Abstract
Adverse influences during pregnancy are associated with a range of unfavorable outcomes for the developing offspring. Maternal psychosocial stress, exposure to infections and nutritional imbalances are known risk factors for neurodevelopmental derangements and according psychiatric and neurological manifestations later in offspring life. In this context, the maternal immune activation (MIA) model has been extensively used in preclinical research to study how stimulation of the maternal immune system during gestation derails the tightly coordinated sequence of fetal neurodevelopment. The ensuing consequence of MIA for offspring brain structure and function are majorly manifested in behavioral and cognitive abnormalities, phenotypically presenting during the periods of adolescence and adulthood. These observations have been interpreted within the framework of the “double-hit-hypothesis” suggesting that an elevated risk for neurodevelopmental disorders results from an individual being subjected to two adverse environmental influences at distinct periods of life, jointly leading to the emergence of pathology. The early postnatal period, during which the caregiving parent is the major determinant of the newborn´s environment, constitutes a window of vulnerability to external stimuli. Considering that MIA not only affects the developing fetus, but also impinges on the mother´s brain, which is in a state of heightened malleability during pregnancy, the impact of MIA on maternal brain function and behavior postpartum may importantly contribute to the detrimental consequences for her progeny. Here we review current information on the interaction between the prenatal and postnatal maternal environments in the modulation of offspring development and their relevance for the pathophysiology of the MIA model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The internal and external environment of the mother during pregnancy is critically important for the development of the mammalian fetus, determining its path to health and disease. The intrauterine period encompasses extensive developmental processes, from the implantation of the zygote, the differentiation of precursor cells and organogenesis, to the final functional organ development of an expansive growth of the fetus [1, 2]. This unique transformation from fertilized egg to new intact organism most impressively includes the generation and development of the nervous system, which starts with the formation of the neural tube, the establishment of the main structures of the brain and involves the coordinated differentiation and migration of neural cells as prerequisite for the initial wiring with other neurons, finally leading to the construction of the first neural networks [1, 2]. However, a fundamental part of brain development occurs after birth, with an expeditious expansion of neuropil and glial cells accompanying the remodeling and experience-dependent refinement of existing neural circuits and the creation of new connections [3,4,5].
In order to accommodate the developing fetus and responding to its changing and growing demands throughout gestation, the pregnant female also has to undergo significant anatomical and physiological changes [6,7,8]. These dynamic processes, enabling a healthy pregnancy and providing the conditions for optimal development of the offspring, also serve to prepare the mother for the requirements of the post-partum period. Most importantly, the period of matrescence involves a high level of neuroplastic adaptions in the female brain, which accompany her changing throughout pregnancy to motherhood [9]. The modulatory mechanisms of structural and functional neural rearrangements enable the full range of care and nurturing behavior a mother displays towards her newborn immediately after birth, which are pivotal for their survival and ability to thrive.
This enormous capacity for brain development, dynamic adaptation and refinement of present neural architecture and functional circuitry, demand a high degree of plasticity [10]. The underlying malleability comes at the price of heightened sensitivity to external influences and consequently, presents a window of vulnerability to environmental adversities, of both mother and child [2]. As such, infections of the mother during pregnancy leading to maternal immune activation (MIA) are known to hamper the integrity and/ or quality of fetal development [2, 11,12,13] and are associated with a range of negative consequences for offspring social, emotional and cognitive functions [11, 14,15,16]. Direct mother-placental-fetal transmission exists for some infections agents, such as the Zika virus, Toxoplasma gondii, rubella virus, cytomegalovirus and the herpes simplex virus [17,18,19,20,21]. However, activation of the maternal immune system is common to all infections leading to a cascade of pathophysiological events which finally disrupt the tightly controlled balance between the maternal and fetal compartments. To explore the underlying principles, different MIA animal models exist which have jointly contributed to a robust body of knowledge demonstrating that activation of the maternal immune system is a shared pathophysiological mechanism that mediates the consequences of several infectious and non-infectious conditions during pregnancy on offspring brain development.
The present review will assess how infection-mediated MIA impacts the maternal–fetal interface during pregnancy and the perinatal maternal physiological adaptations, both of which can, presumably independently and jointly, exert life-long and even generation-spanning effects on offspring health.
1.1 Fetal programming
The fine-tuned series of spatially and temporally constrained events required for optimal fetal development are intrinsically dependent on a balanced intrauterine environment that ensures the adequate supply of macro- and micronutrients, oxygen, hormones, and other regulators of guidance and growth. The fetus is entirely reliant on the maternal organism and communicatory mechanism that enable the most beneficial allocation of resources, matching its demand at a given time point in development. In most mammals the placenta is the main site for maternal–fetal exchange. The placenta not only constitutes a passive interface and transport system, but it is also actively engaged in dynamically modulating supply in response to changing maternal and fetal signals [22]. During evolution, this mechanism has evolved to maintain equilibrium between fetal growth and maternal survival, especially under conditions of scarcity.
As offspring largely grow close to their parents, this also prepares the fetus for the external conditions, which it will face upon birth. Consequently, the fetal organism adapts its blueprint of developmental processes in response to the changes communicated via the placenta, which mirror the environment its mother is confronted with [2].
This process of “fetal programming” was first described by David Barker in the context of inappropriate nutritional support in utero and the “programming” of offspring metabolic characteristics [23]. The corresponding “fetal origins hypothesis” postulates that this developmental programming in response to adverse influences during the intrauterine period determines the risk for the development of diseases later in the life. Although originally focused on the effects of inadequate nutrition in the prenatal period and its association with metabolic derangements, other early life environmental influences have since then been shown to have persistent health effects [24,25,26,27,28,29,30]. Epidemiological studies and work in animal models have revealed a role for maternal alcohol use, smoking, stress exposure and infection and an individual’s risk for a broad spectrum of pathologies in adult life, which importantly include neurological and psychiatric illnesses [31,32,33,34,35,36,37,38,39,40,41,42].
Within the framework of the “fetal origins hypothesis”, fetal programming can induce a variety of effects, ranging from (epigenetic) modulation of gene expression or long-term hormonal changes, to adjustments in metabolism, as well as to changes in basic biological functions like receptor cell density or sensitivity [43].
These molecular, cellular, structural and functional changes in the offspring organism are characterized by their persistency and long latency until the manifestation of disease, often triggered or “unmasked” by a second stimulus later in life [44,45,46].
In the following section we will focus on gestational infection and the maternal immune activation (MIA) model to exemplify how the impact of the interaction between prenatal adversities and the postnatal environment can shape neurodevelopment to contribute to the emergence of neuropsychiatric disorders.
1.2 Neurodevelopmental consequences of maternal immune activation
Of the many potential adverse events during pregnancy, infections of the mother were among the first for which adverse effects on fetal neurodevelopment manifesting in alterations of offspring brain function have been demonstrated. Evidence is spanning from rubella embryopathy [13, 47] and congenital infections with Toxoplasma gondii [48,49,50,51], to the seminal epidemiological observations that after seasonal outbreaks or epidemics of rubella, influenza, measles, mumps and polio the number of cases of autism spectrum disorders (ASD) and schizophrenia (SZ) increased in the next generation [52,53,54,55,56,57,58,59,60]. Recently, large cohort studies have confirmed an increased risk of psychopathologic conditions in children with a history of infection during prenatal life [61]. In this context, the consequences of the COVID-19 pandemic for the incidence of neurodevelopmental disorders and the probability of psychiatric disorders in the offspring generation yet remain to be determined [62,63,64,65,66].
The establishment and successful application of valid and reliable animal models of MIA in the past 30 years have significantly advanced preclinical research into the neuropathological sequelae of gestational infection on offspring brain structure and function. There are excellent reviews available, contrasting and comparing the various existing MIA paradigms [67,68,69,70,71,72,73,74,75,76]. Herein, we will focus on the most widely used Poly(I:C) (Polyriboinosinic polyribocytidylic acid) model and current insights on potential interactions of the pre- and postnatal environments in shaping offspring neurophenotype.
Poly (I:C) is a double-stranded RNA (dsRNA) analog composed of homo-polymers of inosine and cytidine nucleotides. The immune system of mammals has evolved to recognize dsRNA through Toll Like Receptor 3 (TLR3). Consequently, binding of Poly (I:C) to TLR3 induces a cytokine-associated viral-like acute-phase response by activating the nuclear factor kappa-light-chain-enhancer of activated B cells (NK-kB) and Interferon (IFN) Regulatory Factor (IRF3) pathways [77, 78]. These signaling cascades increase the expression of pro-inflammatory cytokines that can induce cellular and molecular changes in different cell types, including neurons and glia [77]. Indeed, specific cytokines, such as Interleukin-6 (IL-6), interleukin-1ß (IL-1ß), interleukin-17A (IL-17A) [79,80,81] and Tumor Necrosis Factor-α (TNF α) have been shown to be necessary for the effect of Poly (I:C) MIA [77, 78, 82]. Importantly, TLR3 is also abundantly expressed in the placenta [77, 78] and Poly (I:C) can activate the placental immune response [83], suggesting a direct consequence of MIA at the maternal–fetal interface. The dire consequences of MIA on the intrauterine milieu in the fetal compartment are not exclusively meditated by the direct effects of proinflammatory cytokines, but also include a consequence of the concomitant restriction in nutritional and oxygen supply and an increase in reactive oxygen species [67, 78, 84,85,86].
Over the years it has become recognized that several procedural parameters importantly determine the outcome of Poly (I:C) MIA. These include the dosage and source of Poly (I:C) [66, 68, 74, 87, 88], the gestational time point of administration [11, 74, 87, 89,90,91,92], species and strain of the experimental animal [66, 76, 93,94,95] and its environment [15, 96,97,98,99,100,101] and these are comprehensively summarized and discussed elsewhere [46, 75, 76, 78, 99, 102,103,104].
