Abstract
The pervasive presence of microplastics within river ecosystems has a profound and often underestimated detrimental impact. These minuscule yet persistent particles have infiltrated diverse biological habitats, making their detrimental effects on aquatic life increasingly concerning worldwide. The ability of microplastics to accumulate within aquatic organisms further exacerbates this concern. Consequently, the monitoring of microplastic pollution in surface water environments has emerged as a crucial endeavour, offering invaluable insights into the extent of this ecological threat. This study delved into the contamination levels of microplastics within two bivalve species, Perna viridis and Villorita cyprinoides, along the Chandragiri River in Kerala and Karnataka, South-West India, spanning eight distinct locations. The investigation yielded a significant discovery, with a total of 667 microplastic particles extracted from 288 individuals. On average, each individual bivalve harboured approximately 2.31 ± 0.93 microplastic items. Notably, these microplastic particles exhibited a wide range of morphological characteristics, underscoring their diverse origins and pathways into the ecosystem. Furthermore, five polymer types of microplastics were unequivocally confirmed through FTIR-ATR analysis, shedding light on the types of plastics that pose a threat to the riverine ecosystem. The findings of this study contribute to a growing body of evidence highlighting the global threat of microplastic pollution, urging international collaboration and innovative solutions to prevent further contamination and remediate existing microplastic burdens in aquatic environments.
Highlights
• Microplastic contamination is a major concern in river ecosystems.
• Microplastic in two bivalve species were characterized at Chandragiri River India.
• The microplastics had diverse shapes and were composed of five different polymers.
• This study emphasizes the need to tackle river microplastic pollution.
AbstractSection Graphical Abstract
Similar content being viewed by others
1 Introduction
Plastic is a pervasive pollutant that poses a significant threat to the biosphere. Despite being aware of its negative impact, humans have astonishingly relied on this synthetic polymer for over seven decades. The annual production of plastics has surpassed 320 million tons, serving various industries such as food packaging, textiles, healthcare, and cosmetics (Wright and Kelly 2017), which is roughly equivalent to the current human biomass on Earth (Lebreton and Andrady 2019). Driven by factors like convenience and low cost, global plastic production ballooned by a staggering 189 times between 1950 and 2015, raising serious concerns about pollution and waste management (Europe 2016). Although PE and PP dominate marine microplastics, their alarming production growth of 8.7% per year from 1950 to 2012 (Andrady 2017) pales in comparison to the shocking amount of plastic waste entering oceans. Estimates suggest a staggering 4.8–12.7 million tons from land sources alone in 2010 (Jambeck et al. 2015), constituting 1.8–4.7% of global plastic production that year. The consumption rate of plastic has escalated even further due to the global pandemic. Commonly reported plastic contaminants in the environment include polyethylene (PE), polyethylene terephthalate (PET), polystyrene (PS), polypropylene (PP), and polyvinyl chloride (PVC) (Rochman et al. 2013a). Over time, plastic debris breaks down into smaller pieces, forming microscopic particles known as microplastics, typically measuring less than 5 mm in size (Alimi et al. 2018). Microplastics can originate from primary or secondary sources, depending on how they are formed. The accumulation of these fragmented plastics is highly toxic to both terrestrial and aquatic ecosystems, permeating habitats and impacting diverse species of organisms (Rochman et al. 2013b). Additionally, microplastic granules formed from plastic scraps are typically bioinert but have the ability to adsorb various organic pollutants and contaminants, including heavy metals, due to their highly hydrophobic nature (Holmes et al. 2012). Unlike bulky plastic debris, microplastics’ stealthy size allows them to silently invade food webs, acting as miniature Trojan horses that deliver harmful chemicals directly into unsuspecting organisms (Botterell et al. 2019).
Studies conducted worldwide indicate that plastic pollution is widespread and affects all natural habitats. In the oceans alone, it is estimated that there are around 4.85 trillion microplastic particles (Eriksen et al. 2014). Similarly, microplastic contamination in riverine ecosystems is a significant concern that should not be overlooked. Floating plastic debris and fragments are one of the pathways through which microplastics enter freshwater systems and eventually reach the oceans. Microplastics can enter surface water through fluvial floods (Kumar & Varghese 2021) anthropogenic activities, and fisheries, posing a potential risk to biota, particularly those that are part of the food web, as they can be transferred through the trophic chain (Akhbarizadeh et al. 2019). Due to their small size, microplastics are inadvertently ingested by aquatic invertebrates such as zooplankton (Cole et al. 2013), fish (Boerger et al. 2010), shellfish (Gamarro et al. 2020), and filter feeders like mussels (Wegner et al. 2012), molluscs, sea cucumbers (Graham and Thompson 2009; Mohsen et al. 2019), lungworms (Wright et al. 2013; Besseling et al. 2013), and oysters (Sussarellu et al. 2016; Teng et al. 2019).
As bivalves, including Perna viridis (Asian green mussel) and Villorita cyprinoides (Black clam), are widely cultured and consumed as food worldwide, there is a risk of microplastics entering higher trophic levels through their consumption. Therefore, it is essential to investigate the ecotoxicological impacts of microplastic contamination on commonly consumed seafood such as bivalves. Filter feeders, which pass large volumes of water through their gastrointestinal tracts, are particularly vulnerable to microplastic ingestion (Wegner et al. 2012; Cole et al. 2013; Wright and Kelly 2017), which can adversely affect their biological functions. The impacts of microplastic ingestion include negative effects on immunological responses, neurotoxic effects (Avio et al. 2015), accumulation of plastic in respiratory gills (Su et al. 2019) and circulatory systems (Browne et al. 2008; Avio et al. 2015), reproductive disruption (Besseling et al. 2014), and decreased feeding (Besseling et al. 2013). Importantly, the presence of microplastic particles throughout ecosystems exacerbates their bioaccumulation and biomagnification at the cellular level in aquatic biodiversity (Akhbarizadeh et al. 2019). Therefore, biomonitoring of organisms can provide insights into the overall extent of plastic pollution in aquatic ecosystems. Bivalves, in particular, can serve as indicators of common pollutants in riverine ecosystems due to their ability to bioconcentrate and bioaccumulate pollutants above environmental levels. Their specialized filter-feeding mechanism renders bivalves highly vulnerable to microplastic pollution present in their surrounding aquatic ecosystems (Su et al. 2019). Bivalves’ reliance on nonselective filter-feeding combined with their inefficient self-purification and excretion mechanisms leaves them highly susceptible to MP accumulation, placing them among the most vulnerable organisms in the face of MP pollution (Patterson et al. 2021).
