Abstract
End-stage renal disease patients with arteriovenous fistula (AVF) encounter many vascular risks related to reduced or increased arteriovenous fistula flow. Dialysis access-associated steal syndrome is one of the devastating complications that may lead to limb loss. Multiple vascular techniques (surgical and endovascular) can be used to correct this complication. In this article, we present two endovascular approaches. One approach uses a stent graft covering two thirds of anastomosis for arteriovenous fistulas to generate artificial stenosis and divert more flow to the hand. The other approach applies Supera® stent jailing to the arteriovenous fistula to divert more flow to the hand. Over 12 months of follow up, there was no clinical manifestation of dialysis access-associated steal syndrome and the AV fistula continued to function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Dialysis access-associated steal syndrome (DASS) can be a limb-threatening condition characterized by rest pain, sensory or motor dysfunction, and ulceration or gangrene, thus resulting in amputation [1]. DASS is not uncommon, with an incidence of 4–10% in all patients with arteriovenous fistula (AVF), including symptomatic and asymptomatic patients [1,2,3,4]. The risk factors of DASS include brachial artery flow variation, particularly a digital brachial index (DBI) of < 0.6; peripheral vascular disease; female sex; age > 60 years; diabetes; and coronary artery disease [3]. The majority of DASS cases are asymptomatic and have no clinical consequences. Duplex ultrasound previously provided evidence of arterial stenosis and/or the reversal of blood flow; these conditions represent biomarkers to confirm diagnosis [5]. It is also important to consider that AVF produces an obvious “physiological” steal phenomenon which is more evident in elbow/upper-arm AVFs when compared with forearm AVF [6].
The cause of DASS can be related to a pre-AVF, in-AVF or post-AVF, and therefore requires the assessment of all three of these segments. Pre-AV fistulas can be caused by brachial artery stenosis in cases involving arm AV fistulas or even subclavian artery disease. In-AV fistulas can be causes by large anastomosis cross-section area diverting the majority of blood flow to the AV fistula, thus favoring a low resistance bed at the expense of a high resistance bed, for example, the hand in cases involving arm AV fistulas. Post-AV fistulas are mostly caused by severe distal and peripheral vascular disease.
The treatment strategies for DASS are well documented in the surgical literature. However, the role of endovascular approaches is limited to pre- and post-AV fistula disease. Herein, we present two cases in which two endovascular techniques were used to treat DASS with combined causes including in-AVF. These patients were followed-up for 12 months to confirm the patency of these techniques.
2 Case Reports
2.1 Case 1
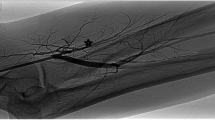
Case 1 was a 59-year-old male patient with type II diabetes mellitus, essential hypertension, coronary artery disease and end stage renal disease. He had received regular renal replacement therapy (hemodialysis: three sessions a week) since July 2018. He underwent left brachiocephalic AVF creation in August 2019; however, on the very next day, he developed DASS and received revision using distal inflow (RUDI) (Fig. 1). The AVF functioned well with a good rate of blood flow. One year later, the patient developed thrombosis of the AV fistula (Fig. 2). Thrombectomy and angioplasty was performed on the cephalic vein (using a CAT D Penumbra Indigo System and an 8 mm balloon) followed by stenting due to significant recoil; this latter procedure involved placement of an 8 × 40 mm absolute self-expandable stent that was post-dilated using an 8 mm balloon. The procedure was completed without immediate complications and was followed by a successful hemodialysis session. During the next hemodialysis session, the patient developed the symptoms and signs of DASS (pain in the arm and hand with digital coldness). He was unable to tolerate the hemodialysis session due to severe pain and hemodialysis was terminated. Subsequently, the patient underwent a left upper limb angiogram, which revealed 60–70% stenosis of the left brachial artery just proximal to the AVF anastomosis with no visible flow to the hand. The flow to the hand improved after manual compression of the AVF, thus confirming the diagnosis of steal syndrome. Pre-dilatation involved a 4 × 21 mm Sprinter® NC balloon up to 12 atm followed by a drug coated balloon (a 4.5 × 20 mm Luminor® iVascular balloon) inflated up to 6 atm for 3 min. However, the flow remained diverted to the AV fistula with no visible flow to the hand on angiography. In addition, the hand still looked ischemic (cold and pale). As the lesion was at the elbow level, we used Supera® 4.5 × 40 mm (Fig. 3). The flow improved markedly to the hand with brisk flow to the AV fistula. A packing technique was used for the Supera® stent around the point of anastomosis.ollowing the procedure, the patient had multiple sessions of efficient hemodialysis; all of these sessions went smoothly. At the 12-month follow up, the patient remained asymptomatic and had no issues related to dialysis access.
