Abstract
Background
We sought to determine whether statin treatment has a protective effect on the outcome of critically ill patients on mechanical ventilation.
Methods
Patients who underwent mechanical ventilation were selected from the MIMIC-III database. Patients with statin usage were allocated into the statin cohort. Patients without any statin use were matched to the statin cohort in a 1:1 ratio by propensity score. To ensure the robustness of the findings, we utilized the gradient boosted model, propensity score analysis, doubly robust estimation and an inverse probability‐weighting model in the statistical procedure.
Results
Before matching, 17,452 patients were enrolled in the non-statin group and 3,999 in the statin group. After matching, 3,363 patients were enrolled in each group. Compared with nonusers, the use of statins was associated with improved 28-day survival in the unmatched cohort (HR 0.85 95% CI 0.80–0.90, p < 0.01) and matched cohort (HR 0.79 95% CI 0.73–0.85, p < 0.01). Statin use was associated with longer ventilator-free days (VFD, 14.93 ± 13.11 vs 12.06 ± 13.26, p < 0.01) and longer ICU-free days (IFD, 13.41 ± 12.14 vs 10.86 ± 12.19, p < 0.01) in the matched cohort. Different types of statins were all associated with significantly improved 28-day survival.
Conclusions
In a population of mechanically ventilated patients, the use of statins may be associated with improved survival, longer VFDs and longer IFDs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
Statins, also known as 3-hydroxy-3 methylblycel coenzyme A (HMG-CoA) reductase inhibitors, play an important role in the prevention of coronary artery disease and ischemic stroke [1, 2]. In addition to their lipid-lowering effects, statins exert pleiotropic effects including reduction of inflammation, immunomodulation, antimicrobial effects, improved endothelial cell function and antithrombotic effects [3,4,5,6,7]. In view of the above mentioned effects of statins, intensivists have investigated whether statins are useful in critically ill patients. In patients with pneumonia, current statin use was associated with reduced mortality [8]. The use or previous use of statins may have reduced the mortality in septic patients in some observational studies [9,10,11]. Preliminary data from animal models have shown a protective effect on sepsisi induced lung injury [12]. The results of a meta-analysis support a similar conclusion [13]. However, some randomized controlled trials have shown the opposite [14,15,16,17,18]. In the Hydroxymethylglutaryl-CoA Reductase Inhibition with Simvastatin in Acute Lung Injury to Reduce Pulmonary Dysfunctin-2 Study (HARP-2), simvastatin compared with placebo was not associated with a reduced mortality in the overall population [18]. In the subset of patients with a hyperinflammatory phenotype, however, simvastatin was shown to reduce all cause mortality [19]. The differences in outcomes between the studies might be due to the characteristics of the different intensive care unit (ICU) populations. A recent retrospective study enrolled all ICU patients who had been prescribed statins and compared outcomes with a matched cohort. The results showed a beneficial association of statin use and 90 day mortality improvement [20]. However, the ICU population in this study may have been healthier or had more self-limiting disease processes than other ICU populations, such as patients with sepsis or acute lung injury/ARDS; this could have resulted in the relatively lower 90-day mortality observed in this study compared to that reported in other studies.
To avoid such patient selection bias and to, moreover, and also to investigate the mortality benefit of statin use in critically ill patients, we enrolled ventilated patients who had taken statins before or during ventilation as our study population. Ventilation is a key life-saving treatment measure for critically ill patients. However, mechanical ventilation is also associated with longer hospital stays, higher costs, and higher mortality rates [21]. Furthermore, ventilation can cause lung injury and other cardiovascular complications [22]. A recently published multicentre study included 3659 mechanically ventilated patients. This study found that the reason for intubation was stroke in 30.5% of the patients. During ventilation, cardiovascular events occurred in 42.6% of the 3659 patients [23]. These data suggest that statins may be able to improve outcomes in ventilated patients.
Whether statin use is associated with lower mortality in critically ill patients requiring ventilation in the ICU remains unclear. Therefore, we designed this observational study to research the potential beneficial effect of statin use among critically ill ventilated patients.
2 Methods
2.1 Study Design and Data Source
This is a retrospective observational study. We analyzed data from a large database: Medical Information Mart for Intensive Care (MIMIC-III). The MIMIC-III database is an openly available dataset developed by the MIT Laboratory for Computational Physiology, comprising deidentified health data associated with nearly 54,000 intensive care unit admissions [24]. The data in the MIMIC-III database consist of comprehensive clinical records of patients admitted to the ICUs of Beth Israel Deaconess Medical Center in Boston, MA, from June 1, 2001 to October 31, 2012. The requirement for institutional review board (IRB) approval from our institution was waived because MIMIC-III is a third-party anonymized publicly available database with pre-existing IRB approval.
