Abstract
Background
The global threat of COVID-19 caused by the SARS-CoV-2 virus has reached a high level and the outbreak has been declared as a pandemic. This disease affects different organs and systems including the central nervous system. In this study, we aimed to clarify the development of neurological complications in patients with COVID-19 and the factors associated with these conditions.
Methodology
Two authors independently searched the Cochrane, Trip, EMBASE, and Google Scholar databases from January 2020 to February 2021. The literature search included studies written in English and related to neurological complications in COVID-19 patients. Then, the two authors independently determined the characteristics and risk of bias of the included studies. Finally, we analyzed the data using odds ratios (ORs) or mean differences (MDs) and 95% confidence intervals (CIs).
Results
This review involved 4401 patients with COVID-19 from six observational studies. Overall, low to moderate heterogeneity was recorded among the included studies. A high risk of bias was not detected in any of the domains studied, although there were some low risks of bias and heterogeneity. Of the included patients, 8.24% developed neurological manifestations, including delirium (84.3%), myalgia (44.8%), headache (37.7%), encephalopathy (31.8%), dizziness (29.7%), dysgeusia (15.9%), anosmia (11.45), acute ischemic stroke (4.6%), cerebrovascular disease (1.78%), and intracerebral hemorrhage (0.5%). The severity of COVID-19 and the association of underlying comorbidity (predominantly hypertension) increased the risk of neurological complications among COVID-19 patients by fourfold (OR 4.30, CI 2.54–7.29 and OR 4.01, CI 1.05–15.36, respectively). Patients with heart diseases, diabetes, and dyslipidemia had a twofold higher risk of developing neurological complications (OR 2.53, CI 1.01–6.33; OR 2.31, CI 1.15–4.65; and OR 2.13, CI 1.52–3.00, respectively).
Conclusion
Our analysis indicated that neurological complications were uncommon in patients with COVID-19. Age, male sex, smoking, the severity of disease, and underlying comorbidity, including hypertension, heart disease, diabetes, and dyslipidemia, were identified as significant risk factors for neurological complications in COVID-19 patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
Since the discovery of SARS-CoV-2 until August 2021, 140 million people have been infected with more than three million deaths all over the world [1,2,3,4]. The global threat of COVID-19 caused by the SARS-CoV-2 virus has reached a high level and the outbreak has been declared by the World Health Organization (WHO) as a pandemic [5]. Upper and lower respiratory symptoms are the most common presentation of COVID-19; however, this disease can affect any of the organs and the central nervous system (CNS). These neurological complications include dizziness, headache, impaired consciousness, encephalopathy, stroke, and delirious manifestations [6]. Brain injury and neurological symptoms might occur via invasion of the neural cell membrane by angiotensin converting enzyme-2 (ACE2). Affects in the CNS could also be caused by viral infection occurring via axonal transport of the virus by peripheral nerves into the brain [6]. An alternative theory is that the CNS becomes inflamed due to nonspecific complications of the systemic disorder [7]. Previous studies showed that COVID-19 patients with chronic diseases are more likely to develop neurological complications [8,9,10]. However, the specific mechanisms responsible for how this virus affects the CNS have yet to be elucidated. Therefore, in this review, we aimed to investigate the development of neurological complications in COVID-19 patients and the mechanisms that occur in these events.
2 Methodology
2.1 Inclusion/Exclusion Criteria
This review involved publications that were written in English and investigated neurological complications in patients with COVID-19 from 2019 until now. We excluded studies that did not confirm COVID-19 infection and studies that involved neurological patients who were subsequently infected with COVID-19. We also excluded case reports and case series that investigated neurological associations with COVID-19 due to the absence of a comparison group.
2.2 Search Methods
Two authors independently searched the Cochrane, Trip, EMBASE, and Google Scholar databases from 2019 until now. In addition, we reviewed the reference lists of the selected articles for further studies. We used the following terms in the search: “COVID-19,” OR “novel corona,” OR “severe pneumonia,” OR “patients infected with corona and neurological manifestation,” OR “neurological complications,” OR “cerebrovascular complications,” OR “encephalopathy,” OR “encephalitis,” OR “delirium,” OR “ischemic stroke,” OR “brain magnetic resonance imaging (MRI).”
