Abstract
There is a void in the knowledge of the acidification status of Eastern Canada's coastal waters. This knowledge is crucial to evaluating the threats posed to marine life, particularly oyster farming, a flagship of New Brunswick seafood production. In this study, we measured the temporal variability of pH and related environmental parameters in three bays of Northeastern New Brunswick. We also evaluated the potential impact of the observed pH levels on the Eastern oyster (Crassostrea virginica Gmelin, 1791), based on the available literature on this species’ sensitivity to acidification. We investigated the presence of inherent cycles of pH with the Fourier transform and the spectral filtering technique. Our results show that pH is highly variable in the studied area, with values ranging from 7.31 to 8.90. A seasonal effect was apparent, as the pH fluctuations were set at the lowest level in winter when the cover of ice and snow on the bay was present. The spectral analysis revealed a clear semidiurnal tidal pattern of pH, this variable being inversely related to the water level in summer and directly related to it in winter. The spectral subtraction of all the tidal components allowed the detection of a circadian rhythm that was not in pace with the alternation between day and night but rather slowly drifted so that the pH troughs occurred at night during the full moon period. Short periodicities of circa 8 and 6 h also existed in two of the three bays. Based on current knowledge of C. virginica’s sensitivity to acidification, this species’ recruitment, growth, and survival are unlikely to be impacted by the present pH levels in the studied area. However, further acidification might overcome the resilience of C. virginica, especially that of the larvae that are produced during the winter in commercial hatcheries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Between 2005 and 2009, massive mortality of oyster larvae (Crassostrea gigas Thunberg, 1793) led to a drop of approximately 80% in the production of oyster seed by Pacific Northwest hatcheries (Mabardy et al. 2016). Wild oyster larvae also experienced a high death rate off the coast of British Columbia (Grossman 2011). Scientists eventually pinpointed ocean acidification as the main cause of this phenomenon (Barton et al. 2012). Various studies showed that ocean acidification is a multi-stressor that causes corrosive conditions for the larvae shells (Barton et al. 2012; Waldbusser et al. 2015a), increases the respiration rate, and reduces the feeding rate of the larvae, leading to an overrun of the larvae energy budget and eventually to death (Waldbusser et al. 2013, 2015b). Fifty-one percent of the shellfish stakeholders who took part in a survey declared that they had personally experienced negative impacts from ocean acidification (Mabardy et al. 2016). This crisis in the bivalve hatchery industry raised awareness of the impacts of ocean acidification on marine fauna and the necessity to develop our knowledge of the acidification process and its consequences on farmed and wild species. In 2014, the National Oceanic and Atmospheric Administration (NOAA) implemented a portal (https://nvs.nanoos.org/ShellfishGrowers, accessed 2 February 2023) giving access to real-time pH and related environmental parameters collected by five monitoring stations along the West Coast of the US. Additional monitoring stations were established afterward and we now have a fair picture of the pH spatiotemporal variability on the north-American West Coast, especially along the US coastline.
In contrast, we know very little about the situation on the Atlantic Coast of Canada which likely differs from that of the West Coast as there is no coastal upwelling bringing CO2-rich deep water to the surface. The basal level of pH in this part of the ocean is therefore higher than in the northwest Pacific. However, sea ice meltwater and the outflow from the Gulf of St-Lawrence and the Labrador and Newfoundland Shelf region discharge acidified water in the northwest Atlantic causing a higher rate of acidification (Blair et al. 2019). To our knowledge, there are only three pH and CO2 monitoring stations on the Canadian east coast, operated by the Atlantic Zone Monitoring Program of the Department of Fisheries and Oceans Canada, all of which are set in deep water energetic hydrodynamic systems, a situation that is quite different from the shallow and relatively calm bays along the coast of northeast New Brunswick. To the best of our knowledge, only one study has described spatiotemporal fluctuations of pH and related physicochemical parameters in eastern Canada coastal waters (Menu-Courey et al. 2019). The acidification portrait is thus very fragmentary for an area that supports approximately 86% of Canadian seafood production (Department of Fisheries and Oceans 2022) and boasts healthy seagrass beds and salt marshes which provide an array of ecosystem services.
The goals of this study were to examine the spatiotemporal variability of water pH and related environmental parameters in three bays of interest for the aquaculture of the Eastern oyster (Crassostrea virginica Gmelin, 1791), which is the principal bivalve species grown in Northeastern New Brunswick (Canada), and to evaluate the level of risk posed by acidification to the oyster farming industry.
2 Study area and methods
2.1 Study sites
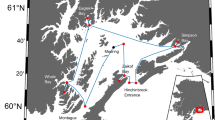
We monitored water pH, temperature, and salinity in three bays situated in northeastern New Brunswick (Canada). The locations of the bays as well as those of the monitoring stations are presented in Fig. 1.
St. Simon Bay has a surface of 17 km2 and connects by a narrow passage of 1.5 km in width to Chaleur Bay, which itself opens out to the Gulf of St. Lawrence. It is a shallow inlet where most areas are < 4 m deep, except for a narrow channel approximately 6 m deep. Eelgrass (Zostera marina Linnaeus) beds cover 98% of the bay surface (Bastien-Daigle et al. 2007). This bay is surrounded by wetlands but receives no significant freshwater inflow. It harbors about 40 active leases dedicated to Eastern oyster aquaculture, distributed over an area of 190 ha, as well as a commercial hatchery for Eastern oysters and Bay scallops (Argopecten irradians Lamarck, 1819).
The Bay of Caraquet covers 40 km2 and connects to Chaleur Bay by a 3.5 km wide opening. It has a depth at low tide < 3 m (Sephton and Boothe 1992) and receives two principal tributaries, Rivière Caraquet and Rivière du Nord. The flow at the head of the Rivière Caraquet varied between 2 and 5 m3 s−1 in June 2018 and between 2 and 2.5 m3 s−1 in July 2018 (Department of Environment and Natural Resources Canada 2022). No data are available for the discharge of the North River but, according to Sephton and Boothe (1992), its catchment is three times smaller than that of Rivière Caraquet. The Bay of Caraquet harbors twenty-eight leases covering 175 ha which are used for oyster growing. It is also one of the three main oyster spat collection sites in New Brunswick. It is thus crucial to build our knowledge of the dynamics of acidification in this area, as larvae are particularly sensitive to low pH.
Tracadie Bay has a surface of 21.4 km2. It is separated from the Gulf of St.Lawrence by barrier islands with two narrow inlets connecting the bay with the Gulf. The northern branch of the bay is < 2 m in depth and receives fresh water from the Petite Tracadie River, which has a discharge of approximately 1—2 m3 s−1 in summer (Deb et al. 2022). In 2016, approximately 75% of cultivated oysters died in some of the aquaculture leases of the northern branch of the bay (Deb et al. 2022). A subsequent study attributed this mass mortality event to the combined effect of environmental hypoxia and endogenous bacterial proliferation (Coffin et al. 2021). Another mortality event occurred at the same site in late August 2019, a few weeks after the end of our monitoring (Tina Rousselle, Dep. Fisheries Aquaculture and Agriculture of New Brunswick, pers. comm.). Nineteen leases covering 137 ha are located in this bay, but oyster farmers have begun to move their operations elsewhere because of the oysters' mortalities.