2.2 Participants
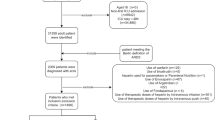
Patients who underwent mechanical ventilation were selected from the MIMIC-III database. The inclusion criteria were as follows: (1) age ≥ 18; (2) patients who were put on mechanical ventilation; and (3) precise outcome data. The exclusion criteria were as follows: (1) age < 18; (2) no mechanical ventilation; (3) no precise outcome data; and (4) statin use after extubation. The ventilation data were extracted from the chartvcents table. Statin usage information was extracted from the prescription table. We selected those who had taken statins before or during ventilation as the statin cohort and those who underwent ventilation without statins as the nonstatin cohort. Those who took statin medicine after extubation were excluded from this study. Then, we performed a propensity matching. Each statin-exposed patient was matched with the closest corresponding nonexposed patient (that is, a patient who was not exposed to statins) at a 1:1 fixed ratio (nearest match cohort).
2.3 Outcomes
The primary outcome was 28-day and in-hospital all-cause mortality. The secondary outcome analyses also included ventilator-free days at 28 days and ICU-free days at 28 days, 60-day survival, 90-day survival and in-hospital survival. VFDs were defined as follows: (1) VFDs = 0 if the subject died within 28 days of mechanical ventilation; (2) VFDs = 28 − x if successfully liberated from ventilation x days after initiation; and (3) VFDs = 0 if the subject was mechanically ventilated for > 28 days. The definition of ICU-free days was similar to that of VFDs. Eight plasma biomarkers were extracted and studied to measure the host responses during the first 10 days after intubation. The HRs of each type of statin were studied by Cox models. The effects of statins in different subgroup populations were analyzed.
2.4 Statistical Methods
The doubly robust estimation method was applied to infer the sensitivity analysis of the primary outcome. “Doubly robust estimation combines a multivariate regression model with a propensity score model to estimate the association and causal effect of an exposure on an outcome” [25, 26]. Usually, the regression model or the propensity score model was applied individually to estimate a causal effect. When the two approaches were built in one estimation model, only one of the two models needs to be correctly specified to obtain an unbiased effect estimator; thus, the term doubly robust analysis. For propensity scores of statin use, a machine learning algorithm named the gradient boosted model (GBM) was employed to maximally correlate with the negative gradient of the predefined loss function. A regression tree was used, and a total of 35 covariates were used in the model so that covariate imbalance between the statin and nonstatin groups was minimized. Confounding covariates included (1) demographic characteristics (sex, age); (2) disease severity score (Simplified Acute Physiology Score); (3) history of coronary artery disease; (4) history of cerebral infarction; (5)primary diagnosis at admission; (6) other comorbidities at ICU admission (congestive heart failure, cardiac arrhythmias, valvular disease, pulmonary circulation, peripheral vascular, other neurological disease, chronic pulmonary, diabetes uncomplicated, diabetes complicated, hypertension, paralysis, hypothyroidism, renal failure, liver disease, peptic ulcer, lymphoma, metastatic cancer, solid tumor, rheumatoid arthritis, coagulopathy, obesity, weight loss, fluid electrolyte, blood loss anemia, deficiency anemia, alcohol abuse, drug abuse, psychoses and depression). Using the propensity score calculated by GBM, the statin cohort and nonstatin cohort were matched at a 1:1 ratio. The matching method was “nearest”, and the caliper value was 0.02. To evaluate the effectiveness of the propensity score model in balancing the two compared groups, the standardized mean difference (SMD) between the statin and nonstatin groups was calculated. Using the estimated propensity scores calculated by the GBM model as weights, an inverse probability weighting (IPW) model was used to generate a weighted cohort [26, 27]. An IPW weighted Cox regression was then built adjusting for the variables that remained unbalanced between the groups. The propensity score was also employed to build the 1:1 matching cohort of the nonstatin cohort.