This study aims to assess the occurrence of microplastic contamination in P. viridis and V. cyprinoides, collected from the Chandragiri River in South-West India. These benthic organisms, particularly mussels, are significantly cultivated and consumed, underscoring the importance of examining potential adverse effects on human health resulting from the ingestion of plastic-contaminated organisms. The proliferation of these species is attributed to the rise in water tourism in Kerala, India. Kerala’s green mussel farmers are increasingly favouring polyethylene farming structures for their resilience, but this trend brings a potential downside: higher microplastic exposure for mussels (FAO 2019). These durable materials raise concerns about MP contamination, putting the mussels at risk of ingesting MPs and ultimately transferring them to human consumers. Thus, the identification of microplastic particles in these bivalve species not only sheds light on the level of microplastic contamination in the human body but also draws attention to the potential presence of microplastic pollution in other species within this surface water environment.
2 Materials and methods
2.1 Study area and sampling
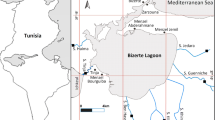
Chandragiri River is mainly known as a lifeline for people in Kasaragod. From ancient times, the livelihood of people has been associated with this River. We can also see various types of historical monuments along the banks of the river. People depend on this River for various purposes like agriculture, fishing, irrigation, tourism, culturing of various organisms etc. So, this River plays a major role in shaping the future of people associated with it. Chandragiri River starts from Koi Nadu and is situated in Kodagu district of Karnataka state. Talakaveri wildlife sanctuary is also situated near to this area. After a long journey of 105 km, finally it reaches the Arabian Sea. The Chandragiri River has a catchment area of 1406 square kilometres, of which 836 square kilometres are in Karnataka and the rest in Kerala. The present study conducted a sampling campaign encompassing eight specific locations along the Chandragiri River. The selected sampling sites was named as Chala, Chengala, Jalsoor, Kasaragod, Kombanadukkam, Sullia, Thalangara, and Thekkil. These sites were chosen due to the prevalent commercial mining and processing activities related to clams, as depicted in Fig. 1. The sampling sites were situated on the riverbanks, which are significantly affected by pollution from various sources, with a focus on areas where clam mining and processing are prominent. Perna viridis samples were specifically collected from Thalangara and Kombanadukkam, while Villorita cyprinoides samples were obtained from the other sampling sites. A minimum of 40 live clams of similar sizes were carefully collected from each sampling site using sterile non-plastic containers and immediately stored at -20 °C for subsequent analysis.
2.2 Sample preparation
The stored samples were thawed and then washed using sterile distilled water. The morphometric measurements of each sample were taken using a digital vernier calliper gauge micrometre, and their weight was recorded using a digital weighing machine. To prevent procedural contamination, all sampling tools and necessary materials underwent a thorough cleaning process with distilled water filtered through a 1.6 µm filter and acetone to minimize the presence of contaminants (Li et al. 2015).
2.3 Pretreatment
Isolation of microplastics from the collected bivalves Perna viridis and Villorita cyprinoides was conducted based on the weight of their soft tissues. The outer shell of each bivalve was carefully opened, and the inner contents, including the soft tissue of approximately 12 individual clams, were transferred into a 1000 ml sterile glass beaker. Triplicates were maintained consistently for each sampling site throughout the study. The beakers containing the triplicates from the 8 sampling sites were covered and pretreated with approximately 200 ml of a 30% hydrogen peroxide (H2O2) solution, following the methods described by Li et al. (2015). The beakers were then incubated at 65 °C for 24 h and then at room temperature for another 48 h to digest the organic matter present (Li et al. 2015). The same procedure was followed for procedural blanks, which did not contain any bivalves.
2.4 Microplastic extraction by salt water density separation
Following the H2O2 treatment, the microplastics released into the dissolved liquid of the soft tissue were separated using floatation and filtration techniques. A concentrated sodium chloride solution (1.2 g/ml) was prepared and approximately 800 ml of this solution was filtered and added to each beaker containing the soft tissue liquid. The resulting solution was thoroughly mixed and left undisturbed for overnight incubation. Subsequently, the overlying water was filtered using a vacuum pump and a 47 mm diameter cellulose nitrate membrane filter (Whatman AE98). After the filtration process, the filter paper containing the captured microplastics was carefully transferred to clean and covered glass Petri dishes for further analysis, as described by Lechner et al. (2014).
2.5 Contamination control process
All those procedures are carried out with maximum care in order to avoid contamination during analysis of samples. Before use all equipment was washed three times with distilled water and acetone is used to clean the exposed laboratory surfaces. Filter papers are checked before the experiment to make sure it was under a microscope. Care was taken to complete the procedures as soon as possible uncontaminated. Aluminium foil was used to cover the beakers during the entire process. 100% cotton made lab coats were used while conducting the experiment. To avoid contamination by air, the laboratory’s ventilation and windows were kept closed at all times. Procedural blanks of 30% hydrogen peroxide solution were also filtered by using Whatman filter paper and it was also observed like other samples.
