Abstract
O-GlcNAc modification is a glycosylation modification that is commonly observed in nuclear and organelle proteins of cells. In recent years, several studies have shown that O-GlcNAc modification plays an important regulatory role in immune cells, which regulates their activity and function and inhibits inflammatory responses. It also enhances immune cells recognition and clearance of pathogens, and improves the host´s antibacterial immune response. Sepsis is a systemic inflammatory response to infection, whose development and progression are regulated by the immune system. Therefore, in the present review, we will discuss the mechanism of O-GlcNAc modification in immune cells and its potential therapeutic value in sepsis, which will be expected to provide new insights and targets for the treatment of sepsis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sepsis is a clinical syndrome that may progress to organ dysfunction by a dysregulated immune response to infection, causing high morbidity and mortality [1]. It is still the main cause of death in most intensive care units, which prompts research on pathogenesis of sepsis to discover new treatments.
Recent studies have shown that activation of pro- and anti-inflammatory responses rapidly occurs at the initial stages of sepsis but pro-inflammatory responses prevail, where innate immune system cells, including monocytes and neutrophils, release substantial amounts of pro-inflammatory cytokines. If sepsis persists, the anti-inflammatory response plays a major role, when the balance between the innate and adaptive immune systems is disrupted and the patient enters into a marked state of immunosuppression. Thus, it can be seen that in the early stage of sepsis, the pro-inflammatory response mediated by a cytokine storm predominates, whereas the late stage is characterized by long-term immunosuppression [2, 3]. The latest research on the subject suggests that such immunosuppression is mediated by programmed gene rearrangements and transcription in macrophages [4]. In recent years, aberrant gene expression for transcriptional control has been widely reported, which may be related to post-translational modifications (PTMs), such as acetylation or methylation [5, 6]. O-linked β-N-acetylglucosamine (O-GlcNAc) is an emerging PTM of protein serine and threonine residues, whose modification is believed to be involved in a variety of cellular life activities, such as transcriptional translation, signal transduction, cellular stress response, and metabolic immunity [7]. It has been shown that the O-GlcNAc cycle acts as metabolic and stress sensor, regulating many key processes that are impaired during sepsis [8]. Taken together, this review discusses mechanisms of innate and adaptive immune system alterations and the function of O-GlcNAc modification during sepsis. It is essential to elucidate the role of O-GlcNAc modification in immune cells and its cross-talk with sepsis to explore its therapeutic potential.
2 Immune Cells in Sepsis
The innate immune system is the first line of defense against invading pathogens, which mainly includes macrophages, neutrophils, and natural killer (NK) cells, whereas the adaptive immune system is mediated by T and B lymphocytes against specific antigens [9]. Dysfunction of the immune system is an important clinical feature of sepsis [Fig. 1]. Based on changes in sepsis progression, it is recognized that sepsis presents two stages, an immune activation stage, followed by a chronic immunosuppression stage. After the patient passes the initial strong pro-inflammatory reaction stage, the function of immune cells, including NK cells, CD4 + and CD8 + T cells, B lymphocytes, and dendritic cells (DC), begins to be impaired. However, apoptosis of neutrophils is delayed during this period, leading to increased recruitment of dysregulated immature neutrophils [10, 11], whose persistent presence causes massive production of reactive oxygen species, leading to extensive tissue damage [12].
In the later stage of sepsis, production, function, and survival of innate and adaptive immune cells are directly affected, leading to widespread immunosuppression, including massive apoptosis of NK cells, CD4 + T cells, B lymphocytes, and mononuclear macrophages, whereas apoptosis of neutrophils is delayed, and expansion of immature neutrophils occurs. In addition, Tregs are the only immune cells that proliferate during sepsis and exacerbate immunosuppression. Moreover, the secretion of cytokines and expression of surface receptors in some immune cells are also altered at this stage
A decreased potential of monocytes to release pro-inflammatory cytokines in the presence of lipopolysaccharide (LPS) and other Toll-like receptor (TLR) agonists and bacterial compounds in septic patients, has been previously reported. In this regard, monocytes from patients under sepsis, showed decreased production of the pro-inflammatory cytokines TNF-α, IL-6, and IL-12, and unimpaired or enhanced potential to release anti-inflammatory mediators, such as IL-10, which is consistent with the phenomenon of endotoxin tolerance [13]. Reduced expression of the human leukocyte antigen HLA-DR is also a phenomenon of endotoxin tolerance [14].
T cells play an important role in the immune system, whose T cell receptors (TCRs) participate in activating a variety of physiological processes after antigen binding. CD4 + T cells reduce the diversity of TCRs in sepsis, further increasing the risk of secondary infections [15]. After TCR activation, naive CD4 + T cells usually differentiate into different populations of effector cells, such as the helper T cells Th1, Th2, and Th17, and regulatory T cells (Tregs), which require the induction by the STATA protein [16]. It has been shown that the production of Th1 and Th2 cell-associated cytokines is reduced in the initial immune response to sepsis and trauma [17]. Other studies have shown that T-bet and GATA3 regulated the response of Th1 and Th2 cells, respectively, and the expression of these two transcription factors significantly decreased in sepsis. In addition, decreased Th17 cell response in sepsis may be due to reduced expression of the Th17 cell-specific transcription factor retinoic acid receptor-associated orphan receptor γt (RORγt) [18]. Unlike T-bet and GATA 3, expression of the Treg-associated transcription factor FOXP3 was not altered [19]. In sepsis, increased Tregs influence prognosis and are often involved in downregulating immune responses and contributing to an immunosuppressive phenotype during sepsis. This inhibitory effect is attributed to the expression and function of the transcription factor Foxp 3 [20].
