Abstract
COVID-19 vaccines have demonstrated significant efficacy in reducing severe symptoms and fatalities, although their effectiveness in preventing transmission varies depending on the population’s age profile and the dominant variant. This study evaluates the impact of the COVID-19 vaccination campaign in the Basque Country region of Spain, which has the fourth highest proportion of elderly individuals worldwide. Using epidemiological data on hospitalizations, ICU admissions, fatalities, and vaccination coverage, we calibrated four versions of an ordinary differential equations model with varying assumptions on the age structure and transmission function. Counterfactual no-vaccine scenarios were simulated by setting the vaccination rate to zero while all other parameters were held constant. The initial vaccination rollout is estimated to have prevented 46,000 to 75,000 hospitalizations, 6,000 to 11,000 ICU admissions, and 15,000 to 24,000 deaths, reducing these outcomes by 73–86%. The most significant impact occurred during the third quarter of 2021, coinciding with the Delta variant’s dominance and a vaccination rate exceeding 60%. Sensitivity analysis revealed that vaccination coverage had a more substantial effect on averted outcomes than vaccine efficacy. Overall, the vaccination campaign in the Basque Country significantly reduced severe COVID-19 outcomes, aligning with global estimates and demonstrating robustness across different modeling approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The Basque Country, a region in northern Spain, faced a significant challenge with the onset of the COVID-19 epidemic due to its aging population. In 2021, 22.9% of the region’s population was at least 65 years old, the fourth highest proportion in the world behind only Monaco, Japan, and Italy [1, 2]. This proportion is projected to rise to 28.2% by 2031 [3]. Additionally, nearly half of its 2 million population are aged 50 and above [4], posing a considerable hurdle against a disease that disproportionately affects the elderly and those with underlying health conditions. Due to the high number of severe cases in the region (see Fig. 1 in Online Resource), the Basque Country, along with the rest of Spain, became the global epicenter of COVID-19 by mid-March 2020 [5]. Authorities responded by implementing a state of alarm, enforcing compulsory home confinement, and closure of educational institutions, restaurants, and non-essential businesses [6], effectively decreasing COVID-19 transmission. The state of alarm was lifted by June 2020, only to be reinstated after four months due to a resurgence of cases and hospitalizations [7]. This second state of alarm was characterized by a less strict set of interventions including curfews and restrictions on social gatherings.
Following a record-speed development of COVID-19 vaccines, vaccination programs in the Basque Country started in December 2020 using the BioNTech/Pfizer, Spikevax/Moderna, Oxford/AstraZeneca, and Janssen/Johnson&Johnson vaccines. The elderly and those with comorbidities were prioritized and, by the end of 2021, more than 80% of the Basque population had already been fully immunized, achieving one of the fastest vaccination rates in the world [8] (see Fig. 2 in Online Resource).
However, real-world effectiveness studies have shown that while COVID-19 vaccinations confer high immunity against severe forms of the disease, vaccinations only confer low to moderate immunity against infection. Additionally, immunity wanes over time and offers lower resistance for the elderly [9,10,11]. The emergence of more infectious variants in 2021 further nuanced the transmission dynamics causing higher rates of immune escape. In the Basque Country, the Alpha and Delta variants of SARS-CoV-2 were respectively dominant during the first and second halves of 2021. Both variants were more transmissible and caused more severe symptoms than the initial wild-type variant [12].
Studies estimating the impact of COVID-19 vaccination have consistently demonstrated that vaccination has significantly reduced hospitalizations and deaths [13,14,15,16,17,18,19]. Watson et al. (2022), using data on COVID-19 deaths and excess mortality for model calibration, estimated that countries that achieved high vaccination rates by the end of 2021 such as Spain and Portugal averted a higher number of deaths compared to countries that had slower vaccination rates [18]. Similarly, He et al. (2022), using COVID-19 mortality data from 12 countries, reported that although the estimated deaths averted by vaccination were lower (< 0.3% of the total population), the conclusion remained the same: faster vaccine implementation led to more deaths being averted [19]. These two studies differ in their model calibration methods. Watson et al. (2022) used a bi-weekly constant reproduction number, while He et al. (2022) estimated the transmission rate using an exponential cubic spline. Despite these methodological differences, both studies quantify the impact of vaccination by considering population-level dynamics such as historical transmission rates and the number of immune and susceptible individuals in the population.
