Abstract
Background
There is no consensus on the cause and effect of systemic chronic inflammation (SCI) regarding chronic obstructive pulmonary disease (COPD). The impact of second-hand smoke (SHS) on COPD has reached inconsistent conclusions.
Methods
The China Kadoorie Biobank cohort was followed up from the 2004–08 baseline survey to 31 December 2018. Among the selected 445,523 participants in the final analysis, Cox and linear regressions were performed to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of tobacco exposure with COPD risk and baseline levels of log-transformed inflammatory factors [βs (95% CIs)], respectively.
Results
Participants were followed up for a median of 12.1 years and 11,825 incident COPD events were documented. Ever-smokers were associated with a higher risk of COPD than non-smokers with non-weekly SHS exposure. A younger age to start smoking, a greater amount of daily tobacco consumption, and deeper inhalation were associated with increased risk of COPD and correlated with elevated levels of plasma high-sensitivity C-reactive protein (hs-CRP, all Ptrend < 0.001) even two years before COPD onset. Among former smokers, COPD risk declined with longer smoking cessation (Ptrend < 0.001) and those quitting smoking for over ten years presented no difference in COPD risk and hs-CRP level from non-smokers [HR (95% CI) = 1.05 (0.89, 1.25), β (95% CI) = 0.17 (− 0.09, 0.43)]. Among non-smokers, weekly SHS exposure was associated with a slightly higher COPD risk [HR (95% CI) = 1.06 (1.01, 1.12)].
Conclusions
Incremental exposure to tobacco smoke was related to elevated SCI level before COPD onset, then an increase in COPD susceptibility. Quitting smoking as early as possible is suggested as a practical approach to reducing COPD risk in smokers. Given the high prevalence of both COPD and SHS exposure, the risk associated with SHS exposure deserves attention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
What is already known on this topic? |
ŸThe association of active smoking with chronic obstructive pulmonary disease (COPD) has been established, while the impact of second-hand smoke (SHS) on COPD has reached inconsistent conclusions. |
ŸThere is no consensus on the cause and effect of systemic chronic inflammation (SCI) regarding COPD. |
What this study adds? |
ŸIncremental exposure to tobacco smoke was related to elevated systemic chronic inflammation before COPD onset. |
ŸFormer smokers quitting for over ten years presented no difference in COPD risk and systemic inflammatory level from non-smokers. |
ŸThe chance that exposure to second-hand smoke increased COPD risk existed. |
How this study might affect research, practice, or policy? |
The study supports the potential justification of the indoor tobacco control policy and the initiative to quit smoking as soon as possible. |
1 Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous pulmonary condition characterized by persistent respiratory symptoms and airflow limitation with extensive under- and misdiagnosis [1]. It poses a serious challenge for public health. It was estimated that there were approximately 391.9 million COPD patients worldwide, with 80.5% living in low- and middle-income countries (LMICs), in 2019 [2].
It’s well established that COPD is accompanied by local inflammation of the airways, parenchyma, and pulmonary vessels [3]. Elevated levels of low-grade systemic chronic inflammation (SCI), indexed by C-reactive protein (CRP) and fibrinogen, also persist in patients with COPD [4]. However, there was no consensus on the cause and effect of SCI regarding COPD [5]. Smoking is a critical environmental pathogenic factor of COPD incidence [6]. and is associated with a broad range of alterations in systemic inflammatory marker levels [7]. Current smokers and self-initiated quitters are ‘natural experiment’ subjects for the inspection of the alteration of SCI status and COPD onset.
Previously published studies have reported that 22–51% of people with COPD have never smoked [8]. However, the 2018 China Adult Tobacco Survey showed that 68.1% of non-smokers were exposed to second-hand smoke (SHS), which is known to contain various toxic and carcinogenic agents [9]. As yet, studies on exposure to SHS and COPD in non-smokers have reached inconsistent conclusions, and most were confined to cross-sectional studies [10,11,12,13]. A comprehensive characterization of the effects of SHS on SCI may provide insight into the association of SHS with COPD.
Based on the China Kadoorie Biobank (CKB) cohort, this study aimed to explore the associations of smoking characteristics and SHS exposure with the risk of COPD within sociodemographic subgroups and to validate their correlations with circulating inflammatory markers.