The primary statistical method of comparison for the time-to-event end points was expressed by Kaplan–Meier curves and tested by the log-rank test. A Cox proportional hazards model was used to estimate the hazard ratio (HR) of 28 day mortality and its associated 95% confidence interval (CI). We included several variables in the model to adjust the 28-day survival, which was based on the top importance calculated by GBM (age, SAPSII score, sex, liver disease, diabetes, obesity, hypertension, renal failure, uncomplicated diabetes, complicated diabetes, coronary disease history, obesity, cerebral infarction history, congestive heart failure, cardiac arrhythmia, other neurological and chronic pulmonary disease). The APACHE III score was not recorded for every patient, so we could not extract all APACHE III scores. The SAPSII was chosen to represent the severity of illness.
Some studies have reported different potencies between statins; for example, simvastatin exerted better antibacterial effects than rosuvastatin, and the latter was found to have a more potent lipid-lowering capacity [28, 29]. Therefore, we analyzed the difference in 28-day survival among statin types in the Cox regression model. To test the efficiency of statins in patients with different profiles, we performed a subgroup analysis. Patient categorical data are presented as percentages, and continuous data are listed as the means with standard deviations (SDs). We used Student’s t tests for continuous variables and chi-square or Fisher’s exact tests for dichotomous variables. Rstudio1.2.1335 (RStudio, Inc., Boston, MA, USA) was used to perform the statistical analyses. The mean (standard deviation) was used for all continuous variables and with two decimal. And rates were compared using counts (percentages), with percentages retaining one decimal.
3 Results
3.1 Baseline Results
All 53,432 cases in the MIMIC-III database were screened. A total of 24,769 individuals who had undergone ventilation met the inclusion criteria. 3999 patients were selected for the statin cohort. A total of 17,065 patients who had not received statin treatment were included in the nonstatin cohort. We constructed a propensity score model by employing the 39 covariates with the GBM. The contributions of individual covariates to the final propensity score are illustrated in Fig. 1. The top covariates include diagnosis, age, history of hypertension, presence of CHF and peripheral vascular disease; such factors would commonly influence the decision regarding whether to prescribe statins. Based on the estimated propensity scores, IPW was applied to standardize the differences between the statin and nonstatin cohorts. As shown in Table 1, most of the covariates of the matched cohorts were balanced between the two cohorts with or without statins. There were 3999 patients who received statin treatment before or during the ventilation time. After matching, there were 3363 patients in each cohort (Table 1). They were similar in age, sex, SAPSII and 36 more variables. The characteristics of the patients are presented in Table 1. There were 2145 cases of atorvastatin, 200 pravastatin, 171 rosuvastatin, 1284 simvastatin and 199 cases who received other statins in the unmatched cohort. In the matched cohort, 1835 patients received atorvastatin, 162 patients received pravastatin, 136 patients received rosuvastatin, 1068 patients received simvastatin, and 162 patients received other statins. Here, patients who used two or more statins were classified as having other statins (Table 2).
3.2 The Primary Outcome and Doubly Robust Analysis
In the overall cohort, the crude 28-day risk of all-cause mortality in statins vs nonstatins was 7753 (44.4%) vs. 1365 (34.1%), p < 0.01. The Kaplan–Meier analysis showed that statin users had a significantly improved 28-day survival (p < 0.01, Fig. 2A). The corresponding unadjusted hazard ratios (HRs) and 95% CIs were 0.85 (0.80–0.90) at 28 days.
Kaplan–Meier curves for all-cause mortality in statin recipients vs. nonrecipients in the unmatched cohorts and propensity score-matched cohorts A Statin use was associated with improved 28 day survival in the unmatched cohort (HR 0.85 95% CI 0.80–0.90); B Statin use was associated with improved 28 day survival in the matched cohort (HR 0.79 95% CI 0.73–0.85)
In the matched cohort. The 28-day mortality was 1599 (47.5%) vs. 1230 (36.6%) in statin users vs. nonstatin patients. The Kaplan–Meier analysis showed that statin users had a significantly improved 28-day survival (p < 0.01, Fig. 2B). The corresponding unadjusted hazard ratios (HRs) and 95% CIs were 0.79 (0.73–0.85) at 28 days.
In the multivariate Cox model, the use of statins was associated with a beneficial effect on 28-day survival in the unmatched cohort (HR 0.73 95% CI 0.68–0.77 Table 3). Under the doubly robust estimation framework, univariate and multivariate regression models were developed to adjust for these unbalanced covariates in the weighted cohort (Table 3). The univariate model showed that the HR of statins was 0.85 (95% CI 0.80–0.90, p < 0.01, Table 3), whereas in the multivariate weighted model, it was 0.85 (95% CI 0.82–0.88, p < 0.01, Table 3).