It has been previously demonstrated that COVID-19 vaccination was cost-saving in the Basque Country during the first half of 2021 [20]. Using a model that accurately predicted COVID-19 outcomes in this region [21, 22], we projected outcomes from January to June 2021 under a no-vaccine scenario, assuming the transmission rate remained consistent with the end of 2020. Other cost-effectiveness studies have employed similar methods for projecting outcomes, though estimates can vary based on assumed vaccine efficacy and coverage [23, 24].
In this study, we aim to retrospectively quantify the impact of vaccination in the Basque Country region of Spain in 2021. The region’s centralized health database and comprehensive vaccination program facilitated thorough assessments of the intervention. Specifically, we calibrated non-age-structured and age-structured versions of a Susceptible-Hospitalized-Asymptomatic/Mild-ICU-Recovered-Deceased model to the COVID-19 dynamics in the Basque Country from 2020 to 2021. To simulate counterfactual no-vaccine scenarios, we used the same parameters describing the 2021 dynamics, except that the vaccination rate parameter was fixed at zero.
2 Materials and Methods
2.1 Transmission Model
We extend a previous model [21, 22, 25] to incorporate vaccination dynamics in 2021. This enhancement allows us to profile transmission dynamics in the Basque Country and simulate a counterfactual scenario without vaccination. The model equations, their full descriptions, and calibration frameworks are available in the Online Resource. Our deterministic model divides the population into susceptible, hospitalized, mild/asymptomatic, ICU admitted, recovered, and deceased compartments. It differentiates between immunity against infection and immunity against hospitalization conferred by the vaccines [25, 26]. Notably, immunity against hospitalization is higher than immunity against infection, aligning with real-world effectiveness studies [9,10,11]. The model also accounts for higher transmission from mild and asymptomatic individuals due to their greater mobility [21, 27]. Recovered and vaccinated individuals gradually lose their immunity over time and become susceptible again. The model diagram is presented in Fig. 1.
Diagram for the SHARUCDV model with age structure. The compartments for the < 50-year-old age group are labeled with a subscript 1 while the compartments for the ≥ 50-year-old age group are labeled with a subscript 2. The arrows indicate the progression from the susceptible (S) compartment to the hospitalized (H), mild and asymptomatic (A), ICU (U), recovered (R), and deceased (D) compartments. Orange arrows and boxes represent the dynamics among the vaccinated (Sv, Hv, Uv). Compartments CA1, CH1, CU1, CA2, CH2, CU2 (not shown in the figure) respectively record the cumulative < 50 y/o mild/asymptomatic cases, < 50 y/o hospitalized, < 50 y/o ICU admitted, 50 + y/o mild/asymptomatic cases, 50 + y/o hospitalized, and 50 + y/o ICU admitted
The SHARUCDV framework evaluates the influence of various factors on COVID-19 outcomes in the Basque Country, including hospitalization, ICU admission, and mortality rates. In this study, we assess four different model configurations by varying assumptions on the transmission function and age stratification:
-
Model 1: No age structure, sigmoidal transmission rate.
-
Model 2: With age structure, sigmoidal transmission rate.
-
Model 3: No age structure, weekly constant transmission rate.
-
Model 4: With age structure, weekly constant transmission rate.
Models 1 and 2 use a sigmoidal transmission rate designed to capture the initial exponential rise, peak, and eventual decline in transmission for each phase of the epidemic (see Sect. 2 of the Online Resource for details). This rate is represented by a decreasing function that starts with a high value and asymptotically approaches a lower final value. In contrast, Models 3 and 4 assume a transmission rate that changes weekly, represented by a piecewise linear function.
Using data on COVID-19 outcomes and vaccination rates from March 2020 to December 2021 [8], we calibrate and validate these models. We then compare their performance to determine which assumptions provide the best fit and most realistic estimates of averted outcomes.