3 The Basic Biological Function of O-GlcNAc
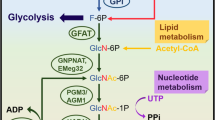
In the early 1980s, Hart et al. [21] found a new form of protein glycosylation detection in the sugar structure on living cell surfaces, which refers to a single N-acetylglucosamine (GlcNAc) connected with the O-glycosidic bond to the hydroxyl group of the serine/threonine of the protein, which is known as O-GlcNAc glycosylation modification. Different from existing glycosylation modifications, O-GlcNAc modifications mainly have the following characteristics [22,23,24,25]: (1) GlcNAc is composed of monosaccharide molecules, which are not further prolonged or modified into more complex sugar structures, (2) except for a few exceptions, it mainly exists in the cytoplasm and nuclear proteins, (3) O-GlcNAc modification is a dynamic and reversible modification process, which is the product of nutrient conversion flow through the hexosamine biosynthesis pathway (HBP). At present, only O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA) have been shown to regulate the modification level of O-GlcNAc, and (4) O-GlcNAc modifications interact with other post-translational modifications of proteins (such as methylation, phosphorylation, and ubiquitination). In conclusion, O-GlcNAc glycosylation modifications are involved in a series of important biological functions, including cell signaling, transcriptional regulation, metabolic regulation, stress, cell cycling, and protein–protein interactions.
4 Cross-Talk Between O-GlcNAc Modification in Immune Cells and Sepsis
O-GlcNAc modification is a nutrient and stress sensor that regulates cellular autotranscription and translation to signal transduction and metabolism. Disruption of O-GlcNAc modification balance will lead to various pathological diseases such as tumors, diabetes, and neurodegenerative disorders [26,27,28]. O-GlcNAc modification in the immune system has also been reported in recent years (Fig. 2) and the role of O-GlcNAc in each immune cell lineage and the relationship with sepsis will be further discussed.
4.1 O-GlcNAc Modification in T Cells in Sepsis
T cells are central to the cell-mediated immune response and are part of the adaptive immune system. T cell progenitors travel from the bone marrow to the thymus and differentiate to early thymic progenitors (ETPs). In the thymus, thymocyte lineage cells combine with T cells and become CD4 and CD8 double negative (DN) cells, and according to different developmental stages, DN thymocytes differentiate into DN1, DN2, DN3, and DN4. DN1 cells are heterogeneous and may differentiate into T cells, NK cells, B cells, and macrophages, whereas TCR recombination occurs from DN2. DN3 cells begin to bind the β chain of TCR to the pre-α chain to form the pre-TCR complex, which mediates β selection and promotes the development of DN3 to DN4. CD4 and CD8 cells are upregulated by DN4 cells, and eventually become double positive (DP) thymocytes, initiating TCR α-expressing TCRαβ complex. DP cells become mature CD4 + /CD8 + T cells by positive and negative selection [29, 30]. During this process, cells at different stages will present dynamic changes in glucose and glutamine intake levels and undergo altered O-GlcNAc levels. For example, levels of protein O-GlcNAc modification are upregulated at the DN3 stage and show a significant decrease from DN4 to DP. In positive selection of DP cells, the level of O-GlcNAc modification increases [31].
Nuclear Factor of Activated T cells (NFAT) and Nuclear Factor kappaB (NF-κB) are two critical transcription factors. O-GlcNAc modification has been found to significantly regulate their function and fate in T cells. Some studies have discovered that, upon T Cell Receptor (TCR) activation, O-GlcNAcylation of NFAT (nfatc1) primarily occurs in the cytosol within the first five minutes post-stimulation and then translocates to the nucleus at later time points. This suggests that O-GlcNAcylation of NFAT may be crucial for its nuclear translocation. The inhibition of O-GlcNAc transferase (OGT) has been shown to decrease TCR-induced interleukin-2 (IL-2) production and the expression of the activation marker CD69, further confirming the role of O-GlcNAcylation in T cells [32]. Golks et al. also found that inhibiting the activity of OGT affects NF-κB transcriptional activity in T cells. They demonstrated that the NF-κB subunit p65 can be modified by O-GlcNAcylation [32, 33]. Another study showed that conditions of hyperglycemia or treatment with PUGNAc enhanced O-GlcNAcylation of the c-Rel subunit of NF-κB at serine residue 350 in T cells. The investigators found that O-GlcNAcylation of c-Rel augmented its binding in the CD28RE region, thereby inducing the expression of proinflammatory molecules such as IL-2, interferon γ (IFNγ), and Granulocyte–Macrophage Colony-Stimulating Factor (GM-CSF) [34]. Bruno et al. discovered that O-GlcNAcylation of DNA fragmentation factor 45 (DFF45) protects proteins from cleavage by caspases during DNA damage-induced T cell apoptosis. This suggests that O-GlcNAcylation may also protect T cells from apoptotic cell death through additional mechanisms [35]. The evolving understanding of these processes indicates potential new avenues for interventions to modulate T cell function in diseases characterized by T cell dysregulation, including sepsis.
Naive CD4 + T cells differentiate into helper T cells (Th1, Th 2, and Th 17) and Tregs [16]. Some studies have found that MiR-15b-mediated downregulation of OGT may be involved in multiple sclerosis (MS) pathogenesis, mainly by inhibiting the differentiation of Th 17 cells [36]. Increased O-GlcNAc levels in naive CD4 + T cells of diet-induced obesity mouse models prompted Th 17 cells to secrete more IL-17A, and elevated O-GlcNAc levels increase lipid ligands for RORγt transcriptional activity [37]. Post-translational modification of TCR activated by O-glcNAc stabilizes FOXP3 and activates STAT5. These regulators work together to control Tregs homeostasis and function [38].
CD8 + T cells are major players in cell-mediated immunity. After acute infection, antigen stimulation induces naive CD8 + T cells to differentiate into effector cells, and the armed effector cells release perforins and granzymes stored in granules and secrete a variety of cytokines (such as IFN-γ and TNF-α) to clear bacterial infection [39, 40]. This process is mediated by phosphorylation of many intracellular signaling proteins. Protein O-GlcNAc modification is a post-translational modification that usually has a synergistic effect with phosphorylation [31]. Aguilar et al. [41] studied O-glcNAc in T cells during bacterial infection, finding that mice infected with Listeria monocytogenes showed elevated levels of O-glcNAc in CD8 + T cells. Taken together, O-GlcNAc modification affects the proliferation, differentiation, and function of T cells by regulating the activity of signal transduction pathways and transcription factors. Many studies have reported significant effects of sepsis in circulating and tissue T cells (as described in the second part of this article). Adequate modulation of T cell immune responses may balance the inflammatory response and facilitate the control of a microbial infection, which is essential for the treatment of sepsis.
