Abstract
Background
Mammography (MG) has demonstrated its effectiveness in diminishing mortality and advanced-stage breast cancer incidences in breast screening initiatives. Notably, research has accentuated the superior diagnostic efficacy and cost-effectiveness of digital breast tomosynthesis (DBT). However, the scope of evidence validating the cost-effectiveness of DBT remains limited, prompting a requisite for more comprehensive investigation. The present study aimed to rigorously evaluate the cost-effectiveness of DBT plus MG (DBT-MG) compared to MG alone within the framework of Taiwan’s National Health Insurance program.
Methods
All parameters for the Markov decision tree model, encompassing event probabilities, costs, and utilities (quality-adjusted life years, QALYs), were sourced from reputable literature, expert opinions, and official records. With 10,000 iterations, a 2-year cycle length, a 30-year time horizon, and a 2% annual discount rate, the analysis determined the incremental cost-effectiveness ratio (ICER) to compare the cost-effectiveness of the two screening methods. Probabilistic and one-way sensitivity analyses were also conducted to demonstrate the robustness of findings.
Results
The ICER of DBT-MG compared to MG was US$5971.5764/QALYs. At a willingness-to-pay (WTP) threshold of US$33,004 (Gross Domestic Product of Taiwan in 2021) per QALY, more than 98% of the probabilistic simulations favored adopting DBT-MG versus MG. The one-way sensitivity analysis also shows that the ICER depended heavily on recall rates, biopsy rates, and positive predictive value (PPV2).
Conclusion
DBT-MG shows enhanced diagnostic efficacy, potentially diminishing recall costs. While exhibiting a higher biopsy rate, DBT-MG aids in the detection of early-stage breast cancers, reduces recall rates, and exhibits notably superior cost-effectiveness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recent studies support mammography’s (MG) effectiveness in reducing breast cancer mortality and late-stage cases during screening [1,2,3]. High breast density, found in 50% of Western and 70% of Asian women, can limit MG’s sensitivity and cancer detection [4, 5]. To address this, additional screening tools like digital breast tomosynthesis (DBT) have emerged, offering potential benefits such as reduced false positives, improved cancer detection, and fewer recalls [6,7,8]. This highlights the evolving nature of breast cancer screening and management.
The DBT is gaining recognition for its improved cancer detection abilities, particularly in women with dense breast tissue [9]. Evidence from the ASTOUND-2 trial, which focused on women with dense breasts and digital MG, revealed an incremental cancer detection rate of 2.83 per 1000 screens [10]. Additionally, a meta-analysis study reported an incremental cancer detection rate of 2.4 cancers per 1000 screens in biennial screening using DBT, with only a marginal increase in recall rates compared to MG [2]. However, limited research has examined the cost-effectiveness of DBT plus MG compared to MG in the context of healthcare economics. Although some studies have compared their cost-effectiveness, only a handful have delved into aspects like recall rates and the broader economic impact, including productivity [9, 11,12,13,14]. Importantly, cost-effectiveness and utility analyses of these screening methods within Asian populations and Taiwan’s National Health Insurance (NHI) program are notably lacking. Therefore, this study aims to fill this gap by investigating the cost-effectiveness of DBT plus MG (DBT-MG) compared to MG alone in breast cancer screening in Taiwan.
2 Materials and Methods
2.1 Research Design
From the perspective of the Taiwanese Government, which encompasses a comprehensive societal viewpoint accounting for both direct and indirect costs, including productivity losses. The Markov model was employed to perform dynamic simulations and estimations based on existing literatures [15,16,17,18,19]. Then, we analyzed the recall rate, cancer detection rate, breast cancer detection rate, treatment cost of each stage, breast cancer and related quality-adjusted life years (QALYs), and other related data. Specifically, the Markov decision model were used to simulate 10,000 times for every two-year cycle. This study was reviewed and approved by the Institutional Review Board of the Kaohsiung Medical University Hospital (KMUHIRB-E(I)-20220012).
