Abstract
Background
Lipodystrophy is a relatively rare, complex disease characterised by a deficiency of adipose tissue and can present as either generalised lipodystrophy (GLD) or partial lipodystrophy (PLD). The prevalence of this disease varies by region. This study aimed to identify the genetic variations associated with lipodystrophy in the southern part of Saudi Arabia.
Methodology
We conducted a retrospective study by recruiting nine patients from six families, recruiting the proband whole exome sequencing results or any other genetic test results, screening other family members using Sanger sequencing and analysing the carrier status of the latter. These patients were recruited from the Endocrinology and Diabetes Clinic at Jazan General Hospital and East Jeddah Hospital, both in the Kingdom of Saudi Arabia.
Result
Eight patients were diagnosed with GLD, and one was diagnosed with PLD. Of the six families, four were consanguineously married from the same tribe, while the remaining belonged to the same clan. The majority of GLD patients had an AGPAT2 c.158del mutation, but some had a BSCL2 c.942dup mutation. The single PLD case had a PPARG c.1024C > T mutation but no family history of the disease. In all families evaluated in this study, some family members were confirmed to be carriers of the mutation observed in the corresponding patient.
Conclusion
Familial screening of relatives of patients with rare, autosomal recessive diseases, such as lipodystrophy, especially when there is a family history, allows the implementation of measures to prevent the onset or reduced severity of disease and reduces the chances of the pathogenic allele being passed onto future generations. Creating a national registry of patients with genetic diseases and carriers of familial pathogenic alleles will allow the assessment of preventive measures and accelerate disease intervention via gene therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Lipodystrophies comprise a rare, complex group of diseases characterised by a deficiency of adipose tissue. These conditions are often misdiagnosed or delayed, largely because of the obscure nature of these disorders [2]. Different versions of lipodystrophy can be distinguished by the distribution of adipose tissue loss as either generalised, localised or partial [3, 15]. The disease can present as generalised lipodystrophy (GLD) or partial lipodystrophy (PLD), which are differentiated based on the extent and location of adipose tissue loss [3, 10, 19]. Both forms can be acquired or have a genetic cause in the case of congenital or familial versions [3, 19]. For example, in familial PLD type 2, subcutaneous fat is lost from the upper and lower limbs, trunk and gluteal regions, but it can accumulate in the face and neck (OMIM entry 151,660) [9, 16]. The global prevalences of GLD and PLD are 0.96 and 1.67 per million individuals, respectively [6]. The disorder is diagnosed partly based on the loss of subcutaneous adipose tissue without evidence of an underlying catabolic state or nutritional deprivation [5]. Loss of adipose tissue can lead to decreased leptin levels, which can interfere with hunger–satiety signals, resulting in hyperphagia [7, 9]. The excess calories consumed from overeating are abnormally and inappropriately stored in the liver and muscle, which causes insulin resistance, hepatic steatosis and hypertriglyceridemia [2, 7]. Therefore, lipodystrophy is associated with a severe form of metabolic syndrome, including not only severe hypertriglyceridemia but also dyslipidemia, extreme insulin resistance and low levels of high-density lipoprotein C [7]. Additionally, to use these symptoms (and loss of subcutaneous fat) to diagnose lipodystrophy, decreased leptin levels can be used as a differential diagnostic test, in which very low fasting serum adiponectin and leptin levels indicate a diagnosis of GLD instead of PLD and correlate with hypertriglyceridemia, high insulin levels (or insulin-resistant diabetes) and low high-density lipoprotein C [10]. Therefore, GLD and PLD can be considered distinctive types of lipodystrophy.