The characteristics of the experimental design undoubtedly shape the response of the maternal system to the immune challenge hereby modulating its impact on the neurodevelopmental processes underlying offspring phenotype. However, the behavioral consequences of MIA for phenotypes related to neuropsychiatric disorders, including the anxiety disorder and depression, as well as autism spectrum disorders and schizophrenia have been repeatedly and robustly demonstrated across a wide range of experimental set-ups and animal species [16, 46, 74,75,76, 78]. As MIA can induce a wide spectrum of behavioral dysfunctions with face validity for distinct psychiatric entities in humans, MIA is not seen as a disease-specific risk factor, but rather as element derailing fetal neurodevelopment and enhancing the predisposition to psychopathology, possibly via as shared mechanisms [46, 76, 105,106,107]. Insights into the underlying mechanisms have substantially increased over the years and led to the identification of systemic, cellular, and molecular derangements used to explain the emotional, social, and cognitive disturbances of MIA offspring. The reader is referred to comprehensive recapitulations of the accumulated body of information on alterations in offspring neural composition, architecture, function and connectivity presented elsewhere [11, 16, 69, 74, 93, 96, 98, 101, 105, 107,108,109,110,111,112,113,114,115,116,117].
Here we would like to specifically point towards the role of epigenetic mechanisms, which are emerging as central regulators of long-term changes in gene expression in the MIA offspring brain. It is conceivable that the alterations in brain structure and function of MIA offspring, persisting beyond the early periods of intrauterine and postnatal brain development, are upheld by modifications of the epigenome driving life-long biased transcriptional programs. Moreover, epigenetic mechanisms are central to the physiological programming of brain development [69, 96, 100, 107, 118,119,120] and deregulations of such are implicated in a range of neuropsychiatric disorders, including those prominently related to gestational infection.
As such, the significance of disturbances of the neuroepigenome and its implications for the disruptions of neural differentiation, migration and circuit formation in the pathophysiology of schizophrenia is increasingly recognized [121,122,123,124,125]. Similarly, epigenetic marks in ASD patients are not only emerging as important diagnostic and prognostic markers but also offer insight into the etiological principles of ASD [126,127,128]. For depressive disorders, also strongly associated with maternal infection during pregnancy epidemiologically [61, 91, 93, 129,130,131], the importance of epigenetic principles in the pathomechanistic events leading to and/ or accompanying disease development has been long recognized [69, 99, 132,133,134,135,136,137,138,139].
With regards to preclinical MIA models, research of the last decade has provided convincing experimental evidence for an impact of early-life exposure to an immunogenic insult on the major epigenetic mechanisms and started uncovering how they can contribute to derailing the trajectories of neurodevelopment. Changes in DNA methylation patterns in the brains of MIA offspring were observed both globally [13, 70, 111, 132, 140, 141] and individually affecting promoter regions of genes with critical roles in neural functions [116, 120, 142]. Similarly, alterations in histone modifications were shown universally in different brain areas of MIA offspring [71, 107] and specifically uncovered in the regulatory regions of selected candidate genes [96, 143]. MIA also leads to persistent changes in gene expression through its effect on miRNAs and their dependent transcriptional networks [139, 144].
Jointly, these observations suggest MIA to directly interfere with the epigenetically determined program of gene expression and its control of neural development and brain function throughout life. In light of the influence of environmental stimuli on epigenetic mechanisms [145,146,147,148], the epigenome may also constitute the molecular interface at which the prenatal influence of an immunogenic stimulation converges with other elements of the postnatal environment. This interaction between MIA and other, additional adversities experienced later in offspring life, is of relevance within the framework of the “double-hit” hypothesis of neurodevelopmental disorders and may importantly determine the heterogeneity of phenotypes observed in the MIA progeny.
1.3 Shaping offspring outcomes after MIA: the modulatory impact of the prenatal environment and the interaction with the postnatal environment
The effect of MIA can be influenced by a variety of factors, such as type and intensity of the immune stimulation, route of administration and gestational time point. It seems plausible, that other crucial factors in this context are variables of the maternal organism. These include the nutritional status of the mother, with the availability of macro- and micronutrients [86, 149,150,151], the maternal microbiome [75, 81, 152, 153], and stress exposure of the mother [37, 154,155,156], all of which can influence the MIA response. The nature of these, and possibly other factors, of the prenatal environment may contribute to the heterogeneity of phenotypes reported across different MIA studies. Indeed, while historically a large degree of variability in outcomes after an experimental manipulation was considered a “weakness” of the paradigm used, it is becoming more appreciated that the wide spectrum of responses obtained in MIA studies harbors a great potential for unraveling the mechanisms controlling the path to pathology, regulating disorder specificity and modulating susceptibility and resilience [75, 78, 111].
Additionally, considering the accepted role of gestational infection as risk factor predisposing to disease development, rather than categorically determining a preformed trajectory to a defined illness, the observed diversity of individual phenotypes may powerfully enhance the translational relevance of MIA models.
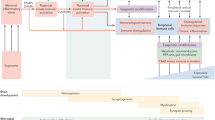
To date, most psychiatric disorders are recognized to be of multifactorial etiology, a consideration that has long been formulated as “dual hit hypothesis” in schizophrenia research [44, 157]. This theory postulates that exposure to a first insult, usually in the form of a genetic predisposition or an adverse environmental exposure prenatally may only induce subthreshold deviations of the physiological trajectories of brain development leading to a latent phenotype in a primed organism. A subsequent interaction with a second “hit” may then induce the manifestation of an apparent phenotype and actual emergence of the disease (Fig. 1.). Specific periods during life are known as “windows of vulnerability” during which environmental adversities can have powerful impacts. This is the result of a highly plastic brain, which undergoes fundamental neural rearrangements and dynamic restructuring requiring heightened malleability, which at the same time also allows for the substantial repercussions of external influences during these periods. As such, adolescence is viewed as a particularly sensitive phase of the mammalian brain development, where environmental impacts can interfere with the ongoing processes of dynamic rewiring [158, 159]. Here, an already “primed” brain is specifically vulnerable to the consequences of trauma, stress and drug abuse as second hits, finally governing the path to pathology [98, 148, 160,161,162]. As such, most of these factors have been studied in the context of gestational infection [163] and mechanistically explored in preclinical MIA models [98, 113, 164, 165].
Interaction between the prenatal and postnatal environments shape for offspring brain and behavior outcomes. Maternal immune activation (MIA) directly disrupts neurodevelopment of the fetus in-utero, while at the same time impinging on the maternal brain during pregnancy. Disturbances of the neural rearrangements in the mother´s brain negatively affect postpartum maternal care behavior which can act as a second hit during postnatal development of the offspring. Additional adverse influences in offspring life, such as drug abuse or stress exposure during adolescence, may further determine an individual´s susceptibility and risk for a specific psychopathology
1.4 Postnatal care behavior and the maternal brain
Developmental psychology has long highlighted the early postpartum period as a phase of remarkable sensitivity when stimuli of the outside world have the capacity to shape the ongoing wiring of the brain in an incomparable manner. Famously postulated in the theory of “imprinting” by Konrad Lorenz, the consequences for behavior are considered to be largely irreversible [166].
In mammals, parental care behavior, mostly maternal care [167,168,169,170], constitutes the strongest external influence during early postnatal life and its critical importance for offspring physiological, psychological and social development is robustly well-established and robustly demonstrated [10, 171,172,173,174,175,176,177,178,179,180,181,182,183]. Displaced or dysfunctional parental care behavior can have severe and long lasting neuropsychiatric and medical consequences for the offspring; indeeed parental maltreatment, abuse, and neglect have been directly associated with irreversible cognitive alterations in children [175, 184,185,186,187,188,189,190].
In rodents, tactile stimulation by licking and grooming of pups is an important component of maternal care behavior that starts directly after birth and continues until weaning. These early somatosensory experiences have a strong relation to and effect on the emotional reactivity to novelty and offspring stress response later in life [171, 176, 183]. Offspring of mothers displaying disruptions in their care behavior, present with increased fearfulness compared to offspring receiving adequate or high maternal care [191, 192]. This augmented anxiety is accompanied by decreased protein and mRNA levels of glucocorticoid receptor (GR) expression within the hippocampal brain region, as well as by an impaired glucocorticoid feedback sensitivity [193]. Alterations of the GR promoter could be directly correlated to a modulation in the reactivity of the hypothalamic–pituitary–adrenal (HPA) axis [191, 192, 194]. Interestingly, cross-fostering of affected offspring to mothers displaying normal maternal care behavior reversed the behavioral effects, as well as the DNA methylation of the GR promoter, indicating the importance of postnatal care and the adverse effects of disruptions during this sensitive period [155, 194].
Furthermore, poor maternal care during the postnatal period was also associated with an adverse effect on the spatial learning and memory ability of offspring, which endured even into later phases of aging [195]. Interestingly, cross-fostering of offspring born to mothers displaying disturbed maternal care behavior, but reared by mothers showing increased maternal care, counteracted this effect [195]. These cross-fostering studies provide evidence for a direct relationship between parental behavior and offspring development. They further suggest that adverse prenatal effects may be reversed by an adequate postnatal environment, and, along this line, a poor postnatal environment may further enhance adverse prenatal influences.
Although epidemiological studies have repeatedly provided strong evidence for an association between maternal infection during pregnancy and neuropsychiatric disorders later in life in the newborn [14, 61]. On the contrary, the effect of gestational infection on the mother’s brain and possible consequences for the bonding and interaction between the mother and her newborn, remains largely unknown. This is surprising, given that the vulnerability of the female brain during the pregnancy and the perinatal period, when structural and functional rearrangements are known to occur [13, 196, 197] is well known. This is also reflected in specific psychiatric pathologies that emerge during this time window, such as postpartum depression (PPD), which severely compromises the mother’s wellbeing and her ability to relate to, and care for her newborn. While the underlying cause is not completely understood and needs further investigation, PPD has been associated with immune dysregulation, as high levels of circulating cytokines have been reported in affected women [198, 199]. Although currently still circumstantial, these observations suggest a possible link between MIA and alterations in the maternal brain, which can then result in the disruption of postnatal maternal care [196]. The relevance of postnatal care behavior and dysfunction thereof, as a possible “second hit” to an immunogenic insult during the prenatal period is, however, only beginning to be explored [74, 99, 196].