4.2 O-GlcNAc Modification in B Cells in Sepsis
B lymphocytes play an essential role in the adaptive immune response. Mature B cells derive from bone marrow hematopoietic stem cells and migrate to the secondary lymphoid organs spleen and lymph nodes, and to the mucosa-associated lymphoid tissue (MALT). Under antigen stimulation, mature B cells proliferate and differentiate into memory B cells and plasma cells [42].
GlcNAc modification is critical in maintaining B cells homeostasis. It has been reported that a decrease in mature B cells is due to OGT deletion, leading to apoptosis. The frequency and number of mature B cells produced in the bone marrow of OGT-knockout mice are significantly reduced, as compared with normal mice. However, the absence of OGT has limited effects on immature B cells, transitional B cells, and marginal B cells. Mechanistically, O-GlcNAcylation of the kinase Lyn at serine 19 prompts its interaction with the kinase Syk in the cascade, activating appropriate BCR signaling [43]. NFATc1 and nuclear transcription factor κB (NF-κB) are modified by O-GlcNAc, which further enhances the extent of B cell activation [32]. In addition, the dynamic interaction of O-GlcNAc and lymphocyte-specific protein-1 (Lsp-1) after BCR crosslinking, leads to B cells apoptosis. The O-GlcNAc of Lsp18 on S209 is critical for PKC-β1 recruitment, which contributes to the phosphorylation of Lsp-1 on S243 [44]. Thus, O-GlcNAc promotes a signal cascade and apoptosis of activated B cells. Apoptosis of memory B cells in sepsis leads to immunosuppression [45]. It may be possible to reverse immune cells depletion by regulating O-GlcNAc modification in sepsis treatment.
4.3 O-GlcNAc Modification in Macrophages in Sepsis
Macrophages are integral components of the innate immune system, playing key roles in pro-inflammatory responses and phagocytosis. Some studies have indicated that Nuclear Factor kappa-B (NF-κB) is a pivotal regulator of the macrophage's inflammatory response, and it has been reported to be modified by O-GlcNAcylation. The interaction between OGT and mSin3a in RAW264.7 cells can interfere with the activation of NF-κB and the expression of the iNOS gene in macrophages stimulated by lipopolysaccharides (LPS) [46]. O-GlcNAc has also been shown to regulate the NF-κB subunit c-Rel in microglia, which drives the formation of c-Rel-p50 heterodimers. Interestingly, LPS was shown to further enhance iNOS production by promoting the interaction between c-Rel and OGT and p50. This increased the O-GlcNAc modification of c-Rel, making it easier for c-Rel to bind to NF-κB [47]. Although both p65 and c-Rel are members of the NF-κB family, they respond differently to O-GlcNAc modification. For instance, Thiamet-G has an anti-inflammatory effect by promoting the O-GlcNAc modification of p65 in microglia and influencing the nuclear localization of p65 [48]. In addition, high-glucose culture conditions can inhibit LPS-induced NF-κB activity in RAW264.7 cells and reduce the O-GlcNAc modification of c-Rel. On the other hand, glucosamine increases the O-GlcNAc modification of c-Rel and enhances the binding to the iNOS promoter under low-glucose conditions [49].
Elevated glucose metabolism of immune cells is a hallmark feature of many inflammatory diseases such as sepsis. Despite glycolytic activity and pentose phosphate pathway activation in macrophages, endotoxin inhibits HBP activity, resulting in reduced levels of protein O-GlcNAcylation. The absence of OGT, a key enzyme of protein O-GlcNAcylation, leads to enhanced innate immune activation and exacerbates septic inflammation. The specific mechanism is that OGT-mediated O-GlcNAcylation of serine-threonine kinase RIPK 3 on threonine 467 prevents the interaction between RIPK 3-RIPK 1 and RIPK 3-RIPK 3 proteins, thus inhibiting the downstream innate immune response and necroptosis signaling, resulting in impaired antibacterial response [50].
Protein O-GlcNAcylation in macrophages determines not only their pro-inflammatory response to bacterial exposure but also their anti-inflammatory response, and STAT 3 is a key transcription factor for inflammation and tissue repair [51]. Several studies have shown that O-GlcNAc of STAT3 at T717 negatively regulates the phosphorylation of STAT3 and reduces IL-10 production [52]. Results from such studies indicate that O-GlcNAc modification in macrophages has a bidirectional regulatory effect on the inflammatory response, which may be closely related to the utilization of glucose by the cells, the inflammatory microenvironment where the cells live, and the regulation of various signaling pathways such as NF-κB and STAT.
As one of the most important cells of the innate immune system, macrophages play an essential role in sepsis and have two main phenotypes, M1 and M2. M1-type macrophages release a large amount of pro-inflammatory mediators, whereas M2-type macrophages mainly promote the secretion of anti-inflammatory factors [53]. In the early stages of sepsis, macrophages polarize towards the M1 phenotype, exhibiting a stage of systemic inflammatory response, whereas in the late stages of sepsis, macrophages polarize toward the M2 phenotype, leading to apoptosis of immune cells and immunosuppression [54]. Therefore, it is of great significance for the treatment of sepsis to precisely regulate the polarization process of macrophages, according to the changes of macrophages in different stages of sepsis.
Studies have suggested that O-GlcNAc may facilitate the polarization of M2 macrophages, which contributes to the resolution of inflammation and tissue repair [48, 55]. A recent study indicated that the treatment with glucosamine (GlcN) diminishes the M1 phenotype of macrophages in animal models of endotoxin-induced lung injury in sepsis. Additionally, the application of Thiamet-G, a specific OGA inhibitor, has been demonstrated to enhance the expression of M2 markers and inhibit NF-κB p65 signaling in microglial cells. This results in a decreased expression of iNOS and Cyclooxygenase-2 (COX-2) in the middle cerebral artery, thus offering neuroprotection in an experimental stroke model [48]. In conclusion, interventions targeting O-GlcNAc may alter the differentiation of M1 and M2 macrophages in tissues, presenting a novel avenue for the treatment of sepsis.