2.2 Markov Decision Modeling Assumptions
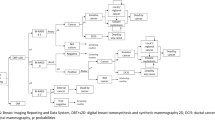
Informed by clinical practices in Taiwan and relevant literature, we developed the status transition diagram of the Markov model utilized in this study (Fig. 1A). The model delineates four states after entering the screening cycle: screening, cancer, missed cancer, and death. In the international medical context, Taiwan’s postoperative or post-treatment breast cancer surveillance strategies advocate ongoing screening [15, 20, 21]. Following breast cancer surgery, patients who have undergone partial or unilateral mastectomy are advised to continue breast screening, although the recommended screening intervals may vary by country and individual case. In addition to biennial breast screening, diagnostic mammography may also be necessary. Our study assumes that all patients will adhere to the recommendation for continued breast imaging examinations following treatment. Additionally, the selection of a 30-year time horizon for our economic evaluation was predicated on Taiwan’s existing breast screening policy, which mandates biennial screenings. This temporal scope captures the period of screening eligibility and accommodates mortality from breast cancer and other causes. In adherence to this policy, all asymptomatic individuals, irrespective of cancer detection status, undergo biennial screening until the conclusion of their lifespans. Natural mortality rates for both screening tools (DBT-MG & MG) were presumed equal for each cycle and were not factored into the model; only breast cancer-related mortality was simulated [15]. Upon transitioning to the death stage, individuals remain in this state indefinitely, precluding further transitions.
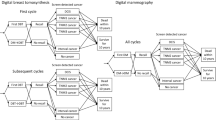
The Markov decision tree model is illustrated in Fig. 1B. Subsequent to screening, outcomes encompass negative results or recalls. Upon recall, results may be negative or necessitate further pathologic diagnosis (path + biop), potentially culminating in a negative breast cancer pathology result. Our premise posits that all asymptomatic screening subjects, irrespective of cancer detection, perpetuate the biennial screening cycle until mortality. Moreover, we posit an absence of post-screening loss to follow-up or untreated individuals in Breast Imaging Reporting and Data System (BI-RADS) categories 0, 4, and 5. Such subjects would undergo subsequent evaluation [22, 23]. To streamline model intricacies, false-negative outcomes with MG are construed as missed cancers. Furthermore, “missed cancer” refers to instances where cancer remains undetected despite the utilization of both DBT-MG and MG screening modalities. It is quantified by the difference in cancer detection rates between the DBT-MG and MG groups [24]. Therefore, in the present study, the DBT-MG group is not included in the category of “missed cancer.” Additionally, in consonance with disparities in cancer detection rates between DBT-MG and MG, we presuppose the absence of stage 0 breast cancer occurrences among missed cancers using MG; other stages (1–4) are redistributed based on national incidence rates. Enhanced screening tools tend to discern a greater proportion of early-stage cancers, potentially yielding cost efficiencies and enhanced clinical outcomes. Breast cancer stages 0 through 4 are encompassed within the model. Following each two-year cycle culmination, in instances where screening results, reexamination, biopsy, and subsequent stages yield negative findings, or where no breast cancer-related mortalities occur, the cycle reverts to breast cancer screening status.
2.3 Transitional Model Parameters
2.3.1 Probability
This study adopted Pan et al. [15] as the primary basis of the screening effectiveness of DBT-MG and MG, such as recall rate, cancer detection rate, biopsy rate, and biopsy positive rate. To our knowledge, this is the first study to compare the results before and after the complete introduction of DBT-MG to replace MG as the breast screening tool in a medical center in Taiwan. DBT-MG and MG were used to diagnose breast cancer at each stage, and the study by Pan et al. [15] calculated the ratio of missed cancer by MG at each stage and the publicly available data of the Health Promotion Administration (Cancer Registry Annual Report 2019), respectively [16]. We assumed that there were no stage 0 breast cancer cases among the missed cancers using MG, and the remaining stages (stages 1–4) were redistributed based on the national incidence (Table 1).
