Abstract
Multiple myeloma is an incurable disease characterized by unregulated growth of malignant plasma cells in the bone marrow (BM). Tumor-induced dysfunction of T-cells may be responsible for immune evasion and failure of immunotherapy. Therefore, a better understanding of the phenotype of T-cells at the tumor site is needed. We assessed the expression of immune regulatory receptors on T-cell subsets from peripheral blood (PB) and BM using multicolor flow cytometry. Paired PB and BM samples were collected from newly diagnosed, treatment-naïve myeloma patients (n = 19) and patients progressing during treatment with the CD38 monoclonal antibody daratumumab alone or in combination with other anti-myeloma drugs (n = 39). We observed that CD4+ T-cells from both PB and BM of patients relapsing on daratumumab have a higher expression of the costimulatory checkpoint receptor DNAM-1. The potential role of DNAM-1+CD4+ T-cells in the development of resistance to daratumumab needs further exploration. We also observed that the inhibitory checkpoint receptor TIGIT is more frequently expressed by BM CD8+ T-cells from myeloma patients than PD-1 and CTLA-4, which supports the hypothesis that TIGIT may play a central role in the immune escape of the malignant plasma cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Multiple myeloma (MM) is an incurable disease characterized by uncontrolled growth of malignant plasma cells in the bone marrow (BM) [1]. As the disease advances, the tumor microenvironment in the BM becomes more permissive for tumor growth [2]. T-cell functions are impaired, possibly due to an increasing concentration of immunosuppressive adenosine in the BM microenvironment. Such impairment is reflected by higher expression of markers of T-cell exhaustion such as programmed death-1 (PD-1) and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) [3,4,5]. Several checkpoint inhibitors that block PD-1, PD-L1 or CTLA-4 have been approved for therapy of solid cancers but, so far, checkpoint inhibitors have not shown convincing efficacy in MM [6,7,8]. T-cell immunoglobulin and ITIM domains (TIGIT) is an inhibitory molecule expressed by lymphocytes that competes with the activating receptor DNAX accessory molecule-1 (DNAM-1) for binding to CD155 and CD112, which is expressed by myeloma cells and other cell types [9, 10]. TIGIT is highly expressed by CD8+ T-cells from MM patients and may play an important, inhibitory role of the T-cell response against MM [11].
Daratumumab (DARA) is a CD38 antibody approved for treatment of MM as monotherapy or in combination with a number of standard of care anti-myeloma drugs. In addition to DARA’s ability to eradicate tumor cells directly by complement dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC) and antibody mediated cellular phagocytosis (ADCP), it can deplete CD38+ regulatory cells of T, B and monocyte origin, which leads to the expansion and activation of cytotoxic T-cells [12]. Chatterjee et al. have shown that CD38 antibodies may directly stimulate T-cell mediated anti-tumor responses, while Marlein et al. showed that CD38-dependent tumor-derived tunneling nanotubes (TNT) are established between bone marrow stromal cells (BMSCs) and MM cells. Mitochondrial transfer via these TNT is a method for the MM cell to provide energy for further tumor growth. This activity was significantly decreased by CD38 antibody in vitro [13, 14]. Additionally, DARA impairs MM cell adhesion to the BMSCs [15].
Despite the multiple modes of action of DARA, the majority of patients receiving this antibody eventually relapse [16]. The mechanisms of resistance are poorly understood. Following the first DARA infusion, there is a very significant reduction of the expression of CD38 on residual MM cells, which is associated with impaired ADCC and CDC activity by DARA [17]. This phenomenon is, however, similar in responding and non-responding patients. A high level of CD38 on MM cells before treatment is associated with a better chance of response to DARA, but it had no impact on progression free survival ([17] and Nijhof personal communication]). In other regards, a low expression of CD38 could be an advantage by preventing the formation of TNT between BMSCs and MM cells, and by preventing the adhesion of MM cells to BMSCs. CDC may also be impaired at the time of progression, due to increased expression of CD55 and CD59 by myeloma cells [17]. Whether impaired T-cell function mediated by checkpoint inhibitors plays a role in the development of resistance to DARA is not known. Therefore, we have studied the expression of inhibitory and costimulatory checkpoint molecules on T-cells isolated from peripheral blood (PB) and BM of patients progressing on a DARA-containing regimen (Daratumumab Refractory Multiple Myeloma patients; DRMM). The results were compared with the profile of treatment-naïve, newly diagnosed myeloma patients (NDMM).
2 Materials and Methods
2.1 Study Population and Sample Collection
Patients diagnosed with MM according to the IMWG guidelines at the Departments of Hematology at Vejle Hospital and Odense University Hospital were included in the study [18]. The patients were either treatment-naïve, newly diagnosed MM patients or those with progressive disease according to the IMWG criteria on a DARA-containing regimen [19]. The study was approved by the regional Ethical Committee (S-20170212). Participation was voluntary, and written informed consent was obtained from all subjects.
