. Cement and Concrete Research, 26, 1199–1202. https://doi.org/10.1016/0008-8846(96)00102-0
Carbon dioxide uptake by MOC-based materials. Applied Science,10, 2254. https://doi.org/10.3390/app10072254
Xu, B. W., Ma, H. Y., Hu, C. L., Yang, S. Q., & Li, Z. J. (2016). Influence of curing regimes on mechanical properties of magnesium oxychloride cement-based composites. Construction and Building Materials, 102, 613–619. https://doi.org/10.1016/j.conbuildmat.2015.10.205
Qin, L., Gao, X. J., Li, W. G., & Ye, H. (2018). Modification of magnesium oxysulfate cement by incorporating weak acids. Journal of Materials in Civil Engineering, 30, 1–9. https://doi.org/10.1061/(asce)mt.1943-5533.0002418
Li, Q. Y., Su, A. S., & Gao, X. J. (2022). Preparation of durable magnesium oxysulfate cement with the incorporation of mineral admixtures and sequestration of carbon dioxide. Science of the Total Environment, 809, 152127. https://doi.org/10.1016/j.scitotenv.2021.152127
Li, Q. Y., Zhang, L. C., Gao, X. J., & Zhang, J. Y. (2020). Effect of pulverized fuel ash, ground granulated blast-furnace slag and CO2 curing on performance of magnesium oxysulfate cement. Construction and Building Materials, 230, 116990. https://doi.org/10.1016/j.conbuildmat.2019.116990
Gu, K., Chen, B., Bi, W. L., & Guan, Y. (2021). Recycling of waste gypsum in preparation of magnesium oxychloride cement (MOC). Journal of Cleaner Production, 313, 127958. https://doi.org/10.1016/j.jclepro.2021.127958
Ba, M. F., Xue, T., He, Z. M., Wang, H., & Liu, J. Z. (2019). Carbonation of magnesium oxysulfate cement and its influence on mechanical performance. Construction and Building Materials, 223, 1030–1037. https://doi.org/10.1016/j.conbuildmat.2019.07.341
Panesar, D. K., & Mo, L. W. (2013). Properties of binary and ternary reactive MgO mortar blends subjected to CO2 curing. Cement and Concrete Composites, 38, 40–49. https://doi.org/10.1016/j.cemconcomp.2013.03.009
Pu, L., & Unluer, C. (2016). Investigation of carbonation depth and its influence on the performance and microstructure of MgO cement and PC mixes. Construction and Building Materials, 120, 349–363. https://doi.org/10.1016/j.conbuildmat.2016.05.067
Mo, L. W., Zhang, F., & Deng, M. (2015). Effects of carbonation treatment on the properties of hydrated fly ash-MgO-Portland cement blends. Construction and Building Materials, 96, 147–154. https://doi.org/10.1016/j.conbuildmat.2015.07.193
Mo, L. W., & Panesar, D. K. (2013). Accelerated carbonation - A potential approach to sequester CO2 in cement paste containing slag and reactive MgO. Cement and Concrete Composites, 43, 69–77. https://doi.org/10.1016/j.cemconcomp.2013.07.001
Andrew, R. M. (2019). Global CO2 emissions from cement production, 1928–2018. Earth System Science Data, 11, 1675-1710
Meldrum, F. C., & Hyde, S. T. (2001). Morphological influence of magnesium and organic additives on the precipitation of calcite. Journal of Crystal Growth, 231, 544–558. https://doi.org/10.1016/S0022-0248(01)01519-6
Pokrovsky, O. S. (1998). Precipitation of calcium and magnesium carbonates from homogeneous supersaturated solutions. Journal of Crystal Growth, 186, 233–239. https://doi.org/10.1016/S0022-0248(97)00462-4
De Silva, P., Bucea, L., & Sirivivatnanon, V. (2009). Chemical, microstructural and strength development of calcium and magnesium carbonate binders. Cement and Concrete Research, 39, 460–465. https://doi.org/10.1016/j.cemconres.2009.02.003
Jang, H., So, S., & Lim, Y. (2022). Carbonation, CO2 sequestration, and physical properties based on the mineral size of light burned MgO using carbonation accelerator. Journal of Cleaner Production, 379, 134648. https://doi.org/10.1016/j.jclepro.2022.134648
