Abstract
Chimeric antigen receptor (CAR) T-cell therapy is novel immunotherapy targeting specifically cancerous cells, and has been shown to induce durable remissions in some refractory hematological malignancies. However, CAR T-cell therapy has adverse effects, such as cytokine release syndrome (CRS), immune effector-associated neurotoxicity syndrome (ICANS), tumor lysis syndrome (TLS), and acute kidney injury (AKI), among others. Not many studies have covered the repercussions of CAR T-cell therapy on the kidneys. In this review, we summarized the available evidence on the safety profile of CAR T-cell therapy in patients with pre-existing renal insufficiency/AKI and in those who develop AKI as a result of CAR T-cell therapy. With a 30% incidence of AKI post-CAR T-cell, various pathophysiological mechanisms, such as CRS, hemophagocytic lymphohistiocytosis (HLH), TLS, serum cytokines, and inflammatory biomarkers, have been shown to play a role. However, CRS is commonly reported as an underlying mechanism. Overall, 18% of patients in our included studies developed AKI after receiving CAR T-cell therapy, and most cases were reversible with appropriate therapy. While phase-1 clinical trials exclude patients with significant renal toxicity, two studies (Mamlouk et al. and Hunter et al.) reported successful treatment of dialysis-dependent patients with refractory diffuse large B-cell lymphoma, and demonstrated that CAR T-cell therapy and lymphodepletion (Flu/Cy) can be safely administered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Chimeric antigen receptor (CAR) T-cell therapy is novel immunotherapy, targeting specifically cancerous cells, and inducing durable remissions in some refractory hematological malignancies like multiple myeloma (MM) [1]. This therapy is promising, as demonstrated in several clinical trials [2]. With its relative safety and efficacy, the Food and Drug Administration (FDA) approved the clinical use of different CAR T-cells, including idecabtagene vicleucel in MM [3], axicabtagene ciloleucel in diffuse large B-cell lymphoma [4], ciltacabtagene autoleucel [5], brexucabtagene autoleucel, lisocabtagene marleucel, and tisgenlecleucel.
CAR T-cell therapy has side effects which include tumor lysis syndrome (TLS) [6], immune effector-associated neurotoxicity syndrome (ICANS) [7], hemophagocytic lymphohistiocytosis (HLH)/ macrophage activation syndrome (MAS) [8], and cytokine release syndrome (CRS) [9], which remarkably affects kidneys. CRS caused by T-cell activation and subsequent release of large amounts of cytokines is experienced by the majority of patients in clinical trials with CAR T-cell therapy [10]. Hypotension, nausea, fever, hypoxia, tachypnea, tachycardia, and pulmonary edema that leads to intravascular depletion are the main clinical presentations of CRS. These cause hemodynamic changes leading to reduced renal flow, ischemia, hypovolemia, and triggering pre-renal AKI [11]. In a case series conducted by Gupta et al., a total of 78 adults received CAR T-cell therapy, and 15 of 78 (19%) developed acute kidney injury (AKI), among which 8 (53%) were due to decreased renal perfusion (resolved in 72 h), 6 (40%) were consequent to acute tubular necrosis (ATN), and 1 developed urinary obstruction. The 60-day mortality and length of hospital stay were higher in those with ATN and obstructive AKI [12].
Similarly, in a retrospective review by Gutgarts et al., 46 adult patients with non-Hodgkin lymphoma (NHL) received treatment with CAR T-cell therapy and reported a cumulative incidence of any grade AKI of 30%, with grades 1/2 and 3 AKI incidences of 21.7%, and 8.7%, respectively [13]. Moreover, no patients developed severe AKI necessitating renal replacement therapy, alongside satisfying kidney function recovery by day 30. That said, phase-1 trials exclude patients with significant renal toxicity. Herein, we aim to summarize all available evidence on the safety profile of CAR T-cell therapy in patients with renal insufficiency/AKI, and to provide insights to encourage the possibility of enrolling this subset of the population in randomized controlled trials and cohort studies.