Samples were obtained between December 2019 and May 2021. Data on patient characteristics and prior treatment were retrospectively obtained from the electronic medical journal and, afterwards, registered in a designated Research Electronic Data Capture (REDCap) database [20, 21].
2.2 Cell Isolation from PB and BM Aspirate
Paired PB and BM samples were obtained from NDMM (n = 19) and DRMM (n = 39). Ten milliliter of each specimen were collected immediately after each other in BD Vacutainer® EDTA blood collection tubes (BD Biosciences, San Jose, CA, USA). Bone marrow mononuclear cells (BMMCs) and PB mononuclear cells (PBMCs) were isolated by density-gradient centrifugation using Ficoll-Paque™ PLUS (GE Healthcare Bio-Sciences AB. Uppsala, Sweden) according to the manufacturer’s instructions. Cells were cryopreserved in a medium of 70% RPMI 1640 with GlutaMAX™ supplement (ThermoFisher Scientific, Waltham, MA, USA), 20% heat-inactivated fetal bovine serum (FBS) (ThermoFisher Scientific), and 10% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO, USA), and kept at − 135 °C until use. The isolation process was initiated less than 24 h after the collection.
2.3 Flow Cytometry
Cryopreserved PBMCs and BMMCs were thawed in a 37 °C water bath and resuspended in phosphate buffered saline (PBS) with 15% heat-inactivated FBS (ThermoFisher Scientific). Concentration and viability were determined using the trypan blue exclusion method and the Countess II Automated Cell Counter (ThermoFisher Scientific). The median viability in PBMC and BMMC were 89% (range 57–98%) and 79% (range 60–90%), respectively. Cells in PBS/0.5% bovine serum albumin (BSA) were treated with Human TruStain FcX (BioLegend, San Diego, CA, USA) according to the manufacturer’s recommendations. Subsequently, monoclonal antibodies and cells were mixed in Brillant Stain Buffer Plus (BD Biosciences) and the suspensions were incubated for 15 min at room temperature. Antibodies (all from BD Biosciences) had been titrated using relevant materials: anti-TIGIT BV421 (clone 741182), anti-CD45 BV650 (clone HI-30), anti-DNAM-1 BB515 (clone DX11), anti-CD3 PerCP-Cy5.5 (clone UCHT-1), anti-CTLA-4 PE (clone BNI3), anti-PD-1 PE-Cy7 (clone EH12.1), anti-CD4 R718 (clone SK3), and anti-CD8 APC-H7 (clone SK1). Two fluorescence-minus-two (FM2) samples were prepared for each sample leaving out anti-DNAM-1 BB515 and anti-PD-1 PE-Cy7 in the first and anti-TIGIT BV421 and anti-CTLA-4 PE in the second as these showed none/minimal spectral overlap. After staining, samples were treated with 2 mL 1× BD Pharm Lyse™ Lysing buffer (BD Biosciences) and washed using PBS/0.5% BSA. Before analysis, 7-AAD (BD Biosciences) was added.
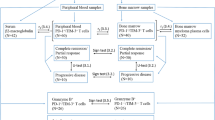
Samples were analyzed immediately after staining on an ACEA NovoCyte Quanteon 4025 flow cytometer (Agilent Technologies, Inc. Santa Clara, CA, USA). Consistency and stability of the instrument were verified on a daily basis using NovoCyte 6 peak QC Particles (Agilent Technologies, Inc.). Compensation was performed using UltraComp eBeads™ (ThermoFisher Scientific) stained with the antibodies and cells stained with 7-AAD. Data were analyzed using FlowJo™ software version 10.7.2 (BD Biosciences). The FM2 controls were used for objective gating of TIGIT, DNAM-1, CTLA-4, and PD-1, and both the percentage of the positive subset and the median fluorescence intensity (MFI) of this positive subset were evaluated. Expression of CD4 and CD8 were analyzed as percentages of the CD3+ lymphocytes. The gating strategy is shown in Fig. 1.
Gating strategy applied in the study shown in a representative MM patient sample. A In the first panel, the cell subset was defined among all events acquired in a forward scatter (FSC)–height (H)/Side scatter (SSC)–height (H) plot. Next, within this cell subset, live cells (7-AAD−) were selected for further analyses. In the following two panels, doublet discrimination was performed; First based on FSC-H and FSC-area (A), and second based on SSC–H and SSC-area (A). Subsequently, in order to identify the lymphocytes, defined as CD45highSSClow, the live single cells were shown in a CD45/SSC plot. Next, the CD3+ T cells were selected in the lymphocyte gate, and in the final panel the CD4+ and CD8+ subsets were defined among the CD3+ T cells. B Panels display the expression of TIGIT, DNAM-1, CTLA-4, and PD-1 in the CD4+ T cell subset. C Panels depict the expression of the same receptors in the CD8+ T cell subset. Red subsets display the results of the FM2 stained sample, which allow for objective gating, while blue subsets show the same sample stained with the nine-color panel
2.4 Statistical Analysis
Both the fraction of cells expressing a given marker and the MFI of each marker on CD4 and CD8 T-cells were expressed as medians. For normally distributed data sets (tested by a q-q plot), an unpaired t test was used for analysis of differences between groups. Otherwise, the data sets were tested using the Mann Whitney U test. The selected statistical tests used for each data set are specified in Tables 2 and 3. p values of less than 0.05 were considered significant. All statistical analyses were performed using STATA version 16.0 for PC (Stata Corp LP, College Station, TX, USA) and GraphPad Prism version 7.05.