Gao, Y. X., Yu, B. Y., Kong, Y. N., Cheng, B. J., Yang, W., Wu, J., & Zhu, X. L. (2022). Effect of carbonisation on the mechanical properties and hydration of magnesium ammonium phosphate cement. Ceramics-Silikáty, 66, 10–18. https://doi.org/10.13168/cs.2021.0047
Hay, R., & Celik, K. (2020). Hydration, carbonation, strength development and corrosion resistance of reactive MgO cement-based composites. Cement and Concrete Research, 128, 105941. https://doi.org/10.1016/j.cemconres.2019.105941
Hu, Z. Q., Guan, Y., Chang, J., Bi, W. L., & Zhang, T. T. (2020). Effect of carbonation on the water resistance of steel slag—magnesium oxysulfate (Mos) cement blends. Materials (Basel), 13, 1–16. https://doi.org/10.3390/ma13215006
Hay, R., & Celik, K. (2022). Enhancing carbonation of magnesium oxide (MgO) cement (RMC)-based composites with calcined limestone. Cement, 9, 100037. https://doi.org/10.1016/j.cement.2022.100037
Guan, Y., Hu, Z. Q., Zhang, Z. H., Chang, J., Bi, W. L., Cheeseman, C. R., & Zhang, T. T. (2021). Effect of hydromagnesite addition on the properties and water resistance of magnesium oxysulfate (MOS) cement. Cement and Concrete Research, 143, 106387. https://doi.org/10.1016/j.cemconres.2021.106387
Wang, D. X., Gao, X. Y., Liu, X. Q., & Zeng, G. (2021). Strength, durability and microstructure of granulated blast furnace slag-modified magnesium oxychloride cement solidified waste sludge. Journal of Cleaner Production, 292, 126072. https://doi.org/10.1016/j.jclepro.2021.126072
Li, Z. H., Zhang, T. S., Hu, J., Tang, Y., Niu, Y. F., Wei, J. X., & Yu, Q. J. (2014). Characterization of reaction products and reaction process of MgO-SiO 2–H2O system at room temperature. Construction and Building Materials, 61, 252–259. https://doi.org/10.1016/j.conbuildmat.2014.03.004
De Blasio, C. (2019). Thermogravimetric analysis (TGA). Green Energy Technologies, 91–102. https://doi.org/10.1007/978-3-030-11599-9_7
Gu, K., Chen, B., Yu, H. F., Zhang, N., Bi, W. L., & Guan, Y. (2021). Characterization of magnesium-calcium oxysulfate cement prepared by replacing MgSO4 in magnesium oxysulfate cement with untreated desulfurization gypsum. Cement and Concrete Composites, 121, 104091. https://doi.org/10.1016/j.cemconcomp.2021.104091
Abdel-Gawwad, H. A., Abd El-Aleem, S., Amer, A. A., El-Didamony, H., & Arif, M. A. (2018). Combined impact of silicate-amorphicity and MgO-reactivity on the performance of Mg-silicate cement. Construction and Building Materials, 189, 78–85. https://doi.org/10.1016/j.conbuildmat.2018.08.171
Hay, R., & Celik, K. (2020). Accelerated carbonation of reactive magnesium oxide cement (RMC)-based composite with supercritical carbon dioxide (scCO2). Journal of Cleaner Production, 248, 119282. https://doi.org/10.1016/j.jclepro.2019.119282
Jagadesh, D., Kubota, N., Yokota, M., Doki, N., & Sato, A. (1999). Seeding effect on batch crystallization of potassium sulfate under natural cooling mode and a simple design method of crystallizer. Journal of Chemical Engineering of Japan, 32, 514–520. https://doi.org/10.1252/jcej.32.514
Ruan, S., & Unluer, C. (2016). Comparative life cycle assessment of reactive MgO and Portland cement production. Journal of Cleaner Production, 137, 258–273. https://doi.org/10.1016/j.jclepro.2016.07.071
Zhang, R. X., Arrigoni, A., & Panesar, D. K. (2021). Could reactive MgO cement be a green solution? The effect of CO2 mineralization and manufacturing route on the potential global warming impact. Cement and Concrete Composites, 124, 104263. https://doi.org/10.1016/j.cemconcomp.2021.104263
Dung, N. T., & Unluer, C. (2020). Influence of accelerated hydration and carbonation on the performance of reactive magnesium oxide concrete. Advances in Cement Research, 32, 78–90. https://doi.org/10.1680/jadcr.17.00186