3.3 Secondary Outcomes and Plasma Biomarkers
The secondary outcomes included VFDs and IFDs. After matching, statin use was associated with more VFDs (14.93 ± 13.11 vs 12.06 ± 13.26) and more IFDs (13.41 ± 12.14 vs. 10.86 ± 12.19) (Table 4). We analyzed the effect of statins on survival in patients with different lengths of stay in the matched cohort. Statin use was associated with improved 60-day (HR 0.81, 95% CI 0.75–0.86, p < 0.001), 90 day (HR 0.81, 95% CI 0.76–0.87, p < 0.001) and in-hospital survival (HR 0.81, 95% CI 0.76–0.87, p < 0.001) in the matched cohort (Table 4).
The results of the biochemical tests all followed a normal distribution and we used box plots to represent these continuous variables and t-tests to calculate the differences between the means. We found no large differences in the matched cohort. Additionally, we found that statin use was not associated with higher aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels after matching (Fig. 3). The WBC and neutrophil counts were significantly lower in the statin cohorts (Fig. 3). However, creatinine was slightly higher in the statin group (Fig. 3).
3.4 Sensitivity and Subgroup Analysis
We further analyzed the different statins and their effect on patients' 28-day survival. All the different kinds of statins were found to be associated with improved 28-day survival of the patients. We compared users of different types of statins to nonusers by the Cox regression model. In the Cox model, all statins improved the survival of ventilated patients. Other statin groiup seemed to be associated with the best outcome (HR 0.48 95% CI 0.35–0.65), followed by rosuvastatin (HR 0.59 95% CI 0.41–0.85), pravastatin (HR 0.65 95% CI 0.49- 0.87) and simvastatin (HR 0.72 95% CI 0.64–0.81). The least-effective statin was atorvastatin, which was associated with decreased 28-day mortality (HR 0.84 95% CI 0.77–0.92). After adjusting for confounders, all statins still resulted in significant improvement in survival compared to nonuse based on this survival function (Fig. 4A).
In the subgroup analysis, we found that statins had a significant interaction effect in subgroups of myocardial infaction. Statin use showed no significant interaction effect in inflammation-related groups, such as the sepsis, or pneumonia subgroups (Fig. 4B).
4 Discussion
This study revealed that statin use was associated with improved 28-day survival and in-hospital survival. All types of statins were associated with reduced reduced mortality. This evidence might indicate a protective effect of statins in patients on mechanical ventilation. The use of statins may also be associated with longer ventilator-free days and longer ICU-free days. The protective effect of statins in critically ill patients may only be applicable to a specific population [19]. We selected ventilated patients for the study for three reasons: (1) ventilated patients represent patients with severe illness and high mortality. Selective bias was avoided; (2) one-third of the ventilated patients would have been intubated for stroke [23]; and (3) ventilated patients had a 40% chance of having a cardiovascular event [23]. Therefore, ventilated patients are a more appropriate choice as a study population for statin exposure factors.
In addition to lowering cholesterol, statins exert pleiotropic effects [3,4,5,6] such as anti-inflammatory, antioxidant, and immunomodulatory effects, particularly in the context of lung disease [30]. Statins may reduce COPD exacerbation [31]. Some observational studies have also suggested that statins may be effective in patients with acute lung injury or acute respiratory distress syndrome [32, 33]. These clinical effects may be mediated by a reduction in pulmonary and systemic inflammation. Simvastatin reduced bronchoalveolar lavage IL-8 by 2.5-fold (P = 0.04) [34]. Statins also showed a protective effect against sepsis. Compared with non-users, simvastatin (HR, 0.72; 95% CI, 0.58–0.90) and atorvastatin (HR, 0.78; 95% CI, 0.68–0.90) users had improved 30 day survival [10]. The current study investigated the anti-inflammatory effect of statins in a cohort of patients with mechanical ventilation, which has not been previously reported. A recent study showed that infections in older adults were associated with prolonged, impaired neutrophil migration. Simvastatin improves neutrophil migration in vivo in healthy individuals and in vitro in milder infectious events but not in severe sepsis, supporting its potential utility as an early intervention in pulmonary infections [35, 36]. Lung injury remains one of the major complications of mechanical ventilation in the ICU. This injury may result from an altered host immune response after mechanical stretch [37]. The use of statin therapy to protect patients with lung injury could therefore be a reasonable strategy, as these drugs may attenuate the host inflammatory response to infection [13], especially within the lungs [3].