2.3.2 Cost
Initially, both direct and indirect costs were computed using an exchange rate of 30:1 for NTD to USD. Subsequently, the direct costs and indirect costs were determined based on the actual treatments received by patients and the reimbursements from the national health insurance, adjusted to 2021 prices using the consumer price index (CPI). Direct costs included direct medical costs of screening, recall, biopsy, and pathological diagnosis, as well as direct medical costs associated with different stages of cancer, as described below. (1) Direct costs of examination: initial examination cost for DBT-MG was $150, whereas MG cost $41.5. The DBT-MG cost was derived from the current self-funded examination rate approved by the Kaohsiung Municipal Government ($150), while the MG cost represented the health insurance reimbursement rate for MG ($41.5). In general, the total cost per screening before being diagnosed with breast cancer was $177.81 for DBT-MG and $171.39 for MG, with a difference of only $6.43. (2) Direct costs of recall: health insurance costs of recall included $588 for breast ultrasonography, $286 for general outpatient diagnosis, and $1374 for diagnostic MG. Based on clinical experience and the health insurance payment review procedures and amendments, we assumed that each recalled screenee would undergo ultrasound reexamination twice within two years, and one in 20 recalled patients would undergo diagnostic MG reexamination four times over two years, etc. (3) Direct costs of biopsy and pathological diagnosis: $526 would be paid in accordance with the payment criteria for the diagnosis of breast cancer according to the Breast Cancer Medical Payment Improvement Program of National Health Insurance (revised on July 1st, 2021). (4) Direct costs associated with different stages of cancer: according to Lin et al. [25], in Taiwan, the lifetime medical expenditures (LME) of breast cancer in different stages were calculated based on the Health Promotion Administration and National Health Insurance Administration data. We hypothesize that due to the lower five-year survival rate and shorter life expectancy associated with Stage 4, the potential medical expenses incurred may also be lower compared to Stage 3.
Additionally, indirect costs included the indirect social costs of recall, biopsy, and pathological diagnosis, as well as treatment at different stages of cancer, as described below. (1) Indirect costs of recall, biopsy, and pathological diagnosis: we assumed that half a day (four hours) of productivity would be lost for a subsequent visit due to recall, and one day (eight hours) of productivity would be lost for pathological diagnosis and biopsy. According to the Employee Salary Survey and Labor Force Participation Rate in Taiwan announced by the Directorate General of Budget, Accounting, and Statistics, the Executive Yuan in 2021 [26], the hourly wage for women was $10, and the average labor force participation rate was 51.49%. The indirect costs of recall and biopsy diagnosis were calculated using the equation “lost labor force hours × hourly wage × labor force participation rate.” (2) Indirect costs of breast cancer at different stages and death: based on Huang et al. study conducted in Taiwan, the three-year indirect costs of breast cancer morbidity and mortality were predicted to be $588 and $5117, respectively [18].
2.3.3 Effectiveness (QALYs)
A QALY quantifies a year lived in perfect health as 1, calculated by multiplying the duration in a health state by its utility value. This study assumed that the QALYs of the screenee with breast cancer was 1 and that of the deceased was 0. Since there were no suitable studies in Taiwan regarding the QALYs of breast cancer for reference, we adopted the QALYs of the first year and beyond at each stage of breast cancer based on an American study conducted by Schousboe et al. [19]. Then, we calculated the utility loss of each cycle (two years per cycle) relative to healthy patients and input the data into the Markov decision model for simulation.
2.4 Cost-Utility Analysis (CUA)
CUA was used to compare the difference in total medical costs and QALYs between the two screening options, and the results were expressed as an incremental cost-effectiveness ratio (ICER). First, we established the Markov decision model, and each state transition probability value, cost, and effectiveness/utility value were pre-set. Second, the results of each stage were summed by simulating the state transition of patients during the course of treatment. The Markov decision tree model was used to simulate the understanding of which option is more cost-effective. Additionally, in the Markov decision model, the ICER of the two screening tools was simulated using a 2% annual discount rate in cost and QALYs based on the literature and willingness to pay (WTP) was set as gross domestic product (GDP) per capita in 2022, namely, $33,004.