3 Results
3.1 Patient Characteristics
A total of 58 MM patients participated in the study: 19 with newly diagnosed treatment-naïve MM (NDMM) and 39 who were progressing on DARA monotherapy or a DARA-containing regimen (DRMM). DRMM had received a median of three prior lines of therapy (Table 1).
3.2 Decreased CD4:CD8-Ratio in the BM of DRMM Patients
In the BM, the median CD4:CD8-ratios were 1.10 (range 0.31–14.31) for NDMM and 0.55 (range 0.13–5.78) for DRMM, p = 0.0014. When measured in PB, the NDMM had a median CD4:CD8-ratio of 1.26 (range 0.54–24.90), while this ratio in the DRMM was 1.19 (range 0.16–8.10), p = 0.07.
3.3 Expression of Checkpoint Molecules by CD4+ T-Cells
In order to reveal divergent expression patterns of the receptors CTLA-4, DNAM-1, PD-1, and TIGIT on CD4+ T cells from both PB and BM, a comparison between the two groups of MM patients was performed. Both the percentage of the positive subsets and the receptor expression level by MFI, were taken into consideration.
Almost all CD4+ T-cells (> 90%) from PB and BM of both groups of patients expressed DNAM-1, but the level of expression (MFI) of DNAM-1 was higher in DRMM compared with NDMM, both in the PB and BM (median MFI: 58,739 versus 40,206, p = 0.0110 in PB; 54,257 versus 35,240, p = 0.0015 in BM) (Tables 2 and 3, Fig. 2). When comparing NDMM to the six DRMM patients who had received DARA monotherapy, we found the same differences, although non-significant due to the small sample size. The percentage of CD4+CTLA-4+ T cells was very low in all samples, hence the numbers of acquired positive events were small. Nonetheless, this subset was statistically larger in both PB and BM of DRMM as compared to NDMM (median 0.66% versus 0.48%, p = 0.0346 in PB, median: 1.035% versus 0.66%, p = 0.0125 in BM) (Table 2). When analyzing the percentages of CD4+ T cells expressing either PD-1 or TIGIT in PB and BM samples of NDMM and DRMM, there was no statistical difference (Table 2). Moreover, we did not find any difference between the groups when evaluating the expression level of these receptors, as determined by MFI of PD-1 and TIGIT on the CD4+PD-1+ T cells and the CD4+TIGIT+ T-cells, respectively (Table 3).
When looking only at the NDMM, we found that the MFI of PD-1 (median MFI: 4060 versus 3006, p = 0.002) and TIGIT (median MFI: 23,235 versus 21,008, p = 0.040) on CD4+ T-cells from the BM was higher in patients with a poor response to the first line of treatment (n = 4) compared to patients with a partial or better response (n = 10) according to the IMWG guidelines [19]. Furthermore, the percentage of TIGIT+CD4+ T-cells in PB was higher in patients with a poor response (median 24.2% versus 15.1%, p = 0.036). None of the patients received DARA in their first line of treatment. Due to the sample size, these data need to be interpreted with caution.
3.4 Expression of Checkpoint Molecules by CD8+ T-Cells
Similar to the CD4+ T-cells, the majority of CD8+ T-cells (70–80%) expressed DNAM-1 (Table 2). In contrast to CD4+ T-cells, there was no difference in the MFI of CD8+DNAM-1+ in PB and BM between the NDMM and DRMM groups (median 32,891 versus 33,407, p = 0.9015 in PB; median 28,172 versus 27,497, p = 0.8377 in BM) (Table 3). Like the CD4+ T-cells, the percentage of CD8+CTLA-4+ T-cells was very low, and the median MFI of CTLA-4 on the CD8+CTLA-4+ T-cells was also low (median 1039 versus 1083, p = 0.1316 in PB; median 1055 versus 1072, p = 0.4462 in BM) (Table 3). As for CD4+ T-cells, the percentages of CD8+ T cells expressing either PD-1 or TIGIT in PB and BM samples of NDMM and DRMM were not statistically different (Table 2). Furthermore, we did not find any difference between the groups when analyzing the expression level of these receptors as determined by the MFI of PD-1 and TIGIT on the CD8+PD-1+ T cells and the CD8+TIGIT+ T-cells, respectively (Table 3).