The various forms of lipodystrophy can be differentiated based on whether they are acquired or inherited. Moreover, there is phenotypic and genetic heterogeneity, even among the congenital or familial forms of lipodystrophy [19]. Different mutations in the same gene can give rise to different characteristic features, and mutations in multiple genes can cause congenital or familial lipodystrophy [3]. For example, peroxisome proliferator-activated receptor gamma (PPARG) is a nuclear receptor involved in insulin resistance, lipid metabolism and inflammation, and mutations in the corresponding gene are linked to PLD [21]. Mutations in the genes encoding lamin A (LMNA), cell death-inducing dffa-like effector C (CIDEC) and perilipin 1A (PLIN1A) are associated with PLD, whereas mutations in genes encoding 1-acylglycerol-3-phosphate-O-acyltransferase 2 (AGPAT2), caveolin 1 (CAV1) and polymerase-I-and-transcript release factor (PTRF), as well as in the Berardinelli–Seip congenital lipodystrophy 2 gene (BSCL2), have been found in patients with congenital GLD [15]. Overall, a variety of mutations in diverse genes are present in different populations, including rare or even novel mutations [12]. For example, in Saudi Arabia, mutations in PTRF have been discovered in patients with lipodystrophy, including the novel PTRF c.550G > T; p.Glu184*, a nonsense mutation that truncates the protein [12]. Another case report determined that the AGPAT2 c.158del/p.Gly53Alafs*8 mutation caused lipodystrophy in two Saudi siblings [11].
This research focused on the genetics associated with lipodystrophy in the Saudi population and determined how implementing familial carriers screening for rare disease (RD) could reduce future incidences while furthering understanding of lipodystrophy’s genetic background and comparing it to mutations presented worldwide.
2 Materials and Methods
2.1 Ethical Approval
The Institutional Review Board of the Jazan Health Research Ethics Committee, Ministry of Health, Saudi Arabia, approved this study. Approval number IRB-No.2275 was received for one year starting September 2022. Project number IFPNC-008–141-2020 was funded by the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
2.2 Study Design
This study was conducted retrospectively on patients diagnosed with GLD or PLD. The data included genetics and clinical phenotypics, which were recruited from Jazan General Hospital. One patient and family members were from East Jeddah Hospital, Kingdom of Saudi Arabia. The inclusion criteria were all patients diagnosed with GLD or PLD, and the initial diagnosis was confirmed with molecular genetics using next-generation sequencing technology at a commercial laboratory accredited by the College of American Pathologists. We further screened other family members, recorded any phenotype and obtained consent for genetic testing using Sanger sequencing. We excluded patients with no initial genetic testing in their primary diagnosis of GLD or PLD.
2.3 Data Collection
The data, including patient demographics, family history, and genetic test results, were collected from the diabetes clinic to confirm inheritance patterns. We took a 5 ml blood sample in an EDTA tube and sent it to the Centre of Excellence in Genomic Medical Research (CEGMR) at King Abdulaziz University. The received blood was processed, and the molecular derivatives were isolated and stored in the biobank at CEGMR for genomic studies. DNA was extracted using QIAAMP genomic DNA according to the manufacturer’s instructions (QIAGEN, USA). A NanoDrop spectrophotometer (Thermo Fisher Scientific, USA) was used to measure the concentration and purity of the DNA for quality assurance. The high-quality DNA was diluted to a concentration of 50–100 ng/μl for Sanger sequencing. Variants were classified according to the American College of Medical Genetics and Genomics (ACMG) guidelines. The reference genome used for the analysis was GRCh38/hg38, and Sanger data interpretation was carried out through finchTV. The families’ histories and pedigrees were used to hypothesise the zygosity and inheritance modes. Finally, segregation analysis was performed on other family members for the same mutations using Sanger sequencing to identify potential carriers.
3 Result:
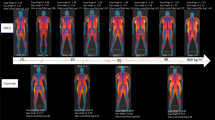
Six families were recruited from the diabetes clinic. Their phenotypic profiles are found in Supplementary Table 1. Each family’s proband was followed up to check the pattern of inheritance within the family. The first patient was female, as indicated by the number L.F01.3 in Fig. 1. She had an abnormal facial shape, abnormal foot morphology, falangist abnormality of the hand, decreased body weight, a depressed nasal bridge, hirsutism, macrotia, micrognathia, a sacral dimple and a sloping forehead. Genetic testing revealed a mutation in AGPAT2 c.158del, causing p.(Gly53Alafs*8), NM_006412.4.
The second patient was also from the same tribe as family 1 and a homozygous carrier for a mutation in AGPAT2 c.158del causing p.(Gly53Alafs*8), NM_006412.4. The girl, aged 22 years (Fig. 2), had decreased adipose tissue, hepatomegaly, hyperglycaemia, hyperinsulinemia and hypertriglyceridemia. Her parents were first-degree cousins from the mother’s side, and her sister, 16 years old, developed the same phenotype and showed the same mutation. Notably, the father, his twin sons and grandchild showed the same genotype with no phenotype, apart from the father being diagnosed with diabetes.
