Abstract
Background
Poor sleep quality is a global public health concern. This study aimed to identify the risk factors for sleep disorders and clarify their causal effects.
Methods
Data were obtained from the National Health and Nutrition Examination Survey (NHANES) and Mendelian randomization (MR)-Base databases. Baseline characteristics of individuals with and without sleep disorders were compared. A multivariate logistic regression analysis was performed to calculate the effects of each variable on sleep disorders. Causal effects of blood lead levels and hypertension on sleep disorders were assessed using MR analysis.
Results
In total, 3660 individuals were enrolled in the study. The prevalence of self-reported sleep disorders was 26.21%. Serum lead level, serum mercury level, serum retinol level, prevalence of hypertension, and daily vigorous work duration were significantly higher for those in the sleep disorders group than the control group. After adjusting for various covariates, the effects of serum lead and hypertension on sleep disorders were stable from logistic regression models 1–4. MR analysis showed that blood lead levels were causally related to the risk of sleep disorders (odds ratio (OR) = 1.09, 95% confidence interval (CI) 1.01–1.17, P = 0.030). There was no causal link between elevated blood pressure and sleep disorders (OR = 0.99, 95% CI 0.94–1.04, P = 0.757). Goodness-of-fit tests and sensitivity analyses were used to verify the reliability of the results.
Conclusions
Blood lead is positively and causally associated with an increased risk of sleep disorders. These findings provide a novel perspective regarding sleep protection. Taking effective measures to reduce lead exposure may significantly improve sleep health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Good sleep quality has been widely recognized as an important indicator for maintaining physical health and optimal brain function [1, 2]. The consensus of the American Academy of Sleep Medicine and Sleep Research Society indicates that adults should sleep seven or more hours per night regularly to maintain optimal health [2]. Nonetheless, poor sleep has become a worldwide public health issue in today’s society [3,4,5]. A meta-analysis conducted on the general population from Netherlands, United Kingdom, and United States revealed that the prevalence of sleep disorders ranges from 9.6 to 19.4% [6]. Therefore, identifying risk factors for sleep disorders may help to alleviate sleep disturbance and improve sleep-related health problems including depression, cognitive dysfunction, Alzheimer’s disease, dry eye disease, obesity, and cardiovascular diseases [7,8,9,10].
Sleep health may be influenced by many factors such as sociodemographic status, health status, individual behaviors, psychological conditions, and environmental factors [3,4,5, 11]. With advancements in industrialization and urbanization, heavy metal pollution has become a global environmental problem. Lead is a well-established environmental neurotoxicant [12]. Previous studies investigating the negative effects of lead have mainly focused on nervous system damage caused by lead exposure. However, the association between blood lead and sleep disorders has not yet received sufficient attention.
A cross-sectional study of 40 female workers exposed to lead showed that most had poor sleep quality [13]. Another cross-sectional study reported a positive association between serum lead levels and sleep duration in premenopausal women, while this association was negative in postmenopausal women [14]. Thus, conflicting findings exist regarding the association between blood lead and sleep disorders in different populations. A cross-sectional study conducted in Mexican children aged 6–8 years showed that increased blood lead levels were associated with decreased sleep duration [15]. Similarly, another study conducted in China showed that elevated blood lead levels among children aged 3–5 years were associated with an increased risk of sleep problems in early adolescence [16]. However, studies in children relied mostly on parental reports, which may lead to inaccurate findings. Meanwhile, owing to the nature of cross-sectional studies, causality cannot be inferred [17]. Therefore, strong evidence supporting the causal link between lead exposure and sleep disorders is still lacking. The same issue also exists in the association between hypertension and sleep disorders. As the pace of life has accelerated and pressure on individuals has increased, researchers have noted a high degree of hypertension and sleep disorders comorbidity [18,19,20]. However, whether the association is causal remains undetermined. Therefore, to fill these gaps, further research is required to explore causal risk factors for sleep disorders among large-scale populations.