2.2 Model Calibration and Estimation of Averted Outcomes
To allow the model to accurately describe the dynamics of the COVID-19 epidemic, we calibrated the model to data by parameter estimation. We used the fminsearchbnd function [28] in MATLAB 2023a to find parameter values that minimized the squared error between the model output and the observed data for cumulative hospitalizations (CH ), ICU admissions (CU ), and deaths (D). A prior sensitivity analysis (see Sect. 3 of Online Resource) revealed which parameters are influential to the cumulative outcomes, and thus are good candidates for parameter estimation [29, 30]. We reduced the bias of the algorithm in minimizing errors for data with higher orders of magnitude (in this case, hospitalizations) by introducing weights to the cost function. This was done by taking the means of each data and assigning weights equal to the ratio of the maximum mean to the mean of the respective data. The details on the initial parameter guesses and boundaries for estimation are available in Table 3 in the Online Resource.
By applying the model calibration methods described here, we were able to obtain good agreement between model output and data on COVID-19 hospitalizations, ICU admissions, and deaths in the Basque Country from 2020 to 2021 (see Sect. 4 of Online Resource). This was true for all of our four models.
After calibrating the models and obtaining a profile of the epidemic, we estimated the hospitalizations, ICU admissions, and deaths averted by COVID-19 vaccination by simulating a counterfactual no-vaccine scenario for each model. Specifically, we ran the models from January 1 2021 to December 31 2021 using the calibrated set of parameter values except that the vaccination rate is fixed at zero for the entire period. The number of averted outcomes is estimated as the differences in cumulative hospitalizations, ICU admissions, and deaths between the no-vaccine scenario and the data. We refer the reader to Sect. 5 of the Online Resource for the corresponding state values in the scenarios without vaccination.
3 Results
3.1 Impact of COVID-19 Vaccination
The impact of COVID-19 vaccination in the Basque Country in 2021 was substantial (Fig. 2), with our estimates indicating a significant reduction in hospitalizations, ICU admissions, and deaths. Across all four models, COVID-19 vaccination was estimated to have averted between 46 and 75 thousand hospitalizations, 6 to 11 thousand ICU admissions, and 15 to 24 thousand deaths. In percentage terms, this translates to a reduction of hospitalizations by 76–84%, ICU admissions by 73–84%, and deaths by 79–86% in 2021.
Estimates of hospitalizations, ICU admissions, and deaths averted by COVID-19 vaccination in the Basque Country region of Spain in 2021. The left graph shows the number of outcomes in thousands while the right graph shows the percentage of outcomes averted, computed as (T − D)/T, where T is the total outcomes in the counterfactual scenario without vaccination and D is the observed data. The four models have varying assumptions on the time-varying transmission rate and age dynamics
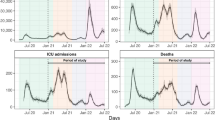
Our analysis identified a critical period in mid-2021, specifically from July to August, where a significant surge in hospitalizations would have occurred if not for vaccination efforts. This surge coincided with the emergence of the Delta variant, renowned for its heightened transmissibility and severity compared to earlier variants [12]. The efficacy of vaccination in reducing hospitalization rates emphasizes the critical importance of vaccination campaigns in managing the spread of highly transmissible variants.
All four models consistently projected a substantial increase in hospitalizations from July to August 2021 in the absence of vaccination, as shown in Fig. 3. The consistency observed across all models strengthens the reliability of our findings and provides additional confidence in the effectiveness of vaccination campaigns as a key strategy in controlling the spread of COVID-19 and mitigating their impact on public health.
Introducing age structure into the model led to lower estimates for averted hospitalizations and ICU admissions (Fig. 2). Models incorporating age structure (Models 2 and 4) estimated that vaccination averted 46 thousand hospitalizations, 6 thousand ICU admissions, and 15 to 17 thousand deaths, compared to 75 thousand hospitalizations, 9 to 11 thousand ICU admissions, and 20 to 24 thousand deaths averted in non-age-structured models (Models 1 and 3). This discrepancy can be attributed to the overall reduction in hospitalization rates, particularly driven by the lower hospitalization rate among younger age groups. In the absence of vaccination, younger age groups were identified as the primary source of cases, contributing to a lower overall hospitalization rate for the population.
Interestingly, variations in assumptions regarding the transmission rate (sigmoidal or weekly constant) did not yield significant differences in estimated averted outcomes. Despite differences in transmission rate assumptions, both approaches demonstrated good agreement between model outputs and observed data. This suggests robustness in the estimated impact of vaccination across different transmission rate scenarios.