Animal models may have further explained the protective effect of statins against lung injury. Statins increase glucocorticoid receptor expression in alveolar macrophages and downregulate NF-κB activation, which is associated with an increased number of alveolar macrophages [12]. Two other animal models of mechanical ventilation-induced lung injury also support these findings [38, 39]. The protective effect may be due to the anti-inflammatory effect of statins. Prior statin use was associated with a lower baseline IL-6 levels, and continued atorvastatin treatment in this cohort was associated with improved survival [40]. Statins have been shown to reduce vascular leakage and inflammation in animal models of lung injury [41]. Statins may also attenuate lung injury by downregulating the expression of inflammatory cytokines [42, 43]. Our previous study demonstrated the lung-protective effect of statins caused by reducing in inflammatory cell infiltration [44]. In addition, statins may have direct antibacterial effects and modulate bacterial virulence [45,46,47]. Sarah et al. showed that prior exposure to physiological nanomolar serum concentrations of simvastatin confers significant cellular resistance to the cytotoxicity of pneumolysin, demonstrating how statins contribute to the reduced pathology observed in the context of pneumonia and other bacterial infections [48].
The results of the subgroup analysis showed no interaction between statin treatment in the pneumonia subgroup and the sepsis subgroup. This is consistent with other negative findings obtained in pneumonia and sepsis [14,15,16,17,18]. In a randomized controlled trial (RCT) with more than one year of follow-up, there was no significant difference in cumulative survival between the rosuvastatin and placebo groups (58 vs 61%; p = 0.377) [49]. Simvastatin therapy was not significantly associated with the difference in 28-day mortality (22.0 and 26.8%; P = 0.23) among patients with ARDS [18]. The conflicting results may be due to the different study populations. These RCTs have focused on patients with severe conditions such as severe ARDS and sepsis. Similarly, the subgroup analyses in this study showed that statins were not effective in the context of pneumonia, sepsis or respiratory failure. Sapey stated that statins may improve neutrophil migration and may have a protective effect in milder infectious events but not in severe sepsis or ARDS [35]. The reason for this controversial evidence may be that statins may have immunomodulatory effect only in milder diseases instead of in severe inflammatory diseases such as ARDS. However, new RCTs investigating the effects in milder infectious diseases such as ventilator-associated lung injury may be needed to prove this hypothesis. About 30–40% of the cases included were patients with myocardial infarction and cerebrovascular disease. Statins are known to improve outcomes in patients with cardiovascular and cerebrovascular disease; therefore, in the subgroup analysis, we focused on people with myocardial infarction and cerebral infarction. Our findings showed a significant association between statin and reduced mortality in patients on ventilators, regardless of the presence of myocardial infarction or cerebral infarction. There was a significant interaction effect of statin use on and between subgroups. This was because statin use resulted in a greater benefit for patients with myocardial infarction.
The current study has several limitations. The main limitation of this study is that its observational nature without randomisation precludes a definitive conclusion on the benefit of statins. However, a randomised controlled trial of the effect of long-term statin treatment on the outcome of patients on mechanical ventilation would require a large number of participants. For the time being, observational data may remain the best available evidence to investigate the effect of statins on patients on mechanical ventilation. Second, because of the retrospective design of this study, patient selection bias may be unavoidable. Third, the lack of data on potential confounders was a limitation that could not be overcome. Therefore, our results should be interpreted with caution. Regarding the host response, the assessment of inflammatory cytokines may provide different insights. The relationship of statin dose and duration with survival was not analysed here because we included different types of statins and the doses of the different statins were not comparable. Finally, we included patients on mechanical ventilation with different diagnoses. Even with subgroup analyses, we cannot conclude that statins are specifically effective in a particular population. Further studies in different populations or RCTs are needed to validate our findings.
5 Conclusions
Our study suggests that statin use may be associated with reduced mortality in ventilated patients. In addition, statin use may be associated with reduced ventilator use and length of stay in the ICU.
Data Availability
The data can be available by contacting the corresponding author Jiang Du by email gowindj@163.com.