1.5 Adaptations of the maternal brain during pregnancy and the impact of MIA
Pregnancy is accompanied by drastic physiological adaptations of the mother, including changes in the cardiovascular system [6, 200], the respiratory system [201, 202], the endocrine system [203,204,205,206], as well as neural alterations [13, 207]. Changes in women’s brain are necessary to allow the mother to form attachments with the baby, appropriately respond to their infant’s needs or detect environmental threats.
The endocrine system plays an important role during pregnancy, parturition and the postnatal period, and allows the mediation of neurological adaptations and behavioral changes [208]. During the prenatal period, progesterone levels increase enabling the implantation of the egg, as well as the maintenance of the pregnancy by inhibiting contractions and thereby preventing preterm labor [209, 210]. Furthermore, progesterone has an suppressive effect on the innate immune response [211, 212], which is important, as during pregnancy the maternal immune system has to tolerate paternal alloantigens expressed in the fetal, and also in placental tissues [213]. Regarding neural adaptions, progesterone also influences hippocampal dendritic spines [214] and acts protectively in the maternal brain [215].
The formation and density of dendritic spines in hippocampal neurons [167, 214, 216,217,218,219], as well as neurogenesis in the dentate gyrus [220], and long-term potentiation [221], are further molded by estradiol. Increased levels of estradiol are known to specifically affect brain areas directly related to maternal care behavior [222], such as the medial preoptic area (mPOA) [223], amygdala [224], hypothalamus [225, 226], olfactory system [227, 228], parietal, and prefrontal cortex [168].
The onset of parturition and initiation of maternal care is further supported by the release of oxytocin [229], which directly affects cell proliferation in the dentate gyrus [156, 230] and hippocampal long-term potentiation [231] in the female brain. Lastly, the hormone prolactin, which is secreted during the beginning of pregnancy and after parturition, mediates hippocampal neurogenesis [232, 233] and neuroprotection [234, 235] in the maternal brain. The precise timing of hormone exposure is fundamental for allowing a healthy pregnancy and adequate offspring care and is not yet well understood. Adverse events experienced by a pregnant female can not only harm the fetus directly, but also disturb maternal health and maternal care post-partum, thereby constituting a negative influence on offspring development postnatally [179].
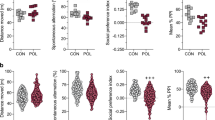
We are only beginning to understand the dire consequences of immune stimulation on the female brain during pregnancy, a period of high malleability and augmented sensitivity of the female brain. This aspect has so far also remained largely unexplored in MIA animal models. We have recently provided first evidence that Poly (I:C) administration substantially impacts the central neural circuits mediating maternal care behavior in the female brain to fundamentally disrupt mother-infant interactions postpartum [196]. For this study we have used the most commonly used MIA Poly (I:C) protocol based upon the application of Poly (I:C) at gestational day 12.5 (20 mg/kg, i.p.) which has not increased the number of pregnancies resulting in abortion or absorption of the fetuses (unpublished data). These observations complement earlier results that showed altered maternal care behavior in MIA dams [96, 99]. Importantly, these studies have shown that the adverse consequences of the immunogenic stressor during pregnancy on postpartum care are not limited to the female experiencing the immune activation herself, but can be passed on her progeny [96, 99]. As such, these reports are in-line with the transgenerational transmission of MIA effects on offspring brain and behavior [61, 69, 116, 132], with the underlying mechanisms only beginning to be unraveled and likely involving changes in the epigenetic signatures [61, 69].
Jointly these data suggest always considering the variable “maternal care” in preclinical models aimed at studying the effects of perinatal risk factors.
The MIA mouse model holds significant translational value for understanding the impact of gestational infection on offspring neurodevelopmental disorders [105]. It provides a robust model system for investigating the underlying mechanisms and exploring potential therapeutic interventions [236]. However, careful consideration should be given to the limitations of the model and the challenges of translating findings obtained in experimental animals to human conditions. Humans have a complex and extended gestational period, allowing for greater interactions between the maternal immune system and the developing fetus [237]. Additionally, human brain development is rather intricated and protracted, continuing until several years after birth, being affected by social and other environmental influences later in life [3, 158, 238]. These factors may result in differences in the manifestation and severity of neurodevelopmental disorders caused by exposure to MIA. Another limitation is the heterogeneity of neurodevelopmental disorders in humans. The MIA mouse model, like any animal model, provides a reductionist representation of the complexity of human conditions to allow for experimental testing of specific hypotheses but cannot fully capture the diversity of clinical presentations and underlying diverse etiologies seen in patients. Integrating findings from animal models with clinical studies in humans will help establishing a more comprehensive understanding of the role of MIA in neurodevelopmental disorders [75].
Where, when, and how the modulatory impact of maternal care interconnects with the consequences of the immunogenic stimulus itself, remains an important topic for future investigations. We have found that neurons of the hypothalamic mPOA express receptors for cytokines induced as a consequence of Poly (I:C) stimulation and in the course of viral and bacterial infections, such as Ifnar1 and Ifnar2. Additionally, mPOA neurons also express tlr3 [196]. Thus, without further experimental data currently available, it can only be speculated whether the adverse effect of gestational infection on the maternal brain occurs directly through the contact with the pathogen, or indirectly as a result of downstream activation and release of cytokines by non-neuronal cells, including microglia or astrocytes.
Similarly, the molecular mediators, and functional interfaces at which the influences of the prenatal and postnatal environment converge to give rise to the complexity of deviations in neural development and the wide spectrum of resulting phenotypes, remains to be explored.
2 Concluding remarks
The complexity of the mechanisms mediating the consequences of activation of the maternal immune system during pregnancy regulates the outcomes for offspring neurodevelopment on a wide spectrum from health to disease. The intricate interrelationship between factors of the prenatal and postnatal environments and their respective sensitivities to further external disruption, may shape an individual’s susceptibility and resilience to determine the risk for a particular pathology later in life.
3 Future directions
The direct impact of immune activation on the female brain during the highly sensitive period of pregnancy shall be a topic for future research in the field. It is important to advance the understanding of the underlying cellular and molecular principles, shaping plastic alterations of neuroarchitecture and functional neurocircuitries. An aspect to consider in this context, is also the timing of the immune activation and how it relates to the neural rearrangements during pregnancy. Here it will be pivotal to determine the translational implications of the data obtained in the MIA mouse model to the human situation, under consideration of the differences in terms of the gestational timelines, and corresponding maternal and fetal developments.
Exploring how MIA impinges on the mother´s brain will be important and relevant for improving maternal health and well-being postpartum and for optimal development of the offspring.
Data availability
Not applicable.
Code availability
Not applicable.
References
Lipsitt LP, Rovee-Collier C. Prenatal and infant development: overview. In: Smelser J, Baltes PB, editors. International encyclopedia of the social & behavioral sciences. Oxford: Pergamon; 2001. p. 11994–7.
Kenner C, Altimier LB, Boykova MV. Fetal development: environmental influences and critical periods. Compr Neonatal Nurs Care. 2019. https://doi.org/10.1891/9780826139146.0001.
Semple BD, Blomgren K, Gimlin K, et al. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106–107:1–16. https://doi.org/10.1016/J.PNEUROBIO.2013.04.001.
Johnson MH. Functional brain development in humans. Nat Rev Neurosci. 2001;27(2):475–83. https://doi.org/10.1038/35081509.
Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. https://doi.org/10.1016/S0149-7634(03)00005-8.
Tkachenko O, Shchekochikhin D, Schrier RW. Hormones and hemodynamics in pregnancy. Int J Endocrinol Metab. 2014;12:14098. https://doi.org/10.5812/IJEM.14098.
Haddad-Tóvolli R, Claret M. Metabolic and feeding adjustments during pregnancy. Nat Rev Endocrinol. 2023;2023:1–17. https://doi.org/10.1038/s41574-023-00871-y.
Grattan DR, Ladyman SR. Neurophysiological and cognitive changes in pregnancy. Handb Clin Neurol. 2020;171:25–55. https://doi.org/10.1016/B978-0-444-64239-4.00002-3.
Elyada YM, Mizrahi A. Becoming a mother-circuit plasticity underlying maternal behavior. Amsterdam: Elsevier Ltd.; 2015.
Feldman R. The adaptive human parental brain: implications for children’s social development. Trends Neurosci. 2015;38:387–99. https://doi.org/10.1016/J.TINS.2015.04.004.
Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neurosci. 2007;13:241–56. https://doi.org/10.1177/1073858406296401.
Lombardo MV, Moon HM, Su J, et al. Maternal immune activation dysregulation of the fetal brain transcriptome and relevance to the pathophysiology of autism spectrum disorder. Mol Psychiatry. 2018;23:1001–13. https://doi.org/10.1038/mp.2017.15.
Bergdolt L, Dunaevsky A. Brain changes in a maternal immune activation model of neurodevelopmental brain disorders. Oxford: Pergamon; 2019.
Han VX, Patel S, Jones HF, Dale RC. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat Rev Neurol. 2021;17:564–79. https://doi.org/10.1038/s41582-021-00530-8.
Meyer U, Schwendener S, Feldon J, Yee BK. Prenatal and postnatal maternal contributions in the infection model of schizophrenia. Exp Brain Res. 2006;173:243–57. https://doi.org/10.1007/s00221-006-0419-5.