The third patient (L.F03.4, Fig. 3) had pigmentation, delayed speech and language development, Type II diabetes mellitus, elevated hepatic transaminase, hepatic failure, intrauterine growth retardation, renal tubular dysfunction and specific learning disabilities. The genetics test indicated a c.942dup mutation in the BSCL2 gene p.(Leu315Alafs*23) NM_001122955.4. The patient belonged to a consanguineous marriage. Both parents were confirmed heterozygous, and another sister had the same phenotype and genotype.
The third family’s pedigree with consanguineous marriage. The proband is indicated by a black arrow. Two daughters (L.F03.5 and L.F03.6) were confirmed to be homozygous for c.942dup in BSCL2, while the parents and a sibling (L.F03.1, L.F03.2 and L.F03.3) were confirmed to be heterozygous, with L.F03.4 being an unaffected daughter
.
The fourth case was a nine-year-old female from a consanguineous marriage, with AGPAT2 c.158del, NM_006412.4 causing a change in the protein p.(Gly53Alafs*8) in homozygous form. Both parents were found to be heterozygous, as indicated in Fig. 4.
The fifth family had two children diagnosed and treated with leptin in the diabetes clinic and were confirmed to be homozygous for a mutation in AGPAT2 c.158del (see Fig. 5), causing a change in the protein p.(Gly53Alafs*8), NM_006412.4.
The fifth family’s pedigree with consanguineous marriage. The proband is indicated with a black arrow and red colour. The two daughters in the red circles (L.F05.3 and L.F05.4) were confirmed to be homozygous for c.1558del in AGPAT2, and the parents and one daughter (L.F05.5) were all confirmed to be heterozygous
The sixth case was a 32-year-old female with severe hypertriglyceridemia and dyslipidemia as indicated in Fig. 6. She had uncontrolled diabetes mellitus and recurrent pancreatitis. No additional fat was seen in the physical examination. No other family member carried the same phenotype, and the genetics test revealed a heterozygous likely pathogenic c.1024C > T mutation in the PPARG gene p.(Gln342Ter), NM_015869.5, which is associated with familial PLD.
4 Discussion
There are more than 7362 RDs recognised worldwide [16, 17], with the prevalence found to be one in 2000 healthy individuals. These lifelong conditions require prolonged hospitalisation, rehabilitation and expensive medications that cause financial burdens on healthcare providers and emotional burdens on patients and their families [22]. Notably, 80% of RDs are inherited, and 63% appear in early childhood [23]. In Saudi Arabia, the prevalence of RDs, especially autosomal recessive disorders, is higher due to the high frequency of consanguineous marriages, which increase the chance of offspring inheriting two copies of pathogenic alleles [1, 8].
The Ministry of Health (MoH) in Saudi Arabia has established various programmes, including premarital and newborn screening, to reduce the prevalence of certain genetic diseases [14]. However, these programmes cover only a limited number of the most common conditions [20]. The prevalence of other diseases is still high due to cultural partnering behaviours [4]. Lipodystrophy is an RD with lifelong manifestations.
In this study, we obtained samples from one region, which was limited to patients visiting certain doctors. The other limitation was that not all family members contributed to this study to follow up on the carrier status. Interestingly, all cases were female. However, in Family 2 (Fig. 2), four males carried the same homozygous mutation in the proband with no phenotype representation. This has been observed previously, with clinical findings showing gender differences in lipodystrophy: female patients are more negatively affected than male patients, including earlier onset of severe metabolic abnormalities compared to males (Mcilroy et al. [27]), [24]. This was also observed in familial PLD women who experienced metabolic complications related to insulin resistance more severely than in men (Garg [25]; Hussain and Garg [26]).
Facilitating familial screening for carriers of certain mutations as preventive measures would reduce future incidences and the financial burden of the MoH. In this case, leptin replacement with metreleptin is the golden standard therapy for GLD and PLD [13] at a cost of three million riyals per year for each individual. In this study, we recruited nine individuals diagnosed with GLP and one female with PLD from one region who were treated with Myalept 11.3 MG Cavgene, at a total of 30 million riyals per year, for our study group.