There was no correlation between the expression of checkpoint molecules by CD8+ T-cells and the response to treatment for NDMM.
3.5 TIGIT is the Most Frequently Expressed Immune Checkpoint Receptor on BM CD8+ T-Cells
TIGIT was the most frequently expressed checkpoint receptor on the BM CD8+ T-cells. It was present on significantly more cells than PD-1 (median 82.5% versus 19.25%, p < 0.00001) (Fig. 3). Of the six patients with PD-1 expressed on more than 50% of the BM CD8+ T-cells, one had received no prior treatment, two had received DARA and an immunomodulatory drug, and three had received DARA in other combinations. CTLA-4 was expressed on less than 1% of BM CD8+ T-cells (Fig. 3). For BM CD4+ T-cells, more cells expressed PD-1 than TIGIT (median 26.7% versus 18.4%, p = 0.0131), but the fractions of BM CD4+ T-cells expressing either of these checkpoint receptors were much lower than the percentage of TIGIT-expressing BM CD8+ T-cells.
4 Discussion
The immune regulatory receptors DNAM-1, CTLA-4, PD-1 and TIGIT are expressed by T-cells, and interact with their ligands upon T-cell receptor ligation, resulting in negative or positive regulation of T-cell activity. Here, we examined the presence of these checkpoint molecules on T-cell subsets from NDMM and DRMM, in order to identify changes that correlated with resistance to treatment with DARA.
In this study, we have demonstrated an increased expression of DNAM-1 by CD4+ T-cells from DRMM compared to NDMM. The biological and possible clinical significance of the finding is not known. Allegedly, DNAM-1 is upregulated on activated CD4+ and CD8+ T-cells. It competes with TIGIT for the CD155 receptor found on antigen-presenting cells, tumor cells and virus-infected cells [22]. Specifically, CD155 is expressed on most MM cells [9, 10]. We found that both the percentage of DNAM-1+CD4+ T-cells and the MFI of DNAM-1 on DNAM-1+CD4+ T-cells in PB and BM of DRMM were higher compared to NDMM. Krejcik et al. found that a subpopulation of CD38+CD4+ T regulatory cells (Tregs) that demonstrated a suppressive capacity against cytotoxic T-cells was eliminated by treatment with DARA due to their expression of CD38 [12]. This process correlated with recruitment and activation of CD8+ T-cells and the clinical response to DARA. In our study, almost all CD4+ T-cells expressed DNAM-1, so we are evidently not dealing with a subpopulation of CD4+ Tregs, as these occur in low numbers. To investigate the role of DNAM-1+ CD4+ T-cells in the development of resistance to DARA, an in-depth subset analysis of the CD4+ T-cells and functional studies are needed. In this context, it could be informative to add DARA-treated patients in complete response to the analyses.
We did not observe any differences between NDMM and DRMM with regards to the expression of PD-1. A few patients expressed PD-1 on more than 50% of their BM CD8+ T-cells. These patients had received different DARA-containing regimens. This is in contrast with a number of studies that examined the expression of PD-1 on T-cells from myeloma patients at different stages of the disease. Minnie et al. analyzed T-cells isolated from the BM of mice with MM and found that PD-1 was overexpressed on CD8+ T-cells from mice with relapsed MM (relapse/refractory MM; RRMM) compared to mice with MM in remission [23]. Furthermore, Zelle-Rieser et al. reported that the expression of PD-1 on CD4+ and CD8+ T cells isolated from the BM of RRMM patients was higher compared to T-cells isolated from the BM of healthy donors [5]. One study, with a design very similar to ours, found that the median percentage of CD8+ T-cells expressing PD-1 was higher in the BM of RRMM compared to that in NDMM (35% and 28%, respectively) [24]. Although we were unable to show such differences, we did observe percentages of PD-1 expressing T cell subsets in approximately the same range as reported by Paiva and colleagues (Table 2). The discrepancy between the two studies could be due to the composition of the patient groups analyzed: while the DRMM group in the present study was uniform, consisting only of patients relapsing on DARA, the treatment regimen of the equivalent group in the Paiva study was not described. However, as the FDA did not approve daratumumab until November 2015, probably the group did not hold patients treated with this drug. Thus, from the information available, it seems plausible that there may be a difference in the expression of PD-1 when comparing RRMM with either MM patients in remission or healthy donors, but whether there is a difference when comparing NDMM to RRMM is not yet clarified.