2.2 Environmental parameters
In the Caraquet and Tracadie bays, the instruments were deployed at approximately 1 m below the sea surface at high tide. As the tidal range is very low at these sites (1.32 m in Caraquet Bay and 0.73 m in Tracadie Bay, data retrieved from the website of the Department of Fisheries and Oceans Canada https://www.tides.gc.ca/), the instruments were always submerged despite the shallow depth of the bays. The Caraquet station was situated in the area where the oyster spat collectors are placed by the aquaculturists. The Tracadie station was located in the northern branch of the bay, where mass oyster mortality occurred in 2016. We registered pH and temperature hourly with INW TempHion sensors and data loggers (Seametrics, Kent, WA, USA). Hourly salinity measurements were done with either the INW CT2X conductivity sensor and data logger by Seametrics or the DST CTD conductivity and temperature recorder by Star Oddi (Gardabaer, Iceland). The instruments were retrieved every 1 or 2 weeks, depending on the fouling conditions, to be cleaned and calibrated. Calibration of pH data loggers was done with NBS buffers and the pH values were thereafter mathematically transformed to the seawater scale (pHSWS, in mol kg−1 seawater) using the equation of Millero and Poisson (1981). We used interpolated salinity values to calculate pHSWS from 21 December to 9 January because of technical issues with the salinity logger during this period. We are confident that the estimated pHSWS are very close to the actual values, given that salinity does not vary much in winter and that a salinity discrepancy of 5, for example, would skew the calculated pHSWS by only ≈ 0.03 units. Chlorophyll a concentration (μg L−1) was measured as a phytoplankton biomass proxy once a week on four to six surface water samples with a portable fluorimeter (model Aquafluor 8000–010, Turner Designs, San Jose, CA, USA) calibrated with a solution of chlorophyll a of known concentration. Two samples of water were also collected once a week at each site, killed with a saturated solution of mercuric chloride, and stored at room temperature pending analyses for total alkalinity (TA) following the protocol SOP3b of Dickson et al. (2007). The predicted water levels were retrieved from the website of the Department of Fisheries and Oceans Canada (https://www.tides.gc.ca/) as a descriptor of the tidal cycles.
For the monitoring in St. Simon Bay, we took advantage of the seawater intake of the hatchery to monitor water pH, salinity, and temperature even during the winter months when a cover of ice and snow up to 1 m in thickness would have greatly complicated the regular recovery and maintenance of the instruments. Seawater was pumped from the channel and we collected unfiltered seawater directly at the entrance of the hatchery to supply an opaque 80 L plastic tank at a rate of ≈ 5 L min−1. The tank was covered with a lid to prevent the stimulation of photosynthesis by light. The instruments described above were installed in this tank to register water temperature, salinity, and pH. Once a week, the instruments were retrieved to be cleaned and calibrated, and the tank was thoroughly scrubbed and rinsed with incoming seawater. On one occasion during the summer of 2017 and one during the winter of 2018, we measured the physicochemical parameters near the intake location and found pH and salinity values identical to the first decimal place to those registered in the tank at the same time, while the temperature was 1° C colder than in the tank. In general, the water temperature was ≈ 1° C warmer in the tank than what we know to be typical values at the same study site during winter (Mayrand et al. 2017). In February and March 2018, a Cyclops 7F submersible sensor (Turner Designs) was installed in the 80 L plastic tank to register the in vivo chlorophyll a fluorescence emitted by phytoplankton. In June, discrete samples were analyzed with the portable Turner fluorometer. The pumping system was shut down in June 2018 which caused the end of data collection.
In St. Simon Bay, data were registered from 23 November 2017 to 7 June 2018. In Caraquet Bay, they were collected from 8 June to 8 August 2018, a time frame that includes the period during which C. virginica larvae are normally present in the water column, and in Tracadie Bay from 10 June to 1 August 2019.
2.3 Statistical analyses
We investigated the presence of inherent cycles within the recorded pH series with the Fourier transform (Bracewell and Bracewell 1986). The Fourier transform allows the conversion of a signal from a time domain to a frequency domain representation and vice-versa. To apply the discrete Fourier transform, the temporal sequence must be sampled at a regular time stride and satisfy the stationarity and the ergodic assumptions (Winograd 1978). As the pH series had some irregularities due to measurement breaks during the regular maintenance of the data loggers and some technical issues, we filled in the missing values by linear interpolation (Lepot et al. 2017). We then calculated the first-order differencing of the time series given by
to detrend the data (Canova 1998). The resulting series had a zero mean and a quasi-constant standard deviation and we could therefore assume stationarity and apply the Fourier transform. The pH magnitude spectrum was processed by the Fourier transform equation:
where \(N\) refers to the total \(y[n]\) observations and \(\theta \left[k\right]=\frac{2\pi k}{N}\) stands for the frequency sampling points. Hence, the underlying periodic components are highlighted as predominant peaks at a specific frequency ( \({F}_{cycle})\) and the yielded cycles have a period of \({T}_{cycle}= \frac{1}{{F}_{cycle}}\) .
Since the St. Simon Bay data span over 8 months during which the photoperiod varied between 8: 16 and 16: 8 (L to D) and the water temperature between – 0.57 and 16.8 °C, we ran additional analyses on subsets of data characteristic of winter (1 – 31 January 2018) and early summer (22 May – 2 June 2018).
To filter out the tidal effects from the pH series, we borrowed the spectra filtering technique from the speech enhancement field of research (Vaseghi 1996). This approach is based on the removal of an additive noise, in the present case the water level fluctuations, from a given signal. All the short- and medium-term tidal constituents are thus filtered out, namely the overtide cycles (< 9 h) that may be produced in shallow water environments (Rickerich et al. 2022), the semidiurnal (12.42 h) and diurnal (24.83 h) cycles, as well as the fortnight cycle (14.77 d) which produces spring and neap tides. The noise signal is written as:
where \({x}_{r}(t)\) is the residual component of the original signal \(x\left(t\right)\) and \(n(t)\) is the additive noise, with the assumption that both residual and noise signals are uncorrelated and stationary. As the estimation of the filtered signal is processed in the frequency domain, the Fourier transform is applied to (3):
where \(X(f)\), \({X}_{r}(f)\) and \(N(f)\) are the spectra of \(x\left(t\right)\), \({x}_{r}(t)\) and \(n(t)\), respectively. The basic spectral subtraction operation is given by:
where \({\theta }_{x}(\omega )\) is the phase of the original noise signal and \(\omega =2\pi f\). The estimated residual signal \({\widetilde{x}}_{r}(t)\) is the inverse Fourier transform of (5). Thus, we rewrite the pH spectrum as:
where \(Y\left(\theta \right)\) is the spectrum of the original pH series, \(T\left(\theta \right)\) the tidal constituents spectrum estimated from the water level time series, and \({Y}_{{pH}_{R}}\left(\theta \right)\) the assumed residual pH spectrum. The latter is the simple subtraction of the water level spectrum from the original pH spectrum, as used in Simms et al. (2022). Hence, the inverse Fourier transform of \({Y}_{{pH}_{R}} (\theta )\) yields the detrended \({Y}_{{pH}_{R}}\) variations representation. The Fourier transform of \({Y}_{{pH}_{R}}\) allows the detection of the remaining cycles that might be concealed by the strong tidal effects. The residual pH spectrum, without the tidal effects, is abbreviated as pHR in the following text. All the spectral analyses were performed using Python 3.8.5 with SciPy libraries.
Finally, two-tailed Pearson correlation tests were run to describe the relationship between pH and water level.
3 Results
The three sites showed highly variable pHSWS not only on a seasonal scale, as for St. Simon Bay, but also over the summer months, as for the Tracadie and Caraquet sites (Table 1). Particularly high pH values, over 8.9, were reached in Tracadie Bay. In St. Simon Bay (Fig. 2a), the formation of the snow and ice cover (hereafter “pack ice-snow”) began around mid-December 2017. The pack ice-snow gradually melted during the second half of April 2018, as depicted by the slump of salinity. The melting period and the subsequent warming of water in May coincided with a series of strong fluctuations in pH. In summer, the general aspect of the pH temporal fluctuations was similar for the bays of Caraquet and Tracadie, except for the sustained high pH episode that occurred during the second half of July in Tracadie (Fig. 2b, c). Total alkalinity was higher and less variable in Tracadie than in Caraquet, and chlorophyll a concentration was moderate at both sites, as shown in Table 2. In St. Simon Bay, chlorophyll a was detectable even in winter although at low levels.