2.3 Outcome Measures
The outcome measures were the development of neurological complications in COVID-19 patients including CNS manifestations (e.g., headache, encephalopathy, and stroke), peripheral nervous system (PNS) impairment (e.g., dysfunction of taste, dysfunction of smell, neuropathy), and skeletal muscle manifestations (e.g., myalgia).
The confirmation of COVID-19 disease was based on WHO guidelines [11]. Patients were classified as having severe COVID-19 based on the American Thoracic Society Infectious Disease guidelines [12]. The level of consciousness was assessed using the Richmond Agitation-Sedation Scale [13]. The assessment of neurological complications was confirmed by (1) the onset of neurological manifestations within 6 weeks of acute COVID-19 infection, and (2) either the detection of SARS-CoV-2 RNA in a given sample or detection of the antibody for the infection with no evidence of other commonly associated causes [14].
2.4 Data Collection and Extraction
The abstracts of screened articles were reviewed independently by two authors (NR, NM). Articles fitting the inclusion criteria were acquired and their characteristics were determined independently. These characteristics included the study setting, duration, and design, participant age and sex, and outcome measures. Any disagreements were resolved by discussion.
2.5 Assessment of the Risk of Bias
Two authors (NR, NM) independently judged the risk of bias for the included studies. The risk of bias was graded as high, low, or unclear, as recommended by The Newcastle Ottawa Scale for nonrandomized studies [15].
2.6 Assessment of the Quality of Evidence
The quality of evidence for each outcome measure was judged as high, moderate, low, or very low according to the GRADE approach (Grading of Recommendations Assessment, Development, and Evaluation) [16].
2.7 Measures of Treatment Effect
A random effect model was created in Collaboration C. Review manager (RevMan) version 5.3. The Nordic Cochrane Centre: Copenhagen, Denmark 2014 software [17] and utilized for data analysis. Odds ratios (ORs) or mean differences (MDs) with 95% confidence intervals (CIs) were utilized for dichotomous and continuous data, respectively.
2.8 Dealing with Heterogeneity
The I2 test was used to test the heterogeneity of the studies included in our analysis [18].
3 Results
3.1 Results of Search
Two hundred and sixteen potentially relevant articles were identified; 94 of these remained after the exclusion of duplicates. The abstracts of these articles were subsequently appraised with regards to the inclusion criteria. Eighteen full text articles were evaluated for eligibility; six of these met the inclusion criteria. A PRISMA flow diagram is shown in Fig. 1 and provides further details of the search method used and the studies included.
3.2 Included Studies
The review included six studies [19,20,21,22,23,24] (four retrospective, one prospective, and one retrospective cohort studies). Two authors independently extracted key characteristics from the selected studies, including study title, journal, study design, duration, setting, aim, participant age, sex, number, and outcome measures (Table 1).
3.3 Trial Participants
The review included 4401 COVID-19 patients; 2496 were male. The mean age of the patients reported by Li et al. [19], Guan et al. [23] and Liotta et al. [20] were 75.7 ± 10.8, 48.9 ± 9.6, and 58.5 ± 16.9 years, respectively. The median age of the patients was 63 years (range 34–87 years), 62 years (range 52–70 years) and 69 years (range 66–78 years) in the studies reported by Kandemirli et al. [22], Helms et al. [24], and Merkler et al. [21], respectively.
3.4 Risk of Bias Among the Included Studies
Overall, the included studies recorded a low risk of bias for most of the studied domains and no high risk of bias was recorded for any domain. The risk of bias was unclear for the “prospective calculation of the study size” domain in the studies reported by Guan et al. [23], Helms et al. [24], Kandemirli et al. [22] and Merkler et al [21]. In addition, an unclear risk of bias was recorded for the “inclusion of consecutive patients” domain reported by Guan et al [23], the “unbiased assessment of study endpoint” domain reported by Kandemirili et al. [22], and the “comparable, adequate and contemporary control” domain reported by Merkler et al. [21] (Fig. 2).