Several clinical trials have investigated the use of PD-1 or PD-L1 inhibitors in MM. Monotherapy with PD-1 inhibitors did not induce clinical response in MM [7, 8]. Furthermore, phase 3 studies, which evaluated the combination of a PD-1 inhibitor with immunomodulatory drugs (IMIDs) were stopped prematurely, due to an increased frequency of serious adverse events in the experimental arm [25, 26]. In a phase 2 clinical trial, the PD-L1 inhibitor durvalumab was used as an add-on to DARA, for patients who recently progressed on a DARA-containing treatment, in an attempt to reverse the resistance to DARA [27]. None of the 18 patients treated in the trial obtained a partial response or better. Interestingly, the investigators compared PD-1 expression on the patients’ T cells at baseline (i.e. the time point of progression on daratumumab) and after 6 weeks of treatment with durvalumab and DARA, but found no difference in expression. These data are in line with our results, indicating that the PD-1—PD-L1 axis may not be the key signaling pathway driving the development of resistance to daratumumab.
Like for PD-1, we found no difference in the expression of TIGIT when comparing NDMM to DRMM, but observed that TIGIT was the inhibitory receptor most frequently expressed by CD8+ T cells. In contrast to our findings, Minnie and colleagues reported that the frequency of TIGIT expression was higher on CD8+ T-cells from mice with relapsed MM compared to MM in remission or healthy mice [23]. Nevertheless, studies in humans have demonstrated that compared to PD-1 and CTLA-4, TIGIT was more frequently expressed on CD8+ T cells from the MM patients thus concurring with our data [11, 28]. Collectively, this suggests that TIGIT may play a major role as an immune regulator of cytotoxicity. The position as a receptor of importance is supported by studies showing that MM mice treated with anti-TIGIT mAbs lived significantly longer [11, 23]. In further support of the importance of TIGIT in MM, Neri et al. found upregulation of TIGIT on T-cells from DARA non-responders compared with DARA responders [29]. In the BM of DRMM patients, we found an excess of CD8+ T-cells, but despite of their presence, the disease was not controlled, maybe because of checkpoint inhibition most likely mediated by TIGIT. Anti-TIGIT mAbs in MM are in currently recruiting clinical trials, both as monotherapy and in combination with anti-MM drugs.
CTLA-4 was expressed at a low level by the T-cells analyzed, and even though there was a significant difference in its expression when comparing NDMM to DRMM, we do not consider this difference to be clinically significant. Other studies confirm our observations [11, 27]. A combination of the PD-1 inhibitor nivolumab and the CTLA-4 inhibitor ipilimumab was tested in vivo in 7 MM patients. None of these patients had any objective response [30]. This supports our interpretation that CTLA-4 is not a key immune regulatory receptor in MM.
Multiple checkpoint molecules exist besides the four which we examined here. High expression of the checkpoint receptor CD200 in AML is associated with poor overall survival, but treatment with an anti-CD200 antibody can restore the AML immune response [31, 32]. Pochard et al. found that low expression of CD200 in MM patients was associated with a good response to DARA [33]. Future combination studies of checkpoint inhibitors are required to clarify if these observations will be clinically important.
The patients in the DRMM group were included when they progressed on DARA with or without other anti-MM drugs. It is a limitation of this study that the DRMM group is not totally uniform, but it does reflect the real world situation where DARA is administered in different combinations. When we examined the findings regarding DNAM-1 expression on CD4+ T-cells in the subpopulation of patients receiving monotherapy with DARA to the rest of the DARA-exposed population, we found no obvious difference. We chose NDMM as a control group to avoid the impact of other drugs given before DARA on the BM microenvironment. Brauneck et al. found that CD8+ T-cells from NDMM showed an increased expression of PD-1 and TIGIT compared to healthy controls [34]. As we did not include a group of healthy controls, we were unable to perform the same comparison; however, we found no correlation between the expression of checkpoint molecules on CD8+ T-cells and response to first line treatment. In contrast, our small dataset on this group of untreated patients may indicate that the expression of PD-1 and TIGIT on CD4+ T-cells could play a role. Further studies with larger sample sizes are needed. Note that none of the NDMM patients received DARA in the first line of treatment, but all were eligible for DARA in their second line of treatment, as recommended by local guidelines in Denmark.
5 Conclusions
In conclusion, our study supports the hypothesis that TIGIT may play a more important role as an immune checkpoint in MM than PD-1 and CTLA-4, and that anti-TIGIT mAbs could be effective in MM. Whether or not the DNAM-1+CD4+ T-cells play a role in the development of resistance to DARA needs further exploration and characterization of the involved T-cells.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
References
Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111(6):2962–72. https://doi.org/10.1182/blood-2007-10-078022.
Dhodapkar MV. MGUS to myeloma: a mysterious gammopathy of underexplored significance. Blood. 2016;128(23):2599–606. https://doi.org/10.1182/blood-2016-09-692954.
