Abstract
Chordoma is a rare bone tumor often present in the skull base and spine. In addition, it is not sensitive to radiotherapy that surgical resection is of great significance for the treatment of chordoma. Residual tumors that cannot be surgically removed usually lead to tumor recurrence. Studies have shown that chordoma will be accompanied by multiple gene mutations, such as PDGFR, EGFR, HER2, VEGFR, and mTOR, and interact with the host immune system to promote tumor progression. Targeted therapy and immunotherapy can improve the prognosis of chordoma patients to some extent. This review focuses on the clinical trials related to targeted therapy, immunotherapy, and chemotherapy of chordoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
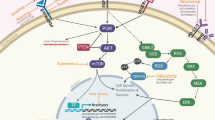
Chordoma is a rare bone tumor, which originates from the residual notochord, accounting for about 1%–4% of bone malignant tumors. Although histologically, it is considered to be a low-grade tumor, it has a high recurrence, and its clinical progress is very similar to malignant tumors (Walcott et al. 2012). The incidence rate of chordoma is 0.08/100000, with more males than females. The peak time of incidence is 55–60 years old, which rarely affects children and adolescents. The primary site is mainly in the skull base, active spine, and sacrum. It is locally invasive, insensitive to radiotherapy, occult, and invades bone and nerve structures. Surgical treatment is the main choice (Bryukhovetskiy et al. 2014). However, due to the complex anatomical structure of the spine, sacrum, and skull base and the relatively large tumor volume, complete surgical resection is difficult, the postoperative recurrence and metastasis rate is very high, and it is not sensitive to radiotherapy. Even with high-dose radiotherapy, the effect on tumor control is not significant. Chordoma is a genetic heterologous tumor, and the chromosomal region is often out of balance. Highly repetitive sequences of the Brachyury gene can be found in tumor samples from patients with familial chordoma (Bruce and Winklbauer, 2020; Dridi et al. 2021; Martin and Kimelman, 2010; Otani et al. 2018; Robinson et al. 2020; Shen et al. 2011; Yang et al. 2009; Zhong et al. 2018), which appears in almost all sporadic chordomas. RTKs are key targets in the occurrence and development of chordoma (Hu et al. 2014), and their mutations can lead to protein imbalance. Therefore, mutation analysis and immunohistochemistry can help to determine the best inhibitor. But interestingly, mutations in molecular targets, such as overexpression (Tamborini et al. 2010), are not always driven by the activation of corresponding signal pathways and may come from the structural regulation of molecular networks. The driving gene of chordoma is not clear, and the gene regulation tends to be a complex reticular structure. At the same time, chordoma interacts with surrounding immune cells such as lymphocytes and macrophages. The immune environment also plays an important role in the occurrence and development of tumors.
According to the latest NCCN guidelines, the initial treatment is surgery with or without radiotherapy, and the adjuvant treatment is radiotherapy or reoperation for the positive margin of the tumor. For patients with local recurrence and metastasis recurrence, treatments like surgery, radiotherapy, and systemic treatment can be considered, including chemotherapy, targeted therapy, and immunotherapy. The median survival time of patients is about 5 to 10 years, and the five-year survival rate is about 67%. Therefore, effective adjuvant therapy is urgently needed to improve the prognosis of patients (Jones et al. 2014). The current clinical trials of targeted therapy and immunotherapy have achieved certain clinical benefits, and the safety is acceptable (Fujii et al. 2016), which brings a new dawn to the treatment of patients. Therefore, this review focuses on summarizing the relevant preclinical and clinical trials in the recent five years and systematically combs the clinical management data related to the drug treatment of chordoma.
2 Targeted therapy
NCCN guidelines recommend that targeted therapy can be used for the systemic treatment of chordoma. Imatinib (Adenis et al. 2013; Baldi et al. 2022; Hindi et al. 2015; Stacchiotti et al. 2009, 2018), dasatinib (Verma et al. 2020), sorafenib (Schuetze et al. 2017; Bompas et al. 2015), sunitinib, and lapatinib (Lebellec et al. 2016a) have shown significant clinical efficacy. Thalidomide (Stacchiotti et al. 2013; Chay et al. 2011; Rehlinghaus et al. 2012) is effective for drug-resistant chordomas, but severe toxicity limits its clinical application. Bevacizumab can be used as a drug of choice for EGFR-negative chordomas after erlotinib resistance.