3.5 Outcome Measures
Of the 4401 subjects included in this analysis, 364 (8.27%) had neurological manifestations. In the study reported by Li et al. [19], 10 out of 219 patients (4.6%) developed acute ischemic stroke and 1 (0.5%) suffered from an intracerebral hemorrhage. In Helms et al [24], 118 out of 140 (84.3%) patients developed delirium with cognition disturbances. In Kandemirli et al [22], 118 out of 235 (50%) patients developed neurological symptoms; 44% of those who underwent brain magnetic resonance image showed acute findings. These findings included subcortical and deep white matter signal intensity abnormalities in the frontal lobe in four patients, the parietal lobe in three patients, the occipital lobe in four patients, the temporal lobe in one patient, the insular cortex in three patients, and the cingulate gyrus in three patients. In Liotta et al. [19], 215 out of 509 (42.2%) patients developed neurological manifestations, including myalgia (44.8%), headache (37.7%), encephalopathy (31.8%), dizziness (29.7%), dysgeusia (15.9%), and anosmia (11.4%); strokes, movement disorders, motor and sensory deficits, ataxia, and seizures accounted for 0.2% to 1.4% of cases. In the study reported by Guan et al. [23], 30 out of 1690 (1.78%) developed cerebrovascular disease, including multiple lobe infiltration, delirium, or the loss of consciousness. In Merkler et al. [21], 31 (1.6%) of the 1916 patients had an acute ischemic stroke.
Figure 3 shows a forest plot of the mean age across the included studies. Analysis included three studies involving 2318 participants. Old age was identified as a statistically significant risk factor for developing neurological complications in COVID-19 patients (MD 18.35, CI 9.39–27.32). Significant heterogeneity was also noted (I2 = 93%, P < 0.00001).
Figure 4 shows a forest plot of factors associated with the development of neurological complications in COVID-19 patients. The significant risk factors for neurological associations were as follows: severity of COVID-19 (OR 4.30, CI 2.54–7.29, I2 = 39%), hypertension (OR 4.01, CI 1.05–15.36, I2 = 77%), heart disease (OR 2.53, CI 1.01–6.33), diabetes (OR 2.31, CI 1.15–4.65), dyslipidemia (OR 2.13, CI 1.52–3.00), smoking (OR 1.77, CI 1.21–2.59), and male sex (OR 1.71, CI 1.24–2.36). Low levels of nonsignificant heterogeneity were recorded for the analysis of disease severity (I2 = 39%, P = 0.15), dyslipidemia (I2 = 0%, P = 0.42), smoking (I2 = 0%, 0.74), and male sex (I2 = 10%, P = 0.35). However, considerable and significant heterogeneity was recorded for hypertension (I2 = 39%, P = 0.004), diabetes (I2 = 63%, P = 0.03), and heart disease (I2 = 76%, P = 0.002). Malignancy, chronic kidney disease (CKD), and a history of neurological diseases were not significant risk factors (OR 1.23, CI 0.41–3.66; OR 1.24, CI 0.55–2.79; OR 2.13, CI 0.66–6.88, respectively). Figure 5 shows a forest plot of the pooled estimate of the significant risk factors for neurological association among COVID-19 patients.
4 Discussion
4.1 Summary of the Main Results
The current review included 4401 COVID-19 patients from six observational studies. Of these, 8.24% developed neurological complications, including delirium (84.3%), myalgia (44.8%), headache (37.7%), encephalopathy (31.8%), dizziness (29.7%), dysgeusia (15.9%), anosmia (11.45), acute ischemic stroke (4.6%), cerebrovascular disease (1.78%), and intracerebral hemorrhage (0.5%). Movement disorders, motor and sensory deficits, ataxia, and seizures, were uncommon and accounted for 0.2–1.4% of cases. In COVID-19 patients, the severity of COVID-19 disease, along with underlying comorbidity, but particularly hypertension, increased the risk for neurological complications by approximately fourfold (OR 4.30, CI 2.54–7.29 and OR 4.01, CI 1.05–15.36 respectively). Heart disease, diabetes, and dyslipidemia increased the risk by approximately twofold (OR 2.53, CI 1.01–6.33; OR 2.31, CI 1.15–4.65; and OR 2.13, CI 1.52–3.00, respectively). Other recorded risk factors were old age (MD 18.35, CI 9.39–27.32), male sex (OR 1.71, CI 1.24–2.36) and smoking (OR 1.77, CI 1.21–2.59). Malignancy, CKD, and a history of neurological diseases were not significant risk factors (OR 1.23, CI 0.41–3.66; OR 1.24, CI 0.55–2.79; OR 2.13, CI 0.66–6.88, respectively).