Kwon M, Kim CG, Lee H, Cho H, Kim Y, Lee EC, Choi SJ, Park J, Seo IH, Bogen B, Song IC, Jo DY, Kim JS, Park SH, Choi I, Choi YS, Shin EC. PD-1 blockade reinvigorates bone marrow CD8(+) T cells from patients with multiple myeloma in the presence of TGFβ inhibitors. Clin Cancer Res. 2020;26(7):1644–55. https://doi.org/10.1158/1078-0432.Ccr-19-0267.
Horenstein AL, Quarona V, Toscani D, Costa F, Chillemi A, Pistoia V, Giuliani N, Malavasi F. Adenosine generated in the bone marrow niche through a CD38-mediated pathway correlates with progression of human myeloma. Mol Med. 2016;22:694–704. https://doi.org/10.2119/molmed.2016.00198.
Zelle-Rieser C, Thangavadivel S, Biedermann R, Brunner A, Stoitzner P, Willenbacher E, Greil R, Jöhrer K. T cells in multiple myeloma display features of exhaustion and senescence at the tumor site. J Hematol Oncol. 2016;9(1):116. https://doi.org/10.1186/s13045-016-0345-3.
Abril-Rodriguez G, Ribas A. SnapShot: immune checkpoint inhibitors. Cancer Cell. 2017;31(6):848.e1. https://doi.org/10.1016/j.ccell.2017.05.010.
Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, Millenson MM, Cohen AD, Schuster SJ, Lebovic D, Dhodapkar M, Avigan D, Chapuy B, Ligon AH, Freeman GJ, Rodig SJ, Cattry D, Zhu L, Grosso JF, Garelik MBB, Shipp MA, Borrello I, Timmerman J. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34(23):2698–704. https://doi.org/10.1200/jco.2015.65.9789.
Ribrag V, Avigan DE, Green DJ, Wise-Draper T, Posada JG, Vij R, Zhu Y, Farooqui MZH, Marinello P, Siegel DS. Phase 1b trial of pembrolizumab monotherapy for relapsed/refractory multiple myeloma: KEYNOTE-013. Br J Haematol. 2019;186(3):e41–4. https://doi.org/10.1111/bjh.15888.
Lozano E, Mena MP, Díaz T, Martin-Antonio B, León S, Rodríguez-Lobato LG, Oliver-Caldés A, Cibeira MT, Bladé J, Prat A, Rosiñol L, Fernández de Larrea C. Nectin-2 expression on malignant plasma cells is associated with better response to TIGIT blockade in multiple myeloma. Clin Cancer Res. 2020;26(17):4688–98. https://doi.org/10.1158/1078-0432.Ccr-19-3673.
El-Sherbiny YM, Meade JL, Holmes TD, McGonagle D, Mackie SL, Morgan AW, Cook G, Feyler S, Richards SJ, Davies FE, Morgan GJ, Cook GP. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res. 2007;67(18):8444–9. https://doi.org/10.1158/0008-5472.Can-06-4230.
Guillerey C, Harjunpää H, Carrié N, Kassem S, Teo T, Miles K, Krumeich S, Weulersse M, Cuisinier M, Stannard K, Yu Y, Minnie S, Hill G, Dougall W, Avet-Loiseau H, Teng M, Nakamura K, Martinet L, Smyth M. TIGIT immune checkpoint blockade restores CD8 + T cell immunity against multiple myeloma. Blood. 2018. https://doi.org/10.1182/blood-2018-01-825265.
Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, Syed K, Liu K, van de Donk NW, Weiss BM, Ahmadi T, Lokhorst HM, Mutis T, Sasser AK. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–94. https://doi.org/10.1182/blood-2015-12-687749.
Chatterjee S, Daenthanasanmak A, Chakraborty P, Wyatt MW, Dhar P, Selvam SP, Fu J, Zhang J, Nguyen H, Kang I, Toth K, Al-Homrani M, Husain M, Beeson G, Ball L, Helke K, Husain S, Garrett-Mayer E, Hardiman G, Mehrotra M, Nishimura MI, Beeson CC, Bupp MG, Wu J, Ogretmen B, Paulos CM, Rathmell J, Yu XZ, Mehrotra S. CD38-NAD(+)axis regulates immunotherapeutic anti-tumor T cell response. Cell Metab. 2018;27(1):85-100e8. https://doi.org/10.1016/j.cmet.2017.10.006.
Marlein CR, Piddock RE, Mistry JJ, Zaitseva L, Hellmich C, Horton RH, Zhou Z, Auger MJ, Bowles KM, Rushworth SA. CD38-driven mitochondrial trafficking promotes bioenergetic plasticity in multiple myeloma. Cancer Res. 2019;79(9):2285–97. https://doi.org/10.1158/0008-5472.CAN-18-0773.
Ghose J, Viola D, Terrazas C, Caserta E, Troadec E, Khalife J, Gunes EG, Sanchez J, McDonald T, Marcucci G, Kaur B, Rosenzweig M, Keats J, Rosen S, Krishnan A, Satoskar AR, Hofmeister CC, Pichiorri F. Daratumumab induces CD38 internalization and impairs myeloma cell adhesion. Oncoimmunology. 2018;7(10): e1486948. https://doi.org/10.1080/2162402x.2018.1486948.