2.1 PDGFR inhibitor
Imatinib is a specific tyrosine kinase inhibitor targeting PDGFR, and it is the first targeted therapeutic drug for myeloma. NCCN recommends imatinib monotherapy for the first-line treatment of chordoma with local recurrence or metastasis. For drug-resistant patients, imatinib, cisplatin, or mTOR inhibitors can be used.
A phase II clinical experiment was conducted by stacchiotti, s et al. (Schonegger et al. 2005), 50 patients with PDGFR-positive chordomas were recruited. All patients were treated with imatinib. After treatment, the disease stability rate was 70%, the median progression-free survival (mPFS) time was nine months, and the median overall survival (mOS) time was 34 months. Hindi et al. (2015). Conducted a retrospective study and recruited patients with PDGFR-positive chordoma, who were given 800 mg of imatinib every day. Among them, 46 patients were able to evaluate the treatment response. The median treatment time was seven months, and the median follow-up time was 24.5 months. 74% of the patients had stable disease (SD). The mPFS was 9.9 months and the mOS was 30 months. More than 70% of patients had tumor growth arrest. The 18 months progression-free survival (PFS) rate of patients was 20%. Side effects from common to rare include rash, fluid retention edema, chronic anemia, fatigue, as well as subacute brain edema, which a few patients could have and affects the vital signs of patients significantly.
Dasatinib is a PDGFR and Src inhibitor, which is recommended by NCCN guidelines for the first-line treatment of locally recurrent and metastatic chordoma. In the phase II clinical trial (Verma et al. 2020), the investigators recruited patients with locally recurrent and metastatic chordomas to receive dasatinib for first-line treatment, and 32 patients could evaluate the treatment response. Patients had a two-year survival rate of 43% and a five-year survival rate of 18%. Fatigue, fever, and anorexia are common adverse events.
Sunitinib is a PDGFR and VEGFR-targeted tyrosinase inhibitor. In a single-center case review study (Stacchiotti et al. 2012), after receiving sunitinib, the patients with metastatic chordoma achieved partial remission according to the RECIST 1.1 standard. The duration of remission can reach 27 months, and no serious adverse reactions have been reported.
2.2 EGFR inhibitor
Lapatinib is a reversible bispecific inhibitor of EGFR and HER2. Lapatinib can be used in the treatment of locally recurrent or metastatic drug-resistant chordomas. In 2022, the second edition of NCCN guidelines was changed from class 2B recommendations to class 2A recommendations. In phase II clinical trial (Lebellec et al. 2016a), 18 patients with EGFR-positive chordoma were included, and lapatinib was used alone for 1500 mg/day until the disease progressed or toxicity became intolerable. Among them, 6 patients (33.3%) had PR and 7 patients (38.9%) had SD, which were evaluated by the Choi standard, with an mPFS of six months, and the mPFS evaluated by RECIST standard was 8 months, The clinical benefit rate was 22%.
Erlotinib (Stacchiotti et al. 2018) is the most commonly used EGFR inhibitor, and NCCN guidelines recommend a single drug for the treatment of recurrent refractory chordoma. A case report showed that there were symptoms and radiological reactions to erlotinib after imatinib treatment of metastatic sacral chordoma progressed, and the imaging showed partial remission at two months. The rash is the most common treatment-related adverse reaction in patients. However, in another clinical study (Lipplaa et al. 2016), a 69-year-old patient with EGFR-positive imatinib refractory sacral chordoma and lung metastasis used erlotinib after disease progression. The patient received the combination treatment of imatinib and everolimus plus metformin. Despite the short-term clinical benefits, the patient experienced rapid clinical deterioration. And he died after 8 weeks of treatment.