4.4 O-GlcNAc Modification in Neutrophils in Sepsis
Neutrophils are the first cells to respond to injury and infection in the body. They fight bacterial infection through bacterophagy and secretion of a variety of antibacterial factors in innate immunity. Impairment of neutrophil migration is closely related to the poor prognosis of sepsis [56].. It has been reported that in animal models and patients with advanced sepsis in the immunosuppressive stage, neutrophils function is significantly altered, including impaired bacterial clearance, and decreased reactivity, ROS production, and neutrophils recruited to infected tissues [57].
O-GlcNAc may be critical for the function of neutrophils. Earlier studies have found that levels of O-glcNacylated protein in neutrophils significantly increased within two minutes of treatment with N-formylmethionine-leucine-phenylalanine (fMLP), and increased O-glcNacylated promoted neutrophils chemotactic and cell migration [58, 59]. GlcN supplements HBP by bypassing the rate-limiting enzyme, glutamine-fructose-6-phosphoramidotransferase (GFAT1), leading to an increase in UDP-GlcNAc synthesis and, consequently, promoting O-GlcNAcylation [60]. Moreover, GlcN elevates the activity of Rac, a crucial small GTPase that regulates neutrophil mobilization, and initiates downstream MAPK signaling [61, 62]. This suggests that O-GlcNAc is inducible in neutrophils and could play a significant role in managing dynamic neutrophil activity. Such insights offer a fresh perspective for the treatment of sepsis.
4.5 O-GlcNAc Modification in NK Cells in Sepsis
NK cells are a type of cytotoxic lymphocytes, which participate in cell innate immunity and initiate and expand inflammatory responses. The cells play an important role in defense against viral infection; maintaining the normal function of NK cells facilitates recognition and killing of virus-infected cells.
Yao et al. [63] found that O-GlcNAc modification in NK cells attenuated their cytotoxicity by affecting their signaling, whereas the GST-sHLA-G1 α chain inhibited the level of O-GlcNAc modification in NK cells. Other studies have shown that NK group2D (NKG 2D) is a receptor by which NK cells recognize and subsequently kill virus-infected cells expressing the stress-induced peptide–major histocompatibility complex class I complexes [64].The cytotoxicity of NKG2D and NKG2D-mediated NK cells to cancer cells is regulated to a certain extent by the transcription factor enhancer of zeste homolog 2(EZH2) [65]. EZH2 is a histone methyltransferase that after its activity is inhibited, not only accelerates NK cells maturation but also improves their cytotoxic activity. Five O-GlcNAc modification sites have been identified on EZH2, which play an important role in the stability and function of this protein [66,67,68].
Another transcription factor that regulates NK cell function through O-GlcNAc modification is c-Myc. It has been shown that c-Myc promotes the expression of granzyme B in NK cells and enhances the cytotoxic activity of NK cells against virus-infected cells, whereas O-GlcNAc acylation contributes to maintaining the stability and function of c-Myc. It is well known that UDP-GlcNAc is a substrate for O-GlcNAc modification, which is produced by glycolysis in the glutamine metabolic pathway. As an important metabolite, glutamine is widely involved in biological processes such as energy metabolism and signaling in immune cells, which indicates that depletion of glutamine leads to a decrease of UDP-GlcNAc levels in NK cells, which in turn, induces a rapid degradation of c-Myc, thus affecting its function and cytotoxicity [69]. Therefore, O-GlcNAc modification may enhance the immune response of NK cells, facilitate the removal of infected cells, and control the progression of sepsis, which provides a new direction for the treatment of sepsis.
Feinberg et al. [70] stimulated NK cells with IL-2 and IL-15, resulting in the enhancement of O-GlcNAc of several cellular proteins. OSMI-1 has been used to inhibit O-glcN acylation, and reduced expressions of TNF-α, IFN-γ, and NK cell receptors were observed in NK cells. In sepsis, NK cytotoxic function in patients is weakened and cytokine secretion is reduced. In addition, the impaired function of NK to produce IFN-γ during sepsis may be a key factor leading to the increase of secondary infections [71, 72]. Therefore, regulating the number and function of NK cells during sepsis may be a potential therapeutic target.
5 Discussion and Summary
Sepsis is a severe clinical manifestation of an infection, characterized by abnormal activation of the immune system and deregulation of the inflammatory response. LPS, the main components of the outer membrane of Gram-negative bacteria, can trigger inflammatory responses. Studies have found that LPS, produced by different constituents of the human gut microbiome, can either stimulate or inhibit TLR4, NF-κB activation, and endotoxin tolerance. Notably, the structure and function of LPS produced by different bacterial species vary, even between different regions. This interspecific difference in LPS structure correlates with its varying ability to trigger an innate immune response [73]. Several animal models have demonstrated the potential of using O-glcNAc modification to control the immune response against infection. For example, in an LPS-induced sepsis mouse model, GlcN regulated the O-GlcNAc of nucleoplasmic proteins, inhibiting MAPK and NF- κB signaling in the lungs of septic mice, thus alleviating the systemic inflammatory response to infection [55]. In the zebrafish model, GlcN also exerts a protective effect against sepsis by regulating the nuclear-cytoplasmic protein O-GlcNAc glycosylation modification [55]. The OGT is a key enzyme of the protein O-GlcNAcylation. Li et al. [50] found significantly increased mortality in LPS-induced sepsis mice, whose mechanism may be related to the O-GlcNAc glycosylation modification at T467 on RIPK 3. Preventing the interaction between RIPK 3-RIPK 1 and RIPK 3-RIPK 3 proteins, inhibits an exacerbated inflammatory response. The absence of O-GlcNAc glycosylation modification will cause RIPK 3 phosphorylation, stimulating an inflammatory response and necrosis signaling. Treatment of LPS-induced septic shock rats with the OGA inhibitor NButGT showed reduced mortality [74]. Silva et al. [75] also reported systemic and local inflammatory responses, observing significant increases in IL-1β, IL-6, and TNF-α levels. Furthermore, LPS increased neutrophils infiltration in the lungs, leading to significant mortality. Treatment with glucosamine or thiamine-G significantly reduced the systemic inflammatory response. In addition, GlcN and ThG reduced LPS-induced inflammatory cytokines produced by bone marrow-derived macrophages and NF-κB activation in macrophages. In conclusion, acute increases in O-GlcNAc, reduced systemic inflammation and cardiovascular dysfunction and improved survival in experimental sepsis models. It is clear that modification of proteins by O-linked N-acetylglucosamine (O-GlcN acylation) affects many key processes that are altered during sepsis (Table 1), and targeting O-GlcNAc acylation inhibits the life-threatening exacerbated immune response in sepsis, which may lead to enhanced treatments or improvement in the prognosis of sepsis.