2 Methods
We performed a literature review using PubMed, Cochrane databases, and Google Scholar using the following keywords: CAR T-cell therapy, adoptive immunotherapy, renal failure, end-stage renal disease, and acute kidney injury. Articles suitable for extraction from the literature searches were case report, case series, editorials, and retrospective reviews. We included studies published in English and reporting data on the safety of CAR-T cell therapy in patients with AKI or renal failure. Non-human studies and studies in languages other than English were excluded. There are currently no published randomized controlled trials available in the defined population. We reviewed the relevant findings and added the information to the data extraction sheet. We used pooled analysis to report some commonly reported data across studies.
3 Results
3.1 Baseline Characteristics
A total of 252 patients (male = 166, female = 86) included in the analysis were treated with CAR T-cell therapy and enrolled across 9 studies from 2016 to 2021. The mean age of the sample was 48 (19–86). The diseases treated with CAR T-cell therapy included NHL (n = 129), diffuse large B-cell lymphoma (DLBCL, n = 120), post-transplant lymphoproliferative disorder (PTLD, n = 1), B-cell ALL (n = 1) and Burkitt Lymphoma (n = 1). Cyclophosphamide (Cy) with fludarabine (Flu) is a common non-myeloablative conditioning regimen and was utilized in 6 of the studies. One study added rituximab to Cy/Flu for one patient, and another reported only using bendamustine for three patients. Three of the studies did not report their conditioning regimen. The type of CAR T-cell therapy used in the analysis includes tisagenlecleucel alone (Lee et al., Melilli et al., Acharya et al., De Nattes et al.), tisagenlecleucel and axicabtagene ciloleucel (Gupta et al., Gutgarts et al.), axicabtagene ciloleucel alone (Farooqui et al. and Mamlouk et al.), axicabtagene ciloleucel and lisocabtagene maraleucel (Hunter et al.). In some cases, due to persistent CD19 antigen, the patient received a 28-day course of a bispecific CD19-directed CD3 T-cell engager antibody construct (blinatumomab), started 6 weeks after the third CAR-T cell infusion, and stopped after negative CD19 antigen at the time of course completion (Acharya et al.).
Baseline creatinine before CAR T-cell therapy ranged from 0.5 to 2 mg/dL. The mean creatinine values pre-therapy for all nine studies was 0.8 mg/dL. In one study, two subjects had ESRD and were receiving hemodialysis (Hunter et al.) AKI was described in 70% of the studies, with the exception of Hunter et al.’s, where creatinine level was not reported due to concomitant ESRD. Table 1 shows patients’ characteristics and kidney profiles before and after receiving CAR-T cell therapy, respectively.
3.2 Clinical Outcomes
The average creatinine levels after therapy ranged between 0.8 and 2 mg/dL, with one patient presenting with 6 mg/dL. For another patient who presented with levels of 11 mg/dL, steroids helped decrease this to 1.5 mg/dL. The CAR T-types which are effective in renal disease are axicabtagene ciloleucel and lisocabtagene maraleucel. It was usually preceded by lymphodepletion chemotherapy with Flu and Cy. Among 252 patients, 46/252 (18%) developed AKI after receiving CAR T-cell therapy. However, 80% developed CRS following CAR T-cell therapy in the included studies, while n = 135/250 (54%) developed ICANs. Most cases of AKI were reported with the use of axicabtagene ciloleucel therapy. We also found that AKI resolved in most cases with supportive care and adequate hydration.
4 Discussion
CAR T-cell therapy was highly effective in patients with renal failure, both with and without pre-existing kidney disease. It can be safely administered with lymphodepletion chemotherapy. According to Hunter et al. [14], patients with end-stage renal disease achieved complete response post CAR T-cell therapy, making it a strong consideration in treating renal disease patients. In our pooled analysis, about 18% developed AKI post-CAR T-cell therapy, whereas most patients reported improved blood urea nitrogen and creatinine levels post-therapy. Three patients from the Mamlouk et al. [15] study, received prior renal transplant for other causes, and thus were excluded from the analysis. Additionally, baseline renal function was not reported in Hunter et al. [14] due to both patients having been diagnosed with ESRD before starting. The improved renal function and kidney injury in patients with pre-existing renal failure emphasize the safety of CAR T-Cell therapy in renal failure.