The spectral analysis presented in Table 3 reveals a clear semidiurnal tidal pattern of pH fluctuations, with a period close to 12.42 h at the three sites. Pearson’s correlation test showed that in summer the water level and pH were inversely correlated in the three bays (Caraquet, r = - 0.183, P < 0.01, N = 1228; Tracadie, r = - 0.091, P < 0.01, N = 1144; summer subset in St. Simon, r = - 0.260, P < 0.01, N = 263). In contrast, these two parameters were directly correlated in the winter subset data from St. Simon Bay (r = 0.495, P < 0.01, N = 717). Periods close to 24 h were also detected, which might be related to the diurnal tide cycle (24.83 h) and/ or to the diel cycle (24 h). This circadian cycle, with a peak at \({F}_{cycle}\cong 0.01 mHz\), was the only one that remained once the spectral subtraction of all the components of the water level spectrum from the original pH series has been done. The pHR cycle was neither consistently synchronized with the day-night cycle but rather slowly drifted. Plotting the data obtained for St. Simon Bay against the lunar phases reveals that the ≈ 24 h cycle of pHR shifted following the lunar cycle (Fig. 3). During the full moon, the lowest pHR values occurred around midnight, when the moon was at its highest position in the sky, and the highest pHR values were around noon. Afterward, the ≈ 24 h cycle drifted irregularly until the troughs of pHR were noted around noon during the new moon phase and again around midnight during the following full moon. In St. Simon Bay, this pattern was consistent for each lunar cycle from December 2017 to April 2018. The data collected during the summer in the Caraquet Bay did not show such a consistent effect of the lunar cycle on pHR, as one trough took place around 5 h 00 during the June full moon and the other around midnight during the July full moon (results not shown). As for the bay of Tracadie, we were not able to subtract efficiently the water level effects from the original pH spectrum and the resulting circadian pattern of pHR is not clear enough to allow for a reliable analysis. Finally, short cycles were detected in the original pH series from St. Simon and Caraquet bays, with periods of approximately 8 h and 6 h.
4 Discussion
In the following discussion, for comparison purposes, we converted pH values to the seawater scale whenever they were expressed on the National Bureau of Standard (NBS) scale in the cited publications. On the other hand, since the difference between pH expressed on the total scale (pHT) and the seawater scale is only approximately 0.01 pH unit, we did not convert the published data given as pHT.
4.1 Spatio-temporal fluctuations of pH
The ranges of pH that we measured at the three sites stand among the widest values reported by Carstensen and Duarte (2019) in their analysis of 83 coastal ecosystems across the world. Most of the pHT ranges reported by these authors were inferior to 0.8 for interannual sets of data, and 0.6 for seasonal ones. The range of 1.32 we noted in the bay of Tracadie compares to the second-largest noted by Carstensen and Duarte (2019), in the Baltic Sea.
The fact that our study sites were located in an area that has a temperate climate partly explains the wide range of pH we observed. Firstly, marked seasonal changes in water temperature such as those recorded in St. Simon Bay affect the solubility of CO2. According to Li and Tsui (1971), the solubility constant of CO2 in water with a chlorinity of 29 increases by a factor of 1.6 from 20 to 4 °C. As more gas is solubilized at low temperatures, more H2CO3 is produced and dissociates in HCO3− and H+, causing a decrease in pH (Orr et al. 2005). Consequently, in winter, water temperature circa 0 °C allowed the solubilization of large amounts of CO2 leading to pH values lower than those measured in early summer when temperature rapidly rose. Likewise, Donham et al. (2022) found that periods of cold waters were characterized by low pH values compared with periods of warm waters. Secondly, CaCO3 precipitation and the augmentation of salinity in the pockets of brine that are embodied in the ice cause pCO2 in brine to become supersaturated with respect to the atmosphere. CO2 can then diffuse to the underlying water through brine channels (Rysgaard et al. 2007; Geilfus et al. 2012) and cause a decline in pH. Biotic factors might also have contributed to reducing the pH during winter. There is evidence of intense microbial respiration in the water column and the sediments of ice-covered lakes which leads to the accumulation of CO2 under the ice (reviewed by Denfeld et al. (2018)) and consequently to acidification. In addition, the rate of photosynthesis and thus the consumption of CO2 was likely slowed down by the attenuation of light through the thick layer of ice and snow (up to 100 cm) that covered St. Simon Bay from mid-December 2017 to mid-April 2018, as well as by the cold conditions since the photosynthesis rate is directly related to temperature with a Q10 varying from 2 to 5 (reviewed by Davison (1991)). Indeed, Baumann and Smith (2018) concluded from their review of 16 coastal shallow-water habitats that the balance between photosynthesis and respiration rate is a major governor of pH conditions.
The transition period we observed in April in St. Simon Bay showed marked fluctuations of pH that may be explained by the decoupling of ice-snow melting and water warming and its effect on physicobiological processes. The release of fresh water that has been trapped in the pack ice-snow (either as the result of salt extrusion during the freezing process or of snow accumulation) may lower the pH of surface seawater in two ways. Firstly, it augments the solubility of CO2 by reducing the salinity, although the solubility constant of CO2 does not respond as strongly to salinity as it does to temperature (Li and Tsui 1971). Secondly, it directly adds acidic water to the bay as the pH of precipitations (rain and snow) in the studied area is generally lower than 7 and even occasionally falls below the threshold of 5.5 which determines acid precipitations (Mayrand, unpublished data, and Miramichi River Environmental Assessment Committee (2018)). And yet a steep augmentation of pH occurred during the melting period in April, similar to the increase of 0.20 to 0.55 pH units during the melting of annual pack ice reported by Castrisios et al. (2018) in their study of the carbonate chemistry in the Antarctic. The authors attributed this phenomenon to the photosynthetic activity of the liberated bottom-ice algae. Indeed, Geilfus et al. (2012) measured chlorophyll a concentrations in bottom sea ice up to 1330 μg L-1 during pack ice melting. In our study, the release of acidic freshwater by the process of melting might thus have been overcompensated by the activation of algae newly exposed to increasing levels of irradiance as the ice-snow cover gradually disappeared. It is to be noted that photosynthetic capacity and efficiency are not necessarily related to chlorophyll a concentration (Harding et al. 1981; Legendre et al. 1988; Aristegui and Harrison 2002) and thus moderate concentrations such as those typical of spring in St. Simon Bay do not preclude an enhanced photosynthetic activity. As water temperature in May rose from circa 0 to 12 - 16 °C, a decoupling between photosynthetic and respiration rates might have led to the observed gradual decline in pH. Even though water warming reduces the solubility of CO2, it also favors CO2 production by respiration over CO2 consumption by photosynthesis (Baumann and Smith 2018) and thus causes a decrease in pH. Indeed, the slope of the temperature dependant increase in respiration is steeper than that in photosynthesis in the upper ocean (reviewed by Boscolo-Galazzo et al. (2018)). The resulting divide between winter and spring mean levels of pH is visible at St. Simon Bay (Fig. 2a). The studies of Hagens and Middelburg (2016) and Baumann and Smith (2018) corroborate our interpretation as these authors concluded that pH seasonality in midlatitude systems is driven mainly by temperature and by dissolved inorganic carbon fluctuations caused by the production (through photosynthesis) and consumption (through respiration) of organic matter.