Given the importance of O-GlcNAcylation in immune responses to infections, an intriguing question arises: can O-GlcNAc directly modulate the virulence of certain pathogens to either promote symbiotic relationships or escalate pathogenic interactions. In some microbial infections, particularly those caused by Gram-positive bacteria, the presence of GlcNAc stimulates the production of toxins and virulence factors, thereby intensifying the severity of the infection. This phenomenon is observed in Pseudomonas aeruginosa infections [80]. Indeed, GlcNAc is a ubiquitous structural component present on the cell surface, serving as a constituent of the bacterial cell wall peptidoglycan (also known as mucopeptides), fungal and parasite cell wall chitin, and extracellular matrix glycosaminoglycans in animal cells. For instance, peptidoglycan, a major component of the bacterial cell wall, is a network formed by alternating GlcNAc and N-acetylmuramic acid (MurNAc) residues. The peptidoglycan matrix stabilizes the bacterial cell wall via cross-linking of polypeptide chains [81, 82].. The remodeling of peptidoglycan is crucial for various cellular processes and is implicated in bacterial pathogenesis. Peptidoglycan deacetylase, which removes the acetyl moiety from N-acetylglucosamine (NAG), plays a critical role in bacterial persistence within host cells and the virulence of Legionella. This deacetylation influences the correct localization and function of the type IV secretion system, a mechanism found in bacteria and pathogens [83]. Moreover, secreted virulence factors that participate in peptidoglycan editing can control type IV secretion and intracellular survival of Legionella pneumophila through deacetylation. Some studies have reported that chemical modifications of glycan chains are involved in peptidoglycan remodeling, and O-acetylation of MurNAc residues can occur in most Gram-positive (Streptococcus pneumoniae, Staphylococcus aureus, Listeria) and Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa) [84].
Similarly, fungal and parasite cell walls contain chitin, a polymer composed of N-acetylglucosamine molecules. Chitin strengthens and stabilizes cell walls and plays a role in environmental interactions [85]. For example, during Cryptococcus C. neoformans infection, Th2 cell induction relies on mammalian chitinase, which breaks down chitin, suggesting a role for chitin fragments, and possibly GlcNAc, in the immune response [86].
Other studies have found that the presence of GlcNAc inhibits the synthesis of type 1 hair adhesin, thereby reducing the adhesion potential of bacteria to host cells, and possibly reducing the risk of urinary tract infection [87]. GlcNAc also reduces the production of Curli fibers, which play an important role in biofilm formation, adhesion, and the internalization of Escherichia coli by epithelial cells [88]. In conclusion, elevated GlcNAc levels during inflammation may signal to bacteria, suggesting activated host defense [89], and in-depth investigation of the relationship between GlcNAc and bacteria will allow us to better understand microbial pathogenesis and provide important clues for the treatment of sepsis.
Sepsis is a systemic inflammatory clinical syndrome caused by the immune system in response to invading pathogens, and early events in the inflammatory process involve the activation of neutrophils, monocytes/macrophages, and lymphocytes, which leads to the production and secretion of pro-inflammatory mediators, responsible for most of the pathophysiological changes in sepsis. O-GlcNAcylation regulates the maturation, division, and activation of different immune cells, thus exerting anti-infection effects. Recent increasing reports indicate that O-GlcN acylation plays a key role in the immune system during infection. Most studies have explored this using in vitro models or mouse models. However, marginal information is known about its importance for human infection. More basic research and clinical evidence are needed to support it. It is believed that with the application of more new detection technologies and the addition of research forces, the potential effects of O-GlcNAc glycosylation modification in the immune system in the treatment of sepsis will be elucidated.
Data Availability
Not applicable.
Abbreviations
- PTMs:
-
Post-translational modifications
- O-GlcNAc:
-
O-linked β-N-acetylglucosamine
- NK:
-
Natural killer
- DC:
-
Dendritic cells
- LPS:
-
Lipopolysaccharide
- TLR:
-
Toll-like receptor
- TCRs:
-
T cell receptors
- Tregs:
-
Regulatory T cells
- RORγt:
-
Retinoic acid receptor-related orphan receptor γt
- GlcNAc:
-
N-acetylglucosamine
- HBP:
-
Hexosamine biosynthesis pathway
- OGT:
-
O-GlcNAc transferase
- OGA:
-
O-GlcNAcase
- ETPs:
-
Early Thymic Progenitors
- DN:
-
Double negative
- DP:
-
Double positive
- MS:
-
Multiple sclerosis
- MALT:
-
Mucosa-associated lymphoid tissue
- NF-κB:
-
Nuclear transcription factor κB
- Lsp-1:
-
Lymphocyte-specific protein-1
- GlcN:
-
Glucosamine
- COX-2:
-
Cycloceuxy-2
- fMLP:
-
N-formylmethionine-leucine-phenylalanine
- EZH2:
-
Enhancer of zeste homolog 2
References
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016. https://doi.org/10.1001/jama.2016.0287.
Torres LK, Pickkers P, van der Poll T. Sepsis-Induced Immunosuppression. Annu Rev Physiol. 2022. https://doi.org/10.1146/annurev-physiol-061121-040214.
Hotchkiss RS, Coopersmith CM, McDunn JE, et al. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009. https://doi.org/10.1038/nm0509-496.
Cao S, Schnelzer A, Hannemann N, et al. The transcription factor FRA-1/AP-1 controls lipocalin-2 expression and inflammation in sepsis model. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.701675.
Santarsiero A, Convertini P, Todisco S, et al. ACLY nuclear translocation in human macrophages drives proinflammatory gene expression by NF-κB acetylation. Cells. 2021. https://doi.org/10.3390/cells10112962.
Lorente-Sorolla C, Garcia-Gomez A, Català-Moll F, et al. Inflammatory cytokines and organ dysfunction associate with the aberrant DNA methylome of monocytes in sepsis. Genome Med. 2019. https://doi.org/10.1186/s13073-019-0674-2.