2.5 Sensitivity Analysis
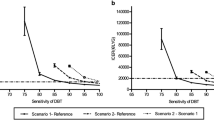
The sensitivity analysis was conducted to identify which risk or uncertainty sources have the most significant potential impact on breast cancer. The descriptions of related settings are as follows: (1) one-way sensitivity analysis: with all uncertainties maintained under baseline conditions, the degree to which the uncertainty of each factor affects the terminal objective analyzed. A tornado diagram was used as the analysis tool. All sensitive parameters were ranked according to their sensitivities to reflect the impact of each sensitive parameter on the result. The examination effects of the two screening tools in this study (recall rate, biopsy rate, positive predictive value, cancer detection rate, etc.) were measured with parameters presented in the range of the available national literature. Parameters, such as total medical costs and QALYs value of each stage and state underwent ± 20% variation based on the national data and literature. The results are presented in the tornado diagram. (2) Probabilistic sensitivity analysis (PSA): the model variables show a probability distribution. The parameters were randomly sampled using Monte Carlo simulation. The distribution of cost and utility in each quadrant was analyzed by the scatter plot. Additionally, the cost-utility benefits of DBT-MG became more pronounced with increasing screening cycles. However, the enhancement in cost-utility from 20 to 30 consecutive years was not as notable as from 10 to 20 consecutive years, suggesting a dose effect on the screening policy. It further underscores the importance of continued implementation of breast cancer screening policies to achieve better outcomes spontaneously. We also conduct the sensitivity analysis of different screening cycles of DBT-MG and MG for breast cancer screening, spanning 10-year, 20-year, and 30-year time horizons. WTP thresholds and ICERs were checked to determine whether the best breast screening option could be available under the highest cost-utility rate and within the acceptable payment range of screening subjects.
Descriptive and inferential statistical analyses were performed using IBM SPSS 23. The cost-effectiveness acceptability (CEA) and sensitivity analysis were performed using TreeAge Pro Healthcare 2021. Statistical significance was set at α = 0.05.
3 Results
According to the results of the Markov decision model (Table 2), after 10,000 simulations and 15 cycles for a total of 30 years, the cumulative cost of DBT-MG was $7861.14, and MG was $7621.73. The incremental cost was $239.42. Regarding the effectiveness, DBT-MG was 22.9942 QALYs, and MG was 22.9541 QALYs. The incremental effectiveness was 0.0401 QALYs. The ICER of DBT-MG vs. MG was $5971.5764/QALYs, which was far lower than the set WTP. This finding suggests that applying DBT-MG is more cost-effective than MG to biennial breast screening. Additionally, the Markov cost-utility analysis results of five cycles (10 years), 10 cycles (20 years), and 15 cycles (30 years) were simulated with different screening years (Table 2). All ICERs were lower than the WTP for the screening cycles from 10 to 30 years. As the screening cycle increased, the cost-utility results of DBT-MG became more accentuated. However, the increase in cost utility from 20 to 30 consecutive years was not as significant as from 10 to 20 consecutive years.
In the comparative assessment of Net Monetary Benefits (NMBs) between the two screening tools, an incremental rise in the WTP threshold corresponded with an augmentation in the NMBs for both strategies (Fig. 2). Despite this trend, the differential magnitude between the two approaches remained relatively stable. Critically, the NMB associated with the DBT-MG exceeded that of the MG, underscoring the enhanced cost-utility of the integrated screening methodology.
After 10,000 Monte Carlo simulations, 10,000 points were presented in the incremental cost-utility scatter plot (Fig. 3A). The findings indicate an 83.7% probability that it is located in the cost-effective zone in the first quadrant (lower than the WTP); i.e., it is, therefore, cost-effective. Moreover, there was a 14.78% probability that it is located in the cost-saving zone of the fourth quadrant. Therefore, DBT-MG had a total probability of more than 98.48% as the more cost-effective breast screening option. Additionally, the results showed that the probability of the cost-effectiveness of MG decreases with an increasing WTP threshold, while that of DBT increase with an increasing WTP threshold (Fig. 3B). When the WTP is greater than the crossing WTP value of the acceptance curve of DBT-MG, which is $5971.5764/QALYs, the probability of the cost-effectiveness of DBT-MG is greater than that of MG.
In the context of cost and QALY estimations, input parameters were anchored to the central estimates of the Markov decision tree model, with a permissible range of ± 20%. An exhaustive one-way sensitivity analysis was conducted to evaluate the impact of individual variables on the ICER, as delineated in Fig. 4. The outcomes of this analysis demonstrated a spectrum of ICERs, spanning from − $9020.36/QALYs to $25660.23/QALYs. Notably, the most influential determinants on the ICERs encompassed the recall rate associated with DBT-MG, the pathological biopsy rate for MG, MG’s recall rate, the pathological biopsy rate for DBT-MG, the biopsy’s positive predictive value 2 (PPV2) for MG, and DBT-MG’s biopsy PPV2.