According to one study [18], there is low renal toxicity for anti-B-cell maturation antigen (BCMA) CAR T-cell therapy. Another clinical trial illustrated safe renal outcomes for MM patients post-CAR T-cell therapy [19]. CD8 + CAR T-cells can target the autoreactive pathogenic B cells. The CAAR T-cells (chimeric autoantigen receptor T cells) that express relevant autoantigens can attract autoreactive B cells and target them successfully. These experiments provide a wide array of the possible efficacy of CAR T-cell therapy in kidney transplantation and immune diseases [20].
Post CAR T-cell therapy, various pathophysiological mechanisms have been shown to play a role, such as CRS, HLH, tumor lysis syndrome, serum cytokines, and inflammatory biomarkers [12, 13, 16]. CRS is usually considered the main cause of kidney injury post-therapy due to the underlying cytokine cascade, which increases third spacing, worsening hypotension, and vascular permeability, while the circulating cytokines cause cardiac toxicity leading to diminished cardiac output and cardiorenal syndrome. Acute cardiomyopathy from CRS promotes hypotension and exacerbates hypoperfusion in the kidney [8, 9, 11]. Hypotension, nausea, fever, hypoxia, tachypnea, tachycardia, and pulmonary edema that leads to intravascular depletion are the main clinical presentations of CRS. CRS can cause hemodynamic changes leading to reduced renal flow, ischemia, hypovolemia, and triggers pre-renal AKI [11, 16]. The prolonged decreased kidney perfusion and pre-renal AKI then progresses to acute tubular necrosis (ATN) [12, 21]. Of note, in Nattes et al.’s case report, the etiology of kidney damage, AKI, and transplant rejection might have been due to either CAR T-cell therapy or T-cell-mediated rejection [17]. According to Teachey et al., specific cytokines, including IL-6, soluble IL-6 receptor, interferon-γ, and soluble gp130, are major predictors of CRS after CAR T-cell therapy [22]. Cytokines may directly affect the kidney via intra-renal inflammation and have direct tubular toxicity [23]. IL-6 has been implicated as a key factor in developing systemic adverse effects in CRS [23]. In the case of AKI and chronic kidney disease (CKD), IL-6 increases fibroblast growth factor 23 levels, which may contribute to phosphaturia and hypophosphatemia, thus affecting renal function [24].
Patients receiving CAR T-cell therapy can develop fulminant HLH and present with increased lactate dehydrogenase, hyperuricemia, IL-10, IL-6, and IFN-γ. The ATN, acute interstitial nephritis (AIN), and thrombotic microangiopathy related to HLH, together with capillary leakage and cytokine-mediated vasodilation, trigger pre-renal ischemia [25, 26]. TLS after CAR T-cell therapy for refractory chronic lymphocytic leukemia is also a cause of AKI, according to Porter et al. [27]. In TLS, the damage to large amounts of tumor cells leads to rapid release of intracellular substances such as potassium, uric acid, calcium, and phosphorus results in a series of metabolic disorders [28]. The treatment with anti-CD19-CAR T cells leading to TLS causes phosphate and uric acid to precipitate and block renal tubules leading to renal tubular injury [29]. Another proposed mechanism is when cytokines produced by infiltrated interstitial and glomerular lymphocytes after CAR T-cell therapy activate podocytes and renal tubular epithelial cells [30]. In turn, podocytes increase cytokine production, such as tumor necrosis factor α, IL-8, and IL-6, which leads to kidney injury [31].
While there have been isolated cases of severe renal impairment post Flu exposure [37,38,39], most are in context of TLS, and the incidence of AKI remains less than 5% [36]. Special considerations for patients with pre-existing renal impairment given Flu treatment should be addressed, as 60% of each Flu dose is excreted in the urine [35]. Dose-reduced Flu (Table 2) has shown reasonably equivalent exposure while maintaining a higher index of safety [35]. Despite occasions of creatinine increasing up to threefold, the worsening renal function is amenable to recovery with proper hydration [32]. One retrospective study has shown that dose-reduced Flu in cases of underlying renal impairment does not affect PFS and OS when compared to patients with previously normal renal function [33]. Thus, lymphodepletion dosing regimens can be personalized to avoid renal failure without compromising eligibility or response to CAR-T cell therapy. However, it is important to note that a new study has shown optimal lymphodepletion dosing (greater than 13.8 mg x h/L) results in lower relapse rates and improved survival after CAR-T cell therapy [34]. There is no clear consensus on exactly how much to reduce Flu doses in patients with underlying renal impairment. Most studies suggest a 20–25% reduction for mild impairment and up to a 50% for moderate to severe impairment [40,41,42].