Silva-Aguiar RP, Peruchetti DB, Pinheiro A, et al. O-GlcNAcylation in renal (Patho)physiology. Int J Mol Sci. 2022. https://doi.org/10.3390/ijms231911260.
Ferron M, Cadiet J, Persello A, et al. O-GlcNAc stimulation: a new metabolic approach to treat septic shock. Sci Rep. 2019. https://doi.org/10.1038/s41598-019-55381-7.
Qiu Y, Tu GW, Ju MJ, et al. The immune system regulation in sepsis: from innate to adaptive. Curr Protein Pept Sci. 2019. https://doi.org/10.2174/1389203720666190305164128.
Boomer JS, Green JM, Hotchkiss RS. The changing immune system in sepsis: is individualized immuno-modulatory therapy the answer? Virulence. 2014. https://doi.org/10.4161/viru.26516.
Nedeva C, Menassa J, Duan M, et al. TREML4 receptor regulates inflammation and innate immune cell death during polymicrobial sepsis. Nat Immunol. 2020. https://doi.org/10.1038/s41590-020-0789-z.
Nguyen GT, Green ER, Mecsas J. Neutrophils to the ROScue: mechanisms of NADPH oxidase activation and bacterial resistance. Front Cell Infect Microbiol. 2017. https://doi.org/10.3389/fcimb.2017.00373.
Vatanen T, Kostic AD, et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. 2016;4:07.
Hynninen M, Pettilä V, Takkunen O, et al. Predictive value of monocyte histocompatibility leukocyte antigen-DR expression and plasma interleukin-4 and -10 levels in critically ill patients with sepsis[J]. Shock. 2003. https://doi.org/10.1097/01.shk.0000068322.08268.b4.
Unsinger J, McGlynn M, Kasten KR, et al. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol. 2010. https://doi.org/10.4049/jimmunol.0903151.
Machacek M, Slawson C, Fields PE. O-GlcNAc: a novel regulator of immunometabolism. J Bioenerg Biomembr. 2018. https://doi.org/10.1007/s10863-018-9744-1.
Greathouse KC, Hall MW. Critical Illness-induced immune suppression: current state of the science. Am J Crit Care. 2016. https://doi.org/10.4037/ajcc2016432.
Pachot A, Monneret G, Voirin N, et al. Longitudinal study of cytokine and immune transcription factor mRNA expression in septic shock. Clin Immunol. 2005. https://doi.org/10.1016/j.clim.2004.08.015.
Bhan C, Dipankar P, Chakraborty P, et al. Role of cellular events in the pathophysiology of sepsis. Inflamm Res. 2016. https://doi.org/10.1007/s00011-016-0970-x.
von Knethen A, Heinicke U, Weigert A, et al. Histone deacetylation inhibitors as modulators of regulatory T cells. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21072356.
Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes evidence for O-linked GlcNAc. J Biol Chem. 1984;259(5):3308–17.
Lockridge A, Hanover JA. A nexus of lipid and O-Glcnac metabolism in physiology and disease. Front Endocrinol (Lausanne). 2022. https://doi.org/10.3389/fendo.2022.943576.
Sharma NS, Saluja AK, Banerjee S. Nutrient-sensing and self-renewal: O-GlcNAc in a new role. J Bioenerg Biomembr. 2018. https://doi.org/10.1007/s10863-017-9735-7.
Chatham JC, Zhang J, Wende AR. Role of O-linked N-acetylglucosamine protein modification in cellular (patho)physiology. Physiol Rev. 2021. https://doi.org/10.1152/physrev.00043.2019.
Yang X, Qian K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat Rev Mol Cell Biol. 2017. https://doi.org/10.1038/nrm.2017.22.
Parker MP, Peterson KR, Slawson C. O-GlcNAcylation and O-GlcNAc cycling regulate gene transcription: emerging roles in cancer. Cancers (Basel). 2021. https://doi.org/10.3390/cancers13071666.
Liu C, Dong W, Li J, et al. O-GlcNAc modification and its role in diabetic retinopathy. Metabolites. 2022. https://doi.org/10.3390/metabo12080725.
Park J, Lai M, Arumugam TV, et al. O-GlcNAcylation as a therapeutic target for Alzheimer’s disease. Neuromo-lecular Med. 2020. https://doi.org/10.1007/s12017-019-08584-0.
Swamy M, Pathak S, Grzes KM, et al. Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nat Immunol. 2016. https://doi.org/10.1038/ni.3439.
Shah DK, Zúñiga-Pflücker JC. An overview of the intrathymic intricacies of T cell development. J Immunol. 2014. https://doi.org/10.4049/jimmunol.1302259.
van der Laarse S, Leney AC, Heck A. Crosstalk between phosphorylation and O-GlcNAcylation: friend or foe. FEBS J. 2018. https://doi.org/10.1111/febs.14491.
Golks A, Tran TT, Goetschy JF, et al. Requirement for o-linked N-acetylglucosaminyltransferase in lymphocytes activation. EMBO J. 2007. https://doi.org/10.1038/sj.emboj.7601845.
Ma Z, Chalkley RJ, Vosseller K. Hyper-o-GlcNAcylation activates nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) signaling through interplay with phosphorylation and acetylation. J Biol Chem. 2017. https://doi.org/10.1074/jbcM116.766568.
Ramakrishnan P, Clark PM, Mason DE, et al. Activation of the transcriptional function of the NF-κB protein c-Rel by o-GlcNAc glycosylation. Sci Signal. 2013. https://doi.org/10.1126/scisignal.20040.
Johnson B, Opimba M, Bernier J. Implications of the O-GlcNAc modification in the regulation of nuclear apoptosis in T cells. Biochim Biophys Acta. 2014. https://doi.org/10.1016/j.bbagen.2013.09.011.
Liu R, Ma X, Chen L, et al. MicroRNA-15b suppresses 17th differentiation and is associated with pathogenesis of multiple sclerosis by targeting O-GlcNAc transferase. J Immunol. 2017. https://doi.org/10.4049/jimmunol.1601727.
Machacek M, Saunders H, Zhang Z, et al. Elevated o-GlcNAcylation enhances pro-inflammatory Th17 function by altering the intracellular lipid microenvironment. J Biol Chem. 2019. https://doi.org/10.1074/jbc.RA119.008373.