The National Health and Nutrition Examination Survey (NHANES) is a large, nationally representative survey of American civilians that provides comprehensive data on health and nutrition [21]. Data from the NHANES have the potential to facilitate an investigation of the association between risk factors and sleep disorders at the epidemiologic level. Mendelian randomization (MR) is an epidemiological method that uses genetic instrumental variables (IVs) to infer the causal effect of exposure on outcomes [22]. Application of the MR method can help to mitigate the potential challenges of residual confounding and reverse causality. This is because genetic variants are randomly assorted during gamete formation and are not influenced by environmental or lifestyle factors [23, 24]. Therefore, the combined application of NHANES and MR analyses provides reliable and unbiased estimates of the causal effects of risk factors on sleep disorders.
In light of this background, the present study aimed to comprehensively explore risk factors for sleep disorders based on 2017–2018 cross-sectional data of NHANES and clarify the causal effect of risk factors on sleep disorders based on MR analysis. These findings may provide novel insights into the prevention of sleep disorders.
2 Methods
2.1 Data Source
The NHANES is a cross-sectional survey that aims to examine the health and nutritional status of the US population [25]. Data for this cross-sectional study were obtained from the 2017–2018 cycles of NHANES. Genome-wide association study (GWAS) datasets for MR analysis were obtained from the National Human Genome Research Institute and European Bioinformatics Institute’s (NHGRI-EBI) GWAS catalog in the MR-Base database [26, 27]. The GWAS IDs for lead levels in the blood, hypertension, and sleep disorders are GCST002831, GCST007707, and finn-b-SLEEP, respectively [28, 29]. Details of the GWAS datasets are given in Supplementary Table 1. No informed consent was required because all data analyzed in this study were publicly available.
2.2 Covariates and Outcome
For the cross-sectional study, covariates including demographic, examination, laboratory, and questionnaire data from the 2017–2018 cycles of the NHANES were defined as covariates. Participants were asked whether they had trouble sleeping via the sleep disorder questionnaire [30], with yes answers indicating a sleep disorders and no answers indicating the absence of a sleep disorders. The answers, “Do not know” and “Refused,” indicated that data regarding the presence or absence of a sleep disorders were missing. For MR analysis, blood lead levels and hypertension were considered exposures, with sleep disorders considered an outcome.
2.3 Statistical Analyses
Baseline characteristics of groups with and without sleep disorders were compared. Shapiro–Wilk test and Levene’s test were used to verify the normality and homogeneity of variance of the baseline data, respectively. The independent-sample t test was used to compare the differences in the measurement covariates meeting assumptions of normality and homogeneity of variance. And the data were presented as mean ± standard deviation (\(\bar{x }\)±s). If normality or homogeneity of variance was not met, differences were assessed using the Wilcoxon rank-sum test, with data presented as the median and interquartile range (IQR). Categorical data were compared using the chi-square test. Multivariate logistic regression was performed to calculate the odds ratio (OR) and 95% confidence interval (CI) for the effects of each variable on sleep disorders. The goodness of fit of the model was evaluated using the Hosmer–Lemeshow test, omnibus test of model, and prediction accuracy.
For MR analysis, single-nucleotide polymorphisms (SNPs) associated with exposures (P < 5 × 10–8) without linkage disequilibrium were selected as IVs. Inverse variance weighted (IVW), MR-Egger, weighted median, and weighted mode methods were applied to assess the causal effect of blood lead on sleep disorders. MR is based on three conditional assumptions including relevance, independence, and exclusion restriction. Cochran’s Q statistic was used to assess the heterogeneity. The MR-Egger regression intercept was used to examine horizontal pleiotropy. The leave-one-out sensitive analysis was performed to verify the robustness of the results. MR analyses were performed using the TwoSampleMR R package. The present study protocol and details had not been registered.
