Abstract
Objective
Mitochondrial DNA (mtDNA) reportedly has diagnostic and predictive value in critically ill patients. This study evaluated the diagnostic and predictive value of mtDNA in patients undergoing continuous venovenous haemofiltration (CVVH).
Methods
We consecutively enrolled 41 patients who were treated with CVVH from September 2018 to December 2019. Prefilter, postfilter, and ultrafiltrate samples were collected before the initiation of CVVH (T0) and 6 and 12 h after CVVH. The total mass removal rate (Mtr), total mass adsorption rate (Mad), plasma clearance (PC), and sieving coefficient (SC) were calculated based on the mass conservation principle.
Results
The plasma mtDNA concentration in patients at T0 prefilter was higher than that in healthy volunteers [13.77 (12.45–15.86) vs. 1.24 (1.15–1.34) ng/mL, P < 0.001]. Prefilter, but not postfilter or ultrafiltrate, mtDNA decreased during CVVH (P = 0.02), with a total CVVH clearance of 25.9%. The postfilter and ultrafiltrate mtDNA levels were lower than the prefilter level at each time point (P < 0.05 for all). The Mtr, Mad, PC, and SC did not change over time (P > 0.05 for all).
Conclusions
Plasma mtDNA in critically ill patients was significantly affected by CVVH.
Trial registration: Retrospectively registered on 20 August 2019 at ClinicalTrials.gov (NCT04083482).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Damage-associated molecular patterns (DAMPs) are endogenous molecules that are released by host cell death; these endogenous danger signals are derived from immune cells that are activated by damaged or necrotic tissues [1, 2]. DAMPs can cause an innate immune response or directly or indirectly initiate an adaptive immune response [2, 3]. A variety of DAMPs, including high mobility group box 1 (HMGB1), heat shock protein 70 (HSR70), and interleukin (IL)-1α, have been identified [4,5,6]. They can be used as agonists or antagonists of Toll-like receptors (TLRs) and participate in the signal cascade reactions of conditional TLRs and NOD-like receptors [7,8,9,10].
Mitochondria are sources of cell energy and centres of free radical metabolism, and they play important roles in cell apoptosis, calcium regulation, the cell cycle, and signal transduction. Mitochondrial DNA (mtDNA) is the genetic material of mitochondria. Mitochondrial function is closely related to the quantity and quality of mtDNA. Only a small part of the mtDNA is stable, while the remaining parts are unstable without histone wrapping. mtDNA can replicate itself via a process carried out in a semi-reserved manner and without introns [11]. mtDNA is in a state of continuous synthesis throughout the cell cycle. Such a dynamic process is susceptible to interference from external factors and has poor stability. Damaged mitochondria can be digested by the lysosomal system, while mtDNA escaping autophagy can autonomously mediate the inflammatory reaction [12]. The aberrant release of mtDNA during cellular stress and injury is an increasingly recognized trigger of inflammatory responses in human diseases. In recent years, scholars have found that endogenous mtDNA that is released as a result of trauma, shock, sepsis, etc., is an important DAMP [13,14,15].
Continuous renal replacement therapy (CRRT) is a common technique for rescuing severely ill patients in intensive care units (ICUs). This technique helps remove inflammatory factors, metabolic wastes, and poisons, correct acid‒base electrolyte imbalances, and perform other renal replacement functions. Continuous venovenous haemofiltration (CVVH) is the most common blood purification method and can remove medium- and small-molecule solutes from the blood. The principle involves convection, that is, liquid flows from a high-pressure chamber to a low-pressure chamber through a semipermeable membrane, and solutes move along with it. The transmembrane pressure is the driving force. The corresponding amount of liquid and electrolytes should be replenished before or after filtration to compensate for the filtered liquid and electrolytes and maintain the balance of the body’s internal environment. The molecular weight of mtDNA is 15.7 ~ 19.5 kb, and it can theoretically be eliminated by CVVH; however, the evidence is currently insufficient. A small-sample study reported that CVVH could reduce blood mtDNA concentrations from 717.7 pg/mL to 237.5 pg/mL in patients with severe acute pancreatitis [16]. In the present study, we aimed to investigate the impact of CVVH on mtDNA concentrations in critically ill patients.