Nobre, J., Ahmed, H., Bravo, M., Evangelista, L., De Brito, J. (2020). Magnesia (MgO) production and characterization, and its influence on the performance of cementitious materials: A review. Materials, 13, 4752. https://doi.org/10.3390/ma13214752
Sahoo, N., Kumar, A., & Samsher. (2022). Review on energy conservation and emission reduction approaches for cement industry. Environment and Behaviour, 44, 100767. https://doi.org/10.1016/j.envdev.2022.100767
Dung, N. T., Hay, R., Lesimple, A., Celik, K., & Unluer, C. (2021). Influence of CO2 concentration on the performance of MgO cement mixes. Cement and Concrete Composites, 115, 103826. https://doi.org/10.1016/j.cemconcomp.2020.103826
Taj, K., Akturk, B., & Ulukaya, S. (2023). Influence of carbonation curing and nano-silica incorporation on compressive strength and micro-structural development of binary RMC-based systems. Journal of Building Engineering, 66, 105856. https://doi.org/10.1016/j.jobe.2023.105856
Hay, R., Khalil, A., Otchere, C., Alanis, S., & Celik, K. (2023). Proportioning, carbonation, performance assessment, and application of reactive magnesium oxide cement-based composites with superplasticizers. Journal of Materials in Civil Engineering, 35, 1–12. https://doi.org/10.1061/(asce)mt.1943-5533.0004552
Dung, N. T., & Unluer, C. (2017). Sequestration of CO2 in reactive MgO cement-based mixes with enhanced hydration mechanisms. Construction and Building Materials, 143, 71–82. https://doi.org/10.1016/j.conbuildmat.2017.03.038
Wu, H. L., Zhang, D., Ellis, B. R., & Li, V. C. (2018). Development of reactive MgO-based Engineered Cementitious Composite (ECC) through accelerated carbonation curing. Construction and Building Materials, 191, 23–31. https://doi.org/10.1016/j.conbuildmat.2018.09.196
Ruan, S., & Unluer, C. (2017). Influence of mix design on the carbonation, mechanical properties and microstructure of reactive MgO cement-based concrete. Cement and Concrete Composites, 80, 104–114. https://doi.org/10.1016/j.cemconcomp.2017.03.004
Sun, Q., Ba, M. F., Zhang, D. L., Zhou, S. S., Zhang, N., & Wang, F. (2022). Effects of citrate acid, solidum silicate and their compound on the properties of magnesium oxysulfate cement under carbonation condition. Construction and Building Materials, 330, 127136. https://doi.org/10.1016/j.conbuildmat.2022.127136
Monkman, S., & Shao, Y. X. (2010). Carbonation curing of slag-cement concrete for binding CO2 and improving performance. Journal of Materials in Civil Engineering, 22, 296–304. https://doi.org/10.1061/(asce)mt.1943-5533.0000018
Morrison, J., Jauffret, G., Galvez-Martos, J. L., & Glasser, F. P. (2016). Magnesium-based cements for CO2 capture and utilisation. Cement and Concrete Research, 85, 183–191. https://doi.org/10.1016/j.cemconres.2015.12.016
Acknowledgements
This study was supported by The Hong Kong Polytechnic University through a University Financial Support for Awardees of Major Renowned Funding and Award Schemes awarded to the corresponding author.
Author information
Authors and Affiliations
Contributions
Conceptualization: M. Aminul Haque, Jian-Guo Dai and Xiao-Ling Zhao. Data collection and writing: M. Aminul Haque. Review & editing: Jian-Guo Dai and Xiao-Ling Zhao. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Authors declare that there is no conflict of interest on the information reported in this review manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haque, M.A., Dai, JG. & Zhao, XL. Magnesium cements and their carbonation curing: a state-of-the-art review. Low-carbon Mater. Green Constr. 2, 2 (2024). https://doi.org/10.1007/s44242-023-00033-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44242-023-00033-3