2.6 Identification of isolated microplastics
The microplastic particles collected on the filter paper from all sampling sites were examined and photographed using a Magnus MLXi—Tr plus microscope, equipped with a Magcam DC-5 and a magnification range of 40-100X. Microplastic identification was based on the distinctive features previously reported, including type, color, and size, and the observed microplastics were classified accordingly. The type and quantity of microplastics were determined based on this microscopic analysis, following the methods outlined by Li et al. (2015).
2.7 Verification of Polymer using FTIR-ATR
FTIR spectrum analysis (Perkin-Elmer Spectrum-2 FTIR) was used to verify the microscopically identified plastic particles. For transparent and semi-transparent objects, the transmittance mode was utilized, while the attenuated total reflection mode (ATR) was applied for opaque objects. The spectrum range spanned from 4000 to 675 cm^1, with a collection time of approximately 3 s and 16 co-scans conducted for each measurement. The aperture size varied depending on the particle’s size, ranging from 50 × 50 mm to 150 × 150 mm (Jung et al. 2018). The spectral data obtained from this process were then compared with FTIR databases and spectral libraries to determine the polymer type. The identification of the polymer type in the detected microplastics was performed using an open-source tool called Openspecy, (www.openspecy.org) with a Pearson coefficient threshold set at > 0.8
3 Results and discussion
In this study, a total of 288 bivalve samples were collected from eight different locations in Chandragiri River to investigate microplastic contamination. the micro plastic pollution in these sample location areas remains relatively less studied. The selected sampling sites, strategically chosen from both urban and rural areas, represent a spectrum of pollutant sources. These include river discharge, industrial effluent, fishing activity, and domestic waste, offering a comprehensive picture of environmental contamination. Substantial volumes of pollutants are consistently introduced into marine areas through river discharges and sewage effluents from both domestic and industrial origins. While intensive maritime activities, such as fishing and tourism, contribute significantly to economic and social advancement, they can contribute directly or indirectly to the heightened release of contaminants. Investigating these specific environments will unquestionably yield supplementary data essential for a more comprehensive assessment of microplastic quantities and a deeper understanding of their dynamics and distribution in river water. The unique filter-feeding mechanism of bivalves exposes them to the surrounding microplastic pollution. Accidental ingestion of microplastics can elicit adverse health effects in bivalves, leading to notable alterations in their digestive system and potentially causing a decrease in their filter-feeding activity (Browne et al. 2008; Wang et al. 2019). A previous study conducted in Goa, West Coast of India, investigated the presence of microplastics in seafood and reported an average microplastic concentration of 3.2 ± 1.8 MPs/g body weight in the soft tissue (Saha et al. 2021). Similarly, a preliminary study conducted at the fishing harbor of Chennai, Southeast coast of India, examined the evidence of microplastics in Perna viridis and found ingested polystyrene polymers (Naidu 2019). In vivo experiments on P. viridis demonstrated the accumulation of weathered polyethylene and microscopic PVC particles, indicating the intake of microplastics with different colors and raising concerns about oxidative stress and structural changes (Vasanthi et al. 2021).
V. cyprinoides (black clam) has been the subject of several studies highlighting its susceptibility to lead toxicity and its after-effects, and it has also been used as a biomonitoring species to assess trace metal pollution in the Cochin backwaters (Neethu et al. 2020).
However, there are limited reports available on microplastics in V. cyprinoides. Our findings revealed the presence of four morphologically diverse microplastic particles, including beads, fragments, film, and fibres in P. viridis, and three morphologically diverse microplastic particles, including beads, fragments, and fibres, in V. cyprinoides. When considering the polymer types identified in both bivalves, there is only one difference in a specific polymer type, while the remaining polymers are more or less the same. Besides variations in microplastic abundance, no significant differences were observed in the polymer type or morphology of plastic particles between both bivalves, suggesting that external factors influence these characteristics. However, the study confirms that both V. cyprinoides and P. viridis are major contributors to identify the presence of submicron microplastic pollutants in freshwater systems.
The overall mean abundance of microplastic content in the 288 bivalves examined in this study was found to be 2.31 ± 0.93 items/individual. Microplastics were classified into four morphometric types – beads, fibres, films, and fragments (Amelia et al. 2021) – based on their shapes and sizes. And their polymer composition, determined by FTIR analysis, along with mean abundance are summarized for each sampling location in Table 1. Average bivalve lengths and average soft tissue weights are provided in Table 2 for reference. To contextualize our findings, Table 3 presents a comparison with similar studies on microplastic abundance in bivalves. Limited research has been conducted on the accumulation of microplastics in Perna viridis and Villorita cyprinoides. When compared to previous studies by Mathalon and Hill (2014), Li et al. (2015), Naji et al. (2018), and Dowarah et al. (2020), Imasha and Babel (2023) the total mean abundance reported in this study is relatively lower. However, it is significantly higher than the studies conducted by Cauwenberghe and Janssen (2014), Phuong et al. (2018), Cho et al. (2019), and Hermabessiere et al. (2019). The variation in microplastic abundance is likely influenced by several external factors, such as regional plastic pollution rates, population indices, climatic and geographical factors, and population habits.