4.2 Quality of Evidence
Overall, we downgraded the quality of evidence by one level for all outcome measures due to the observational design of the included studies. In Table 2, we determined the mean difference (MD) among participants of included articles regarding different aspects, including mean age and male sex. The heterogeneity in the mean age of the included articles showed to be significant (p < 0.0001). This revealed that difference of mean age in the included studies is large. Low to moderate insignificant heterogeneity was recorded for the pooled estimate for male sex, the severity of COVID-19, smoking, dyslipidemia, CKD, and malignancy (I2 = 10%, 39, 0, 0, 57, and 60%, P > 0.05 respectively); thus, the quality of evidence for the pooled estimate of these outcomes was judged to be moderate. However, we judged the quality of evidence to be low for the pooled estimate of the following outcomes: mean age, hypertension, diabetes, heart disease and a history of neurological diseases. We downgraded the evidence by one more level due to the considerable significant heterogeneity recorded in these analysis (I2 = 93%, 77, 63, 76, and 69%, P < 0.05 respectively); this heterogeneity could be explained by differences among participants in the background variables and methods used for neurological diagnosis and case definition (Table 2).
4.3 Overall Completeness and Applicability of Evidence
All of the studies included in this review prespecified and determined neurological complications in COVID-19 patients as the primary outcome measure. Furthermore, most studies investigated and reported the following risk factors: male sex, the severity of COVID-19, hypertension, diabetes, heart disease, malignancy, and CKD. Mean age, dyslipidemia, and smoking were reported by two studies only.
4.4 Potential Bias Encountered During the Review Process
For the current review, two authors independently and systematically screened major databases. In addition, the authors independently extracted and data and performed analysis. The authors solved any disagreement or variation in judgment by discussion. Therefore, it is unlikely that this approach could have introduced bias in the review.
4.5 Agreements and Disagreements with Other Studies and Reviews
4.5.1 Frequency of Neurological Complications in COVID-19 Patients
In this review, we recorded neurological complications in 8.24% of patients with COVID-19, including acute ischemic stroke (4.6%), intracerebral hemorrhage (0.5%), cerebrovascular disease (1.78%), delirium (84.3%), myalgia (44.8%), headache (37.7%), encephalopathy (31.8%), dizziness (29.7%), dysgeusia (15.9%), and anosmia (11.45). Motor and sensory disorders, ataxia, and seizures were rare (accounting for 0.2–1.4% of cases). Recent studies have reported that some COVID-19 patients develop neurological diseases in addition to the more common upper and lower respiratory symptoms, including viral encephalitis, meningoencephalitis, ischemic stroke, and hemorrhagic stroke [8,9,10, 25, 26]. Furthermore, Mao et al. [8] investigated hospitalized COVID-19 patients and found that 36.4% had nervous system manifestations in the CNS (24.8%), PNS (8.9%), and skeletal muscle injury (10.7%). These authors also reported that the most common CNS manifestations were dizziness (16.8%) and headache (13.1%). The most frequently reported PNS symptoms were taste (5.6%) and the impairment of smell (5.1%).
Similarly, Moriguchi et al. [9] and Xiang et al. [10] reported two cases of encephalitis in COVID-19 patients. Rothstein et al. [27] recorded ischemic stroke and intracranial hemorrhage in 2.4 and 0.9% of hospitalized COVID-19 patients, respectively. Furthermore, Guillain–Barre syndrome has been identified in COVID-19 patients in many case reports [28,29,30].
However, Ghannam et al. [31] recorded higher figures for the pooled estimate of neurological complications in COVID-19 patients, including cerebrovascular insults (48.8%), neuromuscular disorders (28%), and encephalitis (23%). These high figures could be explained by the low number of included participants and the different methods used for case definition.
4.5.2 Possible Mechanisms Underlying Neurological Complications
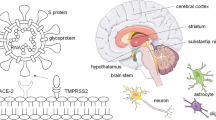
Inflammation of the CNS in COVID-19 patients could be caused by nonspecific complications of systemic disorder [6]. An alternative proposal theorized that inflammation of the CNS could be caused by viral infection via the axonal transport of the virus via the peripheral nerves into the brain [7]. Brain injury and neurological symptoms might occur because of the invasion of neural cell membranes by ACE2. The spike protein of the coronavirus is known to bind to neuronal ACE2 receptors in the brain and attaches to target cells. Then, the spike protein is activated by serine protease TMPRSS2, thus allowing the virus to enter neurons [32]. In addition, endothelial cells in the cerebral blood vessels might be attacked by the virus through ACE2 receptors, thereby disrupting the blood–brain barriers and promoting invasion of the brain tissue and neurons [33, 34].