3.2 Sensitivity Analysis of Averted Outcomes
The influence of each model parameter on the number of averted outcomes was measured using a robust sensitivity analysis [29]. This helps to identify factors that are significantly associated with the total averted hospitalizations, ICU admissions, and deaths. The full details of the sensitivity analysis are available in the Online Resource. In this analysis, the full models incorporating vaccination dynamics were used, with initial state values approximating those of January 1, 2021, marking the onset of the vaccination campaign. Table 8 in the Online Resource summarizes the influence of each parameter on the total averted outcomes. Additionally, figures showing the sensitivity values and the associated p-values for each model are available in the Online Resource.
When considering vaccination dynamics in Models 1 and 3, where the entire population is treated as a single cohort, we find that the vaccination rate and vaccine efficacies against infection and hospitalization are all significantly associated with the increase in the number of averted outcomes. Here, we observe higher sensitivity values from the vaccination rate compared to the vaccine efficacies (see Figs. 31 and 33 of Online Resource). This observation suggests that during the initial stages of the vaccination campaign when vaccine supply was limited, inoculation using vaccines of lower efficacy (such as Oxford/AstraZeneca and Janssen/Jonhson&Johnson), rather than waiting for additional supplies of vaccines of higher efficacy mRNA vaccines (BioNTech/Pfizer and SpikeVax/Moderna), would have resulted in a greater reduction in hospitalizations, ICU admissions, and deaths.
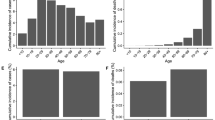
The sensitivity analysis conducted on the age-structured models (Models 2 and 4) demonstrates that vaccine efficacy does not hold equal importance for both age groups (see Fig. 4). Specifically, efficacy against infection is found to be significant only for the younger age group, while efficacy against hospitalization is significant solely for the older age group. Despite these differences, vaccinating individuals across all age groups contributes significantly to averting adverse outcomes. However, targeting vaccination efforts toward individuals aged 50 years and older would yield a more substantial impact in reducing hospitalizations, ICU admissions, and deaths. This underscores the importance of prioritizing vaccination for older age groups to effectively mitigate the burden of severe illness and mortality associated with COVID-19.
Sensitivity (Partial Rank Correlation Coefficients [PRCC]) and the corresponding p values of the parameters of the SHARUCDV model with weekly constant transmission rate and no age-structure (Model 4) to total averted hospitalizations, ICU admissions, and deaths during the initial stages of vaccination. Each bar represents the PRCC and corresponding p value every ten days over a 90-day period starting January 1, 2021. The results for other models are available in the Online Resource
4 Discussion
Our results reveal that COVID-19 vaccination substantially reduced hospitalizations, ICU admissions, and deaths in the Basque Country region of Spain in 2021. We estimate that vaccination averted between 45,800 and 75,100 hospitalizations, 5,500 to 10,600 ICU admissions, and 14,700 to 24,300 deaths. These findings highlight the critical role of COVID-19 vaccination in preventing a healthcare crisis, especially in the face of heightened transmission of variants that cause more severe symptoms.
We observed the most pronounced impact of vaccination during the third quarter of 2021, when more than 60% of Basque residents had already completed the primary series of vaccinations. During this period, school closures and increased outdoor activities due to higher temperatures likely contributed to fewer close contacts. However, given the virus’ lack of seasonality [31, 32] and the increase in international travel to the Basque Country [33], the emergence of the Delta variant as the dominant strain likely played a greater role in driving higher transmission in the region (see Sect. 4 of Online Resource).
Our estimates of averted deaths are aligned with the higher range of estimates in similar studies [18, 19]. Specifically, we estimate that COVID-19 vaccination protected 0.6–1.0% of the Basque population from death in 2021. Two key factors may explain the high number of averted deaths in the Basque Country. First, the region achieved a high vaccination coverage of 82% by the end of 2021, marking one of the fastest vaccination rates globally [34]. Second, the Basque Country has one of the oldest populations in the world, standing as a particularly vulnerable population to the virus [1,2,3]. The positive association of vaccination rate and percentage of the elderly population with a higher estimate of averted deaths is illustrated in Fig. 5, which compares the results from this study, He et al. (2022) [19], and selected countries from Watson et al. (2022) [18].