2 Materials and Methods
2.1 Participants
The ethical committee of the First Peoples’ Hospital approved this study. The study was retrospectively registered with the US National Institutes of Health Clinical Trials Register (NCT04083482). Informed consent was obtained from all study participants or their legal representatives. Patients who were treated with CVVH at our institution were consecutively recruited for the study between August 1, 2018, and December 31, 2019. Ten healthy volunteers were recruited as a control group.
2.2 Inclusion, Exclusion, and Exit Criteria
Consecutive adult patients aged > 18 years who were treated with CVVH at our ICU were assessed for inclusion.
Patients with the following conditions were excluded: (1) liver function Child‒Pugh score > III; (2) chronic kidney disease; (3) malignant tumour; (4) a history of radiotherapy and chemotherapy; (5) pregnancy; (6) transplantation at any time; (7) immunodeficiency diseases such as acquired immune deficiency syndrome; and (8) refusal to participate in this study.
Patients exited the study due to any of the following reasons: (1) had a CVVH duration < 12 h, (2) received a blood transfusion during CVVH, (3) had missing data, or (4) requested withdrawal at any time.
2.3 CVVH Procedure
Vascular access was first established in the femoral or internal jugular vein with an 11- to 14-Fr double-lumen catheter. We used a Fresenius 4008S CRRT plus machine (Fresenius Medical Care, Bad Homburg v.d.H., Germany) or a Prismaflex machine (Gambro AB, Lund, Sweden) for CVVH. The haemodiafiltration membranes that were used during treatment were a Fresenius AV600S (Fresenius Medical Care) or Prismaflex M150 (Gambro AB) membrane. The CVVH parameters were as follows: blood flow rate, 180–220 mL/min and replacement fluid infusion rate, 1,800–2,200 mL/h. The replacement solution formula included solutions A and B. Solution A contained normal saline, 5% glucose solution, water for injection, magnesium sulfate, sodium bicarbonate, and potassium chloride; solution B contained calcium gluconate. We used heparinization, local heparinization, or citric acid for anticoagulation according to the patient’s coagulation function. There was no specific requirement before or after dilution.
2.4 Data Collection
Patient demographic data, including age, sex, aetiological factors, and underlying diseases, were obtained at recruitment. Basic laboratory tests, including white blood cell count and C-reactive protein and procalcitonin levels, were recorded at CVVH initiation. Clinical data that were required for calculating sequential organ failure assessment (SOFA) and acute physiology and chronic health evaluation II (APACHE II) scores to evaluate disease severity were also collected.
2.5 Detection of mtDNA
Prefilter, postfilter, and ultrafiltrate samples were obtained at the beginning of CRRT and again after 6 and 12 h (T0, T6h, and T12h, respectively). mtDNA was extracted using a mitochondrial DNA isolation kit (BioVision, Milpitas, CA, USA) following the manufacturer’s standard protocol. The plasma mtDNA concentration was measured by determining the level of the human mitochondrial cytochrome B (h-mt-cytB) gene using a real-time quantitative PCR assay performed on a 7500 real-time PCR system (ABI-7500, Applied Biosystems, Foster City, CA, USA). The primers for the h-mt-cytB gene were as follows: forward: 5′-CTA GGC GAC CCA GAC AAT TAT AC-3′ and reverse: 5′-TTA GGG ACG GAT CGG AGA AT-3′. The absolute mtDNA concentration was calculated according to the standard curve. Each sample was run in triplicate, and the mean value was used for further analysis.
2.6 Calculations
The following formulas that were used to calculate the plasma mtDNA clearance were based on the mass conservation principle.
SA1bbreviations used in the formulas: HCT: Haematocrit, Ci: Concentration in the inlet plasma before adding the replacement fluid (ng/mL), Co: Concentration in outlet plasma (ng/mL), Cuf: Concentration in the ultrafiltrate (ng/mL), Qb: Inlet blood flow rate (mL/min), Qi: Inlet plasma flow rate (mL/min), Qo: Outlet plasma flow rate (mL/min), Quf: Ultrafiltration flow rate (mL/min), Mi: Mass inlet rate (ng/min), Mo: Mass outlet rate (ng/min), Muf: Mass ultrafiltration rate (ng/min), Mtr: Mass removal rate (ng/min), Mad: Mass adsorption rate (ng/min), PC: Plasma clearance, SC: Sieving coefficient.