R studio and SPSS software were used to perform statistical analyses and two-sided P < 0.05 was considered as statistically significant.
3 Results
3.1 Baseline Data of Included Individuals
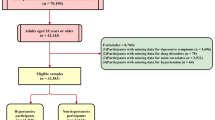
A total of 9254 participants were identified from the 2017–2018 cycles of the NHANES. After excluding 2212 participants younger than 18 years old, 87 pregnancies, and 3098 with missing sleep questionnaire data, 3660 individuals were ultimately enrolled in the current study. The overall prevalence of self-reported sleep disorders was 26.21%. Univariate analysis showed that differences in serum lead, hypertension, serum mercury, retinol, and vigorous work between the two groups were statistically significant. The serum lead level, serum mercury level, serum retinol level, prevalence of hypertension, and the proportion of vigorous work in the sleep disorders group were significantly higher than those in the control group. Baseline characteristics of the included participants are presented in Table 1.
3.2 Multivariate Logistic Regression
Variables with statistical significance in the univariate analysis were further included in the multivariate logistic regression (model 1). The crude model was adjusted for demographic factors, social status, and lifestyle habits. Hosmer–Lemeshow goodness-of-fit tests were not significant (P > 0.05) indicating a good fit of the multivariate logistic regression models (Supplementary Table 2). All the models were statistically significant (Omnibus P < 0.0001). After adjusting for covariates, effects of serum lead level and hypertension on sleep disorders were determined to be stable when applying Models 1–4 (Table 2). Therefore, elevated blood lead and hypertension may be risk factors for sleep disorders.
3.3 MR Analysis
Because a cross-sectional study cannot provide definite information about cause–effect relationships, we attempted to assess the direction of causality based on MR analysis. According to selection criteria, 9 SNPs were selected as IVs to assess the causal relationship between blood lead and sleep disorders. And 56 SNPs were used to assess the causal relationship between hypertension and sleep disorders. The results of the IVW method showed that blood lead levels are causally related to the risk of sleep disorders (ORIVW = 1.09, 95% CI 1.01–1.17, P = 0.030). The scatter plot also indicated that the risk of sleep disorders increases with the increase of blood lead (Fig. 1a). No causal link between elevated blood pressure and sleep disorders was identified (ORIVW = 0.99, 95% CI 0.94–1.04, P = 0.757) (Fig. 1b).
3.4 Sensitivity Analysis for MR Results
Cochran’s Q and MR-Egger intercept tests confirmed the absence of heterogeneity and horizontal pleiotropy in MR analysis (Supplementary Table 3 and 4). Leave-one-out analysis showed that the results remained stable when SNPs were removed individually, indicating the reliability of MR estimates (Supplementary Figs. 1 and 2).
4 Discussion
In the present study, we investigated risk factors for sleep disorders using data of 2017–2018 NHANES participants. Furthermore, we applied the MR approach to assess causal effects of blood lead levels and hypertension on sleep disorders. Results from the observational research showed that serum lead levels were positively related to sleep disorders risk after adjusting for various confounding factors, and the MR analysis further proved that the association was causal. Therefore, high levels of blood lead were directly associated with an increased risk of sleep disorders. Although we found that hypertension was also correlated with sleep disorders, hypertension was not the cause of sleep disorders. To the best of our knowledge, this is the first large-sample study examining the causal association between blood lead and sleep disorders among the real-world population and at the genetic level. Goodness-of-fit tests and sensitivity analyses verified the reliability of our results.