Abbreviations
- MIMIC-III:
-
Medical Information Mart for Intensive Care III
- LOS:
-
Length of stay
- VFDs:
-
Ventilator-free days
- ARDS:
-
Acute respiratory distress syndrome
- ICU:
-
Intensive care unit
- IRB:
-
Institutional review board
- SAPSII:
-
Simplified acute physiology score II
- CI:
-
Confidence interval (CI)
- SD:
-
Standard deviation
- HR:
-
Hazard ratio
- AST:
-
Aminotransferase
- ALT:
-
Alanine aminotransferase
- RCT:
-
Randomized controlled trial
- WBC:
-
White blood cell
References
Willeit P, Ridker PM, Nestel PJ, Simes J, Tonkin AM, Pedersen TR, Schwartz GG, Olsson AG, Colhoun HM, Kronenberg F, et al. Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet. 2018;392(10155):1311–20.
Cohen JS. Statin therapy after stroke or transient ischemic attack. N Engl J Med. 2006;355(22):2368.
Krishna RK, Issa O, Saha D, Macedo FY, Correal B, Santana O. Pleiotropic effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors in pulmonary diseases: a comprehensive review. Pulm Pharmacol Ther. 2015;30:134–40.
Liao JK, Oesterle A. The pleiotropic effects of statins - from coronary artery disease and stroke to atrial fibrillation and ventricular tachyarrhythmia. Curr Vasc Pharmacol. 2018;22:140.
Mihos CG, Santana O. Pleiotropic effects of the HMG-CoA reductase inhibitors. Int J Gen Med. 2011;4:261–71.
Rohilla A, Rohilla S, Kumar A, Khan MU, Deep A. Pleiotropic effects of statins: A boulevard to cardioprotection. Arab J Chem. 2016;9:S21–7.
Terblanche M, Almog Y, Rosenson RS, Smith TS, Hackam DG. Statins and sepsis: multiple modifications at multiple levels. Lancet Infect Dis. 2007;7(5):358–68.
Nielsen AG, Nielsen RB, Riis AH, Johnsen SP, Sørensen HT, Thomsen RW. The impact of statin use on pneumonia risk and outcome: a combined population-based case-control and cohort study. Crit Care. 2012;16(4):R122.
Al Harbi SA, Tamim HM, Arabi YM. Association between statin therapy and outcomes in critically ill patients: a nested cohort study. BMC Clin Pharmacol. 2011;11:12.
Lee CC, Lee MG, Hsu TC, Porta L, Chang SS, Yo CH, Tsai KC, Lee M. A population-based cohort study on the drug-specific effect of statins on sepsis outcome. Chest. 2017;153:805.
Wan YD, Sun TW, Kan QC, Guan FX, Zhang SG. Effect of statin therapy on mortality from infection and sepsis: a meta-analysis of randomized and ob servational studies. Crit Care. 2014;18(2):R71.
Takano K, Yamamoto S, Tomita K, Takashina M, Yokoo H, Matsuda N, Takano Y, Hattori Y. Successful treatment of acute lung injury with pitavastatin in septic mice: potential role of glucoco rticoid receptor expression in alveolar macrophages. J Pharmacol Exp Ther. 2011;336(2):381–90.
Tleyjeh IM, Kashour T, Hakim FA, Zimmerman VA, Erwin PJ, Sutton AJ, Ibrahim T. Statins for the prevention and treatment of infections: a systematic review and meta-analysis. Arch Intern Med. 2009;169(18):1658–67.
Kruger PS, Harward ML, Jones MA, Joyce CJ, Kostner KM, Roberts MS, Venkatesh B. Continuation of statin therapy in patients with presumed infection. Am J Respir Crit Care Med. 2011;183(6):774–81.
Singh RK, Agarwal V, Baronia AK, Kumar S, Poddar B, Azim A. The effects of atorvastatin on inflammatory responses and mortality in septic shock: a single-center, randomized controlled trial. Indian J Crit Care Med. 2017;21(10):646–54.
Nagendran M, McAuley DF, Kruger PS, Papazian L, Truwit JD, Laffey JG, Thompson BT, Clarke M, Gordon AC. Statin therapy for acute respiratory distress syndrome: an individual patient data meta-analysis of r andomised clinical trials. Intensive Care Med. 2017;43(5):663–71.
National Heart L, Blood Institute ACTN, Truwit JD, Bernard GR, Steingrub J, Matthay MA, Liu KD, Albertson TE, Brower RG, Shanholtz C, et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370(23):2191–200.
McAuley DF, Laffey JG, O’Kane CM, Perkins GD, Mullan B, Trinder TJ, Johnston P, Hopkins PA, Johnston AJ, McDowell C, et al. Simvastatin in the acute respiratory distress syndrome. N Engl J Med. 2014;371(18):1695–703.