Renal damage after CAR-T cell therapy is often witnessed. In all the trials of CD-19–directed CAR-T cells, more than 40% of patients depicted CRS regardless of the CAR-T cell construct or the disease [27, 43,44,45]. The rise in serum creatinine was evident, particularly 7–10 days post-infusion, [27, 45, 46]. This was similar in patients with Burkitt lymphoma and TLS has also been evident in the initial trials of CAR-T cell therapy for chronic lymphocytic leukemia, where elevations in uric acid, lactate dehydrogenase, phosphorus levels, and AKI were noted around 22 days after CAR-T cell infusion [27].
Despite the post-CAR T-cell-induced renal damage, patients can present with borderline AKI before receiving the therapy, and their creatinine stays within manageable range, especially with axicabtagene ciloleucel [15, 47]. The majority of patients with B-cell lymphomas also present with pre-CAR-T cell therapy AKI [13], though the incidence of AKI after CAR-T cell therapy is low, unless an ICU admission or grade 3–4 CRS occurs [13].
Kidney complications are associated with various cancer therapies, especially CAR T-cell therapy. Each CART product must be evaluated individually for its toxicity profile to minimize adverse events [12, 49]. Axicabtagene ciloleucel is associated with higher toxicity, and indirectly increases the risk of AKI through CRS mechanisms [50]. Tisagenlecleucel has a reduced inflammatory profile, lower toxicity rate, and low rates of AKI (5%) [49, 51]. The two series evaluating patients who received axicabtagene ciloleucel found higher rates of AKI (23%), severe CRS (13%), and overall CRS (83%) [12, 13].
Previous nephrotoxic medication exposures before post-CART AKI development are common in these patients and include medications like vancomycin, acyclovir, ibuprofen, trimethoprim-sulfamethoxazole, and intravenous contrast. Patients developing AKI usually receive tocilizumab for CRS and dexamethasone [13]. The pre-renal AKI cohort receives supportive care, avoids nephrotoxic agents, uses intravenous fluids and hemodynamic support, with the condition resolving within 72 h [12]. In comparison, ATN and obstructive AKI have high mortality rates [12]. Kidney replacement therapy is mostly fatal, especially in ATN, but post-renal AKI is responsive to IV fluids [12, 13].
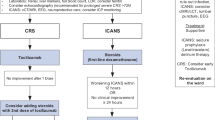
While mild and moderate CRS are self-limiting and can be managed with supportive care [52], severe CRS requires corticosteroids, tocilizumab, or siltuximab, either with corticosteroids or alone [52, 53]. Tocilizumab is also indicated for catecholamine-dependent vasodilatory shock and severe CRS. It improves blood pressure and prevents multiple organ failure [54]. Gutgarts et al. [13] reported normal kidney function within 30 days post-CAR T-cell therapy in patients with NHL. Similarly, Hunter et al. [14] reported the successful treatment of two dialysis-dependent patients diagnosed with refractory-DLBCL with CAR T-cell therapy.
To prevent pre-renal AKI in these patients, intravenous fluids, and vasopressors to maintain renal perfusion and systemic hemodynamics are helpful [55]. In TLS, hydration, allopurinol, and alkalinization prevent AKI for low-risk patients, while in the high-risk cohort, hydration and rasburicase help lower uric acid levels, [56]. HLH cases require aggressive immunosuppression involving corticosteroids and anti-IL-6 therapy (tocilizumab or siltuximab) with supportive care [57]. If immunosuppression fails to reduce toxicity, etoposide should be considered [57, 58]. For HLH management, intravenous immunoglobulin has also been recommended [59]. There is a need to identify various biomarkers that can not only predict AKI earlier but can assist in categorizing long-term risks for CKD in patients with post-CAR T-cell therapy [12, 49].