4.5.3 Risk Factors for the Development of Neurological Complications
In this review, we investigated factors associated with the development of neurological complications in COVID-19 patients. The severity of COVID-19 disease and underlying comorbidity (mainly hypertension) increased the probability of the risk by approximately fourfold (OR 4.30, CI 2.54–7.29 and OR 4.01, CI 1.05–15.36, respectively). Heart disease, diabetes, and dyslipidemia increased the risk by approximately twofold (OR 2.53, CI 1.01–6.33; OR 2.31, CI 1.15–4.65; and OR 2.13, CI 1.52–3.00, respectively). Other recorded risk factors were old age (MD 18.35, CI 9.39–27.32), male sex (OR 1.71, CI 1.24–2.36), and smoking (OR 1.77, CI 1.21–2.59). On the other hand, malignancy, CKD, and a history of neurological disease were not significant risk factors (OR 1.23, CI 0.41–3.66; OR 1.24, CI 0.55–2.79; OR 2.13, CI 0.66–6.88, respectively). These findings were consistent with those of Mao et al. [8] who found that patients with severe COVID-19, along with those with underlying disorders, especially hypertension, developed significant higher percentages of acute cerebrovascular disease (mainly ischemic stroke) (45.5 vs. 30.2%, P = 0.02 and 5.7 vs. 0.8%, P = 0.03 respectively). Similarly, Rothstein et al. [27] found that ischemic stroke was more prevalent in hypertensive, diabetic, and elderly patients with COVID-19. Bekelis et al. [35] reported that COVID-19 patients who develop ischemic stroke were associated with a higher case-fatality rate (OR 10.50, CI 3.54–31.18) and had an increased risk for discharge to rehabilitation (OR 2.45, CI 0.81–1.25). Similarly, Ojo et al. [36] reported similar risk factors for both severe COVID-19 and ischemic stroke. Another study also found that COVID-19 patients who developed ischemic stroke had at least one stroke-associated comorbidity [37].
Along the same lines, Ghannam et al. [31] found that the mean age of COVID-19 patients who developed neurological complications was 62.3 years; moreover, 62.2% of these were males.
Similarly, Kennedy et al. [38] reported that the pooled incidence of intracranial hemorrhage among patients with COVID-19 was 0.7% (CI 0.5–0.9); most patients were elderly men (66%) diagnosed with hypertension (54%).
Similarly, a prospective study identified risk factors associated with the development of delirium among COVID-19 patients including an older age (adjusted relative risk [aRR] 1.51, CI 1.17–1.95), living in a nursing home (aRR 1.23, CI 0.98–1.55), and both vision and hearing impairment (aRR 1.98, CI 1.54–2.54 and aRR 1.10, CI 0.78–1.55, respectively). However, in contrast with our findings, a past history of neurological disease, such as stroke, Parkinson disease, and previous use of psychoactive medication, were all identified as significant risk factors (aRR 1.47, CI 1.15–1.88; aRR 1.88, CI 1.30–2.58; and aRR 1.42, CI 1.11–1.81, respectively) [39].
Similar risk factors for neurological complications in COVID-19 patients were reported by Pun et al. [40], including an older age (OR 1.13, CI 1.03–1.25), a higher Simplified Acute Physiology Score (SAPS) (OR 1.17, CI 1.07–1.29), smoking or alcohol abuse (OR 1.37, CI 1.13–1.67), and disease severity, such as invasive mechanical ventilation (OR 1.48, CI 1.17–1.87). These authors also identified the following risk factors: vasopressors (OR 1.25, CI 1.10–1.43), the use of restraints (OR 1.32, CI 1.16–1.50), antipsychotics (OR 1.59, CI 1.36–1.85, P < 0.0001), sedative benzodiazepine infusions (OR 1.59, CI 1.33–1.91), and continuous opioid infusions (OR 1.39, CI 1.21–1.60). Family visitation, however, was associated with a 27% lower risk of delirium (OR 0.73, CI 0.63–0.84, P < 0.0001).