Calfee CS, Delucchi KL, Sinha P, Matthay MA, Hackett J, Shankar-Hari M, McDowell C, Laffey JG, O’Kane CM, McAuley DF, et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018;6(9):691–8.
Kyu OhT, Song IA, Lee JH, Lim C, Jeon YT, Bae HJ, Jo YH, Jee HJ. Preadmission Statin Use and 90-day Mortality in the Critically Ill: A Retrospective Association Study. Anesthesiology. 2019;131(2):315–27.
Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38(10):1947–53.
Tobin MJ. Mechanical ventilation. N Engl J Med. 1994;330(15):1056–61.
Russotto V, Myatra SN, Laffey JG, Tassistro E, Antolini L, Bauer P, Lascarrou JB, Szuldrzynski K, Camporota L, Pelosi P, et al. Intubation practices and adverse peri-intubation events in critically Ill patients from 29 Countries. JAMA. 2021;325(12):1164–72.
Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, Moody B, Szolovits P, Celi LA, Mark RG. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035.
Mccaffrey DF, Griffin BA, Almirall D, Slaughter ME, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32(19):3388–414.
Feng M, McSparron JI, Kien DT, Stone DJ, Roberts DH, Schwartzstein RM, Vieillard-Baron A, Celi LA. Transthoracic echocardiography and mortality in sepsis: analysis of the MIMIC-III database. Intensive Care Med. 2018;44(6):884–92.
Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–64.
Kamat SA, Gandhi SK, Davidson M. Comparative effectiveness of rosuvastatin versus other statin therapies in patients at increased risk of failure to achieve low-density lipoprotein goals. Curr Med Res Opin. 2007;23(5):1121–30.
Masadeh M, Mhaidat N, Alzoubi K, Al-Azzam S, Alnasser Z. Antibacterial activity of statins: a comparative study of atorvastatin, simvastatin, and rosuvastatin. Ann Clin Microbiol Antimicrob. 2012;11:13.
Chopra V, Flanders SA. Does statin use improve pneumonia outcomes? Chest. 2009;136(5):1381–8.
Blamoun AI, Batty GN, DeBari VA, Rashid AO, Sheikh M, Khan MA. Statins may reduce episodes of exacerbation and the requirement for intubation in patients with COPD: evidence from a retrospective cohort study. Int J Clin Pract. 2008;62(9):1373–8.
O’Neal HR, Koyama T, Koehler EAS, Siew E, Curtis BR, Fremont RD, May AK, Bernard GR, Ware LB. Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011;39(6):1343–50.
Mansur A, Steinau M, Popov AF, Ghadimi M, Beissbarth T, Bauer M, Hinz J. Impact of statin therapy on mortality in patients with sepsis-associated acute respiratory distress syndrome (ARDS) depends on ARDS severity: a prospective observational cohort study. Bmc Med. 2015;13:68.
Craig TR, Duffy MJ, Shyamsundar M, McDowell C, O’Kane CM, Elborn JS, McAuley DF. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am J Respir Crit Care Med. 2011;183(5):620–6.
Sapey E, Patel JM, Greenwood HL, Walton GM, Hazeldine J, Sadhra C, Parekh D, Dancer RCA, Nightingale P, Lord JM, et al. Pulmonary infections in the elderly lead to impaired neutrophil targeting, which is improved by simvastatin. Am J Respir Crit Care Med. 2017;196(10):1325–36.
Sapey E, Patel JM, Greenwood H, Walton GM, Grudzinska F, Parekh D, Mahida RY, Dancer RC, Lugg ST, Howells PA, et al. Simvastatin improves neutrophil function and clinical outcomes in pneumonia: a pilot randomised controlled trial. Am J Respir Crit Care Med. 2019;196:1325.
Charles PE, Tissieres P, Barbar SD, Croisier D, Dufour J, Dunn-Siegrist I, Chavanet P, Pugin J. Mild-stretch mechanical ventilation upregulates toll-like receptor 2 and sensitizes the lung to bacterial lipopeptide. Crit Care. 2011;15(4):R181.
Maller HC, Hellwig K, Rosseau S, Tschernig T, Schmiedl A, Gutbier B, Schmeck B, Hippenstiel S, Peters H, Morawietz L, et al. Simvastatin attenuates ventilator-induced lung injury in mice. Crit Care. 2010;14(4):143.
Siempos II, Maniatis NA, Kopterides P, Magkou C, Glynos C, Roussos C, Armaganidis A. Pretreatment with atorvastatin attenuates lung injury caused by high-stretch mechanical ventilation i n an isolated rabbit lung model. Crit Care Med. 2010;38(5):1321–8.