Szabo AG, Klausen TW, Levring MB, Preiss B, Helleberg C, Breinholt MF, Hermansen E, Gjerdrum LMR, Bønløkke ST, Nielsen K, Kjeldsen E, Iversen KF, Teodorescu EM, Dokhi M, Kurt E, Strandholdt C, Andersen MK, Vangsted AJ. The real-world outcomes of multiple myeloma patients treated with daratumumab. PLoS One. 2021;16(10): e0258487. https://doi.org/10.1371/journal.pone.0258487.
Nijhof IS, Casneuf T, van Velzen J, van Kessel B, Axel AE, Syed K, Groen RW, van Duin M, Sonneveld P, Minnema MC, Zweegman S, Chiu C, Bloem AC, Mutis T, Lokhorst HM, Sasser AK, van de Donk NW. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. 2016;128(7):959–70. https://doi.org/10.1182/blood-2016-03-703439.
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos M-V, Kumar S, Hillengass J, Kastritis E, Richardson P, Landgren O, Paiva B, Dispenzieri A, Weiss B, LeLeu X, Zweegman S, Lonial S, Rosinol L, Zamagni E, Jagannath S, Sezer O, Kristinsson SY, Caers J, Usmani SZ, Lahuerta JJ, Johnsen HE, Beksac M, Cavo M, Goldschmidt H, Terpos E, Kyle RA, Anderson KC, Durie BGM, Miguel JFS. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–48. https://doi.org/10.1016/s1470-2045(14)70442-5.
Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, Blade J, Richardson P, Orlowski R, Siegel D, Jagannath S, Facon T, Avet-Loiseau H, Lonial S, Palumbo A, Zonder J, Ludwig H, Vesole D, Sezer O, Munshi NC, San Muguel J, International Myeloma Workshop Consensus P. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18):4691–5. https://doi.org/10.1182/blood-2010-10-299487.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. https://doi.org/10.1016/j.jbi.2008.08.010.
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95: 103208. https://doi.org/10.1016/j.jbi.2019.103208.
Shibuya A, Shibuya K. DNAM-1 versus TIGIT: competitive roles in tumor immunity and inflammatory responses. Int Immunol. 2021. https://doi.org/10.1093/intimm/dxab085.
Minnie SA, Kuns RD, Gartlan KH, Zhang P, Wilkinson AN, Samson L, Guillerey C, Engwerda C, MacDonald KPA, Smyth MJ, Markey KA, Vuckovic S, Hill GR. Myeloma escape after stem cell transplantation is a consequence of T-cell exhaustion and is prevented by TIGIT blockade. Blood. 2018;132(16):1675–88. https://doi.org/10.1182/blood-2018-01-825240.
Paiva B, Azpilikueta A, Puig N, Ocio EM, Sharma R, Oyajobi BO, Labiano S, San-Segundo L, Rodriguez A, Aires-Mejia I, Rodriguez I, Escalante F, de Coca AG, Barez A, San Miguel JF, Melero I. PD-L1/PD-1 presence in the tumor microenvironment and activity of PD-1 blockade in multiple myeloma. Leukemia. 2015;29(10):2110–3. https://doi.org/10.1038/leu.2015.79.
Mateos MV, Orlowski RZ, Ocio EM, Rodríguez-Otero P, Reece D, Moreau P, Munshi N, Avigan DE, Siegel DS, Ghori R, Farooqui MZH, Marinello P, San-Miguel J. Pembrolizumab combined with lenalidomide and low-dose dexamethasone for relapsed or refractory multiple myeloma: phase I KEYNOTE-023 study. Br J Haematol. 2019;186(5):e117–21. https://doi.org/10.1111/bjh.15946.
Usmani SZ, Schjesvold F, Oriol A, Karlin L, Cavo M, Rifkin RM, Yimer HA, LeBlanc R, Takezako N, McCroskey RD, Lim ABM, Suzuki K, Kosugi H, Grigoriadis G, Avivi I, Facon T, Jagannath S, Lonial S, Ghori RU, Farooqui MZH, Marinello P, San-Miguel J. Pembrolizumab plus lenalidomide and dexamethasone for patients with treatment-naive multiple myeloma (KEYNOTE-185): a randomised, open-label, phase 3 trial. Lancet Haematol. 2019;6(9):e448–58. https://doi.org/10.1016/s2352-3026(19)30109-7.
Frerichs KA, Verkleij CPM, Dimopoulos MA, Marin Soto JA, Zweegman S, Young MH, Newhall KJ, Mutis T, van de Donk N. Efficacy and safety of durvalumab combined with daratumumab in daratumumab-refractory multiple myeloma patients. Cancers (Basel). 2021. https://doi.org/10.3390/cancers13102452.