Estimates of deaths averted by COVID-19 vaccination as a percentage of total population (y-axis), coverage of vaccination by the end of 2021 (x-axis, top graph) [4, 34], and proportion of the population aged 65 and above [1, 2], 2021 (x-axis, bottom graph) from He et al. (2022) [19], this study, and selected countries from Watson et al. (2022) [18]
The assumed levels of transmission in the counterfactual scenario, derived through model calibration to data in this study, largely influence the estimates of averted outcomes [18]. High vaccination rates allowed many countries to relax restrictions, leading to higher transmission and, consequently, higher estimates of averted outcomes. Additionally, our sensitivity analyses (refer to Sect. 3.2) consistently identified the transmission rate as the most influential parameter on averted outcomes, surpassing even the impact of vaccination. This indicates that vaccination would not have had such a substantial impact if transmission rates had been low.
The sensitivity analysis of the age-structured models further revealed that prioritizing the vaccination of the older population had a greater impact on reducing hospitalizations and deaths compared to a more uniform vaccination program. As a result, this strategy led to significant cost savings and enhanced the cost-effectiveness of the vaccination policy [20].
The robustness of the estimates of averted outcomes to changes in the assumption for the transmission rate function—whether sigmoidal or weekly constant—is an important finding. It suggests that the model’s predictive power on the effectiveness of vaccination strategies are not heavily dependent on the specific form of the transmission rate function. This flexibility in modeling transmission dynamics enhances the applicability of the approach to different scenarios and settings. In particular, the use of weekly constant transmission rates (Models 3 and 4) proved to be more straightforward and faster to implement, highlighting the practical benefits of simplifying the transmission rate function. By assuming weekly constant rates, the retrospective model calibration is streamlined, making it more accessible and efficient for decision-makers and public health authorities. This simplification facilitates quicker assessment of the potential impact of vaccination campaigns and other interventions on disease outcomes.
Our results offer valuable inputs for estimating the cost-effectiveness of COVID-19 vaccination efforts, providing a solid foundation for economic evaluations [20, [35– 37]. Typically, COVID-19 cost-effectiveness models rely heavily on forecasting and involve numerous assumptions about disease dynamics, which can limit their scope and accuracy [23, 24]. Our approach circumvents many of these limitations by explicitly using empirical data from 2021 to simulate a counterfactual scenario with no vaccines administered while maintaining the same magnitude of transmission. This approach significantly reduces the number of model assumptions, enhancing the reliability and applicability of our findings for cost-effectiveness analyses.
However, our estimates of averted outcomes are nonetheless constrained by the remaining assumptions inherent in our model. Presently, the model only considers the immunity provided by the primary series of vaccination. Expanding the model to include immunity from partial vaccination [25] and booster doses would enhance its realism. We anticipate that integrating immunity from partial and booster vaccinations into the model would likely result in higher estimates of averted outcomes, as increased population immunity would require an upward adjustment of transmission rates to align with the observed outcomes in 2021.
Additionally, improving the precision of parameter values would further enhance the accuracy of our model estimates. For example, refining the relative infectiousness of asymptomatic/mild cases (\(\:\varphi\:\)) could involve measuring the differences in transmission or number of contacts between hospitalized and non-hospitalized individuals. Moreover, using a time-dependent vaccine efficacy might improve the realism of our model, particularly given the lower vaccine efficacy observed against the Delta variant [12]. To address this concern, we incorporated waning immunity into the model and adopted a conservative range of vaccine efficacy values: six months from the last dose for the BioNTech/Pfizer and SpikeVax/Moderna vaccines [9], 20 weeks from the last dose for the Oxford/AstraZeneca vaccine [11], and 28 days from the last dose for the Janssen/Johnson&Johnson vaccine [10].
Our model can also be further refined by subdividing it to accommodate more age groups, accounting for further differences in hospitalization and death rates. Due to the lack of information on contact rates between each age group in the Basque Country and vaccine effectiveness for smaller age groups, we strategically chose to split the population into two age groups (< 50 and ≥ 50 years old). Our observations indicate that adding age structure to the model and defining higher hospitalization rates for older age groups resulted in lower estimates of averted outcomes. It is therefore crucial to investigate further the impact of including more age groups on the estimates of total averted outcomes.