Kruger P, Bailey M, Bellomo R, Cooper DJ, Harward M, Higgins A, Howe B, Jones D, Joyce C, Kostner K, et al. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Medicine. 2013;187(7):743–50.
Jacobson JR, Barnard JW, Grigoryev DN, Ma SF, Tuder RM, Garcia JG. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288(6):1026–32.
Melo AC, Valenca SS, Gitirana LB, Santos JC, Ribeiro ML, Machado MN, Magalhaes CB, Zin WA, Porto LC. Redox markers and inflammation are differentially affected by atorvastatin, pravastatin or simvastatin administered before endotoxin-induced acute lung injury. Int Immunopharmacol. 2013;17(1):57–64.
Shyamsundar M, Mckeown STW, O’Kane CM, Craig TR, Brown V, Thickett DR, Matthay MA, Taggart CC, Backman JT, Elborn JS. Simvastatin Decreases Lipopolysaccharide-induced Pulmonary Inflammation in Healthy Volunteers. Am J Respir Crit Care Med. 2009;179(12):1107–14.
Du J, Zhu Y, Meng X, Xie H, Wang J, Zhou Z, Wang R. Atorvastatin attenuates paraquat poisoning-induced epithelial-mesenchymal transition via downregulating hypoxia-inducible factor-1 alpha. Life Sci. 2016;179:1107.
Hennessy E, Adams C, Reen FJ, O’Gara F. Is There Potential for Repurposing Statins as Novel Antimicrobials? Antimicrob Agents Chemother. 2016;60(9):5111–21.
Ting M, Whitaker EJ, Albandar JM. Systematic review of the in vitro effects of statins on oral and perioral microorganisms. Eur J Oral Sci. 2016;124(1):4–10.
Ribeiro NQ, Costa MC, Magalhes TFF, Carneiro HCS, Oliveira LV, Fontes ACL, Santos JRA, Ferreira GF, Araujo GRS, Alves V, et al. Atorvastatin as a promising anticryptococcal agent. Int J Antimicrob Agents. 2017;49(6):695–702.
Statt S, Ruan JW, Hung LY, Chang CY, Huang CT, Lim JH, Li JD, Wu R, Kao CY. Statin-conferred enhanced cellular resistance against bacterial pore-forming toxins in airway epithelial cells. Am J Respir Cell Mol Biol. 2015;53(5):689–702.
Dinglas VD, Hopkins RO, Wozniak AW, Hough CL, Morris PE, Jackson JC, Mendez-Tellez PA, Bienvenu OJ, Ely EW, Colantuoni E, et al. One-year outcomes of rosuvastatin versus placebo in sepsis-associated acute respiratory distress syndrome: prospective follow-up of SAILS randomised trial. Thorax. 2016;71(5):401–10.
Acknowledgements
This article has already appeared as a preprint on both research squares and research gates. However, all authors of this article warrant that the article has not been published in other journals.
Funding
Sponsored by the Interdisciplinary Program of Shanghai Jiaotong University (Project Number 06N1801046). Additionally, sponsored by Clinical Research Innovation Plan of Shanghai General Hospital (Project Number CTCCR-2018C10).
Author information
Authors and Affiliations
Contributions
DC wrote the manuscript, HZ wrote the manuscript and collected the data from the database. QL and LW collected the data from the MIMIC database, washed the data and performed the statistical analysis. QL and DC contributed to data collection and data analysis in this work. QL designed this research, revised the manuscript and analyzed the data. JD designed this research, wrote the manuscript, analyzed the data and drew all the figures in this study. JD takes responsibility for (is the guarantor of) the content of the manuscript, including the data and analysis (Original Research).
Corresponding authors
Ethics declarations
Conflict of Interest
All the authors declare that they have no potential financial or ethical conflict of interest regarding the contents of this manuscript.
Ethical Approval
The requirement for institutional review board (IRB) approval from our institution was exempted because MIMIC-III is a third-party anonymized publicly available database with pre-existing IRB approval.
Consent to Participate
Consent to participate was waived because of the retrospective design of the study.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, D., Zhang, H., Wang, L. et al. Statin Use is Associated with Reduced Mortality in Mechanically Ventilated Patients: A Retrospective Propensity-Matched Analysis of MIMIC-III Database. Intensive Care Res 3, 112–122 (2023). https://doi.org/10.1007/s44231-023-00037-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44231-023-00037-4