3.1 Size, morpho type composition and color of micro plastics detected in bivalves
In this study the size of the micro plastics detected were in the range of 100 μm to 1.5 mm. And the majority of particles were found in the range between 100 μm to 300 μm. Small size of micro plastics makes them appear like food for lower trophic organisms (Lusher et al. 2013). Fragments (43%) are detected as the most abundant morphotype of micro plastic particles from the bivalves that were analyzed. And it was followed by beads (37%), fibres (18%), and films (2%) (Fig. 2A). Fragments are reported more in 5 out of 8 sampling locations (Fig. 3). Various studies regarding the micro plastic pollution in aquatic organisms elucidates fragments as their abundant micro plastic morphological type (Karami et al. 2018; Cho et al. 2019; Akhbarizadeh et al. 2019). Abundance of fragments was also observed in fish species. According to various studies, there is an abundance of fragment type micro plastics in the south-west coast of India (James et al. 2020). On the other hand, fragment type micro plastic could have entered freshwater systems from various sources like auto mobile parts, consumer goods, degradation of damped plastics, packaging materials, personal care products etc. Sources of fibres to the river are likely to be disintegration of fishing nets and ropes and laundry washing water etc. film type micro plastic are recorded very less in this study, and we suspect that the film particle detected in this study had originated from the breakdown of plastic carry bags. Presence of micro beads is detected in personal care products and scrubbers (Reethu et al. 2023). Micro plastics extracted from bivalve species coming from Chandragiri River, Kerala varied in 9 colors (Fig. 2B). This elucidates that the digestion analysis with 30% hydrogen peroxide didn’t change the colors of micro plastic particles (Karami et al. 2018). The diversity in colors present in detected micro plastic particles also suggests that they have different origins (Sathish et al. 2019). It also indicates the origin points of degraded plastic materials (Nelms et al. 2018). In this study 9 different colors of micro plastic particles are identified that are green, blue, orange, violet, yellow, red, black, transparent and pink. The abundant color observed for all bivalve species was transparent (31%), followed by red (16%), blue (15%), green (13%), violet (11%), pink (7%), black (3%), and yellow (2%), orange (2%). Plastic carry bags, packaging products, fishing lines are considered as the main source for transparent micro plastics (Cole et al. 2013; Amrutha and Warrier 2020). Colored plastics are obtained from various sources like cloths, consumer goods, bags, bottles etc. Morphological characters of micro plastics play a major role in micro plastic ingestion processes that are performed by lower trophic organisms (Lusher et al. 2013; Nelms et al. 2018).
Characteristics of microplastics identified a) Percentage of morphotypes composition of microplastics from Chandragiri River b) Percentage of Diversity of colour shades in microscopically observed microplastics c) Percentage of Polymer composition including Polyethylene, Polyethylene terephthalate, Polypropylene, Polyester, Acrylonitrile Butadiene Styrene
3.2 Polymer composition of microplastics in Chandragiri River
Our visual inspection identified 667 potential microplastic particles. Subsequent Fourier-transform infrared spectroscopy with attenuated total reflectance (FTIR-ATR) analysis confirmed all suspicious particles to be genuine microplastics. Importantly, procedural blanks also yielded negative results, devoid of any microplastic contamination, demonstrating the meticulousness and effectiveness of our contamination control protocols. The obtained spectra (Fig. 4) were analyzed using the Openspecy identification tool (www.openspecy.org), which is commonly used for diagnosing IR spectra (Cowger et al. 2021). The study identified five different types of polymers in the microplastic particles. The detected polymer types were polypropylene (31.86%), acrylonitrile butadiene styrene (17.03%), polyethylene terephthalate (24.23%), polyethylene (14.98%), and polyester (11.89%) (Fig. 2C). It is worth noting that a previous study conducted on bivalves (Li et al 2015) did not identify any polypropylene content. However, in this study, polypropylene was found to be the most abundant polymer type. The high abundance of polypropylene and polyethylene microplastics in aquatic environments has been reported in various studies conducted in different geographical locations (Mathalon and Hill 2014; Abidli et al. 2019; Cho et al. 2019; Wang et al. 2020). The increased global production of polypropylene materials contributes to their abundance in microplastic pollution. Polypropylene is commonly used in the manufacturing of bottles, pipes, tubs, plastic bags, ropes used in aquaculture, and carpets. Non-woven cloth materials, which often contain polypropylene sheets, can also be a source of contamination. Polyethylene polymer is used in the production of bags, bottles, toys, and personal care products. Polyethylene terephthalate and polyester polymers are primarily used in the textile industry, and the washing of clothes containing these polymers near rivers can lead to the release of polymer fibres into the water.
Polyethylene terephthalate is also commonly found in brushes, bottles, motor parts, and packaging materials. It has extensive applications in the medical field for manufacturing artificial internal organs, pharmacy prescription bottles, and other medical devices. Polyester, on the other hand, is a significant synthetic fibre used in the production of fishing gear. Acrylonitrile butadiene styrene, a rigid plastic type, is widely used in the manufacturing of toys, domestic products and fishing gears.
The presence of diverse morphotypic and colored microplastic particles in the river can originate from various sources (Fig. 5) such as tourism, improper disposal of plastic waste, automobiles, domestic care products, and natural disasters like flooding and stormwater events (Kumar & Varghese 2021). Previous studies have consistently reported the abundance of polypropylene and polyethylene microplastic polymers in the guts of fish from South India (James et al. 2020). The low density of these particles allows them to float and be easily transported by water currents. Bivalves, due to their filter-feeding activity, are particularly exposed to the floating and sedimented microplastic particles present in their surroundings, leading to the process of bioaccumulation of microplastics in the ecosystem (Karami et al. 2018; Cho et al. 2019; Akhbarizadeh et al. 2019).
3.3 Microplastic abundance in Chandragiri River
A total of 667 microplastic particles were identified in both bivalve species collected from eight sample locations in Chandragiri River, with a mean abundance of 2.315 ± 0.93 items/individual (Table 1, Fig. 6). Microplastic particles (less than 5mm) of various sizes and shapes were detected in each of the eight sampling locations.