The lack of a national registry for RDs, which can be linked to a global registry to prioritise drug development and research, is urgently needed to monitor the number of cases and disease allele associations [18]. Knowing that particular variations, such as AGPAT2 c.158del/p.Gly53Alafs*8, are reported only in the Saudi population has led to the proposition of a founder effect [11]. This will accelerate preventive measures in specific regions or focus on certain families to avoid passing the pathogenic allele to future offspring. Implementing a carrier screening for individuals with a family history will prevent increases in the disease rate. If individuals know that they are carriers, this will allow for screening partners before marriage to avoid partnering with another carrier with the same or other mutations in different locations or genes, which can increase the chance of having a child with the phenotype. Furthermore, for Autosomal Domenant carriers, family planning through a genetic counsellor can assess the chances of healthy offspring.
The clinical findings show that there are regular gender differences in lipodystrophy; female patients are more negatively affected than male patients (Mcilroy et al., [27]). Women with familial PLD experience metabolic complications related to insulin resistance more severely than do men. These findings suggest that women who are both regionally and generally obese may also have more severe metabolic aftereffects of insulin resistance (Garg [25]; Hussain and Garg 26).
5 Conclusion
This study focused on lipodystrophy, an RD that requires high-cost and prolonged treatment. Given that this disease runs in families, cases increase yearly due to consanguineous marriages. It is alarming that this rare condition could become common within a few years, and it is essential to implement preventive measures to avoid increases in allele frequency in our population. Therefore, a national registry is needed to document the incidence rate in the kingdom, and it is highly recommended that screening programmes for families with RD or ultra-rare diseases be implemented along with educational programmes to promote healthy marriages.
Data Availability
Data are available upon request by contacting the PI.
References
Aleissa M, et al. Common disease-associated gene variants in a Saudi Arabian population. Ann Saudi Med. 2022;42(1):29–35. https://doi.org/10.5144/0256-4947.2022.29.
Araújo-Vilar D, Santini F. Diagnosis and treatment of lipodystrophy: a step-by-step approach. J Endocrinol Invest. 2019;42(1):61–73. https://doi.org/10.1007/s40618-018-0887-z.
Bagias C, et al. Familial partial lipodystrophy (FPLD): recent insights. Diabetes Metab Syndr Obes Targets Ther. 2020;13:1531–44. https://doi.org/10.2147/DMSO.S206053.
Bittles AH, Black ML. Consanguinity, human evolution, and complex diseases. Proc Natl Acad Sci USA. 2010;107(SUPPL. 1):1779–86. https://doi.org/10.1073/pnas.0906079106.
Brown RJ, et al. The diagnosis and management of lipodystrophy syndromes: a multi-society practice guideline. J Clin Endocrinol Metab. 2016;101(12):4500–11.
Chiquette E, et al. Estimating the prevalence of generalized and partial lipodystrophy: findings and challenges. Diabetes Metab Syndr Obesity Targets Ther. 2017;10:375.
Diker-Cohen T, et al. Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. J Clin Endocrinol Metab. 2015;100(5):1802–10.
Eissa M, et al. Genetic carrier screening for disorders included in newborn screening in the Saudi population. J Biochem Clin Genet. 2021;4(May):70–5. https://doi.org/10.24911/jbcgenetics/183-1614266028.
Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350(12):1220–34.
Haque WA, et al. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab. 2002;87(5):2395–8.
Hummadi A, et al. Congenital generalized lipodystrophy in two siblings from Saudi Arabia: a case report. Clin Case Rep. 2022;10(4):1–5. https://doi.org/10.1002/ccr3.5720.
Jelani M, et al. Novel nonsense mutation in the PTRF gene underlies congenital generalized lipodystrophy in a consanguineous Saudi family. Eur J Med Genet. 2015;58(4):216–21. https://doi.org/10.1016/j.ejmg.2015.02.002.
Meral R, et al. Endogenous leptin concentrations poorly predict metreleptin response in patients with partial lipodystrophy. J Clin Endocrinol Metab. 2022;107(4):E1739–51. https://doi.org/10.1210/clinem/dgab760.
MOH-Premarital Screening Program (2022) Premarital Screening. Available at: https://www.moh.gov.sa/en/HealthAwareness/Beforemarriage/Pages/default.aspx. Accessed 23 May 2023
Nolis T. Exploring the pathophysiology behind the more common genetic and acquired lipodystrophies. J Hum Genet. 2014;59(1):16–23. https://doi.org/10.1038/jhg.2013.107.