Despite these model limitations, a bootstrap analysis of one of our models (Sect. 6 of Online Resource) revealed that our estimates of averted outcomes are identifiable and robust even when introducing uncertainty [38]. Additionally, applying the same methodology to variations of our models where ICU admission is a direct infection outcome [21] (Sect. 8 of Online Resource) further affirms that our estimates of averted outcomes are robust to slight modifications in the models.
This study assesses the impact of COVID-19 vaccination in preventing severe outcomes and fatalities in the Basque Country region of Spain in 2021. Through scenario simulations comparing the year of 2021 with and without vaccination, our results underscore the utility of mathematical modeling in estimating the impacts of health interventions. Furthermore, it emphasizes the crucial role of proactive vaccination initiatives in controlling the rise of adverse outcomes and highlights the importance of sustained vaccination efforts in mitigating the effects of future disease outbreaks.
Data Availability
The COVID-19 data used in this study were provided by the Basque Health Service (Osakidetza). Our data agreement stipulates that the data cannot be shared publicly. Aggregates of the data are publicly available in https://opendata.euskadi.eus/catalogo/-/evolucion-del-coronavirus-covid-19-en-euskadi/.
References
EUSTAT. Statistical overview of elderly persons. [Accessed 09-April-2024]. https://en.eustat.eus/estadisticas/tema_1280/opt_1/ti_statistical-overview-of-elderly-persons/temas.html
World Bank. Population ages 65 and above (% of total population). [Accessed 09-April-2024]. https://data.worldbank.org/indicator/SP.POP. 65UP.TO.ZS
World Health Organization. Case Study: The Age-friendly Programme in Basque Country (Euskadi). [Accessed 19-June-2024].
EUSTAT. Población. [Accessed 09-April-2024]. https://es.eustat.eus/bancopx/spanish/id_2212/indiceRR.html
Minder R. Spain becomes latest epicenter of coronavirus after a faltering response. [Accessed 09-April-2024]. https://www.nytimes.com/2020/03/13/world/europe/spain-coronavirus-emergency.html
Departamento de Salud, Euskadi. Boletin Oficial del País Vasco, Decreto 36.2020. [Accessed 09-April-2024]. https://www.euskadi.eus/normativa-de-medidas-excepcionales-adoptadas-por-el-nuevo-coronavirus-covid-19/web01-a2korona/es/
La Moncloa. El Gobierno decreta un estado de alarma para dar amparo constitucional pleno a las medidas contra la pandemia necesarias en las CC.AA. [Accessed 27-May-2024]. https://www.lamoncloa.gob.es/presidente/actividades/Paginas/2020/251020estado-alarma.aspx
OPEN DATA EUSKADI. Evolución del coronavirus (COVID-19) en Euskadi. [Accessed 09-April-2024]. https://opendata.euskadi.eus/catalogo/-/evolucion-del-coronavirus-covid-19-en-euskadi/
Lin DY, Gu Y, Wheeler B, Young H, Holloway S, Sunny SK, et al. Effectiveness of Covid-19 vaccines over a 9-Month period in North Carolina. N Engl J Med. 2022;386(10):933–41. https://doi.org/10.1056/nejmoa2117128.
Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Final analysis of efficacy and safety of single-dose Ad26.COV2.S. N Engl J Med. 2022;386(9):847–60. https://doi.org/10.1056/nejmoa2117608.
Katikireddi SV, Cerqueira-Silva T, Vasileiou E, Robertson C, Amele S, Pan J, et al. Two-dose ChAdOx1 nCoV-19 vaccine protection against COVID-19 hospital admissions and deaths over time: a retrospective, population-based cohort study in Scotland and Brazil. Lancet. 2022;399(10319):25–35. https://doi.org/10.1016/s0140-6736(21)02754-9.
Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infec. 2022;28(2):202–21. https://doi.org/10.1016/j.cmi.2021.10.005.
Vilches TN, Abdollahi E, Cipriano LE, Haworth-Brockman M, Keynan Y, Sheffield H, et al. Impact of non-pharmaceutical interventions and vaccination on COVID-19 outbreaks in Nunavut, Canada: a Canadian Immunization Research Network (CIRN) study. BMC Public Health. 2022;22(1). https://doi.org/10.1186/s12889-022-13432-1.