5 Conclusion
The incidence of AKI in those receiving CAR T-cell therapy ranges from 50 to 90%. The range is determined by the types of CAR T-cells used and the underlying pathophysiological mechanisms. These include CRS, HLH, TLS, and inflammatory cytokines. Also, other factors, such as lymphodepleting therapy (fludarabine), dehydration, and nephrotoxic agents, may potentiate pre-renal or other renal injuries. Though phase 1 clinical trials exclude patients with significant renal toxicity, two studies have demonstrated that CAR T-cell therapy and lymphodepletion (Flu/Cy) can be safely administered. While there is no clear consensus on how much to reduce Flu doses in patients with underlying renal impairment, most studies suggest a 20–25% reduction for mild impairment and up to a 50% reduction for moderate to severe impairment, without significantly compromising the response to CAR T-cell. Additionally, the majority of post-CAR T-cell therapy-related AKI remains reversible by treating the underlying cause, adequate hydration, supportive care, and tocilizumab or siltuximab. That being said, the safety data we present in the defined population are limited; however, they should pave the way for including patients with renal insufficiency in prospective cohort studies and clinical trials of CAR T-cell therapy to determine its long-term safety and efficacy profiles.
Abbreviations
- CAR T-cell therapy:
-
Chimeric antigen receptor T-cell therapy
- CRS:
-
Cytokine release syndrome
- ICANS:
-
Immune effector-associated neurotoxicity syndrome
- TLS:
-
Tumor lysis syndrome
- AKI:
-
Acute kidney injury
- ATN:
-
Acute tubular necrosis
- MM:
-
Multiple myeloma
- NHL:
-
Non-Hodgkin lymphoma
- DLBCL:
-
Diffuse large B-cell lymphoma
- PTLD:
-
Post-transplant lymphoproliferative disorder
- ALL:
-
B-cell acute lymphoblastic leukemia
- Cy:
-
Cyclophosphamide
- Flu:
-
Fludarabine
- CKD:
-
Chronic kidney disease
References
Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–44. https://doi.org/10.1056/NEJMoa1707447.
Klyuchnikov E, Bacher U, Kroll T, et al. Allogeneic hematopoietic cell transplantation for diffuse large B cell lymphoma: who, when and how? Bone Marrow Transplant. 2014;49(1):1–7. https://doi.org/10.1038/bmt.2013.72.
Munshi NC, Anderson LD Jr, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705–16. https://doi.org/10.1056/NEJMoa2024850.
Axicabtagene Ciloleucel for Diffuse Large B-Cell Lymphoma: Economic Review Report. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; August 2019.
Chacon A, et al. “Nouvelles AMMs : Ciltacabtagene autoleucel – patient atteint d’un myélome multiple en rechute ou réfractaire après au moins trois lignes de traitements” [New drug approval: Ciltacabtagene autoleucel—in patients with relapsed or refractory multiple myeloma who received 3 or more lines of therapy]. Bull du Cancer. 2022;S0007-4551(22):00267–73. https://doi.org/10.1016/j.bulcan.2022.06.001.
Feldmann A, Arndt C, Koristka S, Berndt N, Bergmann R, Bachmann MP. Conventional CARs versus modular CARs. Cancer Immunol Immunother. 2019;68(10):1713–9. https://doi.org/10.1007/s00262-019-02399-5.
Sheth VS, Gauthier J. Taming the beast: CRS and ICANS after CAR T-cell therapy for ALL. Bone Marrow Transplant. 2021;56(3):552–66. https://doi.org/10.1038/s41409-020-01134-4.
Johnson TS, Terrell CE, Millen SH, Katz JD, Hildeman DA, Jordan MB. Etoposide selectively ablates activated T cells to control the immunoregulatory disorder hemophagocytic lymphohistiocytosis. J Immunol. 2014;192(1):84–91. https://doi.org/10.4049/jimmunol.1302282.
Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24(6):731–8. https://doi.org/10.1038/s41591-018-0041-7.
Sauter CS, Senechal B, Rivière I, et al. CD19 CAR T cells following autologous transplantation in poor-risk relapsed and refractory B-cell non-Hodgkin lymphoma. Blood. 2019;134(7):626–35. https://doi.org/10.1182/blood.2018883421.
Ganatra S, Carver JR, Hayek SS, et al. Chimeric antigen receptor T-cell therapy for cancer and heart: JACC council perspectives. J Am Coll Cardiol. 2019;74(25):3153–63. https://doi.org/10.1016/j.jacc.2019.10.049.