2.3 VEGFR inhibitor
Sorafenib is a kind of tyrosine kinase inhibitor targeting VEGFR and PDGFR. In phase II clinical study conducted by lebellec, l. et al. (Trapani et al. 2017), patients with locally progressive and metastatic chordoma were recruited. All patients were treated with sorafenib, and 12 of them had previously received chemotherapy and molecular-targeted therapy. The 9-month PFS rate was 73.0%, the 12-month overall survival (OS) rate was 86.5%, and the mPFS was 12 months. The common grade 3–4 adverse reactions (AEs) of sorafenib are severe thrombocytopenia and diarrhea. The rate of grade 3 is 77.8% and the rate of 4 AEs is 14.8%, which limits the clinical application of sorafenib. Further analysis showed that having high VEGF levels in the blood is closely related to the poor prognosis of patients.
Thalidomide is also a VEGFR inhibitor. It can be used as a second-line treatment after the treatment failure of the combination therapy of imatinib and rapamycin. One case reported that a male patient with metastatic clival chordoma received multiple rounds of treatment over nine years. From the earliest diagnosis of chordoma, brain and lung metastases appeared in the eighth year. In the ninth year, the patient's intracranial metastases and lung metastases progressed rapidly, and the disease was stable after using the combination treatment of liposomal doxorubicin and thalidomide. Adverse reactions such as rash and fatigue can be tolerated.
Pazopanib is a tyrosine kinase inhibitor targeting VEGFR. In the retrospective study (Stacchiotti et al. 2012) carried out by lipplaa et al., patients with locally advanced or metastatic chordoma received pazopanib. The patients obtained 50% clinical benefit, the disease stabilization time was 14 months, and the mOS was 15 months.
2.4 IGF inhibitor
Linsitinib (Lebellec et al. 2016b) is an inhibitor of IGF-1R and insulin receptors. IGF-1R can be detected in the cell membrane and cytoplasm, which is closely related to the recurrence and metastasis of chordoma. Activation of the IGF signaling pathway often leads to the occurrence and development of chordoma. Benign notochord cells were not detected. Linsitinib combined with erlotinib has a synergistic effect. In a phase I study, a patient received 50 mg Linsitinib once a day combined with 50 mg erlotinib once a day. From 43 months, the measurement of Linsitinib was added to 50 mg twice a day to obtain 18 months of PFS. The disease was stable for five years, and the main adverse reactions were prolonged QT interval and abnormal liver function.
2.5 mTOR inhibitor
Rapamycin is a macrolide immunosuppressant. Sirolimus or everolimus alone have no effect on chordoma. According to NCCN guidelines, imatinib combined with sirolimus can be used for recurrent and refractory chordoma. Rapamycin can inhibit mTOR, and then inhibit the expression of downstream eIF4E, to affect the regulation of the cell cycle, promote apoptosis, and inhibit angiogenesis by inhibiting the expression of VEGFR and HIF-1. In addition to PDGFRb, the mTOR pathway can be activated in chordoma, accompanied by upstream RTK mutations, and affect PI3K/AKT/mTOR feedback circuit. Therefore, combination therapy can be considered.
In one trial, Stacchiotti, S Et al. (Hindi et al. 2015) recruited patients with progressive chordoma. The patient is resistant to imatinib and is accompanied by activation of the mTOR target. They received the combination treatment of imatinib (400 mg/d) and sirolimus (2 mg/d). The average treatment time was 9 months. Using Choi criteria to evaluate the response, 9 patients can evaluate the treatment response, and at 3 months, 7 partial remissions, 1 stable, and 1 progress. Among them, 6 patients were associated with Akt activation, and 7 patients were associated with s6sp6 expression or activation. In another study (Stacchiotti et al. 2009), patients with imatinib-resistant chordoma received the combination therapy of imatinib( 400 mg/d) and everolimus (2.5 mg/d) until disease progression or the occurrence of severely limited toxicity. According to Choi's criteria, the 9 and 12 months PFS rate is 58.8% and 48.1%, respectively. The mOS was 47.1 months. Notably, 60.5% and 30.2% of patients temporarily and permanently stopped treatment due to adverse events.