Liu B, Salgado OC, Singh S, et al. The lineage stability and suppressive program of regulatory T cells require protein O-GlcNAcylation. Nat Commun. 2019. https://doi.org/10.1038/s41467-019-08300-3.
Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009. https://doi.org/10.1146/annurev.Immunol.021908.132706.
Janas ML, Groves P, Kienzle N, et al. IL-2 regulates perforin and granzyme gene expression in CD8+ T cells independently of its effects on survival and proliferation. J Immunol. 2005. https://doi.org/10.4049/jimmunol.175.12.8003.
Lopez Aguilar A, Gao Y, Hou X, et al. Profiling of protein o-GlcNAcylation in murine CD8(+) effector− and memory-like T cells. ACS Chem Biol. 2017. https://doi.org/10.1021/acschembio.7b00869.
Pieper K, Grimbacher B, Eibel H. B-cell biology and development. J Allergy Clin Immunol. 2013. https://doi.org/10.1016/j.jaci.2013.01.046.
Wu JL, Chiang MF, Hsu PH, et al. o-GlcNAcylation is required for B cell homeostasis and antibody responses. Nat Commun. 2017. https://doi.org/10.1038/s41467-017-01677-z.
Wu JL, Wu HY, Tsai DY, et al. Temporal regulation of Lsp1 o-GlcNAcylation and phosphorylation during apoptosis of activated B cells. Nat Commun. 2016. https://doi.org/10.1038/ncomms12526.
Shankar-Hari M, Fear D, Lavender P, et al. Activation—associated accelerated apoptosis of memory B cells in critically ill patients with sepsis. Crit Care Med. 2017. https://doi.org/10.1097/CCM.0000000000002380.
Hwang SY, Hwang JS, Kim SY, et al. O-GlcNAc transferase inhibitsLPS-mediated expression of inducible nitric oxide synthase through an increased interaction with mSin3A in RAW264.7 cells. Am J Physiol Cell Physiol. 2013. https://doi.org/10.1152/ajpcell.00042.2013.
Hwang SY, Hwang JS, Kim SY, et al. O-GlcNAcylation and p50/p105 binding of c-Rel are dynamically regulated by LPS and glucosamine in BV2 microglia cells. Br J Pharmacol. 2013. https://doi.org/10.1111/bph.12223.
He Y, Ma X, Li D, Thiamet G, et al. Mediates neuroprotection in experimental stroke by modulating microglia/macrophage polarization and inhibiting NF-κB p65 signaling. J Cereb Blood Flow Metab. 2017. https://doi.org/10.1177/0271678X16679671.
Hwang JS, Kwon MY, Kim KH, et al. Lipopolysaccharide (LPS)-stimulated iNOS induction is increased by glucosamine under normal glucose conditions but is inhibited by glucosamine under high glucose conditions in macrophage cells. J Biol Chem. 2017. https://doi.org/10.1074/jbc.M116.737940.
Li X, Gong W, Wang H, et al. O-GlcNAc transferase suppresses inflammation and necroptosis by targeting receptor-interacting serine/threonine-protein kinase 3. Immunity. 2019. https://doi.org/10.1016/j.immuni.2019.01.007.
Huynh J, Chand A, Gough D, et al. Therapeutically exploiting STAT3 activity in cancer—using tissue repair as a road map. Nat Rev Cancer. 2019. https://doi.org/10.1038/s41568-018-0090-8.
Li X, Zhang Z, Li L, et al. Myeloid-derived cullin 3 promotes STAT3 phosphorylation by inhibiting OGT expression and protects against intestinal inflammation. J Exp Med. 2017. https://doi.org/10.1084/jem.20161105.
Yunna C, Mengru H, Lei W, et al. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020. https://doi.org/10.1016/j.ejphar.2020.173090.
Chen X, Liu Y, Gao Y, et al. The roles of macrophage polarization in the host immune response to sepsis. Int Immunopharmacol. 2021. https://doi.org/10.1016/j.intimp.2021.107791.
Hwang JS, Kim KH, Park J, et al. Glucosamine improves survival in a mouse model of sepsis and attenuates sepsis-induced lung injury and inflammation. J Biol Chem. 2019. https://doi.org/10.1074/jbc.RA118.004638.
Lerman YV, Kim M. Neutrophil migration under normal and sepsis conditions. Cardiovasc Hematol Disord Drug Targets. 2015. https://doi.org/10.2174/1871529x15666150108113236.
Sônego F, Castanheira FV, Ferreira RG, et al. Paradoxical roles of the neutrophil in sepsis: protective and deleterious. Front Immunol. 2016. https://doi.org/10.3389/fimmu.2016.00155.
Madsen-Bouterse SA, Xu Y, Petty HR, et al. Quantification of o-GlcNAc protein modification in neutrophils by flow cytometry. Cytometry A. 2008. https://doi.org/10.1002/cyto.a.20569.
Kneass ZT, Marchase RB. Neutrophils exhibit rapid agonist-induced increases in protein-associated o-GlcNAc. J Biol Chem. 2004. https://doi.org/10.1074/jbc.M407911200.
Cong R, Sun L, Yang J, et al. Protein O-GlcNAcylation alleviates small intestinal injury induced by ischemia-reperfusion and oxygen-glucose deprivation. Biomed Pharmacother. 2021. https://doi.org/10.1016/j.biopha.2021.111477.
Kneass ZT, Marchase RB. Protein o-GlcNAc modulates motility-associated signaling intermediates in neutrophils. J Biol Chem. 2005. https://doi.org/10.1074/jbc.M414066200.
Cicchetti G, Allen PG, Glogauer M. Chemotactic signaling pathways in neutrophils: from receptor to actin assembly. Crit Rev Oral Biol Med. 2002. https://doi.org/10.1177/154411130201300302.
Yao AY, Tang HY, Wang Y, et al. Inhibition of the activating signals in NK92 cells by recombinant GST-sHLA-G1a chain. Cell Res. 2004. https://doi.org/10.1038/sj.cr.7290215.
Jamieson AM, Diefenbach A, McMahon CW, et al. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002. https://doi.org/10.1016/s1074-7613(02)00333-3.