Perna viridis samples from Kombanadukkam region yielded 102 microplastic particles, with a mean abundance of 2.83 ± 0.17 items/individual. Fibre, bead, and fragment types were present, with fragments again dominating at 66%. Polypropylene, acrylonitrile butadiene styrene, polyethylene terephthalate, and polyester polymers were ubiquitous. Notably, polypropylene-containing plastics were more common, and most of the fibres were made of polyester. Thalangara region recorded a mean abundance of microplastic particles, at 3.61 ± 0.20 items/individual. Bead, film, fibre, and fragment types were observed, with fragments constituting 55%. Four types of plastic polymers were identified: acrylonitrile butadiene styrene, polypropylene, polyethylene terephthalate, and polyethylene.
Clam species Villorita cyprinoides samples were collected from six other locations. Chengala region had a mean abundance of 2.41 ± 0.17 items/individual, with fibre, bead, fragment, and film types identified. Fragments (48%) were the most prevalent, and polyethylene terephthalate, acrylonitrile butadiene styrene, polyester, and polyethylene polymers were present. From Chala region, micro plastic particles were identified at a mean abundance of 1.11 ± 0.05 items/individual from this area. From the identified micro plastic types, fragments were accounted more (55%) and beads, fibre are also present in this sampling area. Polypropylene, polyethylene terephthalate, polyethylene contained micro plastic substances were obtained from here. Sulia’s mean microplastic abundance was 1.22 ± 0.07 items/individual, with bead, fibre, and fragment types observed. As in other locations, fragments (48%) were the most prevalent. Polypropylene, polyethylene terephthalate, and polyethylene polymers were ubiquitous. Thekkil region yielded 75 microplastic particles, with a mean abundance of 2.08 ± 0.18 items/individual. Three types were observed, with beads (56%) outnumbering fibres and fragments. Jalsoor recorded the least number of microplastic particles, with a mean abundance of 0.94 ± 0.97 items/individual. Only two polymers were identified: polypropylene and polyester. Interestingly, this region had the highest proportion of fibre type microplastics (46%). Kasaragod had the highest mean abundance of microplastics (4.27 ± 0.34 items/individual), with bead type (58%) dominating over fibre and fragments. Polypropylene, acrylonitrile butadiene styrene, polyethylene terephthalate, and polyester polymers were identified.
The analysis of different morphotype microplastic abundance by location (Fig. 3) revealed that Kasaragod exhibited the highest mean abundance among the eight sampling locations. This aligns with the Census report (Chandramouli 2011) and Current estimated population of Kasaragod Municipality in 2023 is approximately 74,000, both highlighting this densely populated and urbanized region’s increased propensity for plastic waste generation and subsequent deposition in its surroundings. As per the 2011 Census Report (Chandramouli 2011), the district Kasaragod has shown a continuous increase in the degree of urban concentration from 1991 to 2011 and the increase is rapid between 2001 and 2011 (Ds and Ks 2016). Kasaragod’s urban centre exhibits a striking disparity in microplastic composition compared to surrounding areas. The region’s dense population (3200/km2 compared to the district average of 657/km2; Chandramouli 2011) potentially translates to higher waste generation and accumulation of these specific microplastics.
Moreover, the recent COVID-19 surge has exacerbated plastic pollution with increased use of personal protective equipment (PPE) and face masks containing polyester blends. This trend reinforces the link between human density, plastic consumption, and microplastic contamination, highlighting the need for sustainable alternatives and waste management strategies, particularly in densely populated areas (Reethu et al. 2023).
The abundance of microplastics in the studied area is closely linked to human activities. The areas with higher human population density and proximity to urban centres exhibited greater microplastic abundance. Apart from this according to Census 2011, the urban population in Kerala was 48.50%. The National Population Commission estimates that 92.8% of Kerala will become an urbanised State by 2035.
Microplastic abundance in the Chandragiri River also varied by location, with the highest levels found near the mouth in Kasaragod and the lowest upstream in Jalsoor. This suggests that geography influences microplastic sedimentation, with downstream areas likely receiving microplastic particles carried by the water current. It is worth noting that this study recorded a smaller number of microplastic particles compared to other studies conducted in India, particularly in marine environments such as Pondicherry (Dowarah et al. 2020) and Tuticorin (Patterson et al. 2019). This difference in abundance can be attributed to the focus of this study on a freshwater ecosystem rather than a marine environment.
4 Conclusion
Our study revealed the presence of microplastic contamination in the Chandragiri River, located in Kerala and Karnataka, Southwest India, as evidenced by the total microplastics isolated from two bivalve species, P. viridis and V. cyprinoides. Microplastic pollution was widespread across all eight sampling sites, with an average abundance of 2.31 ± 0.09 microplastics per individual. We identified four different morphotypes of degraded plastic particles, including beads, fragments, fibres, and films. Using the FTIR-ATR method, we determined the polymer composition and found the occurrence of five types of polymers: polypropylene, acrylonitrile butadiene styrene, polyethylene terephthalate, polyethylene, and polyester. In this freshwater ecosystem, we observed a notable prevalence of microplastic fragments containing polypropylene particles, underscoring a clear linkage between human activities and the release of microplastics into the environment. The escalating population growth and urbanization have led to the introduction of diverse plastic waste types into the freshwater system, leading to deleterious consequences on the biological functioning of the water and its associated macro and microflora. Moreover, our investigation revealed the presence of microplastics in heterogeneous colors, such as green, blue, orange, violet, yellow, red, black, transparent, and pink, originating from various sources of plastic disintegration. The 667 microplastic particles isolated from 288 bivalve samples in our study represent only a fraction of the widespread contamination in the Chandragiri River. Further research, including investigations into seasonal variations of microplastics, is needed to develop comprehensive approaches aimed at mitigating additional microplastic pollution and maintaining ecological balance, safeguarding our biosphere.