2.6 Other inhibitors
Patients with chordoma usually show the deletion of the smarcb1 site. Smarcb1 can antagonize histone methyltransferase EZH2 and activate CDKN2A, thereby inhibiting CDK4/6 and Rb signaling pathways and regulating the cell cycle. In a phase I experiment on EZH2 inhibitors (Aleksic et al. 2016), patients obtained 100% clinical benefit. At the same time, chordoma is often accompanied by chromosome 9 variation, and CDKN2A is inactivated, which activates CDK4/6 and Rb signaling pathways and promotes tumor proliferation and metastasis.
In another preclinical experiment, Adrian von witzleben et al. analyzed the immunohistochemistry, genomics, mRNA, and protein expression of the tissue bank of 43 patients with chordoma and confirmed that all cells showed the deletion of CDKN2A and p16, resulting in the general activation of CDK4/6 and Rb pathways. Palbociclib treatment effectively inhibited the growth of tumor cells in vitro. Based on the immunohistochemical expression of CDK4/6/prb (s780), the molecular characteristics of drug response and non-response were defined. Chordomas with higher proliferative activity have higher expression of PRB. The high expression of PRB is related to the increase in the Ki-67 index.
At present, clinical trials related to CDK4/6 inhibitors (NCT03242382 and NCT03110744) are in progress.
3 Immunotherapy
The immune microenvironment of a chordoma has interactions with chordoma in many circumstances, affecting the invasiveness of tumors. New treatments targeting the immune microenvironment have been developed. Zou et al. (Chi et al. 2018) reported that the expression of PD-L1 in tumor tissue is related to the increased level of tumor-infiltrating lymphocytes, while the expression of PD-L1 in tumor-infiltrating lymphocytes is related to the improvement of local recurrence-free survival and OS. In the contrast, the expression of PD-1 on tumor-infiltrating lymphocytes has a close relationship with adverse clinical outcomes (Feng et al. 2015). Ga19 is an apoptosis-inducing factor that interacts with Tim-3 positive T lymphocytes. The expression of Ga19 in chordoma is related to the increase of Tim-3 positive tumor-infiltrating lymphocytes (TILs), which is usually related to poor clinical outcomes. At the same time, the down-regulation of mir-455-5p can promote the expression of ga19. CTLA-4 is also expressed in chordomas and TILs. The high expression of CTLA-4 is related to the poor clinical outcomes of patients (Zhou et al. 2019). Cspg4 can be detected in most chordomas, whose high expression can lead to the metastasis of chordomas (He et al. 2020). EZH2 is a histone methyltransferase. EZH2 can regulate cell differentiation. The activation of EZH2 is related to the initiation and development of chordoma. Patients with chordoma, who is resistant to the standard treatment can benefit from the treatment with a tumor vaccine or immune checkpoint inhibitor (Schoenfeld et al. 2016).
3.1 Immune checkpoint inhibitor
Studies have reported that after the use of pembrolizumab in patients with chordoma who failed the standard treatment, the tumor subsided significantly, and the recovery of facial paralysis occurred during the 6-month follow-up period (Gan et al. 2018). A case report describes a patient with a mutation of the PBRM1 gene diagnosed as metastatic chordoma (Migliorini et al. 2017). After receiving pembrolizumab, the tumor burden was reduced by 30%, and the progression-free survival was more than 9 months. Meanwhile, through the organoid culture of chordoma, it was found that PD-L1 expression in chordoma was related to tumor-infiltrating lymphocytes, but its response to immunosuppressants could not be predicted (Wu et al. 2020).
3.2 Short peptide vaccine
Brachyury is a transcription factor protein product of the T-box gene, which plays an important role in regulating the initiation and metastasis of chordoma and is a driving factor in the occurrence of chordoma. T gene replication and single nucleotide variation can lead to the occurrence and development of chordoma. Studies have confirmed that targeting lethal T genes can improve the local recurrence-free survival and OS of chordoma. In one study (Ao et al. 2022), 11 patients with locally advanced chordoma were treated with the Gi-6301 vaccine (yeast Brachyury vaccine). The mOS of patients was 8.3 months. According to the Choi standard (2007 edition), the clinical benefit rate (CBR) was 70%. The most common adverse reaction was the injection site reaction.