Gupta S, Seethapathy H, Strohbehn IA, et al. Acute kidney injury and electrolyte abnormalities after chimeric antigen receptor T-cell (CAR-T) therapy for diffuse large B-cell lymphoma. Am J Kidney Dis. 2020;76(1):63–71. https://doi.org/10.1053/j.ajkd.2019.10.011.
Gutgarts V, Jain T, Zheng J, et al. Acute kidney injury after CAR-T cell therapy: low incidence and rapid recovery. Biol Blood Marrow Transplant. 2020;26(6):1071–6. https://doi.org/10.1016/j.bbmt.2020.02.012.
Hunter BD, Hoda D, Nguyen A, et al. (2022) Successful administration of chimeric antigen receptor (CAR) T-cell therapy in patients requiring hemodialysis. Exp Hematol Oncol. 2022;11(1):10. https://doi.org/10.1186/s40164-022-00266-1.
Mamlouk O, Nair R, Iyer SP, et al. Safety of CAR T-cell therapy in kidney transplant recipients. Blood. 2021;137(18):2558–62. https://doi.org/10.1182/blood.2020008759.
Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–95. https://doi.org/10.1182/blood-2014-05-552729. ([Published correction appears in Blood 2015 Aug 20;126(8):1048. Dosage error in article text] [published correction appears in Blood. 2016 Sep 15;128(11):1533]).
de Nattes T, Camus V, François A, et al. Kidney transplant T cell-mediated rejection occurring after anti-CD19 CAR T-cell therapy for refractory aggressive burkitt-like lymphoma with 11q aberration: a case report. Am J Kidney Dis. 2022;79(5):760–4. https://doi.org/10.1053/j.ajkd.2021.07.012.
Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726–37. https://doi.org/10.1056/NEJMoa1817226.
Yan Z, Cao J, Cheng H, et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: a single-arm, phase 2 trial. Lancet Haematol. 2019;6(10):e521–9. https://doi.org/10.1016/S2352-3026(19)30115-2.
Ellebrecht CT, Bhoj VG, Nace A, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353(6295):179–84. https://doi.org/10.1126/science.aaf6756.
Shalabi H, Sachdev V, Kulshreshtha A, et al. Impact of cytokine release syndrome on cardiac function following CD19 CAR-T cell therapy in children and young adults with hematological malignancies. J Immunother Cancer. 2020;8(2):e001159. https://doi.org/10.1136/jitc-2020-001159.
Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6(6):664–79. https://doi.org/10.1158/2159-8290.CD-16-0040.
Perazella MA, Shirali AC. Nephrotoxicity of cancer immunotherapies: past, present and future. J Am Soc Nephrol. 2018;29(8):2039–52. https://doi.org/10.1681/ASN.2018050488.
Durlacher-Betzer K, Hassan A, Levi R, Axelrod J, Silver J, Naveh-Many T. Interleukin-6 contributes to the increase in fibroblast growth factor 23 expression in acute and chronic kidney disease. Kidney Int. 2018;94(2):315–25. https://doi.org/10.1016/j.kint.2018.02.026.
Siddall E, Khatri M, Radhakrishnan J. Capillary leak syndrome: etiologies, pathophysiology, and management. Kidney Int. 2017;92(1):37–46. https://doi.org/10.1016/j.kint.2016.11.029.
Malaga-Dieguez L, Ming W, Trachtman H. Direct reversible kidney injury in familial hemophagocytic lymphohistiocytosis type 3. J Am Soc Nephrol. 2015;26(8):1777–80.
Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–33. https://doi.org/10.1056/NEJMoa1103849. (Published correction appears in N Engl J Med. 2016 Mar 10;374(10):998).
Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364(19):1844–54.
Kochenderfer JN, Dudley ME, Carpenter RO, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122(25):4129–39. https://doi.org/10.1182/blood-2013-08-519413.
Van Kooten C, Daha MR. Cytokine cross-talk between tubular epithelial cells and interstitial immunocompetent cells. Curr Opin Nephrol Hypertens. 2001;10(1):55–9. https://doi.org/10.1097/00041552-200101000-00009.