2 Methods
2.1 Study Population
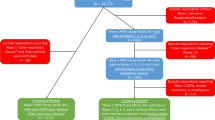
The CKB study was a prospective cohort involving participants from 10 regions covering diverse ranges of geographical divisions, socioeconomic conditions, and spectrums of exposure and disease across China. 512,724 adults aged 30–79 years were enrolled in the baseline survey from June 2004 to July 2008. Details of the CKB study have been described elsewhere [14, 15]. In the present study, participants with any diagnosis of cancer (n = 2132), heart attack or stroke (n = 20,060), COPD (n = 37,057), and asthma or tuberculosis (n = 7949) before enrollment, and those who lacked data on body mass index (BMI, n = 2) at baseline and were right-censored on the same day of study entry (n = 1) were excluded, leaving 445,523 participants for the primary analyses. The Ethical Review Committee of the Chinese Center for Disease Control and Prevention (Beijing, China) and the Oxford Tropical Research Ethics Committee, University of Oxford (Oxford, UK) approved the study. Written informed consent was obtained from all participants.
2.2 Assessment of Exposure and Covariates at Baseline
Information on sociodemographic factors (age, sex, marital status, occupation, household income), lifestyle (tobacco smoking, alcohol drinking, physical activity, frequency of fresh fruit, fresh vegetable, grains, and red meat consumption), indoor air pollution (passive smoking status, cooking and heating fuel use, kitchen ventilation device) and medical history (cancer, heart attack or stroke, COPD, asthma, tuberculosis) was collected through questionnaire-based interviews by trained staff.
Smoking frequency (i.e., never, occasionally, formerly, and currently) at and before the baseline survey was inquired for each participant [16]. Occasional smokers, relatively few in number, were categorized into non-smokers. Ex-smokers who quit smoking due to any physical illness were counted as current smokers to avoid reverse causality bias. Non-smokers were further dichotomized into non-weekly or weekly SHS exposure groups according to their frequency of direct exposure to SHS either at home or in the workplace. Smoking characteristics (i.e., the age of first starting smoking regularly, amount of cigarettes or equivalents consumed per day, and depth of inhalation) were further collected among ever-smokers. For former smokers, information on years since smoking cessation was obtained. Information on cumulative exposure duration (h/w) was further asked among those exposed to SHS weekly. The daily SHS exposure duration (h/d) was additionally calculated by dividing weekly exposure duration (h/w) by exposure frequency (d/w).
Regarding alcohol drinking, participants were inquired about the frequency of alcohol consumption in the past year. Those who did not drink weekly were further asked whether they had a year-long history of weekly drinking previously. Participants were also asked the type and corresponding quantity of drinking for current weekly drinkers. By multiplying the metabolic equivalent tasks (MET) value for a certain type of physical activity by the number of hours that activity is performed each day, the total daily physical activity level (MET-h/d) was determined. The main fuel types for cooking and heating was classified into four categories: clean, solid, and other fuels, as well as no history of cooking or heating. The equipment of ventilation facility was inquired for participants who cooked at home.
Standing height, body weight, and waist circumference (WC) were measured by uniformly trained practitioners using calibrated instruments and standard procedures. Weight in kilograms was divided by the square of height in meters to obtain BMI. Prebronchodilator forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) were measured using spirometers by trained technicians following recommended protocols.
2.3 Measurement of Circulating Inflammatory Markers
For each participant, either fasting or postprandial blood specimen was collected at baseline. A subpopulation was selected from those free of cardiovascular events until 1 January 2014 to measure clinical biochemistry biomarkers, including high-sensitivity CRP (hs-CRP) and fibrinogen. Detailed sampling procedures can be referred to in the previous article [17]. Plasma hs-CRP (mg/L) was assayed using AU680 clinical chemical analyzer (Beckman Coulter Incorporation, UK), and fibrinogen (g/L) was measured by BN Prospec nephelometer analyzer (Siemens, UK) at the Wolfson Laboratory (Clinical Trial Service Unit & Epidemiological Studies Unit, UK). All assays were performed using the manufacturer’s standard reagents, calibrators, and settings.