Mvx-onco-1 (Demaria et al. 2020) is an individualized anti-tumor vaccine, which is composed of irradiated single tumor cells and promotes antigen-presenting cells to present tumor antigens. After 19 months of treatment with the mvx-onco-1 vaccine, the imaging performance of chordoma patients continued to improve.
Car-T (Mach et al. 2016) treatment-related clinical trials are also in progress.
4 Chemotherapy
Cisplatin is an alkylating agent, which acts on the DNA of chordoma cells, affects the expression of genetic material of chordoma cells, and works in synergy with imatinib (Folkert et al. 2019). NCCN guidelines recommend that the combined treatment of imatinib and cisplatin can be used for drug-resistant and relapsed refractory chordomas. At the same time, studies have shown that bortezomib (Whelan et al. 2018; Trucco et al. 2013), camptothecin (Xia et al. 2013), cyclophosphamide, and other chemotherapy drugs mainly act on the u-ch1 cell line, which can reduce the tumor volume of chordoma. Baldi, GGEt al. conducted a retrospective study on cisplatin or in combination with imatinib in the treatment of patients with advanced Brachyury positive chordoma. According to the analysis of all patients, 84.3% of the patients were SD. At the median follow-up of 54.0 months, the mOS was 30.3 months, the mPFS was 8.0 months, the PFS rate was 65.2% in 6 months and 30.3% in 12 months. Among the 22 patients with cisplatin, the best response of 18 patients (81.8%) was SD, 4 patients (18.2%) developed progression, and the mPFS was 8.0 months. Among the 10 patients who received imatinib plus cisplatin, the best response was SD. The SD rate of patients is 90%, 10% of patient occurred disease progression and the mPFS was 9.3 months.
The results of preclinical studies (Adenis et al. 2013) showed that the combination of imatinib mesylate and metronidazole cyclophosphamide had a synergistic anti-angiogenic effect on pericytes and endothelial cells. Imatinib mesylate and metronidazole cyclophosphamide were used to treat chordoma patients, and no dose-limiting toxicity was observed in the phase I experiment. The mPFS of patients was 10.2 months.
At present, the inhibition efficiency of chemotherapeutic drugs on the u-ch2 cell line is not high, which limits its clinical application. In a preclinical study, a biocompatible RNA nanoparticle used as the delivery system (Kato et al. 2011) was constructed. It includes a 3-way junction nanoparticle and an EGFR aptamer for specifically targeting chordoma cells, 3-way junction nanoparticle is particularly targeting EGFR-positive cell line u-ch2. The targeted 3-way junction nanoparticle provides a new treatment method for chordoma.
5 Conclusion
Chordoma is a kind of tumor that can occur in the skull base, spine, and sacral vertebra. It is resistant to radiotherapy and other traditional treatments, and recurrence and metastasis are often unavoidable. At present, molecular targeted therapy based on gene screening and immunohistochemistry has improved the prognosis of patients to a certain extent and has been included in the recommendations of NCCN guidelines. Single-drug targeted therapy can be used for first-line treatment, and combined therapy can be used for patients with drug resistance. At the same time, in recent years, we have also fully studied the immune microenvironment and its genetic and molecular markers of chordoma. On this basis, we have developed personalized immunotherapy, such as immune checkpoint inhibitors and short peptide vaccines. It has been confirmed by clinical trials that patients can obtain certain clinical benefits. At present, it is mainly pre-clinical and phase I and II clinical trials, there is a lack of large randomized controlled multicenter phase III clinical trials, and the safety and effectiveness of the treatment need to be further confirmed. In the future, further research is needed to clarify the treatment-related targets and molecular markers of treatment prognosis. At the same time, clinical trials should be promoted to realize the individualized treatment of patients.