Other factors than seasonality must have been at play as drivers of pH temporal variations since an even wider range of pH was noted during the summer months in the bays of Caraquet and Tracadie, as compared with those noted in St. Simon Bay over three seasons. The range of pH during the summer months was also much broader than those reported by (Menu-Courey et al. 2019) for surface water offshore Eastern New Brunswick from May to November (range ≈ 0.2 unit), which points to a significant influence of the terrestrial environment at our study sites. Indeed, freshwater inputs by rivers are known to cause pH decreases in coastal waters (Salisbury et al. 2008; Carstensen and Duarte 2019), and both Tracadie Bay and Caraquet Bay receive significant river water, as described in the “Study area and methods” section. The proximity of natural and exploited peat bogs (pH circa 4, Mayrand pers. obs. and Surette et al. (2002)) might also have induced acidification episodes through rainwater runoffs. Clausen and Brooks (1983) measured the pH in runoffs from 45 natural peatland watersheds in Minnesota and found two modes, one around 4.8 and the other around 6.0, with a mean of 5.6 (standard deviation not given). As very few studies on the impact of peat bog effluents on coastal water quality are available, this interpretation remains hypothetical but would certainly deserve investigation. Finally, in the case of Tracadie Bay, hydrodynamic traits might explain how high pH values were reached in Tracadie during the summer. We find similarities between this bay and one of the sites monitored by Chou et al. (2018), as pHSWS values as high as 8.90 in Tracadie and 8.77 in the lagoon studied by Chou et al. (2018) were noted. Both sites are semi-enclosed lagoons with low energetic zones surrounded by dense seagrass beds. These characteristics may favor CO2 uptake by photosynthesis and TA generation by sedimentary anaerobic processes and thus lead to an increase in pH. According to the hydrodynamic model named TBR that Deb et al. (2022) built for the bay of Tracadie, our site was located in a stagnation point, which contrasts with the Caraquet and St. Simon bays where highly bi-directional currents predominate (Comeau et al. 2010; Niles et al. 2014). The expected increased settlement of suspended material in such conditions was confirmed by the high (16%) organic content in sediments measured by Deb et al. (2022) and by the presence of a thick (~ 2 m) layer of loose sediments (Rémy Haché, Dep. Aquaculture, Agriculture and Fisheries, New Brunswick, pers. comm). Our hypothesis is reinforced by the fact that features of a reduced environment are periodically present in the vicinity of our station, such as oxygen levels near 0%, sulfur concentration up to 80 ug L−1, and ammonia concentration up to 0.12 mg L−1 (Rémy Haché, pers. comm.). Moreover, the highest TA levels we measured were found in Tracadie Bay (Table 2) and compared with the values of 2726 – 2871 umol kg−1 reported by Chou et al. (2018). Similar chemical conditions in the bottom water of the Chesapeake Bay also led to a local increase in pH at this depth (Cai et al. 2017). Wang et al. (2020) concluded that in summer there may be a build-up of reduced chemical species in the sediments that leads to a rise of TA and pH, and that these reduced chemical species may not be re-oxidized until fall. In Tracadie Bay, sedimentary alkalinization might thus have added up to the effect of CO2 consumption by the nearby eelgrass (Zostera marina) bed to create conditions conducive to high pH values.
The Fourier transform analysis exposed short-period cycles that modulated the pH, in addition to the large temporal and spatial factors discussed above. The semidiurnal tidal rhythm revealed by the periodicity of approximately 12.42 h was present at the three sites. In Caraquet, Tracadie, and St. Simon in early summer, the water pH was inversely related to the water level. Although these relationships were significant, they were rather weak because, as pointed out by Pettay et al. (2020) in their study of the ebb and flow of protons in an estuary system, numerous antagonistic processes modulate the water pH. For example, primary production, carbonate buffering, mineral dissolution, and CO2 degassing lead to an increase in pH while freshwater inputs, aerobic respiration, and nitrification have the opposite effect; the authors concluded that the fluctuations of pH at a given location in the watershed resulted from the combination of these biogeochemical processes and tidal forcing. Our results thus suggest that the ratio of proton sinks to proton sources was higher in the three enclosed bays under study than in the adjacent Chaleurs Bay and Gulf of St. Lawrence, probably owing to the very dense eelgrass beds present at the three sites. Our hypothesis is supported by the fact that the relationship between water level and water pH was reversed during winter in St. Simon Bay when the impact of seagrass beds on CO2 consumption was presumably greatly diminished. Borum et al. (2002) showed that the carbon balance of the macrophyte Laminaria saccharina is slightly negative in winter due to the attenuation of light by the pack ice and the pull out of the seaweeds by ice scouring. In our study, the thick pack ice-snow thus likely reduced the photosynthesis rate in St. Simon Bay, while the incomplete ice cover on the connecting Chaleurs Bay probably allowed significant rates of CO2 consumption by phytoplankton primary production. Indeed, during the winter of 2017- 2018, a maximum of 40% of ice coverage was observed on Chaleurs Bay (Service canadien des glaces 2018). The incoming tide from Chaleurs Bay would thus bring water with a slightly higher pH to St. Simon Bay.
Given the synchronization of the circadian rhythm cycle of pHR with the moon phases that we noted in St. Simon Bay, we can add water pH to the list of variables that are influenced by the lunar cycle in coastal waters, although most likely in an indirect way in the case of pH. Many of the variables reported in the literature as being affected by the lunar cycle are the biomass of various species assemblages and thus have the potential to modulate the water pH through fluctuations of the respiratory CO2 production. For example, during the full moon, the biomass of epipelagic copepods (Hernández-León et al. 2002), as well as that of ichthyoplankton (Díaz-Astudillo et al. 2017) are at their peak, while that of small predatory fish species is strongly reduced (Vergara et al. 2017). Boehm and Weisberg (2005) showed that there are up to 140% more enterococci in coastal water during the full moon spring tides than during the neap tides. Interestingly, Last et al. (2016), who conducted a study in the Arctic, noted a massive vertical migration of zooplankton toward the deep waters during the full moon even when the ice-snow pack was present. The authors concluded that this phenomenon was due to the moonlight intensity rather than the water level since they did not note such a migration during the new moon spring tide. Significant light transmission through combined layers of ice and snow has indeed been measured by Lei et al. (2012). This is congruent with our observations of consistent synchronicity between the pHR circadian fluctuations and the lunar phases during the pack ice-snow period, with troughs occurring around midnight during the full moon but not during the new moon. However, how the moon-driven cycles of various biological variables such as those cited above may affect the pH in coastal waters remains to be explained, as our data do not allow us to make findings in this regard. To the best of our knowledge, this is the first time that synchronicity between the lunar cycle and coastal water pH is reported. According to Díaz-Astudillo et al. (2017), the lunar cycle acts to a lower degree than water temperature (in this case, on ichthyoplankton concentration). Our results support this observation, as the tight synchronicity between the lunar cycle and the timing of the daily pHR troughs noted during the ice-snow pack period was absent during the summer months in Caraquet Bay when the water temperature is higher and much more variable than in winter.
The drifting in time of the diel pH cycle that we describe here might partly explain the discrepancies among the studies as to the period of the day when the peaks and troughs of water pH occur. Indeed, most studies that followed the diel variations in pH did so only over a few days. Short-term monitoring is particularly susceptible to yielding inconsistent results concerning the timing of daily highs and lows of pH, depending on the season and the lunar phase during which the data were collected. A good example of this is provided by the study of Kerrison et al. (2011) who monitored the diel periodicity of water pH at 5 stations during periods of 3 to 10 days at different dates and noted maximal values in mid-morning, mid-afternoon, late-afternoon, or over most of the night, depending on the station. The authors pose the hypothesis that day-night changes of wind-driven water mixing explain these changes in pH periodicity. We might add the possibility that the timing of the diel fluctuation slowly drifted over time which yielded different results as to the period of maximal pH depending on the date at which the data were collected. Other examples of contrasting results are found in Chou et al. (2018) who noted pH peaks at sunset at a given station and during midday at a nearby site a few days later. For their part, Wahl et al. (2018) and Wolfe et al. (2020) observed pH peaks during the day. A significant effect of the day-night cycle on seawater pH has been detected and related to the photosynthesis rate of macrophytes in controlled experiments (Wahl et al. 2018). However, this effect seems to be hidden in the much more complex natural environment which is subjected to the influence of tides, temperature changes, animal respiration, etc., as are the sites monitored in the present study.