2.4 Follow-up and Outcome Ascertainment
The primary outcome in the present study was incident COPD coded J41-J44 by the International Classification of Diseases, 10th revision (ICD-10). Information on the COPD morbidity of participants was ascertained via electronic linkage, using their unique national identification number, with national health insurance hospitalization records (covering about 98% of CKB participants) [18] and the Disease Surveillance Points (DSP) system. Vital status and cause of death were ascertained through reviews of official residential records and death certificates submitted to the local Center for Disease Control and Prevention (CDC), supplemented by medical records and validated verbal autopsy [15]. Participants were followed up from baseline to the date of COPD incidence, death, loss to follow-up, or 31 December 2018, whichever came first.
2.5 Statistical Analysis
Linear and logistic regressions were applied to compare continuous and categorical baseline characteristics according to baseline smoking and SHS exposure status [non-smoker (SHS exposure: non-weekly, weekly), former smoker, current smoker], adjusted for age, sex, and region, where appropriate.
Hazard ratios (HRs) and 95% confidence intervals (95% CIs) for associations of smoking and SHS exposure status with COPD incidence were estimated by Cox proportional hazard regression models, taking non-smokers with non-weekly SHS exposure as the reference group. Cox models were further conducted to evaluate: i) associations of age to start smoking (≤ 18, 19–25, > 25 years), daily cigarette consumption (≤ 10, 11–20, > 20 cigarettes), and depth of inhalation while smoking (down to the mouth, throat, lung) with COPD risk among current smokers, taking non-smokers as the reference; (ii) associations of the duration of smoking cessation (≤ 5, 5–10, > 10 years) with COPD among former smokers, taking non-smokers as the reference; (iii) associations of average daily SHS exposure duration (< 2, 2–4, ≥ 4 h/d) with COPD among those exposed to SHS exposure 6–7 d/w, taking those exposed to SHS less than once a week as the reference group, limited to non-smokers. All Cox models were stratified by baseline age groups (in 5-year intervals), sex, and study areas, with age as the underlying time scale. Potential confounders were adjusted in a stepwise manner. Model 1 included marital status, education, household income, and occupation; model 2 additionally included drinking status [non-weekly, ex-regular, non-daily, daily (< 15, 15–29, 30–59, ≥ 60g/d)], physical activity (MET-h/d), dietary habits (consumption frequency of fresh fruit, fresh vegetable, grains, and red meat), passive smoking (except for associations of SHS exposure with COPD), cooking fuel use, heating fuel use, kitchen ventilation device, BMI, and WC. The p-value for the linear trend was calculated by including the midpoint of each category and treating the variable as continuous in each separate regression model. Subgroup analyses by baseline characteristics, including age, sex, and region, were performed to examine potential effect modifications.
To estimate the correlations between smoking and SHS exposure status with hs-CRP and fibrinogen, linear regression models were applied using the same reference group with the same adjustment in the same population as the above corresponding models, including baseline age, sex, and study areas.
Sensitivity analysis was conducted by further excluding those who developed COPD within the first 2 years of follow-up to minimize reverse causality. All analyses used Stata 15.0 (StataCorp, TX, USA). The significance level was set at 0.05.
3 Results
The current study included 445,523 participants in total. Among them, 68.7% were non-smokers, 2.8% were former smokers, and 28.5% were current regular smokers. Of former and current regular smokers, 93.1% and 95.2% were men, respectively, while men only accounted for 14.9% of non-smokers. Both current regular smokers and former smokers were less educated, less likely to have a household income of ≥ 20,000 yuan and to use ventilation devices for cooking, and more likely to consume alcohol and to use solid fuels for heating, compared with non-smokers. Among non-smokers, those exposed to SHS weekly tended to be younger, female, rural residents, married, drink regularly, and use solid fuels for cooking and heating, compared with those exposed to SHS non-weekly (Table 1).
During a median follow-up of 12.1 (inter-quartile range 11.1–13.1) years, 11,825 incident COPD events occurred. As shown in Table 2, ever-smokers had a higher crude incidence rate than non-smokers, and current regular smokers had the highest incidence (37.0 per 10,000 person-years). After adjusting for potential confounders, the HRs (95% CIs) for COPD among former smokers and current regular smokers were 1.18 (1.05, 1.32) and 1.79 (1.68, 1.90), respectively, as compared with non-smokers with non-weekly SHS exposure. Among non-smokers, weekly SHS exposure was associated with a slightly higher risk of COPD compared with non-weekly exposure [HR (95% CI) = 1.06 (1.01, 1.12)].