2.2 Case 2
Case 2 was a 76-year-old woman known to have hypertension and chronic lung disease (bronchial asthma and a history of treated pulmonary tuberculosis). She had end stage renal disease and was receiving regular renal replacement therapy and had received hemodialysis for three sessions a week since March 2018 via the right internal jugular vein (Permacath). She underwent left brachiobasilic AVF creation in February 2018 but subsequently developed AVF thrombosis on two occasions. In March 2020, she developed severe pain in her left arm and hand after AVF thrombectomy; this procedure was performed in a different hospital. In addition, she had coldness of the digits which lead to an incomplete hemodialysis session consistent with the diagnosis of DASS. She was found to have severe stenosis in the axillary artery (pre-AV fistula cause) and underwent successful percutaneous transluminal angioplasty (PTA) and stenting of the axillary artery. Initially, we attempted to place a drug-coated balloon; however, the patient was eventually stented due to significant recoil (a 5.0 × 80 mm Luminor® drug-coated balloon and an iVolution® self-expandable 5 × 100 mm stent which was post-dilated using a 6 mm balloon and a proximal segment was created with a 7 mm balloon. Angiography showed that there was no flow to the hand. We waited for 10 min to assess the hand coldness; it remained cold and tender. Therefore, we addressed the in-AV fistula by creating an artificial stenosis in this segment which appeared to be aneurysmal. The first two thirds of the anastomosis was covered by a Bentley® covered stent (5 × 57 mm); this resulted in a palpable radial pulse with brisk flow to the AV fistula. This step of the procedure was performed under a magnified field and was controlled by the road map technique in an angiogram to avoid inadvertent coverage of the AV fistula anastomosis. Angiography showed good flow to the radial artery and brisk flow to the AV fistula (Fig. 4). After this procedure, the patient received efficient hemodialysis. A clinical and radiological follow-up by ultrasound at 12 months showed a patent AV fistula with no more symptoms of DASS.
A A baseline angiogram of case 2 showing severe stenosis in the brachial artery (arrow) and no flow towards the hand (arrowhead). B Post-stenting of the brachial artery, the flow was still poor towards the hand (arrow). C Careful landing prior to implantation of the stent graft across two-thirds of the brachiocephalic anastomosis. D Post-stenting, the flow towards the hand had improved markedly, (arrow)
3 Discussion
DASS is a devastating complication that can occur in patients with dialysis-dependent chronic kidney disease. Here, we present two endovascular approaches (the Supera® stent with a packing technique and the Bentley® stent graft covering two-thirds of an AVF anastomosis) to manage the symptoms of these patients while maintaining patency for dialysis access.
In this article, we consider individualized management approaches targeted to the direct causes of DASS. These approaches are based on clinical assessment and radiological findings. In a previous study, Beathard et al. formulated a disease staging system that was based on a patient’s clinical condition in order to select the most suitable management strategy [6]. Multiple surgical therapeutic approaches are used to address the management of DASS, including banding, distal revascularization interval ligation (DRIL), RUDI, proximalization of arterial inflow (PAI), banding and ligation [7]. More recently, researchers reported a surgical technique that is carried out under local anesthesia which uses a short interposition graft to create a stenosis in a hyperfunctioning AV-fistula [8]. Several other techniques have been published in the literature, including the use of stent graft resembling anastoplasty; these techniques were described in detail in a recent review article [9].
In our community, we noted that many patients lost the AV fistula by surgical ligation due to an advanced stage of ischemia that was not recognized initially. In this article, we present two cases in which different endovascular approaches were used to treat DASS. In the first case, we used a novel approach to treat DASS: a Supera® stent with a packing technique. This case was complex and was managed initially in September 2019 by the RUDI procedure. Subsequently, the patient developed another episode of DASS; this was not tolerable by the patient during dialysis. Supera® stent alloy was used to create a pseudo-stenosis towards the AV fistula anastomosis with maintenance of flow to the distal arm. The Supera® stent is a unique technology using an interwoven individual, flexible six nitinol wire connected only at the ends. This stent has high compression resistance, low outwards compression, flexible, and cannot be kinked. Furthermore, this is type of stent is malleable and can be placed in one segment and stretched in another segment, thus allowing flexibility to the jailed side branches. We packed the stent at the juxta-anastomosis level to reduce cell size across the jailed AV fistula anastomosis, thus reducing the steal towards the low resistance vascular bed (the AVF), while maintaining flow towards the hand. Since surgery, the patient remains asymptomatic with patent AVF (for ultrasound, see Fig. 5).