Shoukat A, Vilches TN, Moghadas SM, Sah P, Schneider EC, Shaff J, et al. Lives saved and hospitalizations averted by COVID-19 vaccination in New York City: a modeling study. Lancet Reg Health Am. 2022;5:100085. https://doi.org/10.1016/j.lana.2021.100085.
Ferreira LS, Darcie Marquitti FM, Paixão da Silva RL, Borges ME, Ferreira da Costa Gomes M, Cruz OG, et al. Estimating the impact of implementation and timing of the COVID-19 vaccination programme in Brazil: a counterfactual analysis. Lancet Reg Health Am. 2023;17:100397. https://doi.org/10.1016/j.lana.2022.100397.
Santos CVBd N, TGd, Werneck GL, Struchiner CJ, Villela DAM. Estimated COVID-19 severe cases and deaths averted in the first year of the vaccination campaign in Brazil: a retrospective observational study. Lancet Reg Health Am. 2023;17:100418. https://doi.org/10.1016/j.lana.2022.100418.
Kayano T, Sasanami M, Kobayashi T, Ko YK, Otani K, Suzuki M, et al. Number of averted COVID-19 cases and deaths attributable to reduced risk in vaccinated individuals in Japan. Lancet Reg Health Am West Pac. 2022;28:100571. https://doi.org/10.1016/j.lanwpc.2022.100571.
Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22(9):1293–302. https://doi.org/10.1016/s1473-3099(22)00320-6.
He D, Ali ST, Fan G, Gao D, Song H, Lou Y, et al. Evaluation of effectiveness of global COVID-19 vaccination campaign. Emerg Infect Dis. 2022;28(9):1873–6. https://doi.org/10.3201/eid2809.212226.
Mar J, Ibarrondo O, Estadilla CDS, Stollenwerk N, Antoñanzas F, Blasco-Aguado R, et al. Cost-effectiveness analysis of vaccines for COVID-19 according to sex, Comorbidity and Socioeconomic Status: a Population Study. PharmacoEconomics. 2023. https://doi.org/10.1007/s40273-023-01326-y.
Aguiar M, Ortuondo EM, Van-Dierdonck JB, Mar J, Stollenwerk N. Modelling COVID 19 in the Basque Country from introduction to control measure response. Sci Rep-UK. 2020;10(1). https://doi.org/10.1038/s41598-020-74386-1.
Aguiar M, Van-Dierdonck JB, Mar J, Cusimano N, Knopoff D, Anam V, et al. Critical fluctuations in epidemic models explain COVID-19 post-lockdown dynamics. Sci Rep-UK. 2021;11(1). https://doi.org/10.1038/s41598-021-93366-7.
Fu Y, Zhao J, Han P, Zhang J, Wang Q, Wang Q, et al. Cost-effectiveness of COVID-19 vaccination: a systematic review. J Evid-Based Med. 2023;16(2):152–65. https://doi.org/10.1111/jebm.12525.
Utami AM, Rendrayani F, Khoiry QA, Noviyanti D, Suwantika AA, Postma MJ, et al. Economic evaluation of COVID-19 vaccination: a systematic review. J Glob Health. 2023;13. https://doi.org/10.7189/jogh.13.06001.
Stollenwerk N, Estadilla CDS, Mar J, Van-Dierdonck JB, Ibarrondo O, Blasco-Aguado R, et al. The effect of mixed vaccination rollout strategy: a modelling study. Infec Dis Model. 2023;8(2):318–40. https://doi.org/10.1016/j.idm.2023.03.002.
Aguiar M, Van-Dierdonck JB, Mar J, Stollenwerk N. The role of mild and asymptomatic infections on COVID-19 vaccines performance: a modeling study. J Adv Res. 2022;39:157–66. https://doi.org/10.1016/j.jare.2021.10.012.
Srivasrav AK, Stollenwerk N, Bidaurrazaga Van-Dierdonck J, Mar J, Ibarrondo O, Aguiar M. Modeling the initial phase of COVID-19 epidemic: the role of age and disease severity in the Basque Country, Spain. PLoS ONE. 2022;17(7):e0267772. https://doi.org/10.1371/journal.pone.0267772.