The present study provides direct evidence for the causal effect of blood lead on sleep disorders based on a large-sample cross-sectional study (including adult males and females) and MR analysis. Lead is one of the most toxic metals and potent pollutants [31, 32]. The possible mechanism by which blood lead inducing sleep disorders is that lead may affect the release and reuptake of several neurotransmitters controlled by voltage-gated Ca2+ channels to affect the activity of cerebral cortex [33,34,35]. Fortunately, lead exposure is preventable [36]. Blood levels of lead is a precise estimate of lead exposure [37]. It is generally recognized that environmental pollution is the main cause of human exposure to heavy metals [38]. Pollutants that may expose humans to lead include industrial waste, lead-acid batteries, metal smelting, lead-based paint, leaded gasoline and kerosene, lead-containing water pipes, and contaminated foods [32, 38,39,40,41,42]. Children and pregnant women are more sensitive to lead toxicity than adults [38, 43]. As the subjects in the present study were adults, the impact of lead exposure on sleep disorders may be higher than our estimate. Initiatives aimed at reducing lead exposure, such as strengthening hand hygiene, environmental rehabilitation, and conformity testing of industrial materials and waste, may have significant implications for protecting sleep health. As a result, body energy and immunity can be maintained [44]. We also observed a correlation between hypertension and sleep disorders. Another study that enrolled participants from the 2007–2014 cycles of NHANES also proved that poor sleep patterns were associated with an increased risk of hypertension [20]. However, as is the case with all cross‐sectional studies, associations identified do not necessarily indicate causality [45]. The results of the MR analysis did not support the hypothesis that hypertension causes sleep disorders. This may be because sleep disorders excite the sympathetic nervous system, leading to hypertension [46]. Therefore, further studies are needed to clarify whether sleep disorders are a direct cause of hypertension.
The major strength of this study was the combination of a large-sample cross-sectional investigation and GWAS data. The NHANES is a nationally representative survey and its large sample size is sufficient for providing real-world evidence of the association between blood lead and sleep disorders. MR analysis addresses the inherent effects of residual confounding factors and reverse causality. The combined application of NHANES and MR analyses provides robust and unbiased estimates of the causal effects of blood lead on sleep disorders.
The present study has some noteworthy limitations. First, the diagnosis of sleep disorders was based on self-reported questionnaire. Thus, self-selection, memory, and recall biases may exist. In addition, the populations in the MR analysis were predominantly of European descent; hence, its generalizability to other races may be limited. Another limitation was that we did not explore the specific biological mechanism by which elevated blood lead promote sleep disorders. Further studies are needed to clarify the mechanisms underlying this association.
5 Conclusions
Ultimately, we conclude that blood lead is positively associated with an increased risk of sleep disorders, and that this association is causal. These findings provide a novel perspective for the protection of sleep health. Taking effective measures to reduce lead exposure may significantly improve sleep health.
Availability of Data and Materials
The datasets analyzed during the current study are available in the NHANES and MR-Base database.
Abbreviations
- NHANES:
-
National Health and Nutrition Examination Survey
- MR:
-
Mendelian randomization
- IVs:
-
Instrumental variables
- GWAS:
-
Genome-wide association study
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- SNPs:
-
Single-nucleotide polymorphisms
- IVW:
-
Inverse variance weighted
- BMI:
-
Body mass index
- HR:
-
Heart rate
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- Hb:
-
Hemoglobin
- WBC:
-
White blood cell
- PLT:
-
Platelet
- CRP:
-
C-reactive protein
- HbA1C:
-
Glycosylated hemoglobin
- TB:
-
Total bilirubin
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ALB:
-
Albumin
- BUN:
-
Blood urea nitrogen
- SCr:
-
Serum creatinine
- SUA:
-
Serum uric acid
- UAlb:
-
Urine albumin
- TG:
-
Triglyceride
- TC:
-
Total cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
References
Uchima Koecklin KH, Shimosawa T, Li P. Editorial: endocrine consequences of sleep disorders. Front Endocrinol (Lausanne). 2023;14:1238950. https://doi.org/10.3389/fendo.2023.1238950.
Ohayon M, Wickwire EM, Hirshkowitz M, Albert SM, Avidan A, Daly FJ, et al. National sleep foundation’s sleep quality recommendations: first report. Sleep Health. 2017;3:6–19. https://doi.org/10.1016/j.sleh.2016.11.006.