2.7 Statistical Analysis
Normally distributed data are reported as the mean ± standard deviation (SD), and nonnormally distributed data are expressed as the median (interquartile interval). Categorical variables are presented as frequencies (n, %). The Mann‒Whitney U test was used to compare the difference in mtDNA concentration between the patients and volunteers. The Kruskal‒Wallis H test was used to compare measurements and calculations based on the data. mtDNA changes over time were compared using repeated measures analysis of variance (ANOVA). Differences with P < 0.05 were considered to indicate statistical significance. All the statistical analyses were performed using IBM SPSS Statistics for Windows, version 19.0 (IBM Corp., Armonk, NY, USA).
3 Results
Seventy-six patients were screened in the study. At baseline, forty-one patients were enrolled in the study. Among these patients, three were excluded following a withdrawal by their next of kin, four were excluded because their CVVH duration was less than 12 h, and 12 were excluded because their data were inadequate. Thus, this study included 22 patients (16 males and six females) and ten healthy volunteers (five males and five females) as controls. The participant recruitment flowchart is shown in Fig. 1. The femoral or internal jugular vein was established in all patients. Clinical characteristics and biological data at T0 are shown in Tables 1 and 2.
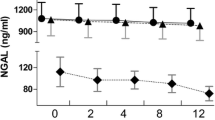
All the plasma and ultrafiltration samples had detectable levels of mtDNA. The patients’ median plasma mtDNA concentration at T0 was significantly higher than that of the healthy volunteers [13.77 (12.45–15.86) versus 1.24 (1.15–1.34) ng/mL, P < 0.001]. The prefilter mtDNA levels significantly decreased during the 12 h of CVVH (P = 0.02, Fig. 2), while the mtDNA concentrations in the postfilter and ultrafiltrate samples remained unchanged (P > 0.05). Furthermore, the mtDNA concentrations in the postfilter and ultrafiltrate samples were significantly lower than those in the prefilter samples at each time point (P < 0.05 for all). The total PC during the CVVH treatment was 25.9%. The mtDNA Mtr, Mad, SC, and PC during CVVH treatment are shown in Fig. 3. These values did not change at the different time points.
Total mass removal rate, mass adsorption rate, sieving coefficient, and plasma clearance of mtDNA during CVVH. The total mass removal rate (a), mass adsorption rate (b), sieving coefficient (c), and plasma clearance (d) did not change over time. mtDNA mitochondrial DNA, CVVH continuous venovenous haemofiltration
4 Discussion
Many studies have been published on the diagnostic and predictive value of mtDNA as a DAMP [17,18,19]. However, these studies have ignored the effect of changes in plasma mtDNA concentrations during CVVH intervention. The present study demonstrated that CVVH significantly affects plasma mtDNA concentration [20].
mtDNA is rich in demethylated CpG sequences, and it can induce an inflammatory reaction. CpG mononucleotides mediate mitogen-activated protein kinase phosphorylation, leading to the upregulation of NF-κB via the TLR9 receptor and thus to the induction of inflammatory reactions [8, 21, 22]. In recent years, many studies have reported increases in plasma mtDNA concentrations in various diseases and have suggested the use of mtDNA as a biomarker of changes in illness severity and prognosis prediction [23,24,25,26]. Oka et al. showed that mtDNA plays an important role in inducing and maintaining inflammation in the heart [27]. Hu et al. demonstrated that plasma mtDNA levels were associated with the occurrence of sepsis, multiple organ dysfunction syndrome, and death in patients with intra-abdominal injuries caused by severe abdominal trauma [28]. Nakahira et al. reported that mtDNA could serve as a viable plasma biomarker in ICU patients [10].