Jiang M, Xu B, Li X, et al. O-GlcNAcylation promotes colorectal cancer metastasis via the miR-101-O-GlcNAc/EZH2 regulatory feedback circuit. Oncogene. 2019. https://doi.org/10.1038/s41388-018-0435-5.
Yin J, Leavenworth JW, Li Y, et al. Ezh2 regulates differentiation and function of natural killer cells through histone methyltransferase activity. Proc Natl Acad Sci US A. 2015. https://doi.org/10.1073/pnas.1521740112.
Chu CS, Lo PW, Yeh YH, et al. O-GlcNAcylation regulates EZH2 protein stability and function. Proc Natl Acad Sci USA. 2014. https://doi.org/10.1073/pnas.1323226111.
Lo PW, Shie JJ, Chen CH, et al. o-GlcNAcylation regulates the stability and enzymatic activity of the histone methyltransferase EZH2. Natl Acad Sci USA. 2018. https://doi.org/10.1073/pnas.1801850115.
Loftus RM, Assmann N, Kedia-Mehta N, et al. Amino acid-dependent cMyc expression is essential for NK cell metabolic and functional responses in mice. Nat Commun. 2018. https://doi.org/10.1038/s41467-018-04719-2.
Feinberg D, Ramakrishnan P, Wong DP, et al. Inhibition of o-GlcNAcylation decreases the cytotoxic function of natural killer cells. Front Immunol. 2022. https://doi.org/10.3389/fimmu.2022.841299.
Guo Y, Patil NK, Luan L, et al. The biology of natural killer cells during sepsis. Immunology. 2018. https://doi.org/10.1111/imm.12854.
Wesselkamper SC, Eppert BL, Motz GT, et al. NKG2D is critical for NK cell activation in host defense against pseudomonas aeruginosa respiratory infection. J Immunol. 2008. https://doi.org/10.4049/jimmunol.181.8.5481.
Vatanen T, Kostic AD, et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. 2016. https://doi.org/10.1016/j.cell.2016.05.056.
Ferron M, Cadiet J, Persello A, et al. O-GlcNAc stimulation: a new metabolic approach to treat septic shock. Sci Rep. 2019. https://doi.org/10.1038/s41598-019-55381-7.
Silva JF, Olivon VC, Mestriner F, et al. Acute increase in o-GlcNAc improves survival in mice with LPS-induced systemic inflammatory response syndrome. Front Physiol. 2019. https://doi.org/10.3389/fphys.2019.01614.
Denis M, Dupas T, Persello A, et al. An o-GlcNAcylomic approach reveals ACLY as a potential target in sepsis in the young rat. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms22179236.
Wang J, Lu X, Zheng K, et al. Glutamine’s protection against brain damage in septic rats via increased protein oxygen-N-acetylglucosamine modification. NeuroReport. 2021. https://doi.org/10.1097/WNR.0000000000001582.
Wu D, Su S, Zha X, et al. Glutamine promotes O-GlcNAcylation of G6PD and inhibits AGR2 S-glutathionylation to maintain the intestinal mucus barrier in burned septic mice. Redox Biol. 2023. https://doi.org/10.1016/j.redox.2022.102581.
Dupas T, Persello A, Blangy-Letheule A, et al. Beneficial effects of O-GlcNAc stimulation in a young rat model of sepsis: beyond modulation of gene expression. Int J Mol Sci. 2022. https://doi.org/10.3390/ijms23126430.
Korgaonkar AK, Whiteley M. Pseudomonas aeruginosa enhances production of an antimicrobial in response to N-acetylglucosamine and peptidoglycan. J Bacteriol. 2011. https://doi.org/10.1128/JB.01175-10.
Park JT, Uehara T. How bacteria consume their own exoskeletons (turnover and recycling of cell wall peptidoglycan). Microbiol Mol Biol Rev. 2008. https://doi.org/10.1128/MMBR.00027-07.
Wheeler R, Chevalier G, Eberl G, et al. The biology of bacterial peptidoglycans and their impact on host immunity and physiology. Cell Microbiol. 2014. https://doi.org/10.1111/cmi.12304.
Boamah D, Gilmore MC, Bourget S, et al. Peptidoglycan deacetylation controls type IV secretion and the intracellular survival of the bacterial pathogen Legionella pneumophila. Proc Natl Acad Sci USA. 2023. https://doi.org/10.1073/pnas.2119658120.
Bonnet J, Durmort C, Jacq M, et al. Peptidoglycan o-acetylation is functionally related to cell wall biosynthesis and cell division in Streptococcus pneumoniae. Mol Microbiol. 2017. https://doi.org/10.1111/mmi.13849.
Bueter CL, Specht CA, Levitz SM. Innate sensing of chitin and chitosan. PLoS Pathog. 2013. https://doi.org/10.1371/journal.ppat.1003080.
Wiesner DL, Specht CA, Lee CK, et al. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog. 2015. https://doi.org/10.1371/journal.ppat.1004701.
Korgaonkar A, Trivedi U, Rumbaugh KP, et al. Community surveillance enhances Pseudomonas aeruginosa virulence during polymicrobial infection. Proc Natl Acad Sci USA. 2013. https://doi.org/10.1073/pnas.1214550110.
Sohanpal BK, El-Labany S, Lahooti M, et al. Integrated regulatory responses of fimB to N-acetylneuraminic (sialic) acid and GlcNAc in Escherichia coli K-12. Proc Natl Acad Sci USA. 2004. https://doi.org/10.1073/pnas.0405821101.
Barnhart MM, Lynem J, Chapman MR. GlcNAc-6P levels modulate the expression of Curli fibers by Escherichia coli. J Bacteriol. 2006. https://doi.org/10.1128/JB.00234-06.
Acknowledgements
The authors would like to express their gratitude to Edit Springs (https://www.editsprings.cn) for the expert linguistic services provided.
Funding
No financial support was provided.
Author information
Authors and Affiliations
Contributions
ZZH collected and wrote the manuscript; YMQ supervised the manuscript and give professional advices. All authors have approved the final and submitted version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Not applicable.
Ethical Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, Z., Qin, Y. O-GlcNAcylation Modification in Immune Cells: Potential Therapeutic Implications of Sepsis. Intensive Care Res 3, 204–214 (2023). https://doi.org/10.1007/s44231-023-00048-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44231-023-00048-1