References
Adenis A, Ray-Coquard I, Italiano A, et al. A dose-escalating phase I of imatinib mesylate with fixed dose of metronomic cyclophosphamide in targeted solid tumours. Br J Cancer. 2013;109(10):2574–8.
Aleksic T, Browning L, Woodward M, et al. Durable response of spinal chordoma to combined inhibition of IGF-1R and EGFR. Front Oncol. 2016;6:98. https://doi.org/10.3389/fonc.2016.00098.
Ao Z, Wu Z, Cai H, et al. Rapid profiling of tumor-immune interaction using acoustically assembled patient-derived cell clusters. Adv Sci. 2022;9(22):2201478. https://doi.org/10.1002/advs.202201478.
Baldi GG, Lo Vullo S, Grignani G, et al. Weekly cisplatin with or without imatinib in advanced chordoma: A retrospective case-series analysis from the Italian Rare Cancers Network. Cancer. 2022;128(7):1439–48.
Bompas E, Le Cesne A, Tresch-Bruneel E, et al. Sorafenib in patients with locally advanced and metastatic chordomas: a phase II trial of the French Sarcoma Group (GSF/GETO). Ann Oncol. 2015;26(10):2168–73.
Bruce AEE, Winklbauer R. Brachyury in the gastrula of basal vertebrates. Mech Dev. 2020;163:103625.
Bryukhovetskiy A, Shevehenko V, Kovalev S, et al. To the novel paradigm of proteome-based cell therapy of tumors: through comparative proteome mapping of tumor stem cells and tissue-specific stem cells of humans. Cell Transplant. 2014;23:S151–70.
Chay WY, Teo M, Sittampalam K, et al. Effective use of thalidomide in the treatment of recurrent metastatic chordoma. J Clin Oncol. 2011;29(16):E477–80.
Chi SS, Fouladi M, Shukla N, et al. Phase 1 study of the EZH2 inhibitor, tazemetostat, in children with relapsed or refractory INI1-negative tumors including rhabdoid tumors, epithelioid sarcoma, chordoma, and synovial sarcoma. Mol Cancer Ther. 2018;17(1 Suppl):A175.
Demaria PJ, Bilusic M, Park D, et al. A randomized, double-blind, phase II clinical trial of GI-6301 (yeast-brachyury vaccine) versus placebo in combination with standard of care definitive radiotherapy in locally advanced, unresectable, chordoma. J Clin Oncol. 2020;38(15 Suppl):11527.
Dridi M, Boutonnat J, Dumollard JM, et al. Patterns of brachyury expression in chordomas. Ann Diagn Pathol. 2021;53:151760.
Feng Y, Shen J, Gao Y, et al. Expression of programmed cell death ligand 1 (PD-L1) and prevalence of tumor-infiltrating lymphocytes (TILs) in chordoma. Oncotarget. 2015;6(13):11139–49.
Folkert IW, Devalaraja S, Linette GP, et al. Primary bone tumors:challenges and opportunities for CAR-T therapies. J Bone Miner Res. 2019;34(10):1780–8.
Fujii R, Friedman ER, Richards J, et al. Enhanced killing of chordoma cells by antibody-dependent cell-mediated cytotoxicity employing the novel anti-PD-L1 antibody avelumab. Oncotarget. 2016;7(23):33498–511.
Gan L, Yang YN, Li Q, et al. Epigenetic regulation of cancer progression by EZH2: from biological insights to therapeutic potential. Biomarker Res. 2018;6:10.
He G, Liu X, Pan X, et al. Cytotoxic T lymphocyte antigen-4 (CTLA-4) expression in chordoma and tumor-infiltrating lymphocytes (TILs) predicts prognosis of spinal chordoma. Clin Transl Oncol. 2020;22(12):2324–32.
Hindi N, Casali PG, Morosi C, et al. Imatinib in advanced chordoma: a retrospective case series analysis. Eur J Cancer. 2015;51(17):2609–14.