References
Abidli S, Lahbib Y, Trigui El, Menif N (2019) Microplastics in commercial molluscs from the lagoon of Bizerte (Northern Tunisia). Mar Pollut Bull 142:243–252. https://doi.org/10.1016/j.marpolbul.2019.03.048
Akhbarizadeh R, Moore F, Keshavarzi B (2019) Investigating microplastics bioaccumulation and biomagnification in seafood from the Persian Gulf: a threat to human health? Food Addit Contam Part A Chem Anal Control Expo Risk Assess 36(11):1696–1708. https://doi.org/10.1080/19440049.2019.1649473
Alimi OS, Farner-Budarz J, Hernandez LM, Tufenkji N (2018) Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Environ Sci Technol 52(4):1704–1724. https://doi.org/10.1021/acs.est.7b05559
Amelia TSM, Khalik WMAWM, Ong MC, Shao YT, Pan HJ, Bhubalan K (2021) Marine microplastics as vectors of major ocean pollutants and its hazards to the marine ecosystem and humans. Prog Earth Planet Sci 8:12. https://doi.org/10.1186/s40645-020-00405-4
Amrutha K, Warrier AK (2020) The first report on the source-to-sink characterization of micro plastic pollution from a riverine environment in tropical India. Sci Total Environ 739:140377. https://doi.org/10.1016/j.scitotenv.2020.140377
Andrady AL (2017) The plastic in microplastics: a review. Mar Pollut Bull 119(1):12–22. https://doi.org/10.1016/j.marpolbul.2017.01.082
Avio CG, Gorbi S, Milan M, Benedetti M, Fattorini D, d’Errico G, Pauletto M, Bargelloni L, Regoli F (2015) Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ Pollut 198:211–222. https://doi.org/10.1016/j.envpol.2014.12.021
Besseling E, Wegner A, Foekema EM, van den Heuvel-Greve MJ, Koelmans AA (2013) Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environ Sci Technol 47(1):593–600 https://europepmc.org/article/med/23181424
Besseling E, Wang B, Lürling M, Koelmans AA (2014) Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ Sci Technol 48:12336–12343 https://pubmed.ncbi.nlm.nih.gov/25268330/
Boerger C, Lattin GL, Moore S, Moore CJ (2010) Plastic ingestion by planktivorous fishes in the North Pacific Central Gyre. Mar Pollut Bull 60(12):2275–2278. https://doi.org/10.1016/j.marpolbul.2010.08.007
Bošković N, Joksimović D, Bajt O (2022) Microplastics in fish and sediments from the Montenegrin coast (Adriatic Sea): similarities in accumulation. Sci Total Environ 850:158074. https://doi.org/10.1016/j.scitotenv.2022.158074
Botterell ZLR, Beaumont N, Dorrington T, Steinke M, Thompson RC, Lindeque PK (2019) Bioavailability and effects of microplastics on marine zooplankton: a review. Environ Pollut 245:98–110. https://doi.org/10.1016/j.envpol.2018.10.065
Browne MA, Dissanayake A, Galloway TS, Lowe DM, Thompson RC (2008) Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.). Environ Sci Technol 42(13):5026–5031. https://doi.org/10.1021/es800249a
Cauwenberghe LV, Janssen CR (2014) Microplastics in bivalves cultured for human consumption. Environ Pollut 193:65–70. https://doi.org/10.1016/j.envpol.2014.06.010
Chandramouli C (2011) Census of India 2011. Retrieved December 07, 2022, from. Office of Registrar General & Census Commissioner, India. https://www.census2011.co.in
Cho Y, Shim WJ, Jang M, Han GM, Hong SH (2019) Abundance and characteristics of microplastics in market bivalves from South Korea. Environ Pollut 245:1107–1116. https://doi.org/10.1016/j.envpol.2018.11.091
Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J, Galloway TS (2013) Microplastic ingestion by Zooplankton. Environ Sci Technol 47(12):6646–6655. https://doi.org/10.1021/es400663f
Cowger W, Steinmetz Z, Gray A, Munno K, Lynch J, Hapich H, Primpke S, De Frond H, Rochman C, Herodotou O (2021) Microplastic spectral classification needs an open source community: open specy to the rescue! Anal Chem 93(21):7543–7548. https://doi.org/10.1021/acs.analchem.1c00123
Cowger W, Gray A, Hapich H, Rochman C, Lynch J, Primpke S, Herodotou O (2020) Open Specy. www.openspecy.org
Dowarah K, Patchaiyappan A, Thirunavukkarasu C, Jayakumar S, Devipriya SP (2020) Quantification of microplastics using Nile Red in two bivalve species Perna viridis and Meretrix meretrix from three estuaries in Pondicherry, India and microplastic uptake by local communities through bivalve diet. Mar Pollut Bull 153:110982. https://doi.org/10.1016/j.marpolbul.2020.110982
Ds SD, Ks L (2016) Urbanization and development in Kerala. Int J Appl Res 2:586–590
Eriksen M, Lebreton LCM, Carson HS, Thiel M, Moore CJ, Borerro JC, Galgani F, Ryan PG, Reisser J (2014) Plastic pollution in the World’s Oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS One 9(12):e111913. https://doi.org/10.1371/journal.pone.0111913
Europe P (2016) Plastics—the facts 2016. An analysis of European latest plastics production, demand and waste data
FAO (2019) The state of food and agriculture 2019: moving forward on food loss and waste reduction. Rome.