Smoking characteristics, including the age of first starting smoking regularly, amount of tobacco consumed per day, and depth of inhalation, were all associated with the risk of COPD among ever-smokers (Ptrend < 0.001) (Fig. 1). Taking non-smokers as the reference, the adjusted HRs (95% CIs) for those who started smoking aged ≤ 18, 19–25, and > 25 years were 2.06 (1.92, 2.20), 1.75 (1.64, 1.87), and 1.35 (1.26, 1.46), respectively (Supplementary Table 1). We observed positive associations of the amount of tobacco smoked per day and depth of inhalation with incident COPD after multivariate adjustment among current smokers (Fig. 1b–c). Compared with non-smokers, the adjusted HRs (95% CIs) for those who had quit smoking for ≤ 5 years, 5–10 years, and > 10 years were 1.37 (1.15, 1.63), 1.27 (1.05, 1.55), and 1.05 (0.89, 1.25), respectively (Supplementary Table 1). No association of SHS exposure duration with COPD was observed among non-smokers.
Association of smoking and second-hand smoke exposure characteristics with risk of chronic obstructive pulmonary disease. HR hazard ratio, CI confidence interval. Values of point estimates for HRs were presented and 95% CI were presented as whiskers. The x-coordinate of each dot was set at the midpoint of the corresponding group in Fig. 1a–b, d–e, where appropriate. Cox models were stratified by age, sex, and study regions, with adjustment for marital status, education, household income, occupation, dietary habit (consumption frequency of fresh fruit, fresh vegetable, grains, and red meat), physical activity, drinking status, passive smoking (except for taking passive smoking as exposure), solid fuel use for cooking, solid fuel use for heating, stoves with chimney/extractor, body mass index, and waist circumference. Never or occasional smokers were the reference group in Fig. 1a–d. Those exposed to second-hand smoke less than once a week among never or occasional smokers were the reference group in Fig. 1e. Ptrend was calculated by including the midpoint of each category and treating the variable as continuous in each separate regression model. All the Ptrend values were < 0.001 except for the duration of second-hand smoke exposure (Ptrend = 0.183)
Supplementary Table 2 showed interactions between smoking or SHS status and sociodemographic characteristics for COPD. Associations of the age to start to smoke (Pint = 0.003), the amount of tobacco consumed (Pint = 0.015), and the depth of inhalation (Pint = 0.006) with COPD risk exhibited greater effect estimates in urban areas compared with the ones in rural areas. Furthermore, among urban non-smokers, there was evidence for a dose–response relationship between SHS exposure duration and COPD (Ptrend = 0.019) and a 45% (95% CI: 6%, 98%) increase in risk for ≥ 4 h SHS exposure per day compared with non-weekly SHS exposure. The number of cases in some subgroups was relatively small, and these subgroup analyses were exploratory.
We observed elevated levels of log-transformed plasma hs-CRP in current regular smokers (β = 0.25; 95% CI 0.15, 0.34) compared to non-smokers with non-weekly SHS exposure. Similar findings were observed concerning smoking characteristics, including a younger age to start smoking, more daily cigarette consumption, and deeper smoking inhalation. No statistically significant correlation was found in weekly SHS exposure and former tobacco exposure, except that years since smoking cessation were marginally correlated with log-transformed hs-CRP level in those quitting smoking for 5–10 years compared with non-smokers (β = 0.32; 95% CI 0.00, 0.64). Smoking and SHS exposure status showed no correlation with the level of log-transformed plasma fibrinogen, except for the amount of tobacco consumed per day among current regular smokers (Ptrend = 0.039), especially among those consumed > 20 cigarettes or equivalents per day (β = 0.03; 95% CI 0.00, 0.05), compared with non-smokers (Fig. 2).