CVVH is commonly used in the ICU to treat critically ill patients. The molecular weight of mtDNA is approximately 15.7 ~ 19.5 kb, and it can theoretically be filtered out by CVVH. Hence, it is particularly important to verify the mtDNA clearance rate during CVVH. A small sample size study reported that 12 h of CVVH could significantly reduce plasma mtDNA concentrations by 67% in patients with severe acute pancreatitis [16]. In our study, after 12 h of CVVH treatment, the plasma mtDNA concentration was significantly decreased by 25.9%. It is evident that CVVH can significantly decrease mtDNA levels and thus reduce their damaging effect. However, mtDNA clearance by CVVH might affect the accuracy of using the mtDNA level as a diagnostic and predictive biomarker in critically ill patients in clinical practice.
This study had several limitations. First, the sample size was small, so our results need to be confirmed by investigating a larger number of patients. Second, we did not assess the effect of anticoagulants on mtDNA clearance by CVVH. Third, our results did not explore the effect of the filtration membrane, CRRT mode or dilution mode on the mtDNA concentration, so the impact of conditions of CRRT should be considered in future studies. Last, this study did not include patients who did not receive CVVH treatment as a control group for ethical reasons.
The results of this study suggest that plasma mtDNA was significantly removed by CVVH. This finding indicates that CVVH plays a therapeutic role by removing the DAMP of mtDNA and that the diagnostic and predictive value of mtDNA for disease severity during CVVH treatment should be considered.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Patel S. Danger-associated molecular patterns (DAMPs): the derivatives and triggers of inflammation. Curr Allergy Asthma Rep. 2018;18(11):63.
Nakahira K, Hisata S, Choi AMK. The roles of mitochondrial damage-associated molecular patterns in diseases. Antioxid Redox Signal. 2015;23(17):1329–50.
Pouwels SD, Heijink IH, ten Hacken NHT, et al. DAMPs activating innate and adaptive immune responses in COPD. Mucosal Immunol. 2014;7(2):215–26.
Coleman LG Jr, Maile R, Jones SW, et al. HMGB1/IL-1β complexes in plasma microvesicles modulate immune responses to burn injury. PLoS ONE. 2018;13(3):e195335.
Malik A, Kanneganti T-D. Function and regulation of IL-1α in inflammatory diseases and cancer. Immunol Rev. 2018;281(1):124–37.
Muth C, Rubner Y, Semrau S, et al. Primary glioblastoma multiforme tumors and recurrence: comparative analysis of the danger signals HMGB1, HSP70, and calreticulin. Strahlenther Onkol. 2016;192(3):146–55.
Choudhary V, Uaratanawong R, Patel RR, et al. Phosphatidylglycerol inhibits toll-like receptor-mediated inflammation by danger-associated molecular patterns. J Invest Dermatol. 2019;139(4):868–77.
Liu Y, Yan W, Tohme S, et al. Hypoxia induced HMGB1 and mitochondrial DNA interactions mediate tumor growth in hepatocellular carcinoma through Toll-like receptor 9. J Hepatol. 2015;63(1):114–21.
Johansson PI, Nakahira K, Rogers AJ, et al. Plasma mitochondrial DNA and metabolomic alterations in severe critical illness. Crit Care. 2018;22(1):360.
Nakahira K, Kyung SY, Rogers AJ, et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med. 2013;10(12):e1001577.
Duggan AT, Stoneking M. A highly unstable recent mutation in human mtDNA. Am J Hum Genet. 2013;92(2):279–84.
Perez-Trevino P, Velasquez M, Garcia N. Mechanisms of mitochondrial DNA escape and its relationship with different metabolic diseases. Biochim Biophys Acta Mol Basis Dis. 2020;1866(6):165761.
Timmermans K, Kox M, Scheffer GJ, et al. Plasma nuclear and mitochondrial DNA levels, and markers of inflammation, shock, and organ damage in patients with septic shock. Shock. 2016;45(6):607–12.
Harrington JS, Choi A, Nakahira K. Mitochondrial DNA in sepsis. Curr Opin Crit Care. 2017;23(4):284–90.
Yamanouchi S, Kudo D, Yamada M, et al. Plasma mitochondrial DNA levels in patients with trauma and severe sepsis: time course and the association with clinical status. J Crit Care. 2013;28(6):1027–31.
Wu L, Xu W, Wang F, et al. Plasma mtDNA analysis aids in predicting pancreatic necrosis in acute pancreatitis patients: a pilot study. Dig Dis Sci. 2018;63(11):2975–82.