As we did in the present work, Mel'nikova (2017) detected cycles with short periods (4.7 and 2.8 h) in her study on the intensity of the bioluminescence field in the Black Sea. She hypothesized that they resulted from endogenous cycles of cellular respiration of the planktonic communities. However, the short pH periodicities of approximately 8 h and 6 h that we noted in Caraquet and St. Simon Bays do not seem to originate from a biological rhythm that would modulate the production of CO2 through respiration, as they are filtered out by the spectral subtraction of the water level spectrum. They might rather represent overtide cycles caused by advection and bottom friction in shallow water environments (Rickerich et al. 2022).
4.2 The potential impacts of acidification on the oyster farming industry
Since the Eastern oyster C. virginica is the predominant marine species farmed in North-East New Brunswick, it is of interest to analyze our data in the context of the physiological tolerance of this species to acidification. Conditions of pH outside the tolerance range of a species impact the physiology of the organisms by disrupting the acid–base homeostasis in the internal fluids; the hydrated hydrogen ion is particularly damaging to proteins and it severely interferes with the activity of enzymes (Hammer 2012). A low environmental pH also decreases the aragonite saturation state in seawater, which in turn leads to a reduced shell growth rate and shell deformities (Waldbusser et al. 2015b). In most marine invertebrate species, the impact of environmental stress varies across life stages (Byrne 2011; Pineda et al. 2012; Pandori and Sorte 2019). We must thus take into account the different stages of development to analyze the suitability of the pH ranges measured in the studied bays. However, it is difficult to pinpoint a precise pH threshold beyond which the animals’ survival and growth begin to decrease in nature, as the duration of the experiments reported in the literature varied from 2 to 11 weeks among the studies and as the animals were exposed to constant acidification levels, while in nature the animals rather face fluctuating pH.
C. virginica spawns when the water temperature reaches 20 °C and larvae are usually observed in the studied area in July and August (Aucoin et al. 2003). In studies using pH values circa 8 as the control conditions, reduced survival, shell growth rate, and larval settlement success started to be noted around pH 7.75 or lower (Miller et al. 2009, 2020; Talmage and Gobler 2011; Gobler and Talmage 2014), although Meyer-Kaiser et al. (2019) did not note reduced settlement rates at pHSWS 7.27. According to our data, the pH conditions noted in July and August (Caraquet and Tracadie bays) are generally suitable for C. virginica larvae since the pH values rarely fall below this 7.75 threshold and generally only for a few hours at a time, whereas the animals studied in the publications cited above were exposed to constant acidified conditions for approximately 3 weeks. Thus, the natural recruitment of Eastern oysters should not be jeopardized under the conditions currently prevailing in the studied bays and a bottleneck effect is unlikely. However, the production of larvae in oyster hatcheries generally takes place in January and February, a period during which the water pH rarely exceeded 7.75 in St. Simon Bay. This does not seem to be problematic for now because the high concentrations of algae which are added as food raise the pH to approximately 8 in the upwellers containing the larvae, and so far the larvae production has never failed at the St. Simon Bay hatchery (Martin Mallet, co-owner and manager of the oyster company L’Étang Ruisseau Bar Ltée, pers. comm.).
As for the juveniles, they begin to suffer an increased mortality rate and a reduced growth rate around pH 7.0 and 7.4 respectively (Young and Gobler 2018; Dodd et al. 2021; Pruett et al. 2021). No mortality has been noted in adults exposed to pH as low as 6.67 (Clements et al. 2018), while Matoo et al. (2013) did not register mortality in adult oysters at pH 7.47 which was the lowest value tested in their experiments. Considering that the lowest pH level we observed in nature was around 7.3 and was reached only during the winter at St. Simon Bay, a season during which the oysters do not grow in any case (Mayrand et al. 2017), it is highly unlikely that juvenile and adult Eastern oysters would be negatively affected by the conditions prevailing in the studied areas. Periods of low salinities such as those we noted in the spring at St. Simon Bay and during the summer at Tracadie and Caraquet might occasionally exacerbate the impact of acidification on juvenile oysters’ mortality. Indeed, Dickinson et al. (2012) have shown that constant low salinity (15 as compared to 30) combined with a reduction of pH from 8.23 to 7.97 increased juvenile mortality by fourfold. However, it is not clear how more realistic conditions exposing the experimental animals to diel fluctuations of pH such as those observed in the natural environment may affect the state of physiological stress. The only two studies that we know of in which juvenile oysters (C. virginica, C. gigas, and Ostrea lurida) were submitted to diel cycling pH (7.70 ± 0.5 and 7.35 ± 0.5 during 5 and 6 weeks) revealed that fluctuating pH conditions may reduce or increase shell growth compared to the static treatment, but do not affect the respiration rate and survival (Keppel et al. 2016; Bednaršek et al. 2022).
With the attention of the scientific community currently focused on ocean acidification, the question as to the point at which pH exceeds the upper critical limit of marine organisms has seldom been addressed. This question is nonetheless relevant since we noted pHSWS values reaching 8.90 in the bay of Tracadie in late July 2019. As far as we know, the study of Huo et al. (2019) is the only one to cover the effect of pHSWS as high as 8.67 and 9.02 on bivalves (in this case, the geoduck clam Panopea japonica, Adams, 1850). Their results indicate that the cut-off value for most health indicators is around pH 9.02, where hatching, larval metamorphosis, and juvenile survival rates fall as low as the values noted at pH 7.07. The authors did not propose a physiological mechanism that might explain the deleterious effect of high pH. In addition to a probable direct effect on the acid–base homeostasis of the animals, we suggest that an indirect repercussion may take place through the increment of the toxic form of ammonia NH3 relative to the more benign form NH4+ with increasing pH. Indeed, the ratio of toxic un-ionized NH3 relative to total ammonia increases with pH. For example, the % of un-ionized NH3 jumps from 6.7% at pHSWS 8.00 to 36.4% at pHSWS 8.90, as calculated with the saltwater ammonia calculator provided by the Department of Environmental Quality of the state of Oregon, at 25 °C and salinity 20 (https://www.oregon.gov/deq/wq/Pages/WQ-Standards-Ammonia.aspx, page consulted on 2022–12-12). As stated previously, ammonia concentrations in water up to 0.12 mg L−1 occasionally occur near the location of our station. This is rather high, considering that the Canadian recommendation (Canadian Council of Ministers of the Environment 2010) for aquatic life in freshwater is 0.019 mg L−1 (no recommendation for marine life is available). Conditions of concurrent high pH and ammonia concentrations could thus create physiologically stressing conditions for the oysters, as well as for the fauna in general. Such conditions might have played a role in the mass mortality of oysters that occurred in Tracadie Bay in 2016, in addition to the asphyxiation and anaerobic bacterial processes demonstrated by Coffin et al. (2021).
In conclusion, this study showed that multiple factors determine the variations of pH in the coastal waters of Northeastern New Brunswick. This leads to a large array of values that vary with the location, the seasons, the overtide and semidiurnal tidal cycles, and the lunar phases. Our study is one of the few that have measured pH in sub-ice waters. By exploiting the discrete Fourier transform, we have shown the existence of pH cycles with various periodicities despite the near-constant water temperature and the attenuation of light by a thick layer of ice and snow. To the best of our knowledge, our study is the first to use the spectral subtraction technique to filter out the tidal components, which has allowed us to bring to light a circadian rhythm of pH that is more strongly related to the phases of the moon than to the day-night cycle, during winter. The temporal drifting of the diel pH cycle over the lunar cycle might explain the inconsistent results reported in the literature regarding the time of the day during which peaks and troughs of pH occur. Based on the available scientific data and local knowledge of C. virginica resistance to acidification, the present ranges of pH values observed at the study sites are generally suitable for the different life stages of this species. However, the levels predicted for 2100 might get near the critical limits of the larval stage in winter, when oyster spat is produced in commercial hatcheries. Although C. virginica can adapt to acidification (Clements et al. 2021), which might help the species and the oyster farming industry to face adversity better than their west coast counterpart, other species may not be able to do so, and further acidification might overcome the resilience of the coastal ecosystem.