Correlations between smoking and second-hand smoke exposure status and log-transformed inflammatory markers. aThose who quit smoking due to illness were classified as current regular smoker. SHS second-hand smoke, CI confidence interval. Point estimates for HRs were presented as dots and 95% CI were presented as whiskers. Linear models were adjusted for age, sex, study regions, marital status, education, household income, occupation, dietary habit (consumption frequency of fresh fruit, fresh vegetable, grains, and red meat), physical activity, drinking status, passive smoking (except for taking passive smoking as exposure), solid fuel use for cooking, solid fuel use for heating, stoves with chimney/extractor, body mass index, and waist circumference. βs (95% CIs) for the correlations between the age to start smoking (≤ 18, 19–25, > 25 years), the daily cigarette consumption (≤ 10, 11–20, > 20 cigarettes/d), and the depth of inhalation while smoking (down to the mouth, throat, lung) and inflammatory marker levels were estimated with never or occasional smokers as the reference group. βs (95% CIs) for the correlations between average daily second-hand smoke exposure duration (< 2, 2–4, ≥ 4 h/d) and inflammatory marker levels among never or occasional smokers were estimated with those exposed to second-hand smoke exposure less than once a week as the reference group. Ptrend was calculated by including the midpoint of each category and treating the variable as continuous in each separate regression model
In the sensitivity analyses, primary results remained substantially unchanged after excluding participants who developed COPD in the first 2 years of follow-up (Supplementary Table 1 & Supplementary Table 3).
4 Discussion
In this population-based cohort study with a median follow-up of 12.1 years, ever-smokers showed a higher risk of COPD compared with non-smokers. In addition, a younger age to start smoking, a larger amount of daily tobacco consumption, and deeper inhalation increased risks of COPD, accompanied by elevated levels of plasma hs-CRP, even two years before the onset of COPD. Among former smokers, COPD risk declined with longer smoking cessation, and those quitting for over 10 years presented consistent hs-CRP levels compared with non-smokers. Among non-smokers, weekly SHS exposure was associated with a slightly higher risk of COPD, and a dose–response relationship between the duration of SHS exposure and COPD was observed in urban residents.
As expected, active smoking, no matter at present or in the past, had an increased risk of COPD in the current study. Inhaled tobacco smoke could cause oxidant-induced injury, reprogramming of the epithelial cells, and a new small airway microenvironment with altered innate immune defenses, dysfunctional cilia, and pathological mucus secretion, leading to the onset and progression of COPD [19]. A recent meta-analysis included population-based studies on COPD worldwide, and found threefold and twofold risk of COPD in current and former smokers as non-smokers, respectively [2]. In addition, risk estimate sizes could be influenced by accumulated amounts of tobacco exposure through daily consumption quantity and age of starting smoking, which was consistent with previous findings [20, 21]. In addition, we also examined the association of self-perceived inhalation depth with the risk of COPD among current smokers. Deeper inhaling of tobacco smoke would be expected to be related to more severe health effects by more direct damage to bronchial epithelial cells.
The literature has many controversies on the hypothesis that pulmonary inflammation leads to ‘overspill’ into the circulation causing SCI [5]. Our studies found the alterations of SCI in smokers even before two years of COPD onset. Interestingly, we observed that the levels of circulating inflammatory markers among those quitting smoking lowered to those among non-smokers within 5 years, while their COPD risk still increased. Those who stopped smoking for over 10 years exhibited no difference in both COPD risk and SCI status from non-smokers, which opened windows of opportunity for COPD prevention by improving changeable behavior and supported the benefit of tobacco cessation previously proposed [22, 23].
The mixed findings about SHS and COPD continue to be debated. The meta-analysis mentioned above found SHS exposure was marginally associated with COPD worldwide [HR (95% CI) = 1.2 (1.0, 1.4)], and the association was no longer significant when divided by high-income countries and LMICs [2]. Our results suggested a slight rise in the risk of COPD for weekly SHS exposure among non-smokers. Furthermore, the duration of SHS exposure was also positively associated with COPD, and a nonnegligible risk increase for a long period of SHS exposure was observed among non-smokers in urban regions.
The key strengths of our study lay in the large-scale prospective design, long follow-up, details of smoking characteristics, and reasonable control of measured confounders. Electronic linkage with national health insurance hospitalization records and the DSP system were combined to ascertain COPD events. Also, comprehensive depictions of smoking and SHS exposure by subgroups were possible.