Hu YP, Mintz A, Shah SR, et al. The FGFR/MEK/ERK/brachyury pathway is critical for chordoma cell growth and survival. Carcinogenesis. 2014;35(7):1491–9.
Jones PS, Aghi MK, Muzikansky A, et al. Outcomes and patterns of care in adult skull base chordomas from the Surveillance, Epidemiology, and End Results (SEER) database. J Clin Neurosci. 2014;21(9):1490–6.
Kato TA, Tsuda A, Uesaka M, et al. In vitro characterization of cells derived from chordoma cell line U-CH1 following treatment with X-rays, heavy ions and chemotherapeutic drugs. Radiat Oncol. 2011;6:116.
Lebellec L, Bertucci F, Tresch-Bruneel E, et al. Circulating vascular endothelial growth factor (VEGF) as predictive factor of progression-free survival in patients with advanced chordoma receiving sorafenib: an analysis from a phase II trial of the french sarcoma group (GSF/GETO). Oncotarget. 2016a;7(45):73984–94.
Lebellec L, Bertucci F, Tresch-Bruneel E, et al. Circulating vascular endothelial growth factor (VEGF) as prognostic factor of progression-free survival in patients with advanced chordoma receiving sorafenib: An analysis from a phase II trial of the French Sarcoma Group (GSF/GETO). Ann Oncol. 2016b. https://doi.org/10.1093/annonc/mdw388.11.
Lipplaa A, Dijkstra S, Gelderblom H. Efficacy of pazopanib and sunitinib in advanced axial chordoma: a single reference centre case series. Clin Sarcoma Res. 2016;6:19.
Mach N, Vernet R, Belkouch MC, et al. MVX-ONCO-1 phase 1 final results of the first personalized cell-based immunotherapy using cell encapsulation technology. Ann Oncol. 2016;27.
Martin BL, Kimelman D. Brachyury establishes the embryonic mesodermal progenitor niche. Genes Dev. 2010;24(24):2778–83.
Migliorini D, Mach N, Aguiar D, et al. First report of clinical responses to immunotherapy in 3 relapsing cases of chordoma after failure of standard therapies. Oncoimmunology. 2017;6(8):e1338235.
Otani R, Mukasa A, Shin M, et al. Brachyury gene copy number gain and activation of the PI3K/Akt pathway: association with upregulation of oncogenic Brachyury expression in skull base chordoma. J Neurosurg. 2018;128(5):1428–37.
Rehlinghaus J, Austein T, Morche M, et al. Progression-free interval of 7 months by Thalidomide treatment in refractory metastatic chordoma of the sacrum. Onkologie. 2012;35:152–152.
Robinson H, Mcfarlane RJ, Wakeman JA. Brachyury: strategies for drugging an intractable cancer therapeutic target. Trends Cancer. 2020;6(4):271–3.
Schoenfeld AJ, Wang X, Wang Y, et al. CSPG4 as a prognostic biomarker in chordoma. Spine J. 2016;16(6):722–7.
Schonegger K, Gelpi E, Prayer D, et al. Recurrent and metastatic clivus chordoma: systemic palliative therapy retards disease progression. Anticancer Drugs. 2005;16(10):1139–43.
Schuetze SM, Bolejack V, Choy E, et al. Phase 2 study of dasatinib in patients with alveolar soft part sarcoma, chondrosarcoma, chordoma, epithelioid sarcoma, or solitary fibrous tumor. Cancer. 2017;123(1):90–7.
Shen J, Li C-D, Yang H-L, et al. Classic chordoma coexisting with benign notochordal cell rest demonstrating different immunohistological expression patterns of brachyury and galectin-3. J Clin Neurosci. 2011;18(1):96–9.
Stacchiotti S, Marrari A, Tamborini E, et al. Response to imatinib plus sirolimus in advanced chordoma. Ann Oncol. 2009;20(11):1886–94.
Stacchiotti S, Longhi A, Ferraresi V, et al. Phase II study of imatinib in advanced chordoma. J Clin Oncol. 2012;30(9):914–20.