Availability of data and materials
The first author, Prof. Elise Mayrand (elise.mayrand@umoncton.ca), can be contacted for access to the data.
References
Aristegui J, Harrison W (2002) Decoupling of primary production and community respiration in the ocean: Implications for regional carbon studies. Aquat Microb Ecol 29:199–209. https://doi.org/10.3354/ame029199
Aucoin F, Doiron S, Nadeau M (2003) Guide d'échantillonnage et d'identification des larves d'espèces à intérêt maricole. Ministère Agriculture, Pêcheries et Alimentation du Gouvernement du Québec
Barton A, Hales B, Waldbusser GG, LangdonFeely CJRA (2012) The Pacific oyster, Crassostrea gigas, shows negative correlation to naturally elevated carbon dioxide levels: Implications for near-term ocean acidification effects. Limnol Oceanogr 57(3):698–710. https://doi.org/10.4319/lo.2012.57.3.0698
Bastien-Daigle S, Hardy M, Robichaud G (2007) Habitat management qualitative risk assessment: water column oyster aquaculture in New Brunswick. Can Tech Rep Fish Aquat Sci no. 2728
Baumann H, Smith EM (2018) Quantifying metabolically driven pH and oxygen fluctuations in US nearshore habitats at diel to interannual time scales. Estuaries Coasts 41(4):1102–1117. https://doi.org/10.1007/s12237-017-0321-3
Bednaršek N, Beck MW, Pelletier G, Applebaum SL, Feely RA, Butler R, Byrne M, Peabody B, Davis J, Štrus J (2022) Natural analogues in pH variability and predictability across the coastal Pacific estuaries: extrapolation of the increased oyster dissolution under increased pH amplitude and low predictability related to ocean acidification. Env Sci Tech 56(12):9015–9028. https://doi.org/10.1021/acs.est.2c00010
Blair JWG, Thomas SJ, Loder JW, Pépin P, Azetsu-Scott K, Ianson D, Hamme RC, Gilbert D, Tremblay J-É, Wang XL, Perrie W (2019) Changes in oceans surrounding Canada. Government of Canada, Ottawa
Boehm AB, Weisberg SB (2005) Tidal forcing of enterococci at marine recreational beaches at fortnightly and semidiurnal frequencies. Envir Sci Tech 39(15):5575–5583. https://doi.org/10.1021/es048175m
Borum J, Pedersen M, Krause-Jensen D, Christensen P, Nielsen K (2002) Biomass, photosynthesis and growth of Laminaria saccharina in a high-arctic fjord. NE Greenland Mar Biol 141(1):11–19. https://doi.org/10.1007/s00227-002-0806-9
Boscolo-Galazzo F, Crichton KA, Barker S, Pearson PN (2018) Temperature dependency of metabolic rates in the upper ocean: A positive feedback to global climate change? Global Planet Change 170:201–212. https://doi.org/10.1016/j.gloplacha.2018.08.017
Bracewell R, Bracewell R (1986) The Fourier transform and its applications. McGraw-Hill, New York
Byrne M (2011) Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Oceanogr Mar Biol Ann Rev 49:1–42
Cai W-J, Huang W-J, Luther GW, Pierrot D, Li M, Testa J, Xue M, Joesoef A, Mann R, Brodeur J, Xu Y-Y, Chen B, Hussain N, Waldbusser GG, Cornwell J, Kemp WM (2017) Redox reactions and weak buffering capacity lead to acidification in the Chesapeake Bay. Nat Commun 8(1):369. https://doi.org/10.1038/s41467-017-00417-7
Canadian Council of Ministers of the Environment (2010) Canadian water quality guidelines for the protection of aquatic life: Ammonia. In: Canadian Council of Ministers of the Environment (ed) Canadian environmental quality guidelines. Winnipeg
Canova F (1998) Detrending and business cycle facts. J Monet Econ 41(3):475–512
Carstensen J, Duarte CM (2019) Drivers of pH Variability in Coastal Ecosystems. Environ Sci Technol 53(8):4020–4029. https://doi.org/10.1021/acs.est.8b03655
Castrisios K, Martin A, Müller MN, Kennedy F, McMinn A, Ryan KG (2018) Response of Antarctic sea-ice algae to an experimental decrease in pH: a preliminary analysis from chlorophyll fluorescence imaging of melting ice. Polar Res 37(1):1438696. https://doi.org/10.1080/17518369.2018.1438696
Chou W-C, Chu H-C, Chen Y-H, Syu R-W, Hung C-C, Soong K (2018) Short-term variability of carbon chemistry in two contrasting seagrass meadows at Dongsha Island: Implications for pH buffering and CO2 sequestration. Estuar Coast Shelf S 210:36. https://doi.org/10.1016/j.ecss.2018.06.006
Clausen JC, Brooks KN (1983) Quality of runoff from Minnesota peatlands: I. A characterization. J Am Water Resour As 19(5): 763–767. https://doi.org/10.1111/j.1752-1688.1983.tb02799.x
Clements JC, Carver CE, Mallet MA, Comeau LA, Mallet AL (2021) CO2-induced low pH in an eastern oyster (Crassostrea virginica) hatchery positively affects reproductive development and larval survival but negatively affects larval shape and size, with no intergenerational linkages. ICES J Mar Sci 78(1):349–359. https://doi.org/10.1093/icesjms/fsaa089
Clements JC, Comeau LA, Carver CE, Mayrand É, Plante S, Mallet AL (2018) Short-term exposure to elevated pCO2 does not affect the valve gaping response of adult eastern oysters, Crassostrea virginica, to acute heat shock under an ad libitum feeding regime. J Exp Mar Biol Ecol 506:9–17. https://doi.org/10.1016/j.jembe.2018.05.005
Coffin MRS, Clements JC, Comeau LA, Guyondet T, Maillet M, Steeves L, Winterburn K, Babarro JMF, Mallet MA, Haché R, Poirier LA, Deb S, Filgueira R (2021) The killer within: Endogenous bacteria accelerate oyster mortality during sustained anoxia. Limnol Oceanogr 66(7):2885–2900. https://doi.org/10.1002/lno.11798
Comeau LA, Sonier R, Lanteigne L, Landry T (2010) A novel approach to measuring chlorophyll uptake by cultivated oysters. Aquacult Eng 43(2):71–77. https://doi.org/10.1016/j.aquaeng.2010.06.002
Davison IR (1991) Environmental effects on algal photosynthesis: temperature. J Phycol 27(1):2–8. https://doi.org/10.1111/j.0022-3646.1991.00002.x
Deb S, Guyondet T, Coffin MRS, Barrell J, Comeau LA, Clements JC (2022) Effect of inlet morphodynamics on estuarine circulation and implications for sustainable oyster aquaculture. Estuar Coast Shelf S 269: 107816. https://doi.org/10.1016/j.ecss.2022.107816
Denfeld BA, Baulch HM, del Giorgio PA, Hampton SE, Karlsson J (2018) A synthesis of carbon dioxide and methane dynamics during the ice-covered period of northern lakes. Limno Oceanogr Lett 3(3):117–131. https://doi.org/10.1002/lol2.10079
Department of Environment and Natural Resources Canada (2022) Historical hydrometric data. https://wateroffice.ec.gc.ca/search/historical_results_e.html?search_type=station_name&station_name=caraquet. Accessed 2 February 2023
Department of Fisheries and Oceans (2022) 2018 Value of Atlantic and Pacific coast commercial landings, by province. https://www.dfo-mpo.gc.ca/stats/commercial/land-debarq/sea-maritimes/s2018pv-eng.htm. Accessed 2 Feb 2023
Díaz-Astudillo M, Castillo MI, Cáceres M, Plaza G, Landaeta MF (2017) Oceanographic and lunar forcing affects nearshore larval fish assemblages from temperate rocky reefs. Mar Bio Res 13:1015–1026. https://doi.org/10.1080/17451000.2017.1335872