Reisinger S, Khan D, Kong E, et al. The Poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol Ther. 2015;149:213–26. https://doi.org/10.1016/j.pharmthera.2015.01.001.
James SH, Sheffield JS, Kimberlin DW. Mother-to-child transmission of herpes simplex virus. J Pediatric Infect Dis Soc. 2014;3:S19. https://doi.org/10.1093/JPIDS/PIU050.
Fisher S, Genbacev O, Maidji E, Pereira L. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J Virol. 2000;74:6808. https://doi.org/10.1128/JVI.74.15.6808-6820.2000.
Andrade JQ, Bunduki V, Curti SP, et al. Rubella in pregnancy: intrauterine transmission and perinatal outcome during a Brazilian epidemic. J Clin Virol. 2006;35:285–91. https://doi.org/10.1016/j.jcv.2005.09.007.
Robbins JR, Zeldovich VB, Poukchanski A, et al. Tissue barriers of the human placenta to infection with Toxoplasma gondii. Infect Immun. 2012;80:418. https://doi.org/10.1128/IAI.05899-11.
Teixeira FME, Pietrobon AJ, de Oliveira LM, et al. Maternal-fetal interplay in Zika virus infection and adverse perinatal outcomes. Front Immunol. 2020;11:175. https://doi.org/10.3389/fimmu.2020.00175.
Ander SE, Diamond MS, Coyne CB. Immune responses at the maternal-fetal interface. Sci Immunol. 2019. https://doi.org/10.1126/sciimmunol.aat6114.
Barker DJP. The fetal origins of adult hypertension. J Hypertens. 1992;10:S39-44. https://doi.org/10.1097/00004872-199212000-00004.
Barouki R, Gluckman PD, Grandjean P, et al. Developmental origins of non-communicable disease: implications for research and public health. Environ Heal. 2012;11:42. https://doi.org/10.1186/1476-069X-11-42.
Langley-Evans SC. Fetal programming of cardiovascular function through exposure to maternal undernutrition. Proc Nutr Soc. 2001;60:505–13. https://doi.org/10.1079/PNS2001111.
Langley-Evans SC. Intrauterine programming of hypertension by glucocorticoids. Life Sci. 1997;60:1213–21. https://doi.org/10.1016/S0024-3205(96)00611-X.
Lindsay RS, Lindsay RM, Edwards CRW, Seckl JR. Inhibition of 11-beta-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertens. 1996;27:1200–4. https://doi.org/10.1161/01.HYP.27.6.1200.
Welberg LAM, Seckl JR, Holmes MC. Inhibition of 11β-hydroxysteroid dehydrogenase, the foetoplacental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur J Neurosci. 2000;12:1047–54. https://doi.org/10.1046/j.1460-9568.2000.00958.x.
Bertram C, Trowern AR, Copin N, et al. The maternal diet during pregnancy programs altered expression of the glucocorticoid receptor and type 2 11beta-hydroxysteroid dehydrogenase: potential molecular mechanisms underlying the programming of hypertension in utero. Endocrinology. 2001;142:2841–53. https://doi.org/10.1210/ENDO.142.7.8238.
Pladys P, Lahaie I, Cambonie G, et al. Role of brain and peripheral angiotensin ii in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr Res. 2004;55:1042–9. https://doi.org/10.1203/01.PDR.0000127012.37315.36.
Liu C, Jiao C, Wang K, Yuan N. DNA methylation and psychiatric disorders. In: Grayson DR, editor. Progress in molecular biology and translational science. Amsterdam: Elsevier Inc.; 2018. p. 175–232.
Zakhari S. Alcohol metabolism and epigenetics changes. Alcohol Res Curr Rev. 2012;35:6–16.
Zoubovsky SP, Williams MT, Hoseus S, et al. Neurobehavioral abnormalities following prenatal psychosocial stress are differentially modulated by maternal environment. Transl Psychiatry. 2022;121(12):1–10. https://doi.org/10.1038/s41398-022-01785-5.
Franco P, Chabanski S, Szliwowski H, et al. Influence of maternal smoking on autonomic nervous system in healthy infants. Pediatr Res. 2000;472(47):215–215. https://doi.org/10.1203/00006450-200002000-00011.
Bhuvaneswar CG, Chang G, Epstein LA, Stern TA. Alcohol use during pregnancy: Prevalence and impact. Prim Care Companion J Clin Psychiatry. 2007;9:455–60. https://doi.org/10.4088/PCC.v09n0608.
Crews FT, Sarkar DK, Qin L, et al. Neuroimmune function and the consequences of alcohol exposure. Alcohol Res Curr Rev. 2015;37:331–51.
Murgatroyd C, Patchev AV, Wu Y, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–66. https://doi.org/10.1038/nn.2436.
DeRosa H, Caradonna SG, Tran H, et al. Got milk? Maternal immune activation during the mid-lactational period affects nutritional milk quality and adolescent offspring sensory processing in male and female rats. Mol Psychiatry. 2022. https://doi.org/10.1038/s41380-022-01744-y.
Yan X, Zhao X, Li J, et al. Effects of early-life malnutrition on neurodevelopment and neuropsychiatric disorders and the potential mechanisms. Prog Neuro-Psychopharmacol Biol Psychiatry. 2018;83:64–75. https://doi.org/10.1016/j.pnpbp.2017.12.016.
Thaler I, Goodman JDS, Dawes GS. Effects of maternal cigarette smoking on fetal breathing and fetal movements. Am J Obstet Gynecol. 1980;138:282–7. https://doi.org/10.1016/0002-9378(80)90249-5.
Mochizuki M, Maruo T, Masuko K, Ohtsu T. Effects of smoking on fetoplacental-maternal system during pregnancy. Am J Obstet Gynecol. 1984;149:413–20. https://doi.org/10.1016/0002-9378(84)90156-X.
Arbeille P, Bosc M, Vaillant TF. Nicotine-induced changes in the cerebral circulation in ovine fetuses. Am J Perinatol. 1992;9:270–4. https://doi.org/10.1055/S-2007-994787.
Kwon EJ, Kim YJ. What is fetal programming?: a lifetime health is under the control of in utero health. Obstet Gynecol Sci. 2017;60:506. https://doi.org/10.5468/ogs.2017.60.6.506.
Bayer TA, Falkai P, Maier W. Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the “Two hit hypothesis.” J Psychiatr Res. 1999;33:543–8. https://doi.org/10.1016/S0022-3956(99)00039-4.
Catuzzi JE, Beck KD. Anxiety vulnerability in women: a two-hit hypothesis. Exp Neurol. 2014;259:75–80. https://doi.org/10.1016/j.expneurol.2014.01.023.
Estes ML, McAllister AK. Maternal immune activation: implications for neuropsychiatric disorders. Science. 2016;353:772–7. https://doi.org/10.1126/science.aag3194.
Dunn PM. Perinatal lessons from the past: Sir Norman Gregg, ChM, MC, of Sydney (1892–1966) and rubella embryopathy. Arch Dis Child Fetal Neonatal Ed. 2007;92:F513. https://doi.org/10.1136/ADC.2005.091405.
Berrébi A, Assouline C, Bessières MH, et al. Long-term outcome of children with congenital toxoplasmosis. Am J Obstet Gynecol. 2010;203:552.e1-552.e6. https://doi.org/10.1016/j.ajog.2010.06.002.
Meenken C, Assies J, Van Nieuwenhuizen O, et al. Long term ocular and neurological involvement in severe congenital toxoplasmosis. Br J Ophthalmol. 1995;79:581–4. https://doi.org/10.1136/BJO.79.6.581.
Setian N, Andrade RSF, Kuperman H, et al. Precocious puberty: an endocrine manifestation on congenital toxoplasmosis. J Pediatr Endocrinol Metab. 2002;15:1487–90. https://doi.org/10.1515/jpem.2002.15.9.1487.
Sever JL, Ellenberg JH, Ley AC, et al. Toxoplasmosis: maternal and pediatric findings in 23,000 pregnancies. Pediatrics. 1988;82:181–92. https://doi.org/10.1542/peds.82.2.181.
Brown AS, Cohen P, Greenwald S, Susser E. Nonaffective psychosis after prenatal exposure to rubella. Am J Psychiatry. 2000;157:438–43. https://doi.org/10.1176/APPI.AJP.157.3.438.
Brown AS, Cohen P, Harkavy-Friedman J, et al. Prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biol Psychiatry. 2001;49:473–86. https://doi.org/10.1016/S0006-3223(01)01068-X.
Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. https://doi.org/10.1523/jneurosci.23-01-00297.2003.
Fatemi SH, Pearce DA, Brooks AI, Sidwell RW. Prenatal viral infection in mouse causes differential expression of genes in brains of mouse progeny: a potential animal model for schizophrenia and autism. Synapse. 2005;57:91–9. https://doi.org/10.1002/SYN.20162.
Tanskanen A, Taipale H, Cannon M, et al. Incidence of schizophrenia and influence of prenatal and infant exposure to viral infectious diseases. Acta Psychiatr Scand. 2021;143:487–94. https://doi.org/10.1111/ACPS.13295.
Sham PC, O’Callaghan E, Takei N, et al. Schizophrenia following pre-natal exposure to influenza epidemics between 1939 and 1960. Br J Psychiatry. 1992;160:461–6. https://doi.org/10.1192/BJP.160.4.461.
Barr CE, Mednick SA, Munk Jorgensen P. Exposure to influenza epidemics during gestation and adult schizophrenia: a 40-year study. Arch Gen Psychiatry. 1990;47:869–74. https://doi.org/10.1001/ARCHPSYC.1990.01810210077012.
Sever JL, Nelson KB, Gilkeson MR. Rubella epidemic, 1964: effect on 6,000 pregnancies. Am J Dis Child. 1965;110:395–407. https://doi.org/10.1001/ARCHPEDI.1965.02090030415009.