Huang BH, Duncan MJ, Cistulli PA, Nassar N, Hamer M, Stamatakis E. Sleep and physical activity in relation to all-cause, cardiovascular disease and cancer mortality risk. Br J Sports Med. 2022;56:718–24. https://doi.org/10.1136/bjsports-2021-104046.
Rogers EM, Banks NF, Jenkins NDM. The effects of sleep disruption on metabolism, hunger, and satiety, and the influence of psychosocial stress and exercise: a narrative review. Diabetes Metab Res Rev. 2023. https://doi.org/10.1002/dmrr.3667.
Liu J, Ghastine L, Um P, Rovit E, Wu T. Environmental exposures and sleep outcomes: a review of evidence, potential mechanisms, and implications. Environ Res. 2021;196: 110406. https://doi.org/10.1016/j.envres.2020.110406.
Kocevska D, Lysen TS, Dotinga A, Koopman-Verhoeff ME, Luijk M, Antypa N, et al. Sleep characteristics across the lifespan in 1.1 million people from the Netherlands, United Kingdom and United States: a systematic review and meta-analysis. Nat Hum Behav. 2021;5:113–22. https://doi.org/10.1038/s41562-020-00965-x.
Mirchandaney R, Asarnow LD, Kaplan KA. Recent advances in sleep and depression. Curr Opin Psychiatry. 2023;36:34–40. https://doi.org/10.1097/yco.0000000000000837.
Pearson O, Uglik-Marucha N, Miskowiak KW, Cairney SA, Rosenzweig I, Young AH, et al. The relationship between sleep disturbance and cognitive impairment in mood disorders: a systematic review. J Affect Disord. 2023;327:207–16. https://doi.org/10.1016/j.jad.2023.01.114.
Wang C, Holtzman DM. Bidirectional relationship between sleep and Alzheimer’s disease: role of amyloid, tau, and other factors. Neuropsychopharmacology. 2020;45:104–20. https://doi.org/10.1038/s41386-019-0478-5.
Li S, Ning K, Zhou J, Guo Y, Zhang H, Zhu Y, et al. Sleep deprivation disrupts the lacrimal system and induces dry eye disease. Exp Mol Med. 2018;50: e451. https://doi.org/10.1038/emm.2017.285.
Wallace DA, Gallagher JP, Peterson SR, Ndiaye-Gueye S, Fox K, Redline S, et al. Is exposure to chemical pollutants associated with sleep outcomes? A systematic review. Sleep Med Rev. 2023;70: 101805. https://doi.org/10.1016/j.smrv.2023.101805.
Neuwirth LS, Phillips GR, El Idrissi A. Perinatal Pb(2+) exposure alters the expression of genes related to the neurodevelopmental GABA-shift in postnatal rats. J Biomed Sci. 2018;25:45. https://doi.org/10.1186/s12929-018-0450-4.
Mohammadyan M, Moosazadeh M, Borji A, Khanjani N, Rahimi MS. Exposure to lead and its effect on sleep quality and digestive problems in soldering workers. Environ Monit Assess. 2019;191:184. https://doi.org/10.1007/s10661-019-7298-2.
Nguyen HD. Interactions between heavy metals and sleep duration among pre- and postmenopausal women: a current approach to molecular mechanisms involved. Environ Pollut. 2023;316: 120607. https://doi.org/10.1016/j.envpol.2022.120607.
Kordas K, Casavantes KM, Mendoza C, Lopez P, Ronquillo D, Rosado JL, et al. The association between lead and micronutrient status, and children’s sleep, classroom behavior, and activity. Arch Environ Occup Health. 2007;62:105–12. https://doi.org/10.3200/aeoh.62.2.105-112.
Liu J, Liu X, Pak V, Wang Y, Yan C, Pinto-Martin J, et al. Early blood lead levels and sleep disturbance in preadolescence. Sleep. 2015;38:1869–74. https://doi.org/10.5665/sleep.5230.