Inevitably, several limitations need to be considered when interpreting the study results. First, we only used tobacco exposure at baseline, which may change during follow-up. However, the harm of smoking may not be eliminated in the short run, as our results in former smokers suggested. Secondly, although we adjusted for various established and potential risk factors of COPD, air pollution was not measured in the present study, resulting in the possibility of residual confounding. Nevertheless, one previous CKB study observed relatively stronger correlation between personal and household PM2.5 levels than correlation between personal and community PM2.5 levels [24]. Generally, the outdoor air pollution was homogenous within regions and some key indoor ambient pollution like heating and cooking was controlled in our study. Thirdly, the information on smoking and SHS exposure was self-reported, which might bias the association, especially in women’s smoking behaviour. The internal dose of nicotine or cotinine may better reflect actual exposure levels. Finally, as this study was observational, it is still necessary to be cautious in inferring causality.
5 Conclusions
The findings of this study suggest that prolonged and incremental exposure to cigarette smoke tended to increase COPD risk and current levels of inflammation before COPD onset. Long-lasting smoking cessation might partly offset the harm of tobacco brought about. These findings support the potential justification of the indoor tobacco control policy and the initiative to quit smoking as soon as possible.
Data Availability Statement
The access policy and procedures are available at http://www.ckbiobank.org.
References
Disease GIfCOL: Global strategy for the diagnosis, management, and prevention of COPD: 2023 report. In.; 2022.
Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, Rudan I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med. 2022;10(5):447–58.
Ge J, Xu Y, Wang C. Internal Medicine (9th edition). Beijing: People’s Medical Publishing House; 2018.
Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138(1):16–27.
Sinden NJ, Stockley RA. Systemic inflammation and comorbidity in COPD: a result of “overspill” of inflammatory mediators from the lungs? Review Evidence Thorax. 2010;65(10):930–6.
[Guidelines for the diagnosis and management of chronic obstructive pulmonary disease (revised version 2021)]. Zhonghua Jie He He Hu Xi Za Zhi 2021, 44(3):170–205.
Shiels MS, Katki HA, Freedman ND, Purdue MP, Wentzensen N, Trabert B, Kitahara CM, Furr M, Li Y, Kemp TJ et al: Cigarette smoking and variations in systemic immune and inflammation markers. Journal of the National Cancer Institute 2014, 106(11).
Yang IA, Jenkins CR, Salvi SS. Chronic obstructive pulmonary disease in never-smokers: risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir Med. 2022;10(5):497–511.
Soleimani F, Dobaradaran S, De-la-Torre GE, Schmidt TC, Saeedi R. Content of toxic components of cigarette, cigarette smoke vs cigarette butts: a comprehensive systematic review. Science Total Environ. 2022;813: 152667.
Lee PN, Forey BA, Coombs KJ, Hamling JS, Thornton AJ: Epidemiological evidence relating environmental smoke to COPD in lifelong non-smokers: a systematic review. F1000Res 2018, 7:146.
Denguezli M, Daldoul H, Harrabi I, Gnatiuc L, Coton S, Burney P, Tabka Z. COPD in nonsmokers: reports from the tunisian population-based burden of obstructive lung disease study. PLoS ONE. 2016;11(3): e0151981.
Smith M, Li L, Augustyn M, Kurmi O, Chen J, Collins R, Guo Y, Han Y, Qin J, Xu G, et al. Prevalence and correlates of airflow obstruction in ∼317,000 never-smokers in China. Eur Respir J. 2014;44(1):66–77.
Tan WC, Sin DD, Bourbeau J, Hernandez P, Chapman KR, Cowie R, FitzGerald JM, Marciniuk DD, Maltais F, Buist AS, et al. Characteristics of COPD in never-smokers and ever-smokers in the general population: results from the CanCOLD study. Thorax. 2015;70(9):822–9.
Chen Z, Lee L, Chen J, Collins R, Wu F, Guo Y, Linksted P, Peto R. Cohort profile: the Kadoorie Study of Chronic Disease in China (KSCDC). Int J Epidemiol. 2005;34(6):1243–9.
Chen Z, Chen J, Collins R, Guo Y, Peto R, Wu F, Li L: China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol 2011, 40(6):1652–1666.
Lv J, Chen W, Sun D, Li S, Millwood IY, Smith M, Guo Y, Bian Z, Yu C, Zhou H et al: Gender-specific association between tobacco smoking and central obesity among 0.5 million Chinese people: the China Kadoorie Biobank Study. PLoS One 2015, 10(4):e0124586.