Deterministic sensitivity analysis—results as tornado plot. Bars indicate the effect of a ± 20% variance of a variable on the ICER. The red segments of the bars represent values that elevate the base case ICER, while the blue segments depict values that reduce the base case ICER. This color-coded scheme aids in interpreting the sensitivity analysis outcomes. DBT digital breast tomosynthesis; MG mammography; ICER incremental cost effectiveness ratio
4 Discussion
In Taiwan, despite the higher initial examination cost of DBT-MG compared to MG, DBT-MG demonstrates superior diagnostic capability, potentially reducing recall costs. Despite a higher biopsy rate, DBT-MG facilitates the detection of more breast cancers, particularly in the early stages. According to existing literature, early breast cancer treatment is associated with lower costs and higher QALYs. Thus, employing DBT-MG aids in the early detection and treatment of breast cancer, potentially reducing subsequent cumulative treatment costs and increasing cumulative QALYs. Simulation-based cost-utility analysis corroborates that while the cumulative cost of DBT-MG rises, cumulative QALYs also increase. With a probability exceeding 98.48% under the WTP, the 30-year simulation indicates that DBT-MG is likely to be cost-effective and cost-saving.
This Markov decision model includes screening, recall, and further pathologic diagnosis stages. The cost of treating breast cancer is high, but the actual cancer detection rate during screening is low (< 1%). Thus, cancer treatment costs have minimal impact in the overall model. The recall rate, biopsy rate, and biopsy positive rate significantly affect the model's outcomes, based on one-way sensitivity analysis.
The NMB metric amalgamates both costs and effectiveness into a unified measure, computed as the product of effectiveness and the WTP threshold, subtracted by costs. In the present study, as the WTP increased, the NMB of both screening strategies also increased. However, reviewing the data in detail, we found that when WTP was set as $33,004, DBT-MG had an NMB of $751,039.34, and MG had an NMB of $749,955.53. Therefore, DBT-MG has relative advantages over MG.
Our study is in line with prior research in the US and Europe, demonstrating the cost-effectiveness benefits of DBT-MG (Table 3) [9, 17, 24, 27, 28]. Notably, it’s the first to integrate societal-level indirect costs, including productivity loss, into the analysis. While data limitations exist, we used Taiwanese medical data supplemented by international literature, expert input, and some assumptions. Our Markov decision model's key parameters, such as transitional probabilities, costs, and QALYs, mainly relied on government sources and Taiwanese literature. These findings strongly support the government’s adoption of DBT-MG as a preferred breast screening policy [9, 17, 24, 27, 28].
In our sensitivity analysis, recall rate, PPV2, and biopsy rate were key factors influencing the ICER, with DBT-MG examination cost being less critical [9, 17, 24, 27, 28]. Probabilistic sensitivity analysis over 15 cycles and 30 years indicated a high likelihood (> 98.48%) of DBT-MG being cost-effective and cost-saving. Previous studies on breast screening tools’ cost-effectiveness vary, but collectively support DBT’s effectiveness. Our study shares similarities with previous work but offers unique insights.
Through further analysis using Pan et al. we found that the breast cancer detection rate of DBT-MG was 8.68‰, while that of MG was 7.55‰ [15]. The early breast cancer detection rate of DBT-MG (cancer detection rate * early breast cancer detection rate) was calculated as 5.31‰, while the early breast cancer detection rate of MG was 3.62‰. Therefore, DBT-MG can detect 1.69 more cases of early breast cancer than MG per 1000 women screened. The total cost per screening before being diagnosed with breast cancer was $177.81 for DBT-MG and $171.39 for MG, with a difference of only $6.43. Overall, if all the 738,974 women screened in 2019 had been screened with DBT-MG and had the same diagnostic capability advantages, the total costs would increase by $4,751,602.82 and about 1248 more women with early-stage breast cancer would be detected [16]. This analysis can also provide a reference for the government to decide on future screening policies.
This study has limitations. It relied on data from a single medical center in Taiwan for DBT-MG and MG [15], which may limit generalizability. Furthermore, some cost calculations were based on assumptions rather than real-world data. QALYs data specific to Taiwanese women is lacking. Additionally, important factors like age, breast density, health status, and family history were not extensively considered, which could be explored in future research.