Wright RD, Beresford MW. Podocytes contribute, and respond, to the inflammatory environment in lupus nephritis. Am J Physiol Renal Physiol. 2018;315(6):F1683–94. https://doi.org/10.1152/ajprenal.00512.2017.
Nunes R, Passos-Coelho JL, Miranda N, Nave M, da Costa FL, Abecasis M. Reversible acute renal failure following single administration of fludarabine. Bone Marrow Transplant. 2004;33(6):671. https://doi.org/10.1038/sj.bmt.1704404. (PMID: 14730341).
Wood AC, Perez AP, Arciola B, Patel K, Johnson G, DiMaggio E, Bachmeier CA, Reid K, Carallo S, Vargas MH, Faramand R, Chavez JC, Shah B, Gaballa S, Khimani F, Elmariah H, Nishihori T, Lazaryan A, Freeman C, Davila ML, Locke FL, Mhaskar R, Bassil C, Jain MD. Outcomes of CD19-targeted chimeric antigen receptor T cell therapy for patients with reduced renal function including dialysis. Transplant Cell Ther. 2022;28(12):829.e1-829.e8. https://doi.org/10.1016/j.jtct.2022.09.009. (Epub 2022 Sep 26. PMID: 36174934; PMCID: PMC9791940).
Fabrizio VA, Boelens JJ, Mauguen A, Baggott C, Prabhu S, Egeler E, Mavroukakis S, Pacenta H, Phillips CL, Rossoff J, Stefanski HE, Talano JA, Moskop A, Margossian SP, Verneris MR, Myers GD, Karras NA, Brown PA, Qayed M, Hermiston M, Satwani P, Krupski C, Keating AK, Wilcox R, Rabik CA, Chinnabhandar V, Kunicki M, Goksenin AY, Mackall CL, Laetsch TW, Schultz LM, Curran KJ. Optimal fludarabine lymphodepletion is associated with improved outcomes after CAR T-cell therapy. Blood Adv. 2022;6(7):1961–8. https://doi.org/10.1182/bloodadvances.2021006418. (PMID:34788386; PMCID:PMC9006295).
Lichtman SM, Etcubanas E, Budman DR, et al. The pharmacokinetics and pharmacodynamics of fludarabine phosphate in patients with renal impairment: a prospective dose adjustment study. Cancer Invest. 2002;20(7–8):904–13. https://doi.org/10.1081/cnv-120005903.
McEvoy GK, editor. American hospital formulary service—drug information. Bethesda: American Society of Health-System Pharmacists; 2001. p. 972–8.
List AF, Kummet TD, Adams JD, et al. Tumorlysis syndrome complicating treatment of chronic lymphocytic leukemia with fludarabine phosphate. Am J Med. 1990;89:388–90.
Macheta MP, Parapia LA, Gouldesbrough DR. Renal failure in a patient with chronic lymphocytic leukaemia treated with fludarabine. J Clin Pathol. 1995;48(2):181–2. https://doi.org/10.1136/jcp.48.2.181.
Tisler A, Pierratos A, Lipton JH. Crescentic glomerulonephritis associated with p-ANCA positivity in fludarabine-treated chronic lymphocytic leukaemia. Nephrol Dial Transplant. 1996;11(11):2306–8. https://doi.org/10.1093/oxfordjournals.ndt.a027155.
Bodge MN, Reddy S, Thompson MS, Savani BN. Preparative regimen dosing for hematopoietic stem cell transplantation in patients with chronic kidney disease: analysis of the literature and recommendations. Biol Blood Marrow Transplant. 2014;20(7):908–19. https://doi.org/10.1016/j.bbmt.2014.02.013. (Epub 2014 Feb 22 PMID: 24565993).
Golightly L, Teitelbaum I, Kizer TH, et al editors. Renal pharmacotherapy: dosage adjustment of medications eliminated by the kidneys. New York: Springer; 2013.
Aronoff GM, Bennett WM, Berns JS, et al. Drug Prescribing in Renal Failure: Dosing Guidelines for Adults and Children, 5th ed, American College of Physicians. 2007.
Carpenter RO, Evbuomwan MO, Pittaluga S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19(8):2048–60. https://doi.org/10.1158/1078-0432.CCR-12-2422.
Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. https://doi.org/10.1056/NEJMoa1407222. (Published correction appears in N Engl J Med. 2016 Mar 10;374(10):998).