Dickinson GH, Ivanina AV, Matoo OB, Poertner HO, Lannig G, Bock C, Beniash E, Sokolova IM (2012) Interactive effects of salinity and elevated CO2 levels on juvenile eastern oysters, Crassostrea virginica. J Exp Biol. 215(1): 29–43. https://doi.org/10.1242/jeb.061481
Dickson AG, Sabine CL, Christian JR (2007) Guide to best practices for ocean CO2 measurements. PICES Special Publication 3
Dodd LF, Grabowski JH, Piehler MF, Westfield I, Ries JB (2021) Juvenile Eastern oysters more resilient to extreme ocean acidification than their Mud crab predators. Geochem Geophys 22(2): e2020GC009180. https://doi.org/10.1029/2020GC009180
Donham EM, Strope LT, Hamilton SL, Kroeker KJ (2022) Coupled changes in pH, temperature, and dissolved oxygen impact the physiology and ecology of herbivorous kelp forest grazers. Global Change Biol 28(9):3023–3039. https://doi.org/10.1111/gcb.16125
Geilfus N-X, Carnat G, Papakyriakou T, Tison J-L, Else B, Thomas H, Shadwick E, Delille B (2012) Dynamics of pCO2and related air-ice CO2 fluxes in the Arctic coastal zone (Amundsen Gulf, Beaufort Sea). J Geophys Res-Oceans 117(C9). https://doi.org/10.1029/2011JC007118
Gobler CJ, Talmage SC (2014) Physiological response and resilience of early life-stage Eastern oysters (Crassostrea virginica) to past, present and future ocean acidification. Conservation Physiology 2(1). https://doi.org/10.1093/conphys/cou004
Grossman E (2011) Northwest oyster die-offs show ocean acidification has arrived. https://e360.yale.edu/features/northwest_oyster_die-offs_show_ocean_acidification_has_arrived. Accessed 2 February 2023
Hagens M, Middelburg JJ (2016) Attributing seasonal pH variability in surface ocean waters to governing factors. Geophys Res Lett 43(24): 12,528–512,537. https://doi.org/10.1002/2016GL071719
Hammer KM (2012) Acid-base regulation and metabolite responses in shallow- and deep-living marine invertebrates during environmental hypercapnia. Dissertation, Norwegian University of Science and Technology
Harding LW, Meeson BW, Prézelin BB, Sweeney BM (1981) Diel periodicity of photosynthesis in marine phytoplankton. Mar Biol 61(2):95–105. https://doi.org/10.1007/BF00386649
Hernández-León S, Almeida C, Yebra L, Arístegui J (2002) Lunar cycle of zooplankton biomass in subtropical waters: biogeochemical implications. J Plankton Res 24(9):935–939. https://doi.org/10.1093/plankt/24.9.935
Huo Z, Rbbani M, Cui H, Xu L, Yan X, Fang L, Wang Y, Yang F (2019) Larval development, juvenile survival, and burrowing rate of geoduck clams (Panopea japonica) under different pH conditions. Aquacult Int 27(5):1331–1342. https://doi.org/10.1007/s10499-019-00389-z
Keppel AG, Breitburg DL, Burrell RB (2016) Effects of co-varying diel-cycling hypoxia and pH on growth in the juvenile Eastern oyster. Crassostrea Virginica Plos One 11(8):e0161088–e0161088. https://doi.org/10.1371/journal.pone.0161088
Kerrison P, Hall-Spencer JM, Suggett DJ, Hepburn LJ, Steinke M (2011) Assessment of pH variability at a coastal CO2 vent for ocean acidification studies. Estuar Coast Shelf S 94(2):129–137. https://doi.org/10.1016/j.ecss.2011.05.025
Last Kim S, Hobbs L, Berge J, Brierley Andrew S, Cottier F (2016) Moonlight drives ocean-scale mass vertical migration of zooplankton during the arctic winter. Curr Biol 26(2):244–251. https://doi.org/10.1016/j.cub.2015.11.038
Legendre L, Demers S, Garside C, Haugen EM, Phinney DA, Shapiro LP, Therriault J-C, Yentsch CM (1988) Circadian photosynthetic activity of natural marine phytoplankton isolated in a tank. J Plankton Res 10(1):1–6. https://doi.org/10.1093/plankt/10.1.1
Lei R, Zhang Z, Matero I, Cheng B, Li Q, Huang W (2012) Reflection and transmission of irradiance by snow and sea ice in the central Arctic Ocean in summer 2010. Polar Res 31(1):17325. https://doi.org/10.3402/polar.v31i0.17325
Lepot M, Aubin J-B, Clemens FHLR (2017) Interpolation in time series: an introductive overview of existing methods, their performance criteria and uncertainty assessment. Water 9(10):796. https://doi.org/10.3390/w9100796
Li Y-H, Tsui T-F (1971) The solubility of CO2 in water and sea water. J Geophysic Res 76(18):4203–4207. https://doi.org/10.1029/JC076i018p04203
Mabardy B, Conway FDL, Waldbusser GG, Olsen CS (2016) The U.S. west coast shellfish industry's perception of and response to ocean acidification. Oregon Sea Grant, Corvallis
Matoo OB, Ivanina AV, Ullstad C, Beniash E, Sokolova IM (2013) Interactive effects of elevated temperature and CO2 levels on metabolism and oxidative stress in two common marine bivalves (Crassostrea virginica and Mercenaria mercenaria). Comp Biochem Phys A 164(4):545–553. https://doi.org/10.1016/j.cbpa.2012.12.025
Mayrand E, Comeau L, Mallet A (2017) Physiological changes during overwintering of the Eastern oyster Crassostrea virginica (Gmelin, 1791). J Molluscan Stud 83(3):333–339. https://doi.org/10.1093/mollus/eyx017
Mel’nikova YB (2017) Evaluation of parameters of a plankton community’s biological rhythms under the natural environment of the Black Sea using the Fourier transform method. Luminescence 32(3):321–326. https://doi.org/10.1002/bio.3181
Menu-Courey K, Noisette F, Piedalue S, Daoud D, Blair T, Blier PU, Azetsu-Scott K, Calosi P (2019) Energy metabolism and survival of the juvenile recruits of the American lobster (Homarus americanus) exposed to a gradient of elevated seawater pCO2. Mar Environ Res 143:111–123. https://doi.org/10.1016/j.marenvres.2018.10.002
Meyer-Kaiser KS, Houlihan EP, Wheeler JD, McCorkle DC, Mullineaux LS (2019) Behavioral response of eastern oyster Crassostrea virginica larvae to a chemical settlement cue is not impaired by low pH. Mar Ecol- Progr Ser 623:13–24. https://doi.org/10.3354/meps13014
Miller AW, Reynolds A, Minton MS, Smith R (2020) Evidence for stage-based larval vulnerability and resilience to acidification in Crassostrea virginica. J Mollus Stud 86(4):342–351. https://doi.org/10.1093/mollus/eyaa022
Miller W, Reynolds A, Sobrino C, Riedel GF (2009) Shellfish face uncertain future in high CO2 world: influence of acidification on oyster larvae calcification and growth in estuaries. PLoS One 4(5): e5661. https://doi.org/10.1371/journal.pone.0005661
Millero FJ, Poisson A (1981) International one-atmosphere equation of state of seawater. Deep-Sea Res 28:625–629. https://doi.org/10.1016/0198-0149(81)90122-9
Miramichi River Environmental Assessment Committee (2018) Interim report
Niles M, Davidson L-A, Nowlan R, Doiron S, Sonier R, Comeau LA (2014) Growing oysters (Crassostrea virginica) using French string technique at an exposed and a sheltered site in Chaleur Bay. Can Tech Rep Fish Aquat Sci, New Brunswick, p 3079
Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, Gnanadesikan A, Gruber N, Ishida A, Joos F, Key RM, Lindsay K, Maier-Reimer E, Matear R, Monfray P, Mouchet A, Najjar RG, Plattner G-K, Rodgers KB, Sabine CL, Sarmiento JL, Schlitzer R, Slater RD, Totterdell IJ, Weirig M-F, Yamanaka Y, Yool A (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437(7059):681–686. https://doi.org/10.1038/nature04095