5 Conclusions
An effective breast screening tool is vital for early cancer detection and reducing the burdens of breast cancer [29]. This study suggests the government should prioritize using DBT-MG for screening, initially for high-risk groups, and then potentially for the entire population, based on the experience with MG screening since 2002. Our 30-year simulation with 15 cycles found DBT-MG to be highly cost-effective at $5971.57/QALYs, well below the WTP threshold. Wider DBT-MG adoption can enhance early breast cancer detection and program efficiency. Notably, the recall rate, biopsy rate, and biopsy positive rate PPV2 impact sensitivity. This study provides valuable insights for healthcare decision-makers and suggests analyzing cost-effectiveness for various risk groups and additional variables in future research.
Availability of Data, Material and Code
All data generated can be found in the published article. The data analyzed can be provided upon request.
Abbreviations
- MG:
-
Mammography
- DBT:
-
Digital breast tomosynthesis
- NHI:
-
National health insurance
- QALYs:
-
Quality-adjusted life years
- BI-RADS:
-
Breast imaging reporting and data system
- LME:
-
Lifetime medical expenditures
- ICER:
-
Incremental cost effectiveness ratio
- WTP:
-
Willingness to pay
- GDP:
-
Gross domestic product
- PSA:
-
Probabilistic sensitivity analysis
- CEA:
-
Cost-effectiveness acceptability
- NMB:
-
Net monetary benefits
- PPV2:
-
Positive predictive value 2
References
Kerlikowske K, Su YR, Sprague BL, et al. Association of screening with digital breast tomosynthesis vs digital mammography with risk of interval invasive and advanced breast cancer. JAMA. 2022;327:2220–30.
Ahmed GY, Al Mutair A, Bashir S, et al. Attitudes and practice of health care providers toward cancer screening: a cross-sectional multicenter study. Saudi Arabia J Epidemiol Glob Health. 2022;12(4):383–9.
Houssami N. Should tomosynthesis replace mammography for breast cancer screening? Lancet Oncol. 2022;23(5):554–5.
Jiang S, Bennett DL, Rosner BA, Colditz GA. Longitudinal analysis of change in mammographic density in each breast and its association with breast cancer risk. JAMA Oncol. 2023;9:808–14.
Bae JM, Kim EH. Breast density and risk of breast cancer in asian women: a meta-analysis of observational studies. J Prev Med Public Health. 2016;49:367–75.
Barton MK. Digital breast tomosynthesis may improve breast cancer detection rates. CA Cancer J Clin. 2013;63(5):291–2.
Lee CI, Chen LE, Elmore JG. Risk-based breast cancer screening: implications of breast density. Med Clin North Am. 2017;101:725–41.
Heindel W, Weigel S, Gerß J, et al. Digital breast tomosynthesis plus synthesised mammography versus digital screening mammography for the detection of invasive breast cancer (TOSYMA): a multicentre, open-label, randomised, controlled, superiority trial. Lancet Oncol. 2022;23(5):601–11.
Sprague BL, Coley RY, Lowry KP, et al. Digital breast tomosynthesis versus digital mammography screening performance on successive screening rounds from the breast cancer surveillance consortium. Radiology. 2023;307(5): e223142.
Tagliafico AS, Mariscotti G, Valdora F, et al. A prospective comparative trial of adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts (ASTOUND-2). Eur J Cancer. 2018;104:39–46.
Blankenburg M, Sánchez-Collado I, Soyemi BO, et al. Economic evaluation of supplemental breast cancer screening modalities to mammography or digital breast tomosynthesis in women with heterogeneously and extremely dense breasts and average or intermediate breast cancer risk in US healthcare. J Med Econ. 2023;26(1):850–61.
Wong FL, Lee JM, Leisenring WM, et al. Health benefits and cost-effectiveness of Children’s Oncology Group breast cancer screening guidelines for chest-irradiated hodgkin lymphoma survivors. J Clin Oncol. 2023;41:1046–58.
Nicosia L, Bozzini AC, Pesapane F, et al. Breast digital tomosynthesis versus contrast-enhanced mammography: comparison of diagnostic application and radiation dose in a screening setting. Cancers (Basel). 2023;15(9):2413.