Meyer S, Weidmann R, Grob A. The mirror’s curse: weight perceptions mediate the link between physical activity and life satisfaction among 727,865 teens in 44 countries. J Sport Health Sci. 2021;10:48–54. https://doi.org/10.1016/j.jshs.2020.01.002.
Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. https://doi.org/10.1161/01.HYP.0000217362.34748.e0.
Jiang L, Xu H. U-shaped relationship between sleep duration and CKD in US adults: data from National Health and Nutrition Examination Survey (NHANES) 2005–2014. Am J Nephrol. 2023. https://doi.org/10.1159/000531440.
Li C, Shang S. Relationship between sleep and hypertension: findings from the NHANES (2007–2014). Int J Environ Res Public Health. 2021. https://doi.org/10.3390/ijerph18157867.
Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr. 2016;7:121–34. https://doi.org/10.3945/an.115.009258.
Bowden J, Holmes MV. Meta-analysis and mendelian randomization: a review. Res Synth Methods. 2019;10:486–96. https://doi.org/10.1002/jrsm.1346.
Richmond RC, Anderson EL, Dashti HS, Jones SE, Lane JM, Strand LB, et al. Investigating causal relations between sleep traits and risk of breast cancer in women: mendelian randomisation study. BMJ. 2019;365: l2327. https://doi.org/10.1136/bmj.l2327.
Skrivankova VW, Richmond RC, Woolf BAR, Davies NM, Swanson SA, Vanderweele TJ, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. 2021;375: n2233. https://doi.org/10.1136/bmj.n2233.
Qato DM, Ozenberger K, Olfson M. Prevalence of prescription medications with depression as a potential adverse effect among adults in the United States. JAMA. 2018;319:2289–98. https://doi.org/10.1001/jama.2018.6741.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018. https://doi.org/10.7554/eLife.34408.
Welter D, Macarthur J, Morales J, Burdett T, Hall P, Junkins H, et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42:D1001–6. https://doi.org/10.1093/nar/gkt1229.
Warrington NM, Zhu G, Dy V, Heath AC, Madden PA, Hemani G, et al. Genome-wide association study of blood lead shows multiple associations near ALAD. Hum Mol Genet. 2015;24:3871–9. https://doi.org/10.1093/hmg/ddv112.
Takeuchi F, Akiyama M, Matoba N, Katsuya T, Nakatochi M, Tabara Y, et al. Interethnic analyses of blood pressure loci in populations of East Asian and European descent. Nat Commun. 2018;9:5052. https://doi.org/10.1038/s41467-018-07345-0.
Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80–90. https://doi.org/10.1177/0748730402239679.
Chandwani S, Kayasth R, Naik H, Amaresan N. Current status and future prospect of managing lead (Pb) stress through microbes for sustainable agriculture. Environ Monit Assess. 2023;195:479. https://doi.org/10.1007/s10661-023-11061-8.
Kumar S, Rahman MA, Islam MR, Hashem MA, Rahman MM. Lead and other elements-based pollution in soil, crops and water near a lead-acid battery recycling factory in Bangladesh. Chemosphere. 2022;290: 133288. https://doi.org/10.1016/j.chemosphere.2021.133288.
Suszkiw JB. Presynaptic disruption of transmitter release by lead. Neurotoxicology. 2004;25:599–604. https://doi.org/10.1016/j.neuro.2003.09.009.
Ji X, Wang B, Paudel YN, Li Z, Zhang S, Mou L, et al. Protective effect of chlorogenic acid and its analogues on lead-induced developmental neurotoxicity through modulating oxidative stress and autophagy. Front Mol Biosci. 2021;8: 655549. https://doi.org/10.3389/fmolb.2021.655549.