In addition, previous studies reported that patients with COVID-19 who were admitted with neurological diseases, including stroke, had significantly higher rates for admission into the intensive care unit [39], in-hospital mortality, incident delirium [41], and discharge to rehabilitation [35].
5 Conclusion
In this review, we identified several significant factors associated with the development of neurological complications in COVID-19 patients, including old age, male sex, smoking, severity of the disease and underlying comorbidities including hypertension, heart disease, diabetes, dyslipidemia. These complications are relatively uncommon. However, during the COVID-19 pandemic, differential diagnosis of the disease should be suspected when confronted with patients with neurological manifestations to avoid delayed diagnosis or misdiagnosis. Early identification of at-risk patients, particularly experiencing comorbid conditions could be important if we are to improve their outcome and prevent further transmission.
Data Availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Abbreviations
- ACE2:
-
Angiotensin converting enzyme-2
- aRR:
-
Adjusted relative risk
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- CNS:
-
Central nervous system
- COVID-19:
-
Coronavirus disease 2019
- HD:
-
Heart disease
- MD:
-
Mean difference
- MRI:
-
Magnetic resonance image
- OR:
-
Odds ratio
- PNS:
-
Peripheral nervous system
- SAPS:
-
Simplified Acute Physiology Score
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- TMPRSS2:
-
Transmembrane protease serine 2
- WHO:
-
World Health Organization
References
Holder K, Reddy PH. The COVID-19 effect on the immune system and mitochondrial dynamics in diabetes, obesity, and dementia. Neuroscientist. 2021;27(4):331–9.
Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2020;19(3):141–54.
Vallamkondu J, John A, Wani WY, Ramadevi S, Jella KK, Reddy PH, Kandimalla R. SARS-CoV-2 pathophysiology and assessment of coronaviruses in CNS diseases with a focus on therapeutic targets. Biochim Biophys Acta Mol Basis Dis. 2020;1866(10): 165889.
Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–4.
Bulut C, Kato Y. Epidemiology of COVID-19. Turk J Med Sci. 2020;50(SI-1):563–70. https://doi.org/10.3906/sag-2004-172.
Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S, et al. Neuropath genesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease. JAMA Neurol. 2020;77(8):1018–27. https://doi.org/10.1001/jamaneurol.2020.2065.
Andrabi MS, Andrabi SA. Neuronal and cerebrovascular complications in coronavirus disease 2019. Front Pharmacol. 2020;11: 570031. https://doi.org/10.3389/fphar.2020.570031.
Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–90. https://doi.org/10.1001/jamaneurol.2020.1127.
Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–8. https://doi.org/10.1016/j.ijid.2020.03.062.
Xiang PX, Xu XM, Gao LL, Wang HZ, Xiong HF, Li RH. First case of 2019 novel coronavirus disease with encephalitis. ChinaXiv. 2020;202003:00015.
Emery SL, Erdman DD, Bowen MD, Newton BR, Winchell JM, Meyer RF, Tong S, et al. Real-time reverse transcription–polymerase chain reaction assay for SARS-associated coronavirus. Emerg Infect Dis. 2004;10:311–6.
Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Disease Society of America. Am J Respir Crit Care Med. 2019;200:e45–67.
Kumar A, Bakhla AK, Gupta S, Raju BM, Prasad A. Etiologic and cognitive differences in hyperactive and hypoactive delirium. Prim Care Companion CNS Disord. 2015. https://doi.org/10.4088/PCC.15br01810.
Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–8.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2019.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924.
Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen; The Nordic Cochrane Centre, The Cochrane Collaboration; 2014.
Higgins JPT, Thompson SG, Deeks JJ. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Li Y, Li M, Wang M, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5: e000431. https://doi.org/10.1136/svn2020-000431.
Liotta EM, Batra A, Clark JR, Shlobin NA, Hoffman SC, Orban ZS, Koralnik IJ. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol. 2020;7(11):2221–30. https://doi.org/10.1002/acn3.51210.
Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H, Lin E, Lantos J, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020;77(11):1366–72. https://doi.org/10.1001/jamaneurol.2020.2730.
Kandemirli SG, Dogan L, Sarikaya ZT, Kara S, Akinci C, Kaya D, et al. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology. 2020;297:E232–5. https://doi.org/10.1148/radiol.2020201697.
Guan W-J, Liang W-H, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. https://doi.org/10.1183/13993003.00547-2020.