Lowry KP, Trentham-Dietz A, Schechter CB, et al. Long-term outcomes and cost-effectiveness of breast cancer screening with digital breast tomosynthesis in the United States. J Natl Cancer Inst. 2020;112:582–9.
Pan HB, Wong KF, Yao A, et al. Breast cancer screening with digital breast tomosynthesis - 4 year experience and comparison with national data. J Chin Med Assoc. 2018;81:70–80.
Health Promotion Administration, Ministry of Health and Welfare (MOHW). Breast X-ray mammography screening reduces the risk of breast cancer mortality by 40%. Statistic Reports in Taiwan. 2021. Available from: https://www.hpa.gov.tw/EngPages/Detail.aspx?nodeid=1051&pid=5957. Accessed 26 Oct 2021.
Wang J, Phi XA, Greuter MJW, et al. The cost-effectiveness of digital breast tomosynthesis in a population breast cancer screening program. Eur Radiol. 2020;30:5437–45.
Huang SY, Chen HM, Liao KH, Ko BS, Hsiao FY. Economic burden of cancers in Taiwan: a direct and indirect cost estimate for 2007–2017. BMJ Open. 2020;10(10): e036341.
Schousboe JT, Sprague BL, Abraham L, et al. Cost-effectiveness of screening mammography beyond age 75 years: a cost-effectiveness analysis. Ann Intern med. 2022;175:11–9.
Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–614.
Khatcheressian JL, Hurley P, Bantug E, et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(7):961–5.
Gwak H, Woo SS, Oh SJ, et al. A Comparison of the prognostic effects of fine needle aspiration and core needle biopsy in patients with breast cancer: a nationwide multicenter prospective registry. Cancers (Basel). 2023;15(18):4638.
Kerlikowske K, Sprague BL, Tosteson ANA, et al. Strategies to identify women at high risk of advanced breast cancer during routine screening for discussion of supplemental imaging. JAMA Intern Med. 2019;179(9):1230–9.
Kalra VB, Wu X, Haas BM, Forman HP, Philpotts LE. Cost-effectiveness of tomosynthesis in annual screening mammography. AJR Am J Roentgenol. 2016;207(5):1152–5.
Lin CN, Lee KT, Chang SM, Wang JD. Cost-effectiveness evaluation of mammography screening program in Taiwan: adjusting different distributions of age and calendar year for real world data. J Formos Med Assoc. 2022;121:633–42.
Directorate General of Budget, Accounting, and Statistics, Executive Yuan. Employee salary survey and labor force participation rate. Statistic Reports in Taiwan. 2021. Available from: https://eng.dgbas.gov.tw/Default.aspx. Accessed 22 Jan 2021.
Lee CI, Cevik M, Alagoz O, et al. Comparative effectiveness of combined digital mammography and tomosynthesis screening for women with dense breasts. Radiology. 2015;274:772–80.
Cressman S, Mar C, Sam J, Kan L, Lohrisch C, Spinelli JJ. The cost-effectiveness of adding tomosynthesis to mammography-based breast cancer screening: an economic analysis. CMAJ Open. 2021;9:E443–50.
Appelman L, Siebers CCN, Appelman PTM, et al. US and digital breast tomosynthesis in women with focal breast complaints: results of the breast US trial (BUST). Radiology. 2023;307: e220361.
Funding
This work was supported by grants through funding from the Ministry of Science and Technology (MOST 108-2410-H-037-006-SS3 & MOST 111-2410-H-037-002-MY3).
Author information
Authors and Affiliations
Contributions
WSC and HYS conceptualized the study, developed the mathematical methods, and contributed to the programming. YTS collected the data. The study was supervised by TW and YTS. WSC, TW, and HYS drafted the original manuscript and has prepared the publication. TW provided expert advice to help refine the analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
As a retrospective study, patient informed consent was waived. This study has obtained Institutional Review Board approval on 02.03.2022 no. KMUHIRB-E(I)-20220012 Taiwan.
Consent for Publication
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chung, WS., Wan, T.T.H., Shiu, Y.T. et al. Cost-Effectiveness Analysis of Digital Breast Tomosynthesis and Mammography in Breast Cancer Screening: A Markov Modeling Study. J Epidemiol Glob Health (2024). https://doi.org/10.1007/s44197-024-00239-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44197-024-00239-z