Pandori LLM, Sorte CJB (2019) The weakest link: sensitivity to climate extremes across life stages of marine invertebrates. Oikos 128(5):621–629. https://doi.org/10.1111/oik.05886
Pettay DT, Gonski SF, Cai W-J, Sommerfield CK, Ullman WJ (2020) The ebb and flow of protons: A novel approach for the assessment of estuarine and coastal acidification. Estuar Coast Shelf S 236: 106627. https://doi.org/10.1016/j.ecss.2020.106627
Pineda MC, McQuaid CD, Turon X, López-Legentil S, Ordóñez V, Rius M (2012) Tough adults, frail babies: an analysis of stress sensitivity across early life-history stages of widely introduced marine invertebrates. PLoS One 7(10): e46672. https://doi.org/10.1371/journal.pone.0046672
Pruett JL, Pandelides AF, Willett KL, Gochfeld DJ (2021) Effects of flood-associated stressors on growth and survival of early life stage oysters (Crassostrea virginica). J Exp Mar Biol Ecol 544: 151615. https://doi.org/10.1016/j.jembe.2021.151615
Rickerich S, Ross L, Valle-Levinson A (2022) Wave enhanced overtides in an idealized tidal inlet system. J Geophys Res- Oceans 127(10): e2022JC018848. https://doi.org/10.1029/2022JC018848
Rysgaard S, Glud R, Sejr M, Bendtsen J, Christensen P (2007) Inorganic carbon transport during sea ice growth and decay: A carbon pump in polar seas. Journal of Geophysical Research 112. https://doi.org/10.1029/2006JC003572
Salisbury J, Green M, Hunt C, Campbell J (2008) Coastal acidification by rivers: a threat to shellfish? Eos 89(50):513–513. https://doi.org/10.1029/2008EO500001
Sephton TH, Boothe DA (1992) Physical oceanographic and biological data from the study of the flushing of oyster (Crassostrea virginica) larvae from Caraquet Bay, New Brunswick. Can Man Rep Fish Aquat Sci no 2162
Service canadien des glaces (2018) Résumé saisonnier. L'est du Canada Hiver 2017-2018
Simms LE, Engebretson MJ, Reeves GD (2022) Removing diurnal signals and longer term trends from electron flux and ULF correlations: A comparison of spectral subtraction, simple differencing, and ARIMAX models. Journal of Geophysical Research: Space 127(2): e2021JA030021. https://doi.org/10.1029/2021JA030021
Surette C, Brun G, Mallet V (2002) Impact of a commercial peat moss operation on water quality and biota in a small tributary of the Richibucto River, Kent County, New Brunswick, Canada. Arch Environ Contam Toxicol 42:423–430. https://doi.org/10.1007/s00244-001-0043-0
Talmage SC, Gobler CJ (2011) Effects of elevated temperature and carbon dioxide on the growth and survival of larvae and juveniles of three species of northwest Atlantic bivalves. PLoS One 6(10):e26941. https://doi.org/10.1371/journal.pone.0026941
Vaseghi SV (1996) Spectral Subtraction. Advanced Signal Processing and Digital Noise Reduction. Vieweg+Teubner Verlag, Wiesbaden, pp 242–260
Vergara C, Quinitio G, Baeck G (2017) Effects of the lunar cycle in the catch composition and total catch of stationary lift nets in the coastal waters of Miagao, Iloilo, the Philippines. J Korean Soc Fish Tech 53:349–356. https://doi.org/10.3796/KSFT.2017.53.4.349
Wahl M, Schneider Covachã S, Saderne V, Hiebenthal C, Müller JD, Pansch C, Sawall Y (2018) Macroalgae may mitigate ocean acidification effects on mussel calcification by increasing pH and its fluctuations. Limnol Ocean 63(1):3–21. https://doi.org/10.1002/lno.10608
Waldbusser GG, Brunner EL, Haley BA, Hales B, Langdon CJ, Prahl FG (2013) A developmental and energetic basis linking larval oyster shell formation to acidification sensitivity. Geophys Res Lett 40(10):2171–2176. https://doi.org/10.1002/grl.50449
Waldbusser GG, Hales B, Langdon CJ, Haley BA, Schrader P, Brunner EL, Gray MW, Miller CA, Gimenez I (2015a) Saturation-state sensitivity of marine bivalve larvae to ocean acidification. Nat Clim Change 5(3):273–280. https://doi.org/10.1038/nclimate2479
Waldbusser GG, Hales B, Langdon CJ, Haley BA, Schrader P, Brunner EL, Gray MW, Miller CA, Gimenez I, Hutchinson G (2015b) Ocean acidification has multiple modes of action on bivalve larvae. PLoS One 10(6): e0128376. https://doi.org/10.1371/journal.pone.0128376
Wang H, Lehrter J, Maiti K, Fennel K, Laurent A, Rabalais N, Hussain N, Li Q, Chen B, Scaboo M, Cai W-J (2020) Benthic Respiration in Hypoxic Waters Enhances Bottom Water Acidification in the Northern Gulf of Mexico. Journal of Geophysical Research: Oceans 125. https://doi.org/10.1029/2020JC016152
Winograd S (1978) On Computing the Discrete Fourier Transform. Math Comput 32(141):175–199. https://doi.org/10.2307/2006266
Wolfe K, Nguyen HD, Davey M, Byrne M (2020) Characterizing biogeochemical fluctuations in a world of extremes: A synthesis for temperate intertidal habitats in the face of global change. Global Change Biol 26(7):3858–3879. https://doi.org/10.1111/gcb.15103
Young C, Gobler C (2018) The ability of macroalgae to mitigate the negative effects of ocean acidification on four species of North Atlantic bivalve. Biogeosciences 15:6167–6183. https://doi.org/10.5194/bg-15-6167-2018
Acknowledgements
We thank the owners and the staff members of the oyster company L’Étang Ruisseau Bar Ltée for their constant support of our research project, including access to their facilities and the sharing of technical and biological knowledge. We are also grateful to Rémy Haché, from the Agriculture, Aquaculture, and Fisheries Department of New Brunswick, for sharing his expertise on oyster farming and his invaluable contribution during the field trips at Tracadie and Caraquet Bays. We thank France Béland for her impeccable work in the laboratory and the field. Thanks are also addressed to Dr. Sid-Ahmed Selouani (Laboratoire de Recherche en Interaction Humain-Système (LARIHS), Université de Moncton) for his advice on the statistical approach. Finally, we are grateful to the anonymous reviewers whose valuable comments and suggestions improved the quality of the paper.
Funding
This study was funded by the Environmental Trust Fund of New Brunswick (projects numbers 160123, 170131, 180308, and 190282), and by the Université de Moncton, campus de Shippagan.
Author information
Authors and Affiliations
Contributions
The conception and design of the study were done by Elise Mayrand, as well as the instruments preparation, deployment, and maintenance, with the help of the technician. E. Mayrand also did the data collection, treatment, and analysis, as well as the figures and the first draft of the manuscript. The conception of the statistical approach, the conduction of the statistical analyses as well as the statistical interpretation were done by Zhor Benhafid, who also commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mayrand, E., Benhafid, Z. Spatiotemporal variability of pH in coastal waters of New Brunswick (Canada) and potential consequences for oyster aquaculture. Anthropocene Coasts 6, 14 (2023). https://doi.org/10.1007/s44218-023-00029-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44218-023-00029-3