Helms J, Kremer S, Merdji H, Schenck M, Severac F, Clere-Jehl R, et al. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit Care. 2020;24:491. https://doi.org/10.1186/s13054-020-03200-1.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Yu T. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13. https://doi.org/10.1016/s0140-6736(20)30211-7.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5.
Rothstein A, Oldridge O, Schwennesen H, Do D, Cucchiara BL. Acute cerebrovascular events in hospitalized COVID-19 patients. Stroke. 2020;51:e219–22. https://doi.org/10.1161/STROKEAHA.120.030995.
Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, Franciotta D, Baldanti F, Daturi R, Postorino P, Cavallini A. Guillain–Barré syndrome associated with SARS-CoV-2. New England Journal of Medicine. 2020;382(26):2574–6.
Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. 2020;76:233–5.
Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain–Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383–4.
Ghannam M, Alshaer Q, Al-Chalabi M, Zakarna L, Robertson J, Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. 2020;267:3135–53. https://doi.org/10.1007/s00415-020-09990-2.
Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–7. https://doi.org/10.1002/path.1570.
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271-280.e8. https://doi.org/10.1016/j.cell.2020.02.052.
Wang Z, Yang Y, Liang X, Gao B, Liu M, Li W, Chen Z, Wang Z. COVID-19 associated ischemic stroke and hemorrhagic stroke: incidence, potential pathological mechanism, and management. Front Neurol. 2020;11:1152. https://doi.org/10.3389/fneur.2020.571996.
Bekelis K, Missios S, Ahmad J, Labropoulos N, Schirmer CM, Calnan DR, et al. Ischemic stroke occurs less frequently in patients with COVID-19: a multicenter cross-sectional study. Stroke. 2020;51:3570–6. https://doi.org/10.1161/STROKEAHA.120.031217.
Ojo AS, Balogun SA, Idowu AO. Acute ischemic stroke in COVID-19: putative mechanisms, clinical characteristics, and management. Neurol Res Int. 2020;2020:7397480. https://doi.org/10.1155/2020/7397480.
Jillella DV, Janocko NJ, Nahab F, Benameur K, Greene JG, Wright WL, et al. Ischemic stroke in COVID-19: an urgent need for early identification and management. PLoS ONE. 2020;15: e0239443. https://doi.org/10.1371/journal.pone.0239443.
Cheruiyot I, Sehmi P, Ominde B, Bundi P, Mislani M, Ngure B, Olabu B, Ogeng’o JA. Intracranial hemorrhage in coronavirus disease 2019 (COVID-19) patients. Neurol Sci. 2021;42(1):25–33. https://doi.org/10.1007/s10072-020-04870-z.
Kennedy M, Helfand BKI, Gou RY, Gartaganis SL, Webb M, Moccia M. Delirium in older patients with COVID-19 presenting to the emergency department. JAMA Netw Open. 2020;3(11): e2029540. https://doi.org/10.1001/jamanetworkopen.2020.29540.
Pun BT, Badenes R, Heras La Calle G, Orun OM, Chen W, Raman R, et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicenter cohort study. Lancet Respir Med. 2021;9(3):239–50. https://doi.org/10.1016/S2213-2600(20)30552-X.
Benussi A, Pilotto A, Premi E, Libri I, Giunta M, Agosti C, et al. Clinical characteristics and outcomes of inpatients with neurologic disease and COVID-19 in Brescia, Lombardy, Italy. Neurology. 2020;95:e910–20. https://doi.org/10.1212/WNL.0000000000009848.
Acknowledgements
The authors would like to thank Dr. Yousef Almutairi (Saudi Ministry of Health) for his assistance to complete this study.
Funding
This study was not funded by any institution or organization and the authors declare that none of the authors have any conflicts to report.
Author information
Authors and Affiliations
Contributions
NR and AK conceptualized the study. NR, AK, and NM wrote the original draft preparation. NM, AF, and KA helped to write and edit the review. NR, AK, AF, and KA contributed resources (scholarly journal articles and research based reviews). All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
Not applicable.
Consent to publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Radwan, N., Mahmoud, N., Alkattan, A. et al. Neurological Associations Among COVID-19 Patients: A Systematic Review and Meta-Analysis. Dr. Sulaiman Al Habib Med J 4, 53–63 (2022). https://doi.org/10.1007/s44229-022-00010-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44229-022-00010-1