West AP, Khoury-Hanold W, Staron M, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520(7548):553–7.
Aswani A, Manson J, Itagaki K, et al. Scavenging circulating mitochondrial DNA as a potential therapeutic option for multiple organ dysfunction in trauma hemorrhage. Front Immunol. 2018;9:891.
Simmons JD, Lee YL, Pastukh VM, et al. Potential contribution of mitochondrial DNA damage associated molecular patterns in transfusion products to the development of acute respiratory distress syndrome after multiple transfusions. J Trauma Acute Care Surg. 2017;82(6):1023–9.
Wu J, Ren J, Liu Q, et al. Effects of changes in the levels of Damage-Associated molecular patterns following continuous Veno-Venous hemofiltration therapy on outcomes in acute kidney injury patients with sepsis. Front Immunol. 2019;9:3052.
Zhang L, Deng S, Zhao S, et al. Intra-peritoneal administration of mitochondrial DNA provokes acute lung injury and systemic inflammation via toll-like receptor 9. Int J Mol Sci. 2016;17(9):1425.
Krychtiuk KA, Ruhittel S, Hohensinner PJ, et al. Mitochondrial DNA and Toll-Like receptor-9 are associated with mortality in critically ill patients. Crit Care Med. 2015;43(12):2633–41.
Singel KL, Grzankowski KS, Khan A, et al. Mitochondrial DNA in the tumour microenvironment activates neutrophils and is associated with worse outcomes in patients with advanced epithelial ovarian cancer. Br J Cancer. 2019;120(2):207–17.
Nakayama H, Otsu K. Mitochondrial DNA as an inflammatory mediator in cardiovascular diseases. Biochem J. 2018;475(5):839–52.
Zhang X, Wu X, Hu Q, et al. Mitochondrial DNA in liver inflammation and oxidative stress. Life Sci. 2019;236:116464.
Hu Q, Ren J, Ren H, et al. Urinary mitochondrial DNA identifies renal dysfunction and mitochondrial damage in Sepsis-Induced acute kidney injury. Oxid Med Cell Longev. 2018;2018:8074936.
Oka T, Hikoso S, Yamaguchi O, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485(7397):251–5.
Hu Q, Ren J, Wu J, et al. Elevated levels of plasma mitochondrial DNA are associated with clinical outcome in Intra-Abdominal infections caused by severe trauma. Surg Infect (Larchmt). 2017;18(5):610–8.
Acknowledgements
We thank the staff at the Department of Critical Care Medicine of the First Peoples’ Hospital of Chenzhou for their assistance in this study.
Funding
This work was supported by the Natural Science Foundation of China (No. 81601708), and the Natural Science Foundation of Hunan Province, China (No. 2018JJ2014), and the Project funded by China Postdoctoral Science Foundation (2019M65018), and the Foundation of Chenzhou Science and Technology Bureau (jsyf2017035), and the Project funded by Hunan Health Committee (B2016200).
Author information
Authors and Affiliations
Contributions
Yujing Wang and Zepeng Duan designed the study, participated in the acquisition of the data, performed the data analysis, and drafted the manuscript. Hua Ling and Qiong Li carried out the biochemical assays and contributed to the conception and design of the work, the analysis and interpretation of the data. Xingui Dai designed the study, guided the data analysis and the use of medical statistics, responded for protocol revisions, and final draft revision. All authors have read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical Approval and Consent to Participate
This study protocol was approved by the Institutional Review Committee on Human Research of the First People's Hospital of Chenzhou T (reference number 2018-013). All participants or their family members gave written informed consent.
Statement of Ethics
The ethical committee of the First Peoples’ Hospital approved this study. The study was registered retrospectively with the US National Institutes of Health Clinical Trials Register (NCT04083482). Informed consent was obtained from all study participants or their legal representatives.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Duan, Z., Ling, H. et al. Plasma Mitochondrial DNA Clearance by Continuous Venovenous Haemofiltration in Critically Ill Patients. Intensive Care Res 4, 143–148 (2024). https://doi.org/10.1007/s44231-024-00069-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44231-024-00069-4