D’Errico J. fminsearchbnd, fminsearchcon. MATLAB Central File Exchange. [Accessed 09-April-2024]. https://www.mathworks.com/matlabcentral/fileexchange/8277-fminsearchbnd-fminsearchcon
Marino S, Hogue IB, Ray CJ, Kirschner DE. A methodology for performing global uncertainty and sensitivity analysis in systems biology. J Theor Biol. 2008;254(1):178–96. https://doi.org/10.1016/j.jtbi.2008.04.011.
de los Reyes AA, Escaner JML. Dengue in the Philippines: model and analysis of parameters affecting transmission. J Biol Dynam. 2018;12(1):894–912. https://doi.org/10.1080/17513758.2018.1535096.
National Center for Immunization and Respiratory Diseases. COVID-19 can surge throughout the year. U.S. Centers for Disease Control and Prevention. [Accessed 07-August-2024].
Saey TH. Here’s why COVID-19 isn’t seasonal so far. ScienceNews. [Accessed 07-August-2024]. https://www.sciencenews.org/article/why-covid-not-seasonal
Eustat. Trips, overnight stays and average travel time (days) of tourists from abroad to the Basque Country by month. 2016–2022. [Accessed 07-August-2024]. https://en.eustat.eus/bankupx/pxweb/en/DB/-/PX_132614_fe_mti_a_03.px/
Mathieu E, Ritchie H, Ortiz-Ospina E, Roser M, Hasell J, Appel C, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5(7):947–53. https://doi.org/10.1038/s41562-021-01122-8.
Marco-Franco J, Pita-Barros P, González-de Julián S, Sabat I, Vivas-Consuelo D. Simplified Mathematical Modelling of uncertainty: cost-effectiveness of COVID-19 vaccines in Spain. Mathematics. 2021;9(5):566. https://doi.org/10.3390/math9050566.
Kohli M, Maschio M, Becker D, Weinstein MC. The potential public health and economic value of a hypothetical COVID-19 vaccine in the United States: Use of cost-effectiveness modeling to inform vaccination prioritization. Vaccine. 2021;39(7):1157–64. https://doi.org/10.1016/j.vaccine.2020.12.078.
Nagano M, Kamei K, Matsuda H, Takahashi C, Yang J, Wada K, et al. Cost-effectiveness analysis of COVID-19 booster vaccination with BNT162b2 in Japan. Expert Rev Vaccines. 2024;23(1):349–61. https://doi.org/10.1080/14760584.2024.2323133.
Chowell G. Fitting dynamic models to epidemic outbreaks with quantified uncertainty: a primer for parameter uncertainty, identifiability, and forecasts. Infec Dis Model. 2017;2(3):379–98. https://doi.org/10.1016/j.idm.2017.08.
Acknowledgements
The authors would like to acknowledge Dr. Ganna Rozhnova of the Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, for her valuable feedback on the initial iterations of our model.
Funding
MA acknowledges the financial support by the Ministerio de Ciencia e Innovacion (MICINN) of the Spanish Government through the Ramon y Cajal grant RYC2021-031380-I. This research is also supported by the Basque Government through the “Mathematical Modeling Applied to Health” (BMTF) Project, BERC 2022–2025 program and by the Spanish Ministry of Sciences, Innovation and Universities: BCAM Severo Ochoa accreditation CEX2021-001142-S / MICIN / AEI / https://doi.org/10.13039/501100011033, and EITB Marathon 2021 call, reference BIO21/COV/001.
Author information
Authors and Affiliations
Contributions
CE, MA, NS, and JM contributed to the study design. CE, MA, and NS developed the transmission model. JM and OI prepared the data for model calibration. CE implemented the model calibration, prepared the figures, and drafted the initial manuscript. All authors read and approved the final version of the manuscript. Furthermore, all authors had full access to the data used in this study and accepted the responsibilities for each submission.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Estadilla, C.D., Mar, J., Ibarrondo, O. et al. Impact of High Covid-19 Vaccination Rate in an Aging Population: Estimating Averted Hospitalizations and Deaths in the Basque Country, Spain Using Counterfactual Modeling. J Epidemiol Glob Health (2024). https://doi.org/10.1007/s44197-024-00286-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44197-024-00286-6