Chen L, Zhang Y, Yu C, Guo Y, Sun D, Pang Y, Pei P, Yang L, Millwood IY, Walters RG, et al. Modeling biological age using blood biomarkers and physical measurements in Chinese adults. EBioMedicine. 2023;89: 104458.
Li J, Zhu L, Wei Y, Lv J, Guo Y, Bian Z, Du H, Yang L, Chen Y, Zhou Y et al: Association between adiposity measures and COPD risk in Chinese adults. Eur Respir J 2020, 55(4).
Christenson SA, Smith BM, Bafadhel M, Putcha N. Chronic obstructive pulmonary disease. Lancet (London, England). 2022;399(10342):2227–42.
Ma L, Huo X, Yang A, Yu S, Ke H, Zhang M, Bai Y: Metal Exposure, Smoking, and the Risk of COPD: A Nested Case-Control Study in a Chinese Occupational Population. International journal of environmental research and public health 2022, 19(17).
Safitri W, Martini S, Artanti KD, Li CY: Smoking from a Younger Age Is the Dominant Factor in the Incidence of Chronic Obstructive Pulmonary Disease: Case-Control Study. International journal of environmental research and public health 2021, 18(11).
Willemse BW, Postma DS, Timens W, ten Hacken NH. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur Respir J. 2004;23(3):464–76.
Godtfredsen NS, Lam TH, Hansel TT, Leon ME, Gray N, Dresler C, Burns DM, Prescott E, Vestbo J. COPD-related morbidity and mortality after smoking cessation: status of the evidence. Eur Respir J. 2008;32(4):844–53.
Chan KH, Xia X, Liu C, Kan H, Doherty A, Yim SHL, Wright N, Kartsonaki C, Yang X, Stevens R et al: Characterising personal, household, and community PM2.5 exposure in one urban and two rural communities in China. Science of The Total Environment 2023, 904:166647.
Acknowledgements
The most important acknowledgment is to the participants in the study and the members of the survey teams in each of the 10 regional centers, as well as to the project development and management teams based at Beijing, Oxford, and the 10 regional centers.
Funding
This work was supported by National Natural Science Foundation of China (82192904, 82192901, 82192900). The CKB baseline survey and the first re-survey were supported by the Kadoorie Charitable Foundation in Hong Kong. The long-term follow-up has been supported by Wellcome grants to Oxford University (212946/Z/18/Z, 202922/Z/16/Z, 104085/Z/14/Z, 088158/Z/09/Z) and grants (2016YFC0900500) from the National Key R&D Program of China, National Natural Science Foundation of China (82388102, 81390540, 91846303, 81941018), and Chinese Ministry of Science and Technology (2011BAI09B01). The UK Medical Research Council (MC_UU_00017/1, MC_UU_12026/2, MC_U137686851), Cancer Research UK (C16077/A29186; C500/A16896) and the British Heart Foundation (CH/1996001/9454), provide core funding to the Clinical Trial Service Unit and Epidemiological Studies Unit at Oxford University for the project. The funders had no role in the study design, data collection, data analysis, interpretation, report writing, or the decision to submit the article for publication.
Author information
Authors and Affiliations
Consortia
Contributions
CY conceptualised and designed the paper. LLM, ZC, and JC, as members of the CKB steering committee, designed and supervised the conduct of the whole study, obtained funding, and together with JL, DS, PP, LY, YC, HD, LLH, XM, and DA acquired the data. LC and HX drafted the manuscript. LC and QW analyzed the data. CY contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content. All authors contributed to and approved the final manuscript. CY is the study guarantor.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Ethics Approval
The study received ethical approval from the Chinese Centre for Disease Control and Prevention (Beijing, China: 005/2004) and the University of Oxford (UK: 025–04).
Patient Consent for Publication
Not applicable.
Additional information
The members of the steering committee and The China Kadoorie Biobank Collaborative Group are listed in the online-only supplemental material.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, L., Xiong, H., Wen, Q. et al. The Role of Active and Passive Smoking in Chronic Obstructive Pulmonary Disease and Systemic Inflammation: A 12-year Prospective Study in China. J Epidemiol Glob Health (2024). https://doi.org/10.1007/s44197-024-00290-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44197-024-00290-w