Yadav M, Green C, Ma C, Robert A, Glibicky A, Nakamura R, Sumiyoshi T, Meng R, Chu Y-W, Wu J, Byon J, Woodard J, Adamkewicz J, Grogan J, Venstrom JM. Tigit, CD226 and PD-L1/PD-1 are highly expressed by marrow-infiltrating T cells in patients with multiple myeloma. Blood. 2016;128(22):2102. https://doi.org/10.1182/blood.V128.22.2102.2102.
Neri P, Maity R, Tagoug I, McCulloch S, Duggan P, Jimenez-Zepeda V, Tay J, Thakurta A, Bahlis N. Immunome single cell profiling reveals T Cell exhaustion with upregulation of checkpoint inhibitors LAG3 and tigit on marrow infiltrating T lymphocytes in daratumumab and IMiDs resistant patients. Blood. 2018;132(Supplement 1):242. https://doi.org/10.1182/blood-2018-99-117531.
Armand P, Lesokhin A, Borrello I, Timmerman J, Gutierrez M, Zhu L, Popa McKiver M, Ansell SM. A phase 1b study of dual PD-1 and CTLA-4 or KIR blockade in patients with relapsed/refractory lymphoid malignancies. Leukemia. 2021;35(3):777–86. https://doi.org/10.1038/s41375-020-0939-1.
Tonks A, Hills R, White P, Rosie B, Mills KI, Burnett AK, Darley RL. CD200 as a prognostic factor in acute myeloid leukaemia. Leukemia. 2007;21(3):566–8. https://doi.org/10.1038/sj.leu.2404559.
Rastogi N, Baker S, Man S, Uger RA, Wong M, Coles SJ, Hodges M, Gilkes AF, Knapper S, Darley RL, Tonks A. Use of an anti-CD200-blocking antibody improves immune responses to AML in vitro and in vivo. Br J Haematol. 2021;193(1):155–9. https://doi.org/10.1111/bjh.17125.
Pochard C, Charles H, Gabellier L, Forneo L, Robert N, Requirand G, Cartron G, Bret C, Moreaux J, Vincent L. Integrative approach to find predictive markers of response to daratumumab in multiple myeloma. EHA Library. 2021; 2021(06/09/21; 325708;EP950)
Brauneck F, Weimer P, Schulzezurwiesch J, Weisel K, Leypoldt L, Vohwinkel G, Fritzsche B, Bokemeyer C, Wellbrock J, Fiedler W. Bone marrow-resident Vδ1 T cells co-express TIGIT with PD-1, TIM-3 or CD39 in AML and myeloma. Front Med (Lausanne). 2021;8:763773. https://doi.org/10.3389/fmed.2021.763773.
Acknowledgements
We gratefully acknowledge Camilla Davidsen, Sara Egsgaard, Nilosa Ushanthan, Xenia Goldberg Borggaard, Bente Sejrskilde Hansen, and Lisbeth Jakobsen for performing the laboratory analyses. Study data were collected and managed using Redcap electronic data capture tools hosted at OPEN, Open Patient data Explorative Network, Odense University Hospital, Region of Southern Denmark.
Funding
This study was funded by grants from The Danish Medical Association Research Foundation (Højmosegårdlegatet 2019–3780/67), The AP Moller Foundation (Fonden til Lægevidenskabens fremme 19-L-0185), The Region of Southern Denmark (Region Syddanmarks Forskningspulje 2019 19/12124/A233), and Overlæge Jørgen Werner Schous og hustru, Else-Marie Schou, født Wonge’s fond.
Author information
Authors and Affiliations
Contributions
Torben Plesner, Katrine Fladeland Iversen and Line Nederby designed the research study. Thomas Lund and Katrine Fladeland Iversen included the patients. Line Nederby performed the flow cytometry. Katrine Fladeland Iversen and Line Nederby analyzed the data. Katrine Fladeland Iversen wrote the paper. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Katrine Fladeland Iversen, Line Nederby, and Thomas Lund declare no conflicts of interest. Torben Plesner is serving as an advisor for Janssen, Celgene, Takeda, Oncopeptides, Genentech, CSL Behring and AbbVie and receives research support from Janssen, Genmab, Celgene, Takeda, Oncopeptides, Genentech, AbbVie and Roche. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Ethics approval
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Regional Committees on Health Research Ethics for Southern Denmark (S-20170212, 02Feb2018).
Consent to participate
All patients included signed the informed consent form, which included consent to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iversen, K.F., Nederby, L., Lund, T. et al. High Expression of the Costimulatory Checkpoint Factor DNAM-1 by CD4+ T-Cells from Multiple Myeloma Patients Refractory to Daratumumab-Containing Regimens. Clin Hematol Int 4, 107–116 (2022). https://doi.org/10.1007/s44228-022-00013-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44228-022-00013-7