Lindquist JM, Plotkin SA, Shaw L, et al. Congenital rubella syndrome as a systemic infection: studies of affected infants born in Philadelphia, U.S.A. Br Med J. 1965;2:1401–5. https://doi.org/10.1136/BMJ.2.5475.1401.
Al-Haddad BJSS, Jacobsson B, Chabra S, et al. Long-term risk of neuropsychiatric disease after exposure to infection in utero. JAMA Psychiat. 2019;76:594. https://doi.org/10.1001/jamapsychiatry.2019.0029.
Oskovi-Kaplan ZA, Buyuk GN, Ozgu-Erdinc AS, et al. The effect of COVID-19 pandemic and social restrictions on depression rates and maternal attachment in immediate postpartum women: a preliminary study. Psychiatr Q. 2021;92:675–82. https://doi.org/10.1007/s11126-020-09843-1.
Hessami K, Norooznezhad AH, Monteiro S, et al. COVID-19 pandemic and infant neurodevelopmental impairment: a systematic review and meta-analysis. JAMA Netw Open. 2022;5:E2238941. https://doi.org/10.1001/jamanetworkopen.2022.38941.
Edlow AG, Castro VM, Shook LL, et al. Neurodevelopmental outcomes at 1 year in infants of mothers who tested positive for SARS-CoV-2 during pregnancy. JAMA Netw Open. 2022;5:E2215787. https://doi.org/10.1001/jamanetworkopen.2022.15787.
Brum AC, Vain NE. Impact of perinatal COVID on fetal and neonatal brain and neurodevelopmental outcomes. Semin Fetal Neonatal Med. 2023;28:101427. https://doi.org/10.1016/j.siny.2023.101427.
Bao M, Hofsink N, Plösch T. LPS versus Poly I: C model: comparison of long-term effects of bacterial and viral maternal immune activation on the offspring. Am J Physiol Integr Comp Physiol. 2022;322:R99–111. https://doi.org/10.1152/ajpregu.00087.2021.
Cieślik M, Gąssowska-Dobrowolska M, Jęśko H, et al. Maternal immune activation induces neuroinflammation and cortical synaptic deficits in the adolescent rat offspring. Int J Mol Sci. 2020;21:4097. https://doi.org/10.3390/IJMS21114097.
Meyer U, Feldon J, Schedlowski M, Yee BK. Immunological stress at the maternal-foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav Immun. 2006;20:378–88. https://doi.org/10.1016/j.bbi.2005.11.003.
Pollak DD, Weber-Stadlbauer U. Transgenerational consequences of maternal immune activation. Semin Cell Dev Biol. 2020;97:181–8. https://doi.org/10.1016/j.semcdb.2019.06.006.
Deng MY, Lam S, Meyer U, et al. Frontal-subcortical protein expression following prenatal exposure to maternal inflammation. PLoS ONE. 2011;6:e16638. https://doi.org/10.1371/JOURNAL.PONE.0016638.
DeRosa H, Smith A, Geist L, et al. Maternal immune activation alters placental histone-3 lysine-9 tri-methylation, offspring sensorimotor processing, and hypothalamic transposable element expression in a sex-specific manner. Neurobiol Stress. 2023. https://doi.org/10.1016/J.YNSTR.2023.100538.
Meyer U, Feldon J. To poly(I:C) or not to poly(I:C): advancing preclinical schizophrenia research through the use of prenatal immune activation models. Neuropharmacology. 2012;62:1308–21. https://doi.org/10.1016/j.neuropharm.2011.01.009.
Otero AM, Antonson AM. At the crux of maternal immune activation: viruses, microglia, microbes, and IL-17A. Immunol Rev. 2022;311:205–23. https://doi.org/10.1111/imr.13125.
Ronovsky M, Berger S, Molz B, et al. Animal models of maternal immune activation in depression research. Curr Neuropharmacol. 2016;14:688–704. https://doi.org/10.2174/1570159X14666151215095359.
Kentner AC, Bilbo SD, Brown AS, et al. Maternal immune activation: reporting guidelines to improve the rigor, reproducibility, and transparency of the model. Neuropsychopharmacology. 2019;44:245–58. https://doi.org/10.1038/s41386-018-0185-7.
Meyer U. Prenatal poly(I:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry. 2014;75:307–15. https://doi.org/10.1016/j.biopsych.2013.07.011.
Haddad FL, Patel SV, Schmid S. Maternal immune activation by poly I: C as a preclinical model for neurodevelopmental disorders: a focus on autism and schizophrenia. Neurosci Biobehav Rev. 2020;113:546–67.
Meyer U. Neurodevelopmental resilience and susceptibility to maternal immune activation. Trends Neurosci. 2019;42:793–806. https://doi.org/10.1016/j.tins.2019.08.001.
Choi GB, Yim YS, Wong H, et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933–9. https://doi.org/10.1126/SCIENCE.AAD0314.
Reed MD, Yim YS, Wimmer RD, et al. IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature. 2020;577:249–53. https://doi.org/10.1038/S41586-019-1843-6.
Kim S, Kim H, Yim YS, et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 2017;549:528–32. https://doi.org/10.1038/NATURE23910.
Zhu X, Levasseur PR, Michaelis KA, et al. A distinct brain pathway links viral RNA exposure to sickness behavior. Sci Rep. 2016;61(6):1–15. https://doi.org/10.1038/srep29885.
Reich D, Thangaraj K, Patterson N, et al. Reconstructing Indian population history. Nature. 2009;461:489–94. https://doi.org/10.1038/nature08365.
Fall CHD. Fetal malnutrition and long-term outcomes. Nestle Nutr Inst Workshop Ser. 2013;74:11–25.
Vuillermot S, Luan W, Meyer U, Eyles D. Vitamin D treatment during pregnancy prevents autism-related phenotypes in a mouse model of maternal immune activation. Mol Autism. 2017;8:1–13. https://doi.org/10.1186/s13229-017-0125-0.
Luan W, Hammond LA, Vuillermot S, et al. Maternal vitamin D prevents abnormal dopaminergic development and function in a mouse model of prenatal immune activation. Sci Rep. 2018;8:1–12. https://doi.org/10.1038/s41598-018-28090-w.
Fortier ME, Luheshi GN, Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res. 2007;181:270–7. https://doi.org/10.1016/J.BBR.2007.04.016.
Missault S, Van den Eynde K, Vanden Berghe W, et al. The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model. Brain Behav Immun. 2014;42:138–46. https://doi.org/10.1016/j.bbi.2014.06.013.
Guma E, do Bordignon PC, Devenyi GA, et al. Early or late gestational exposure to maternal immune activation alters neurodevelopmental trajectories in mice: an integrated neuroimaging, behavioral, and transcriptional study. Biol Psychiatry. 2021;90:328–41. https://doi.org/10.1016/j.biopsych.2021.03.017.
Meyer U, Nyffeler M, Engler A, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–62. https://doi.org/10.1523/JNEUROSCI.0099-06.2006.
Khan D, Fernando P, Cicvaric A, et al. Long-term effects of maternal immune activation on depression-like behavior in the mouse. Transl Psychiatry. 2014;42(4):e363–e363. https://doi.org/10.1038/tp.2013.132.
Ozawa K, Hashimoto K, Kishimoto T, et al. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–54. https://doi.org/10.1016/j.biopsych.2005.07.031.
Babri S, Doosti M-H, Salari A-A. Strain-dependent effects of prenatal maternal immune activation on anxiety- and depression-like behaviors in offspring. Brain Behav Immun. 2014;37:164–76. https://doi.org/10.1016/j.bbi.2013.12.003.
Murray KN, Edye ME, Manca M, et al. Evolution of a maternal immune activation (mIA) model in rats: early developmental effects. Brain Behav Immun. 2019;75:48–59. https://doi.org/10.1016/j.bbi.2018.09.005.
Careaga M, Murai T, Bauman MD. Maternal immune activation and autism spectrum disorder: from rodents to nonhuman and human primates. Biol Psychiatry. 2017;81:391–401. https://doi.org/10.1016/j.biopsych.2016.10.020.
Ronovsky M, Berger S, Zambon A, et al. Maternal immune activation transgenerationally modulates maternal care and offspring depression-like behavior. Brain Behav Immun. 2017;63:127–36. https://doi.org/10.1016/j.bbi.2016.10.016.
Richetto J, Calabrese F, Meyer U, Riva MA. Prenatal versus postnatal maternal factors in the development of infection-induced working memory impairments in mice. Brain Behav Immun. 2013;33:190–200. https://doi.org/10.1016/j.bbi.2013.07.006.
Giovanoli S, Engler H, Engler A, et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339:1100–2. https://doi.org/10.1126/science.1228261.
Berger S, Ronovsky M, Horvath O, et al. Impact of maternal immune activation on maternal care behavior, offspring emotionality and intergenerational transmission in C3H/He mice. Brain Behav Immun. 2018;70:131–40. https://doi.org/10.1016/j.bbi.2018.02.008.
Walker AK, Hawkins G, Sominsky L, Hodgson DM. Transgenerational transmission of anxiety induced by neonatal exposure to lipopolysaccharide: implications for male and female germ lines. Psychoneuroendocrinology. 2012;37:1320–35. https://doi.org/10.1016/j.psyneuen.2012.01.005.
O’Leary C, Desbonnet L, Clarke N, et al. Phenotypic effects of maternal immune activation and early postnatal milieu in mice mutant for the schizophrenia risk gene neuregulin-1. Neuroscience. 2014;277:294–305. https://doi.org/10.1016/j.neuroscience.2014.06.028.
Quagliato LA, de Matos U, Nardi AE. Maternal immune activation generates anxiety in offspring: a translational meta-analysis. Transl Psychiatry. 2021;11:4–9. https://doi.org/10.1038/s41398-021-01361-3.