Gamarro GE, Ryder J, Elvevoll EO, Olsen RL (2020) Microplastics in fish and shellfish – a threat to seafood safety? J Aquat Food Prod Technol 1–9. https://doi.org/10.1080/10498850.2020.1739793
Graham ER, Thompson JT (2009) Deposit- and suspension-feeding sea cucumbers (Echinodermata) ingest plastic fragments. J Exp Mar Biol Ecol 368(1):22–29. https://doi.org/10.1016/j.jembe.2008.09.007
Hermabessiere L, Paul-Pont I, Cassone AL, Himber C, Receveur J, Jezequel R, El Rakwe M, Rinnert E, Rivière G, Lambert C, Huvet A, Dehaut A, Duflos G, Soudant P (2019) Microplastic contamination and pollutant levels in mussels and cockles collected along the channel coasts. Environ Pollut 250:807–819. https://doi.org/10.1016/j.envpol.2019.04.051
Holmes LA, Turner A, Thompson RC (2012) Adsorption of trace metals to plastic resin pellets in the marine environment. Environ Pollut 160:42–48. https://doi.org/10.1016/j.envpol.2011.08.052
Imasha HUE, Babel S (2023) Microplastics contamination in the green mussels (Perna viridis) cultured for human consumption in Thailand. Reg Stud Mar Sci 67:103203. https://doi.org/10.1016/j.rsma.2023.103203
Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL (2015) Plastic waste inputs from land into the ocean. Science 347(6223):768–771. https://doi.org/10.1126/science.1260352
James K, Vasant K, Padua S, Gopinath VKARJ, Babu A, John S (2020) An assessment of microplastics in the ecosystem and selected commercially important fishes off Kochi, south eastern Arabian Sea, India. Marine Pollut Bull 154:111027. https://doi.org/10.1016/j.marpolbul.2020.111027
Jung MR, Horgen FD, Orski SV, Rodriguez VC, Beers KL, Balazs GH, Jones TT, Work TM, Brignac KC, Royer SJ, Hyrenbach KD, Jensen BA, Lynch JM (2018) Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar Pollut Bull 127:704–716. https://doi.org/10.1016/j.marpolbul.2017.12.061
Karami A, Golieskardi A, Choo CK, Larat V, Karbalaei S, Salamatinia B (2018) Micro plastic and meso-plastic contamination in canned sardines and sprats. Sci Total Environ 612:1380–1386. https://doi.org/10.1016/j.scitotenv.2017.09.005
Kolandhasamy P, Su L, Li J, Qu X, Jabeen K, Shi H (2018) Adherence of micro plastics to soft tissue of mussels: a novel way to uptake micro plastics beyond ingestion. Sci Total Environ 610–611:635–640. https://doi.org/10.1016/j.scitotenv.2017.08.053
Kumar AS, Varghese G (2021) Microplastic pollution of Calicut beach - contributing factors and possible impacts. Mar Pollut Bull 169:112492. https://doi.org/10.1016/j.marpolbul.2021.112492
Lebreton L, Andrady A (2019) Future scenarios of global plastic waste generation and disposal. Palgrave Commun 5(1):6. https://doi.org/10.1057/s41599-018-0212-7
Lechner A, Keckeis H, Lumesberger-Loisl F, Zens B, Krusch R, Tritthart M, Glas M, Schludermann E (2014) The Danube is so colourful: a potpourri of plastic litter outnumbers fish larvae in Europe’s second largest river. Environ Pollut 188:177–181. https://doi.org/10.1016/j.envpol.2014.02.006
Li J, Yang D, Li L, Jabeen K, Shi H (2015) Microplastics in commercial bivalves from China. Environ Pollut 207:190–195. https://doi.org/10.1016/j.envpol.2015.09.018
Lusher A, McHugh M, Thompson R (2013) Occurrence of micro plastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar Pollut Bull 67(1–2):94–99. https://doi.org/10.1016/j.marpolbul.2012.11.028
Mathalon A, Hill P (2014) Microplastic fibres in the intertidal ecosystem surrounding Halifax Harbor, Nova Scotia. Marine Pollut Bull 81(1):69–79. https://doi.org/10.1016/j.marpolbul.2014.02.018
Mohsen M, Wang Q, Zhang L, Sun L, Lin C, Yang H (2019) Microplastic ingestion by the farmed sea cucumber Apostichopus japonicus in China. Environ Pollut 245:1071–1078. https://doi.org/10.1016/j.envpol.2018.11.083
Naidu SA (2019) Preliminary study and first evidence of presence of microplastics and colorants in green mussel, Perna viridis (Linnaeus, 1758), from southeast coast of India. Mar Pollut Bull 140:416–422. https://doi.org/10.1016/j.marpolbul.2019.01.024
Naji A, Nuri M, Vethaak AD (2018) Microplastics contamination in molluscs from the northern part of the Persian Gulf. Environ Pollut 235:113–120. https://doi.org/10.1016/j.envpol.2017.12.046
Neethu KV, Nandan SB, Xavier NDD, Jayachandran PR, Anu PR, Midhun AM, Mohan D, Marigoudar SR (2020) A multibiomarker approach to assess lead toxicity on the black clam, Villorita cyprinoides (Gray, 1825), from Cochin estuarine system (CES), southwest coast, India. Environ Sci Pollut Res 28(2):1775–1788
Nelms SE, Galloway TS, Godley BJ, Jarvis D, Lindeque PK (2018) Investigating microplastic trophic transfer in marine top predators. Environ Pollut 238:999–1007. https://doi.org/10.1016/j.envpol.2018.02.016
Patterson J, Jeyasanta KI, Sathish N, Booth AM, Edward JKP (2019) Profiling microplastics in the Indian edible oyster, Magallana bilineata collected from the Tuticorin coast, Gulf of Mannar, Southeastern India. Sci Total Environ 691:727–735. https://doi.org/10.1016/j.scitotenv.2019.07.063
Patterson J, Jeyasanta KI, Laju R, Edward JKP (2021) Microplastic contamination in Indian edible mussels (Perna perna and Perna viridis) and their environs. Mar Pollut Bull 171:112678. https://doi.org/10.1016/j.marpolbul.2021.112678