OMIM (2023) geneMap. https://www.omim.org/statistics/geneMap. Accessed 22 Apr 2023
Orphanet (2023) Orphanet. https://www.orpha.net/consor/cgi-bin/index.php. Accessed 22 Apr 2023
Polyzos SA, Mantzoros CS. Lipodystrophy: Time for a global registry and randomized clinical trials to assess efficacy, safety and cost-effectiveness of established and novel medications. Metab, Clin Exp. 2017;72:4–10. https://doi.org/10.1016/j.metabol.2017.06.003.
Rutkowska L, et al. Familial partial lipodystrophy—literature review and report of a novel variant in pparg expanding the spectrum of disease-causing alterations in FPLD3. Diagnostics. 2022. https://doi.org/10.3390/diagnostics12051122.
Saudi Ministry of Health (2020) Premarital screening. https://www.moh.gov.sa/en/HealthAwareness/EducationalContent/PublicHealth/Pages/PremaritalScreening.aspx. Accessed 7 Oct 2020
Shoaito H, et al. Peroxisome proliferator-activated receptor gamma-ligand-binding domain mutations associated with familial partial lipodystrophy type 3 disrupt human trophoblast fusion and fibroblast migration. J Cell Mol Med. 2020;24(13):7660–9. https://doi.org/10.1111/jcmm.15401.
WHO (2023) Rare diseases. https://www.who.int/standards/classifications/frequently-asked-questions/rare-diseases. Accessed 24 May 2023
Wright CF, FitzPatrick DR, Firth HV. Paediatric genomics: Diagnosing rare disease in children. Nat Rev Genet. 2018;19(5):253–68. https://doi.org/10.1038/nrg.2017.116.
Yildirim Simsir I, et al. Clinical features of generalized lipodystrophy in Turkey: a cohort analysis. Diabetes Obes Metab. 2023;25(7):1950–63. https://doi.org/10.1111/dom.15061.
Garg A. Gender differences in the prevalence of metabolic complications in familial partial lipodystrophy (Dunnigan variety). J Clin Endocrinol Metab. 2000;85(5):1776–1782. https://doi.org/10.1210/jc.85.5.1776.
Hussain I, Garg A. Lipodystrophy syndromes. Endocrinol Metab Clin. 2016;45(4):783–797.
Mcilroy GD et al. Female adipose tissue-specific Bscl2 knockout mice develop only moderate metabolic dysfunction when housed at thermoneutrality and fed a high-fat diet. Scie Rep. 2018;8(1):1–11. https://doi.org/10.1038/s41598-018-36078-9.
Acknowledgements
The authors would like to acknowledge all the healthcare workers and the families involved in this research. The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia, for funding this research work through the project number IFPNC-008-141-2020 and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
NG: conceptualization, supervision, data curation, analysis. MA: conceptualization, designed the study, wrote the IRB and the paper. RQ: samples collection, processing and storage. EH: samples collection, processing and storage. AO: samples collection, processing and storage. AH: patient data, informed consent and samples collection. AN: patient data, informed consent and samples collection. SZ: patient data, informed consent and samples collection. NJ: patient data, informed consent and samples collection. MA: software and data analysis H.B. data receiving and processing. ST: data receiving and processing. SK: funding acquisition, writing, editing, and reviewing the paper. ZM: funding acquisition, editing, and reviewing the paper. AA: project administration and funding acquisition. MQ: supervision, project administration. ACh: supervision, project administration.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Ethics Approval
The Institutional Review Board approved this study at the Jazan Health Research Ethics Committee, Ministry of Health, Saudi Arabia, with approval number IRB-No.2275 received for one year starting September 2022, collaborated with King Abdulaziz University, DSR, Jeddah, Saudi Arabia, the project number IFPNC-008–141-2020.
Informed Consent Statement
The consent was obtained from all the individuals involved in the study.
Consent for Publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abuzenadah, A., Alganmi, N., AlQurashi, R. et al. Familial Screening for the Prevention of Rare Diseases: A Focus on Lipodystrophy in Southern Saudi Arabia. J Epidemiol Glob Health 14, 162–168 (2024). https://doi.org/10.1007/s44197-023-00182-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44197-023-00182-5