Mueller FS, Richetto J, Hayes LN, et al. Influence of poly (I: C ) variability on thermoregulation, immune responses and pregnancy outcomes in mouse models of maternal immune activation. Brain Behav Immun. 2019;80:406–18. https://doi.org/10.1016/j.bbi.2019.04.019.
Estes ML, Prendergast K, MacMahon JA, et al. Baseline immunoreactivity before pregnancy and poly(I:C) dose combine to dictate susceptibility and resilience of offspring to maternal immune activation. Brain Behav Immun. 2020;88:619–30. https://doi.org/10.1016/j.bbi.2020.04.061.
Harvey L, Boksa P. Prenatal and postnatal animal models of immune activation: relevance to a range of neurodevelopmental disorders. Dev Neurobiol. 2012;72:1335–48. https://doi.org/10.1002/DNEU.22043.
Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res. 2011;69:26–33. https://doi.org/10.1203/PDR.0b013e318212c196.
Reisinger SN, Kong E, Khan D, et al. Maternal immune activation epigenetically regulates hippocampal serotonin transporter levels. Neurobiol Stress. 2016;4:34–43. https://doi.org/10.1016/j.ynstr.2016.02.007.
Glass R, Norton S, Fox N, Kusnecov AW. Maternal immune activation with staphylococcal enterotoxin A produces unique behavioral changes in C57BL/6 mouse offspring. Brain Behav Immun. 2019;75:12–25. https://doi.org/10.1016/J.BBI.2018.05.005.
Pineda E, Shin D, You SJ, et al. Maternal immune activation promotes hippocampal kindling epileptogenesis in mice. Ann Neurol. 2013;74:11–9. https://doi.org/10.1002/ANA.23898.
Kreitz S, Zambon A, Ronovsky M, et al. Maternal immune activation during pregnancy impacts on brain structure and function in the adult offspring. Brain Behav Immun. 2019. https://doi.org/10.1016/j.bbi.2019.09.011.
Meyer U, Nyffeler M, Yee BK, et al. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–86. https://doi.org/10.1016/j.bbi.2007.09.012.
Bitanihirwe BK, Peleg-Raibstein D, Mouttet F, et al. Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology. 2010;35:2462–78. https://doi.org/10.1038/npp.2010.129.
Henricks AM, Sullivan EDK, Dwiel LL, et al. Maternal immune activation and adolescent alcohol exposure increase alcohol drinking and disrupt cortical-striatal-hippocampal oscillations in adult offspring. Transl Psychiatry. 2022;121(12):1–8. https://doi.org/10.1038/s41398-022-02065-y.
Tartaglione AM, Villani A, Ajmone-Cat MA, et al. Maternal immune activation induces autism-like changes in behavior, neuroinflammatory profile and gut microbiota in mouse offspring of both sexes. Transl Psychiatry. 2022;121(12):1–10. https://doi.org/10.1038/s41398-022-02149-9.
Kalish BT, Kim E, Finander B, et al. Maternal immune activation in mice disrupts proteostasis in the fetal brain. Nat Neurosci. 2020;242(24):204–13. https://doi.org/10.1038/s41593-020-00762-9.
Weber-Stadlbauer U, Richetto J, Zwamborn RAJ, et al. Transgenerational modification of dopaminergic dysfunctions induced by maternal immune activation. Neuropsychopharmacology. 2020;46:404–12. https://doi.org/10.1038/s41386-020-00855-w.
Shi L, Smith SEP, Malkova N, et al. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav Immun. 2009;23:116–23. https://doi.org/10.1016/J.BBI.2008.07.012.
Maccari S, Krugers HJ, Morley-Fletcher S, et al. The consequences of early-life adversity: neurobiological, behavioural and epigenetic adaptations. J Neuroendocrinol. 2014;26:707–23. https://doi.org/10.1111/jne.12175.
Champagne FA. Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol. 2008;29:386–97. https://doi.org/10.1016/j.yfrne.2008.03.003.
Basil P, Li Q, Dempster EL, et al. Prenatal maternal immune activation causes epigenetic differences in adolescent mouse brain. Transl Psychiatry. 2014;4:e434–e434. https://doi.org/10.1038/tp.2014.80.
Girdhar K, Rahman S, Dong P, et al. The neuroepigenome: implications of chemical and physical modifications of genomic DNA in schizophrenia. Biol Psychiatry. 2022;92:443–9. https://doi.org/10.1016/j.biopsych.2022.04.018.
Karpova N, Sales A, Joca S. Epigenetic basis of neuronal and synaptic plasticity. Curr Top Med Chem. 2017;17:771–93. https://doi.org/10.2174/1568026616666160414124628.
Gejman PV, Sanders AR, Duan J. The role of genetics in the etiology of schizophrenia. Psychiatr Clin. 2010;33:35–66. https://doi.org/10.1016/J.PSC.2009.12.003.
Birnbaum R, Weinberger DR. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat Rev Neurosci. 2017;18(12):727–40. https://doi.org/10.1038/nrn.2017.125.
Purves-Tyson TD, Weber-Stadlbauer U, Richetto J, et al. Increased levels of midbrain immune-related transcripts in schizophrenia and in murine offspring after maternal immune activation. Mol Psychiatry. 2019;263(26):849–63. https://doi.org/10.1038/s41380-019-0434-0.
LaSalle JM. Epigenomic signatures reveal mechanistic clues and predictive markers for autism spectrum disorder. Mol Psychiatry. 2023. https://doi.org/10.1038/s41380-022-01917-9.
Gregory SG, Connelly JJ, Towers AJ, et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;7:1–13. https://doi.org/10.1186/1741-7015-7-62.
Schanen NC. Epigenetics of autism spectrum disorders. Hum Mol Genet. 2006;15:R138–50. https://doi.org/10.1093/hmg/ddl213.
Enayati M, Solati J, Hosseini M-H, et al. Maternal infection during late pregnancy increases anxiety- and depression-like behaviors with increasing age in male offspring. Brain Res Bull. 2012;87:295–302. https://doi.org/10.1016/j.brainresbull.2011.08.015.
Lin YL, Wang S. Prenatal lipopolysaccharide exposure increases depression-like behaviors and reduces hippocampal neurogenesis in adult rats. Behav Brain Res. 2014;259:24–34. https://doi.org/10.1016/J.BBR.2013.10.034.
Gilman SE, Cherkerzian S, Buka SL, et al. Prenatal immune programming of the sex-dependent risk for major depression. Transl Psychiatry. 2016;6:e822–e822. https://doi.org/10.1038/TP.2016.91.
Weber-Stadlbauer U, Richetto J, Labouesse MA, et al. Transgenerational transmission and modification of pathological traits induced by prenatal immune activation. Mol Psychiatry. 2017;22:102–12. https://doi.org/10.1038/mp.2016.41.
Bohacek J, Mansuy IM. Molecular insights into transgenerational non-genetic inheritance of acquired behaviours. Nat Rev Genet. 2015;1611(16):641–52. https://doi.org/10.1038/nrg3964.
Nilsson EE, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of disease susceptibility. Transl Res. 2015;165:12–7. https://doi.org/10.1016/J.TRSL.2014.02.003.
Gapp K, Bohacek J, Grossmann J, et al. Potential of environmental enrichment to prevent transgenerational effects of paternal trauma. Neuropsychopharmacol. 2016;4111(41):2749–58. https://doi.org/10.1038/npp.2016.87.
Bale TL. Epigenetic and transgenerational reprogramming of brain development. Nat Rev Neurosci. 2015;166(16):332–44. https://doi.org/10.1038/nrn3818.
Richetto J, Massart R, Weber-Stadlbauer U, et al. Genome-wide DNA methylation changes in a mouse model of infection-mediated neurodevelopmental disorders. Biol Psychiatry. 2017;81:265–76. https://doi.org/10.1016/j.biopsych.2016.08.010.
Connor CM, Dincer A, Straubhaar J, et al. Maternal immune activation alters behavior in adult offspring, with subtle changes in the cortical transcriptome and epigenome. Schizophr Res. 2012;140:175–84. https://doi.org/10.1016/J.SCHRES.2012.06.037.
Hollins SL, Zavitsanou K, Walker FR, Cairns MJ. Alteration of imprinted Dlk1-Dio3 miRNA cluster expression in the entorhinal cortex induced by maternal immune activation and adolescent cannabinoid exposure. Transl Psychiatry. 2014;49(4):e452–e452. https://doi.org/10.1038/tp.2014.99.
Tsivion-Visbord H, Kopel E, Feiglin A, et al. Increased RNA editing in maternal immune activation model of neurodevelopmental disease. Nat Commun. 2020;111(11):1–13. https://doi.org/10.1038/s41467-020-19048-6.
Garbett KA, Hsiao EY, Kálmán S, et al. Effects of maternal immune activation on gene expression patterns in the fetal brain. Transl Psychiatry. 2012;24(2):e98–e98. https://doi.org/10.1038/tp.2012.24.
Fatemi SH, Reutiman TJ, Folsom TD, et al. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: Implications for genesis of neurodevelopmental disorders. Schizophr Res. 2008;99:56–70. https://doi.org/10.1016/J.SCHRES.2007.11.018.
Tang B, Jia H, Kast RJ, Thomas EA. Epigenetic changes at gene promoters in response to immune activation in utero. Brain Behav Immun. 2013;30:168–75. https://doi.org/10.1016/J.BBI.2013.01.086.
Ronovsky M, Zambon A, Cicvaric A, et al. A role for miR-132 in learned safety. Sci Rep. 2019. https://doi.org/10.1038/S41598-018-37054-Z.
Borgel J, Guibert S, Li Y, et al. Targets and dynamics of promoter DNA methylation during early mouse development. Nat Genet. 2010;42:1093–100. https://doi.org/10.1038/ng.708.