Meng H, Wang L, He J, Wang Z. The protective effect of gangliosides on lead (Pb)-induced neurotoxicity is mediated by autophagic pathways. Int J Environ Res Public Health. 2016;13:365. https://doi.org/10.3390/ijerph13040365.
Braun JM, Hornung R, Chen A, Dietrich KN, Jacobs DE, Jones R, et al. Effect of residential lead-hazard interventions on childhood blood lead concentrations and neurobehavioral outcomes: a randomized clinical trial. JAMA Pediatr. 2018;172:934–42. https://doi.org/10.1001/jamapediatrics.2018.2382.
Wells EM, Navas-Acien A, Herbstman JB, Apelberg BJ, Silbergeld EK, Caldwell KL, et al. Low-level lead exposure and elevations in blood pressure during pregnancy. Environ Health Perspect. 2011;119:664–9. https://doi.org/10.1289/ehp.1002666.
Nain P, Kumar A. Ecological and human health risk assessment of metals leached from end-of-life solar photovoltaics. Environ Pollut. 2020;267: 115393. https://doi.org/10.1016/j.envpol.2020.115393.
Wolfe PJ, Giang A, Ashok A, Selin NE, Barrett SR. Costs of IQ loss from leaded aviation gasoline emissions. Environ Sci Technol. 2016;50:9026–33. https://doi.org/10.1021/acs.est.6b02910.
Coelho SD, Pastorinho MR, Itai T, Isobe T, Kunisue T, Nogueira AJA, et al. Lead in duplicate diet samples from an academic community. Sci Total Environ. 2016;573:603–7. https://doi.org/10.1016/j.scitotenv.2016.08.133.
Almerud P, Zamaratskaia G, Lindroos AK, Bjermo H, Andersson EM, Lundh T, et al. Cadmium, total mercury, and lead in blood and associations with diet, sociodemographic factors, and smoking in Swedish adolescents. Environ Res. 2021;197: 110991. https://doi.org/10.1016/j.envres.2021.110991.
He H, Wei H, Wang Y, Wang L, Qin Z, Li Q, et al. Geochemical and statistical analyses of trace elements in lake sediments from Qaidam basin, Qinghai-Tibet plateau: distribution characteristics and source apportionment. Int J Environ Res Public Health. 2022. https://doi.org/10.3390/ijerph19042341.
Spanier AJ, Mclaine P, Gilden RC. Screening for elevated blood lead levels in children and pregnant women. JAMA. 2019;321:1464–5. https://doi.org/10.1001/jama.2019.2594.
Puścion-Jakubik A, Olechno E, Socha K, Zujko ME. Eating habits during the COVID-19 pandemic and the level of antibodies IgG and FRAP-experiences of Polish School staff: a pilot study. 2022. Foods. https://doi.org/10.3390/foods11030408.
Kimita W, Li X, Ko J, Bharmal SH, Cameron-Smith D, Petrov MS. Association between habitual dietary iron intake and glucose metabolism in individuals after acute pancreatitis. Nutrients. 2020. https://doi.org/10.3390/nu12113579.
Carnethon MR, Johnson DA. Sleep and resistant hypertension. Curr Hypertens Rep. 2019;21:34. https://doi.org/10.1007/s11906-019-0941-z.
Acknowledgements
We thank participants and staff of the NHANES and MR-Base database.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SNC, ZY, and KN: conceived of the study. SNC and MZ: obtained data and conducted statistical analysis. WSZ, XLS, and XBY: provided methodological support. SNC: wrote the original draft. MZ, ZY, and KN: revised and polished the manuscript. All authors reviewed and agreed to the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors have no financial or proprietary interests in any material discussed in this article.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, S., Zhang, M., Zhang, W. et al. The Causal Association Between Blood Lead and Sleep Disorders: Evidence from National Health and Nutrition Examination Survey and Mendelian Randomization Analysis. J Epidemiol Glob Health 14, 462–469 (2024). https://doi.org/10.1007/s44197-024-00199-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44197-024-00199-4