Stacchiotti S, Tamborini E, Lo Vullo S, et al. Phase II study on lapatinib in advanced EGFR-positive chordoma. Ann Oncol. 2013;24(7):1931–6.
Stacchiotti S, Morosi C, Lo Vullo S, et al. Imatinib and everolimus in patients with progressing advanced chordoma: a phase 2 clinical study. Cancer. 2018;124(20):4056–63.
Tamborini E, Virdis E, Negri T, et al. Analysis of receptor tyrosine kinases (RTKs) and downstream pathways in chordomas(dagger). Neuro Oncol. 2010;12(8):776–89.
Trapani D, Conforti F, De Pas TT. EGFR inhibition in a pretreatedsacral chordoma: a role for erlotinib? Case report and a brief review of literature. Transl Med UniSa. 2017;16:30–3.
Trucco MM, Awad O, Wilky BA, et al. A novel chordoma xenograft allows in vivo drug testing and reveals the importance of NF-kappa B signaling in chordoma biology. PLoS One. 2013;8(11):e79950.
Verma S, Vadlamani SP, Shamim SA, et al. Partial response to erlotinib in a patient with imatinib-refractory sacral chordoma. Clin Sarcoma Res. 2020;10(1):28.
Walcott BP, Nahed BV, Mohyeldin A, et al. Chordoma: current concepts, management, and future directions. Lancet Oncol. 2012;13(2):E69–76.
Whelan JS, Davis LE. Osteosarcoma, chondrosarcoma, and chordoma. J Clin Oncol. 2018;36(2):188.
Wu XL, Lin XW, Chen Y, et al. Response of metastatic chordoma to the immune checkpoint inhibitor pembrolizumab: a case report. Front Oncol. 2020;10:565945.
Xia MH, Huang RL, Sakamuru S, et al. Identification of repurposed small molecule drugs for chordoma therapy. Cancer Biol Ther. 2013;14(7):638–47.
Xiao D, Huang YX, Huang SH, et al. Targeted delivery of cancer drug paclitaxel to chordomas tumor cells via an RNA nanoparticle harboring an EGFR aptamer. Colloids Surf B Biointerfaces. 2022;212:112366.
Yang XHR, Ng D, Alcorta DA, et al. T (brachyury) gene duplication confers major susceptibility to familial chordoma. Nat Genet. 2009;41(11):1176–8.
Zhong H, Zhou ZH, Lv GH, et al. Brachyury as prognostic biomarker in chordoma. J Neurosurg. 2018;129(1):273–4.
Zhou JP, Jiang Y, Zhang HY, et al. Clinicopathological implications of TIM3(+) tumor-infiltrating lymphocytes and the miR-455-5p/Galectin-9 axis in skull base chordoma patients. Cancer Immunol Immunother. 2019;68(7):1157–69.
Acknowledgements
The authors acknowledge colleagues in our department for critical reading of the manuscript.
Funding
This work was not financially supported.
Author information
Authors and Affiliations
Contributions
Xue Yang and Parker Li collect data and write articles, while Zhuang Kang and Wenbin Li are responsible for correcting and revising articles. The authors certify that they have participated sufficiently in the work to take public responsibility for the appropriateness of the collection, analysis, and interpretation of the data. This manuscript has not been published in whole or in part nor is it being considered for publication elsewhere. The authors declare that there are no financial or other relationships that might lead to a conflict of interest in the present article. All authors have reviewed the final version of the manuscript and approved it for publication.
Corresponding author
Ethics declarations
Competing interests
Author Wenbin Li is a member of the Editorial Board for Current Medicine. The paper was handled by the other Editor and has undergone rigorous peer review process. Author wenbin Li was not involved in the journal's review of, or decisions related to, this manuscript.
Additional information
Xue Yang and Parker Li collect data and write articles, while Zhuang Kang and Wenbin Li are responsible for correcting and revising articles.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, X., Li, P., Kang, Z. et al. Targeted therapy, immunotherapy, and chemotherapy for chordoma. Curr Med 2, 3 (2023). https://doi.org/10.1007/s44194-022-00017-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44194-022-00017-8