In our second case, we used a Bentley stent graft (BeGraft®, Bentley InnoMed GmbH); this is a 6 French compatible peripheral stent graft. This stent has a low profile, is very flexible, and has a high radial force. This stent is consistent with a micro-porous expanded polytetrafluoroethylene (ePTFE) membrane in a cobalt-chromium stent. The existing literature describes a case in which a stent graft was used to jail two-thirds of an AV fistula anastomosis [10]. In our case, we had three challenges to face when compared to cases described in the literature. First, there was a combination of pre-AVF and in-AVF causes for DASS. Second, there was aneurysmal dilatation at the site of anastomosis that would result in significant malapposition of the stent graft. Third, the presence of anastomosis at the elbow level. The patency of the AV fistula, as well as the effectiveness of this technique, is highly durable according to clinical assessment as the patients still have a functioning AV fistula but without recurrence of DASS symptoms.
4 Conclusion
Dialysis access-associated steal syndrome (DASS) is a devastating complication that commonly ends with ligation of the dialysis access. Here, we describe two novel endovascular approaches with durable results that can treat this complication when surgical options are not feasible.
Data Availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- AVF:
-
Arteriovenous fistula
- AV fistula:
-
Arteriovenous fistula
- DASS:
-
Dialysis access-associated steal syndrome
- DRIL:
-
Distal revascularization interval ligation
- ePTFE:
-
Expanded polytetrafluoroethylene
- PAI:
-
Proximalization of the arterial inflow
- PTA:
-
Percutaneous transluminal angioplasty
- RUDI:
-
Revision using distal inflow
References
Leake AE, Winger DG, Leers SA, Gupta N, Dillavou ED. Management and outcomes of dialysis access-associated steal syndrome. J Vasc Surg. 2015;61(3):754–60. https://doi.org/10.1016/j.jvs.2014.10.038.
Padberg FT Jr, Calligaro KD, Sidawy AN. Complications of arteriovenous hemodialysis access: recognition and management. J Vasc Surg. 2008;48(5):55S-80S. https://doi.org/10.1016/j.jvs.2008.08.067.
Morsy AH, Kulbaski M, Chen C, Isiklar H, Lumsden AB. Incidence and characteristics of patients with hand ischemia after a hemodialysis access procedure. J Surg Res. 1998;74(1):8–10. https://doi.org/10.1006/jsre.1997.5206.
Davidson D, Louridas G, Guzman R, et al. Steal syndrome complicating upper extremity hemoaccess procedures: incidence and risk factors. Can J Surg. 2003;46(6):408–12.
Moghazy KM. Value of color Doppler sonography in the assessment of hemodialysis access dysfunction. Saudi J Kidney Dis Transpl. 2009;20(1):35–43.
Lazarides MK, Staramos DN, Kopadis G, Maltezos C, Tzilalis VD, Georgiadis GS. Onset of arterial steal following proximal angioaccess Immediate and delayed types. Nephrol Dial Transplant. 2003;11:2387–90.
Burrows L, Kwun K, Schanzer H, Haimov M. Haemodynamic dangers of high flow arteriovenous fistulas. Proc Eur Dial Transplant Assoc. 1979;16:686–7.
Lok CE, Huber TS, Lee T, et al. KDOQI clinical practice guideline for vascular access update. Amer J Kid Dise. 2019;2020(75):4–164.
Beathard GA, Spergel LM. Hand ischemia associated with dialysis vascular access: an individualized access flow-based approach to therapy. Semin Dial. 2013;26(3):287–314. https://doi.org/10.1111/sdi.12088.
Papadoulas S, Mulita F, Theodoropoulou T, et al. Short interposition grafting for dialysis-access steal syndrome treatment. BMJ Case Rep. 2022;15:248446. https://doi.org/10.1136/bcr-2021-248446.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All participating authors shared in drafting and revising the manuscript. The corresponding author was responsible for conception, design, drafting the manuscript, revising the manuscript, and final approval.
Corresponding author
Ethics declarations
Conflict of Interest
None of the authors have any conflicts of interest to declare.
Ethical approval and consent to participate
Ethical approval was obtained from Habib Research Center Ethical Committee. Both patients provided informed consent.
Consent for publication
The patients provided consent for their cases to be published.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
AlShammeri, O., AlEidan, I., Budaichieva, A. et al. Novel Endovascular Techniques for Dialysis Access-Associated Steal Syndrome (DASS). Dr. Sulaiman Al Habib Med J 5, 87–92 (2023). https://doi.org/10.1007/s44229-023-00035-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44229-023-00035-0