Zhang TY, Keown CL, Wen X, et al. Environmental enrichment increases transcriptional and epigenetic differentiation between mouse dorsal and ventral dentate gyrus. Nat Commun. 2018;91(9):1–11. https://doi.org/10.1038/s41467-017-02748-x.
Dalton VS, Verdurand M, Walker A, et al. Synergistic effect between maternal infection and adolescent cannabinoid exposure on serotonin 5HT 1A receptor binding in the hippocampus: testing the “two hit” hypothesis for the development of schizophrenia. ISRN Psychiatry. 2012;2012:1–9. https://doi.org/10.5402/2012/451865.
Herbert MR. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr Opin Neurol. 2010;23:103–10. https://doi.org/10.1097/WCO.0b013e328336a01f.
Labrousse VF, Leyrolle Q, Amadieu C, et al. Dietary omega-3 deficiency exacerbates inflammation and reveals spatial memory deficits in mice exposed to lipopolysaccharide during gestation. Brain Behav Immun. 2018;73:427–40. https://doi.org/10.1016/j.bbi.2018.06.004.
Rivera HM, Christiansen KJ, Sullivan EL. The role of maternal obesity in the risk of neuropsychiatric disorders. Front Neurosci. 2015;9:1–16. https://doi.org/10.3389/fnins.2015.00194.
Aguilar-Valles A, Flores C, Luheshi GN. Prenatal inflammation-induced hypoferremia alters dopamine function in the adult offspring in rat: relevance for schizophrenia. PLoS ONE. 2010;5:e10967. https://doi.org/10.1371/journal.pone.0010967.
Vuong HE, Pronovost GN, Williams DW, et al. The maternal microbiome modulates fetal neurodevelopment in mice. Nature. 2020;586:281–6. https://doi.org/10.1038/s41586-020-2745-3.
Sun Z, Lee-Sarwar K, Kelly RS, et al. Revealing the importance of prenatal gut microbiome in offspring neurodevelopment in humans. eBioMedicine. 2023;90:104491. https://doi.org/10.1016/j.ebiom.2023.104491.
Liu D, Diorio J, Tannenbaum B, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–62. https://doi.org/10.1126/SCIENCE.277.5332.1659.
Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–8. https://doi.org/10.1126/science.286.5442.1155.
Leuner B, Caponiti JM, Gould E. Oxytocin stimulates adult neurogenesis even under conditions of stress and elevated glucocorticoids. Hippocampus. 2012;22:861. https://doi.org/10.1002/HIPO.20947.
Maynard TM, Sikich L, Lieberman JA, LaMantia AS. Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophr Bull. 2001;27:457–76. https://doi.org/10.1093/OXFORDJOURNALS.SCHBUL.A006887.
Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl):511–33. https://doi.org/10.1289/EHP.00108S3511.
Kundakovic M, Champagne FA. Early-life experience, epigenetics, and the developing brain. Neuropsychopharmacology. 2015;40:141. https://doi.org/10.1038/NPP.2014.140.
Clarke MC, Tanskanen A, Huttunen M, et al. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am J Psychiatry. 2009;166:1025–30. https://doi.org/10.1176/appi.ajp.2009.08010031.
Fisher HL, Jones PB, Fearon P, et al. The varying impact of type, timing and frequency of exposure to childhood adversity on its association with adult psychotic disorder. Psychol Med. 2010;40:1967–78. https://doi.org/10.1017/S0033291710000231.
Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;912(9):947–57. https://doi.org/10.1038/nrn2513.
Jacobsen H, Walendy-Gnirß K, Tekin-Bubenheim N, et al. Offspring born to influenza A virus infected pregnant mice have increased susceptibility to viral and bacterial infections in early life. Nat Commun. 2021;121(12):1–14. https://doi.org/10.1038/s41467-021-25220-3.
Giovanoli S, Engler H, Engler A, et al. Preventive effects of minocycline in a neurodevelopmental two-hit model with relevance to schizophrenia. Transl Psychiatry. 2016;64(6):e772–e772. https://doi.org/10.1038/tp.2016.38.
Goh JY, O’Sullivan SE, Shortall SE, et al. Gestational poly(I:C) attenuates, not exacerbates, the behavioral, cytokine and mTOR changes caused by isolation rearing in a rat ‘dual-hit’ model for neurodevelopmental disorders. Brain Behav Immun. 2020;89:100–17. https://doi.org/10.1016/J.BBI.2020.05.076.
Lorenz K. Über tierisches und menschliches Verhalten Aus dem Werdegang der Verhaltenslehre Gesammelte Abhandlungen Band II. 1966.
Kinsley CH, Bardi M, Karelina K, et al. Motherhood induces and maintains behavioral and neural plasticity across the lifespan in the rat. Arch Sex Behav. 2008;37:43–56. https://doi.org/10.1007/S10508-007-9277-X.
Glat M, Gundacker A, Cuenca Rico L, et al. An accessory prefrontal cortex–thalamus circuit sculpts maternal behavior in virgin female mice. EMBO J. 2022. https://doi.org/10.15252/embj.2022111648.
Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nat Rev Neurosci. 2007;91(9):11–25. https://doi.org/10.1038/nrn2280.
Numan M. Parental behavior. In: Sala SD, editor. Encyclopedia of behavioral neuroscience. 2nd ed. Amsterdam: Elsevier; 2017. p. 459–73. https://doi.org/10.1016/B978-0-12-809324-5.00400-4.
Champagne FA, Curley JP. Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neurosci Biobehav Rev. 2009;33:593–600.
Paulson JF, Keefe HA, Leiferman JA. Early parental depression and child language development. J Child Psychol Psychiatry Allied Discip. 2009;50:254–62. https://doi.org/10.1111/j.1469-7610.2008.01973.x.
Nelson CA, Zeanah CH, Fox NA. How early experience shapes human development: the case of psychosocial deprivation. Neural Plast. 2019. https://doi.org/10.1155/2019/1676285.
Easterbrooks MA, Bureau J-FF, Lyons-Ruth K. Developmental correlates and predictors of emotional availability in mother-child interation: a longitudinal study from infancy to middle childhood. Dev Psychopathol. 2012;24:65–78. https://doi.org/10.1017/S0954579411000666.
Cogill SR, Caplan HL, Alexandra H, et al. Impact of maternal postnatal depression on cognitive development of young children. Br Med J. 1986;292:1165–7. https://doi.org/10.1136/bmj.292.6529.1165.
Macrì S, Chiarotti F, Würbel H. Maternal separation and maternal care act independently on the development of HPA responses in male rats. Behav Brain Res. 2008;191:227–34. https://doi.org/10.1016/j.bbr.2008.03.031.
Harker A. Social dysfunction: the effects of early trauma and adversity on socialization and brain development. Neurobiol Brain Behav Dev. 2018. https://doi.org/10.1016/B978-0-12-804036-2.00016-9.
Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345:771–6. https://doi.org/10.1126/science.1252723.
Connor KL, Vickers MH, Beltrand J, et al. Nature, nurture or nutrition? Impact of maternal nutrition on maternal care, offspring development and reproductive function. J Physiol. 2012;590:2167–80. https://doi.org/10.1113/jphysiol.2011.223305.
Belsky J, Fearon RMP. Early attachment security, subsequent maternal sensitivity, and later child development: does continuity in development depend upon continuity of caregiving? Attach Hum Dev. 2002;4:361–87. https://doi.org/10.1080/14616730210167267.
Mogi K, Nagasawa M, Kikusui T. Developmental consequences and biological significance of mother-infant bonding. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35:1232–41. https://doi.org/10.1016/j.pnpbp.2010.08.024.
Fleming AS, O’Day DH, Kraemer GW. Neurobiology of mother-infant interactions: experience and central nervous system plasticity across development and generations. Neurosci Biobehav Rev. 1999;23:673–85. https://doi.org/10.1016/s0149-7634(99)00011-1.
Macrì S, Würbel H. Developmental plasticity of HPA and fear responses in rats: a critical review of the maternal mediation hypothesis. Horm Behav. 2006;50:667–80. https://doi.org/10.1016/j.yhbeh.2006.06.015.
McManus BM, Poehlmann J. Parent–child interaction, maternal depressive symptoms and preterm infant cognitive function. Infant Behav Dev. 2012;35:489–98. https://doi.org/10.1016/J.INFBEH.2012.04.005.
Perra O, Phillips R, Fyfield R, et al. Does mothers’ postnatal depression influence the development of imitation? J Child Psychol Psychiatry. 2015;56:1231–8. https://doi.org/10.1111/JCPP.12413.
Trickett PK, McBride-Chang C. The developmental impact of different forms of child abuse and neglect. Dev Rev. 1995;15:311–37. https://doi.org/10.1006/DREV.1995.1012.
Ammerman RT, Cassisi JE, Hersen M, Van Hasselt VB. Consequences of physical abuse and neglect in children. Clin Psychol Rev. 1986;6:291–310. https://doi.org/10.1016/0272-7358(86)90003-6.
Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the adverse childhood experiences (ACE) study. Am J Prev Med. 1998;14:245–58. https://doi.org/10.1016/S0749-3797(98)00017-8.
Gilbert R, Widom CS, Browne K, et al. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373:68–81. https://doi.org/10.1016/S0140-6736(08)61706-7.
McEwen BS. Early life influences on life-long patterns of behavior and health. Ment Retard Dev Disabil Res Rev. 2003;9:149–54. https://doi.org/10.1002/mrdd.10074.
Caldji C, Tannenbaum B, Sharma S, et al. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci. 1998;95:5335–40. https://doi.org/10.1073/pnas.95.9.5335.
Levine S. Influence of psychological variables on the activity of the hypothalamic-pituitary-adrenal axis. Eur J Pharmacol. 2000;405:149–60. https://doi.org/10.1016/S0014-2999(00)00548-3.