Frey NV, Porter DL. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2016;2016(1):567–72. https://doi.org/10.1182/asheducation-2016.1.567.
Fitzgerald JC, Weiss SL, Maude SL, et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med. 2017;45(2):e124–31. https://doi.org/10.1097/CCM.0000000000002053.
Melilli E, Mussetti A, Linares GS, et al. Acute kidney injury following chimeric antigen receptor T-cell therapy for B-cell lymphoma in a kidney transplant recipient. Kidney Med. 2021;3(4):665–8. https://doi.org/10.1016/j.xkme.2021.03.011.
Acharya R, Horn B, Zeng X, Upadhyay K. Collapsing focal segmental glomerulosclerosis and acute kidney injury associated with chimeric antigen receptor T-cell (CAR-T) therapy: a case report. Kidney Med. 2021;3(6):1086–90. https://doi.org/10.1016/j.xkme.2021.06.011.
Lee MD, Strohbehn IA, Seethapathy HS, et al. Acute kidney injury after the CAR-T therapy tisagenlecleucel. Am J Kidney Dis. 2021;77(6):990–2. https://doi.org/10.1053/j.ajkd.2020.08.017.
Pennisi M, Jain T, Santomasso BD, et al. Comparing CAR T-cell toxicity grading systems: application of the ASTCT grading system and implications for management. Blood Adv. 2020;4(4):676–86. https://doi.org/10.1182/bloodadvances.2019000952.
Jaglowski S, Hu Z-H, Zhang Y, et al. Tisagenlecleucel chimeric antigen receptor (CAR) T-cell therapy for adults with diffuse large B-cell lymphoma (DLBCL): real world experience from the center for international blood & marrow transplant research (CIBMTR) cellular therapy (CT) registry. Blood. 2019;134:766.
Hirayama AV, Turtle CJ. Toxicities of CD19 CAR-T cell immunotherapy. Am J Hematol. 2019;94(S1):S42–9. https://doi.org/10.1002/ajh.25445.
Chen F, Teachey DT, Pequignot E, et al. Measuring IL-6 and sIL-6R in serum from patients treated with tocilizumab and/or siltuximab following CAR T cell therapy. J Immunol Methods. 2016;434:1–8. https://doi.org/10.1016/j.jim.2016.03.005.
Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321–30. https://doi.org/10.1182/blood-2016-04-703751.
Joannidis M, Druml W, Forni LG, et al. Prevention of acute kidney injury and protection of renal function in the intensive care unit: update 2017: expert opinion of the Working Group on Prevention, AKI section, European Society of Intensive Care Medicine. Intensive Care Med. 2017;43(6):730–49. https://doi.org/10.1007/s00134-017-4832-y.
Belay Y, Yirdaw K, Enawgaw B. Tumor lysis syndrome in patients with hematological malignancies. J Oncol. 2017;2017:9684909.
Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. https://doi.org/10.1038/nrclinonc.2017.148.
Kakinoki Y, Ishio T, Kimura H, et al. Successful salvage treatment with antithymocyte globulin for patients with early-onset hemophagocytic lymphohistiocytosis refractory to steroid and etoposide therapy following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2020;55(7):1479–82. https://doi.org/10.1038/s41409-020-0802-z.
Carter SJ, Tattersall RS, Ramanan AV. Macrophage activation syndrome in adults: recent advances in pathophysiology, diagnosis and treatment. Rheumatology. 2019;58(1):5–17. https://doi.org/10.1093/rheumatology/key006.
Farooqui N, Sy-Go JPT, Miao J, et al. Incidence and risk factors for acute kidney injury after chimeric antigen receptor T-cell therapy. Mayo Clin Proc. 2022;97(7):1294–304. https://doi.org/10.1016/j.mayocp.2022.05.018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
This manuscript has not been previously published and has not been submitted for publication elsewhere while under consideration. The authors declare no conflict of interest with this manuscript. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. All authors approved the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khan, I., Khan, N., Wolfson, N. et al. Safety of CAR-T Cell Therapy in Patients With Renal Failure/Acute Kidney Injury: Focused Review. Clin Hematol Int 5, 122–129 (2023). https://doi.org/10.1007/s44228-023-00037-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44228-023-00037-7