Phuong NN, Poirier L, Pham QT, Lagarde F, Zalouk-Vergnoux A (2018) Factors influencing the microplastic contamination of bivalves from the French Atlantic coast: location, season and/or mode of life? Mar Pollut Bull 129(2):664–674. https://doi.org/10.1016/j.marpolbul.2017.10.054
Reethu M, Biswajit R, Aravind GH, Rafaz AK, Sandeep K, Sijinkumar AV, Warrier AK (2023) A first report on the spatial and temporal variability of microplastics in coastal soils of an urban town in south-western India: Pre- and post-COVID scenario. Mar Pollut Bull 190:114888. https://doi.org/10.1016/j.marpolbul.2023.114888
Rochman CM, Browne MA, Halpern BS, Hentschel BT, Hoh E, Karapanagioti HK, Rios-Mendoza LM, Takada H, Teh S, Thompson RC (2013a) Classify plastic waste as hazardous. Nature 494(7436):169–171. https://doi.org/10.1038/494169a
Rochman CM, Hoh E, Hentschel BT, Kaye S (2013b) Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: implications for plastic marine debris. Environ Sci Technol 47(3):1646–1654 https://pubs.acs.org/doi/abs/10.1021/es303700s
Saha M, Naik A, Desai A, Nanajkar M, Rathore C, Kumar M, Gupta P (2021) Microplastics in seafood as an emerging threat to marine environment: a case study in Goa, west coast of India. Chemosphere 270:129359. https://doi.org/10.1016/j.chemosphere.2020.129359
Sathish N, Jeyasanta KI, Patterson J (2019) Abundance, characteristics and surface degradation features of microplastics in beach sediments of five coastal areas in Tamil Nadu, India. Mar Pollut Bull 142:112–118. https://doi.org/10.1016/j.marpolbul.2019.03.037
Su L, Deng H, Li B, Chen Q, Pettigrove V, Wu C, Shi H (2019) The occurrence of microplastic in specific organs in commercially caught fishes from the coast and estuary area of east China. J Hazard Mater 365:716–724. https://doi.org/10.1016/j.jhazmat.2018.11.024
Sussarellu R, Suquet M, Thomas Y, Lambert C, Fabioux C, Pernet MEJ, Le Goïc N, Quillien V, Mingant C, Epelboin Y, Corporeau C, Guyomarch J, Robbens J, Paul-Pont I, Soudant P, Huvet A (2016) Oyster reproduction is affected by exposure to polystyrene microplastics. Proc Natl Acad Sci 113(9):2430–2435. https://doi.org/10.1073/pnas.1519019113
Teng J, Wang Q, Ran W, Wu D, Liu Y, Sun S, Liu H, Cao R, Zhao J (2019) Microplastic in cultured oysters from different coastal areas of China. Sci Total Environ 653:1282–1292. https://doi.org/10.1016/j.scitotenv.2018.11.057
Vasanthi RL, Arulvasu C, Kumar P, Srinivasan P (2021) Ingestion of microplastics and its potential for causing structural alterations and oxidative stress in Indian green mussel Perna viridis - a multiple biomarker approach. Chemosphere 283:130979. https://doi.org/10.1016/j.chemosphere.2021.130979
Wang Y, Mao Z, Zhang M, Ding G, Sun J, Du M, Liu Q, Cong Y, Jin F, Zhang W, Wang J (2019) The uptake and elimination of polystyrene microplastics by the brine shrimp, Artemia parthenogenetica, and its impact on its feeding behavior and intestinal histology. Chemosphere 234:123–131. https://doi.org/10.1016/j.chemosphere.2019.05.267
Wang X, Li C, Liu K, Zhu L, Song Z, Li D (2020) Atmospheric microplastic over the South China Sea and East Indian Ocean: abundance, distribution and source. J Hazard Mater 389:121846. https://doi.org/10.1016/j.jhazmat.2019.121846
Wegner A, Besseling E, Foekema EM, Kamermans P, Koelmans AA (2012) Effects of nanopolystyrene on the feeding behavior of the blue mussel (Mytilus edulis L.). Environ Toxicol Chem 31(11):2490–2497. https://doi.org/10.1002/etc.1984
Wright SL, Kelly FJ (2017) Plastic and human health: a micro issue? Environ Sci Technol 51(12):6634–6647. https://doi.org/10.1021/acs.est.7b00423
Wright SL, Rowe D, Thompson RC, Galloway TS (2013) Microplastic ingestion decreases energy reserves in marine worms. Curr Biol 23(23):R1031–R1033. https://doi.org/10.1016/j.cub.2013.10.068
Funding
The authors affirm that they did not receive any funding from external funding agencies for their research.
Author information
Authors and Affiliations
Contributions
Nidhin Krishna K R: Conceived, performed the experimental study and prepared the research article. Poornendhu Jayasree: Assisted in performing the experimental study and writing process of the research article. Dr. Jeyabalan Sangeetha: Conceived, monitored and guided for the experimental study, finalised, edited and reviewed the written article. Jadav Mulji Allabhai: Assisted in performing the experimental study and submission process of the research article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors confirm that the data supporting the findings of this study are available within the article.
Competing interests
The authors declare no conflicts of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Radhakrishnan, N.K.K., Sangeetha, J., Alabhai, J.M. et al. Accumulation of microplastics in bivalves within the Chandragiri River in South-Western India. Anthropocene Coasts 7, 5 (2024). https://doi.org/10.1007/s44218-024-00038-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44218-024-00038-w