Liu D, Diorio J, Tannenbaum B, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic- pituitary-adrenal responses to stress. Science. 1997;277:1659–62. https://doi.org/10.1126/SCIENCE.277.5332.1659/ASSET/F62129BF-CB05-4B6A-ABD3-5CC5D5F458EC/ASSETS/GRAPHIC/SE3675687003.JPEG.
Weaver ICG, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;78(7):847–54. https://doi.org/10.1038/nn1276.
Liu D, Diorio J, Day JC, et al. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;38(3):799–806. https://doi.org/10.1038/77702.
Zambon A, Rico LC, Herman M, et al. Gestational immune activation disrupts hypothalamic neurocircuits of maternal care behavior. Mol Psychiatry. 2022. https://doi.org/10.1038/s41380-022-01602-x.
Crum WR, Sawiak SJ, Chege W, et al. Evolution of structural abnormalities in the rat brain following in utero exposure to maternal immune activation: a longitudinal in vivo MRI study. Brain Behav Immun. 2017;63:50–9. https://doi.org/10.1016/j.bbi.2016.12.008.
Brummelte S, Galea LAM. Postpartum depression: etiology, treatment and consequences for maternal care. Horm Behav. 2016;77:153–66. https://doi.org/10.1016/j.yhbeh.2015.08.008.
Achytes E, Keaton SA, Smart L, et al. Inflammation and kynurenine pathway dysregulation in post- partum women with severe and suicidal depression. Int J Environ Res Public Health. 2020;83:239–47. https://doi.org/10.3390/ijerph18179307.
Gant NF, Worley RJ, Everett RB, MacDonald PC. Control of vascular responsiveness during human pregnancy. Kidney Int. 1980;18:253–8. https://doi.org/10.1038/KI.1980.133.
Wise RA, Polito AJ. Respiratory physiologic changes in pregnancy. Immunol Allergy Clin North Am. 2000;20:663–72. https://doi.org/10.1016/S0889-8561(05)70175-2.
LoMauro A, Aliverti A. Respiratory physiology of pregnancy. Breathe. 2015;11:297–301. https://doi.org/10.1183/20734735.008615.
Lindheimer MD, Barron WM, Davison JM. Osmotic and volume control of vasopressin release in pregnancy. Am J Kidney Dis. 1991;17:105–11. https://doi.org/10.1016/S0272-6386(12)81112-7.
Elsheikh A, Creatsas G, Mastorakos G, et al. The renin-aldosterone system during normal and hypertensive pregnancy. Arch Gynecol Obstet. 2001;264:182–5. https://doi.org/10.1007/S004040000104.
Dôrr HG, Heller A, Versmold HT, et al. Longitudinal study of progestins, mineralocorticoids, and glucocorticoids throughout human pregnancy. J Clin Endocrinol Metab. 1989;68:863–8. https://doi.org/10.1210/JCEM-68-5-863.
Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18:404–33. https://doi.org/10.1210/EDRV.18.3.0300.
Hwang HM, Ku RY, Hashimoto-Torii K. Prenatal environment that affects neuronal migration. Front Cell Dev Biol. 2019;7:1–11. https://doi.org/10.3389/fcell.2019.00138.
Bridges RS. Neuroendocrine regulation of maternal behavior. Front Neuroendocrinol. 2015;36:178–96. https://doi.org/10.1016/j.yfrne.2014.11.007.
Magon N, Kumar P. Hormones in pregnancy. Niger Med J. 2012;53:179. https://doi.org/10.4103/0300-1652.107549.
Zakar T, Hertelendy F. Progesterone withdrawal: key to parturition. Am J Obstet Gynecol. 2007;196:289–96. https://doi.org/10.1016/J.AJOG.2006.09.005.
Dorfman VB, Saucedo L, Di Giorgio NP, et al. Variation in progesterone receptors and GnRH expression in the hypothalamus of the pregnant South American plains vizcacha Lagostomus maximus (Mammalia, Rodentia). Biol Reprod. 2013. https://doi.org/10.1095/BIOLREPROD.113.107995.
Pennacchio GE, Neira FJ, Soaje M, et al. Effect of hyperthyroidism on circulating prolactin and hypothalamic expression of tyrosine hydroxylase, prolactin signaling cascade members and estrogen and progesterone receptors during late pregnancy and lactation in the rat. Mol Cell Endocrinol. 2017;442:40–50. https://doi.org/10.1016/J.MCE.2016.11.029.
La Rocca C, Carbone F, Longobardi S, Matarese G. The immunology of pregnancy: regulatory T cells control maternal immune tolerance toward the fetus. Immunol Lett. 2014;162:41–8. https://doi.org/10.1016/J.IMLET.2014.06.013.
Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. https://doi.org/10.1002/CNE.903360210.
Stein DG, Hoffman SW. Estrogen and progesterone as neuroprotective agents in the treatment of acute brain injuries. Pediatr Rehabil. 2003;6:13–22. https://doi.org/10.1080/1363849031000095279.
Kinsley CH, Trainer R, Stafisso-Sandoz G, et al. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm Behav. 2006;49:131–42. https://doi.org/10.1016/j.yhbeh.2005.05.017.
McEwen BS, Woolley CS. Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain. Exp Gerontol. 1994;29:431–6. https://doi.org/10.1016/0531-5565(94)90022-1.
Yankova M, Hart SA, Woolley CS. Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: a serial electron-microscopic study. Proc Natl Acad Sci U S A. 2001;98:3525–30. https://doi.org/10.1073/PNAS.051624598.
Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–91. https://doi.org/10.1523/JNEUROSCI.10-04-01286.1990.
Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792. https://doi.org/10.1523/JNEUROSCI.19-14-05792.1999.
Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res. 1995;703:26–30. https://doi.org/10.1016/0006-8993(95)01059-9.
Bridges RS. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology. 1984;114:930–40. https://doi.org/10.1210/ENDO-114-3-930.
Keyser-Marcus L, Stafisso-Sandoz G, Gerecke K, et al. Alterations of medial preoptic area neurons following pregnancy and pregnancy-like steroidal treatment in the rat. Brain Res Bull. 2001;55:737–45. https://doi.org/10.1016/S0361-9230(01)00554-8.
Rasia-Filho AA, Fabian C, Rigoti KM, Achaval M. Influence of sex, estrous cycle and motherhood on dendritic spine density in the rat medial amygdala revealed by the Golgi method. Neuroscience. 2004;126:839–47. https://doi.org/10.1016/j.neuroscience.2004.04.009.
Fleming AS, Korsmit M. Plasticity in the maternal circuit: effects of maternal experience on Fos-lir in hypothalamic, limbic, and cortical structures in the postpartum rat. Behav Neurosci. 1996;110:567–82. https://doi.org/10.1037/0735-7044.110.3.567.
Freund-Mercier MJ, Stoeckel ME, Klein MJ. Oxytocin receptors on oxytocin neurones: histoautoradiographic detection in the lactating rat. J Physiol. 1994;480(Pt 1):155–61. https://doi.org/10.1113/JPHYSIOL.1994.SP020349.
Clancy AN, Goldman BD, Bartke A, Macrides F. Reproductive effects of olfactory bulbectomy in the Syrian hamster. Biol Reprod. 1986;35:1202–9. https://doi.org/10.1095/BIOLREPROD35.5.1202.
Liu HX, Lopatina O, Higashida C, et al. Displays of parental mouse pup retrieval following communicative interaction with maternal mates. Nat Commun. 2013. https://doi.org/10.1038/NCOMMS2336.
Lévy F. Neuroendocrine control of maternal behavior in non-human and human mammals. Ann Endocrinol. 2016;77:114–25. https://doi.org/10.1016/J.ANDO.2016.04.002.
Monks DA, Lonstein JS, Breedlove SM. Got milk? Oxytocin triggers hippocampal plasticity. Nat Neurosci. 2003;6:327–8. https://doi.org/10.1038/nn0403-327.
Tomizawa K, Iga N, Lu YF, et al. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci. 2003;6:384–90. https://doi.org/10.1038/NN1023.
Shingo T, Gregg C, Enwere E, et al. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–20. https://doi.org/10.1126/science.1076647.
Leuner B, Sabihi S. The birth of new neurons in the maternal brain: hormonal regulation and functional implications. Front Neuroendocrinol. 2016;41:99. https://doi.org/10.1016/J.YFRNE.2016.02.004.
Torner L. Actions of prolactin in the brain: from physiological adaptations to stress and neurogenesis to psychopathology. Front Endocrinol. 2016. https://doi.org/10.3389/FENDO.2016.00025.
Rivero-Segura NA, Flores-Soto E, De La Cadena SG, et al. Prolactin-induced neuroprotection against glutamate excitotoxicity is mediated by the reduction of [Ca2+]i overload and NF-κB activation. PLoS ONE. 2017;12:e0176910. https://doi.org/10.1371/JOURNAL.PONE.0176910.
Brown AS, Meyer U. maternal immune activation and neuropsychiatric illness: a translational research perspective. Am J Psychiatry. 2018;175:1073–83. https://doi.org/10.1176/appi.ajp.2018.17121311.
Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. Front Neuroendocrinol. 2012;33:267. https://doi.org/10.1016/J.YFRNE.2012.08.006.
Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 2011;31:10937. https://doi.org/10.1523/JNEUROSCI.5302-10.2011.
Funding
This work is supported by the Austrian Science Fund (Fonds für Wissenschaft und Forschung, FWF; Grant no. I 4854 and P 34281 to D.D.P.
Author information
Authors and Affiliations
Contributions
DDP, UW-S and AG conceived the manuscript. AG, LCR, PS, KET, UW-S and DDP wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gundacker, A., Cuenca Rico, L., Stoehrmann, P. et al. Interaction of the pre- and postnatal environment in the maternal immune activation model. Discov Ment Health 3, 15 (2023). https://doi.org/10.1007/s44192-023-00042-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44192-023-00042-5