Abstract
Four hot-gas bypass defrosting configurations for CO2-NH3 cascade blast freezer for application in fish processing firm are numerically investigated. Due to the high moisture content of fish, defrosting is necessary after every 4 to 5 h of batch operation. A thermodynamic model for the cascade system and defrosting was developed to study various defrosting configurations formulated by rearranging the existing compressor to operate as a defrosting compressor and with the addition of an external defrosting compressor. From the simulation findings, it can be summarized that the conventional hot-gas bypass defrosting without defrost compressor is suitable for a high-capacity cascade refrigeration system with more than three evaporators. For low cooling capacity refrigeration systems, a defrosting compressor is necessary to elevate the temperature above the cascade condensing temperature. A dedicated defrosting compressor with a power consumption of 3.1 kW and a modified refrigeration/defrosting compressor with a power consumption of 6.8 kW can deliver 33.3 kW of heating at a temperature of +10 °C (45 bar). Incorporating a desuperheater between the main and defrosting compressors reduces compressor temperature and maintains the lubricating oil stability, without change in defrosting energy consumption and less exergy loss. The defrosting efficiency is obtained in the range of 39.7–42% which is in agreement with published literature.
Similar content being viewed by others
1 Introduction
Seafood processing industries need various temperatures to maintain the quality of their products. The export of marine products from India stood at 1.39 million metric tonnes and was valued at USD 6.73 billion during 2018–2019, with an impressive average annual growth rate of about 10% in recent years [1]. After the fresh catch, fish will be stored in a refrigerated chamber or ice chamber in the fishing vessel. Thereafter, it will be transported for deep freezing and stored in cold storages. All this process requires intense refrigeration and high-energy demand, which contributes to carbon emissions. Ammonia has traditionally been used as the refrigerant in these seafood processing industries. With the increase in residential areas around these industries, more people may be affected if there is an ammonia leakage. Moreover, all ammonia systems are space demanding and operate below atmospheric pressure for deep freezing. These efficient, natural refrigerant-based systems are slowly being replaced by synthetic refrigerants having high GWP. However, India’s nationally determined contributions (NDCs) to phase out HFCs and restrict the global warming temperature to +2 °C lead to the opportunity adopting CO2 as a refrigerant. CO2 has all desirable properties of a good refrigerant such as high vapour density and volumetric capacity for refrigeration. It is non-toxic with A1 safety rating. Ammonia has also excellent properties, but since it is toxic, a viable alternative is to have a cascade system, which limits the filling of ammonia but still utilizes the beneficiary properties.
A complete review of cascade refrigeration systems was presented by M. Pan et al. [2]. These systems can achieve an evaporating temperature as low as −170 °C and have important applications in rapid freezing. The performance of cascade refrigeration system increases with the increase in evaporator temperature, decrease in the condenser outlet temperature (ambient) and maintaining small temperature difference in the cascade heat exchanger. Compared to single-stage and two-stage ammonia refrigeration system, CO2-NH3 cascade system is more efficient for very low evaporating temperatures [3]. Various research has been carried out on CO2-NH3 cascade refrigeration systems, where NH3 and CO2 are used in high-temperature and low-temperature circuit respectively. Lee et al. [4] and Getu and Bansal [5] carried out thermodynamic analyses of CO2-NH3 cascade system and determined the optimal cascade condensing temperature for maximum COP, which depends on evaporating temperature, condensing temperature and cascade heat exchanger temperature differences. Alberto Dopazo et al. [6] theoretically analysed a CO2-NH3 cascade system for a low-temperature application. They developed relevant correlations to serve as guidelines for the design and optimization of a cascade system. Bingming et al. [3] and Dopazo and Fernandez-seara [7] experimentally investigated the performance of CO2-NH3 cascade system. The system was compared with two-stage NH3 and single-stage NH3 systems. They found that below −40 °C evaporator temperature, COP of the cascade system is superior to others. Yilmaz et al. [8] conducted a parametric study on CO2-NH3 cascade system for different operating and ambient conditions. The maximum COP was found in the range of 1.23–2.37. Bellos and Tzivanidis [9] conducted a comparative study of 18 different CO2 cascade systems for yearly operation in weather conditions of Athen, Greece. The result showed that natural refrigerants such as NH3, R290, R600 and R1270 are more appropriate choices according to the energy efficiency and total equivalent warming impact criteria. Saini et al. [10] carried out a comparative analysis of three CO2-NH3 cascade system configurations for application in seafood processing for high ambient conditions. The application involved cooling demand in deep freezing and cold storage. For these improved configurations, they have reported a COP advantage of 11.5–20.3% more than the conventional cascade system.
The deep freezer operating at − 40 °C used in the fish processing is susceptible to frost formation on the surface of evaporators because of the high moisture content of fish. The frost build-up on the evaporator reduces the heat transfer effectiveness; therefore, periodic defrosting is required for the freezers. For a batch process such as in blast and plate freezers, the evaporators have to go through defrost within 4 to 5 h, and for a continuous process such as in tunnel and spiral freezers, it requires defrost after 8 to 12 h of operation. Various defrosting methods used are time-off defrost (water spray/air circulation defrost), electric defrost and hot-gas bypass defrost. J. Klingebiel et al. [11] have experimentally compared three commonly applied defrosting methods such as reverse cycle defrosting, electric heating defrosting and warm brine defrosting and concluded that the efficiency of electric heating defrosting is low and warm brine defrosting incurs high equipment costs. Generally, hot-gas bypass defrosting is considered economical and efficient [11, 12]. However, the hot-gas defrosting method, which is commonly followed in industrial refrigeration systems, cannot be directly adopted in case of cascade refrigeration.
1.1 Hot-gas bypass defrosting
In the hot-gas bypass defrosting method, hot gas from the compressor discharge is redirected to the evaporator. It is generally passed through the outlet of the evaporator in the reverse direction [13]. Hereafter, the hot vapour CO2 condenses inside the evaporator tube and consequently heats the surface of the tube, which results in melting of the frost. The evaporator becomes a condenser during defrosting. Finally, the condensed CO2 will be collected in the low-pressure receiver. Hot-gas defrosting can be carried out when the refrigeration system consists of multi-evaporators so that sufficient vapour is generated for defrosting [14].
Hoffenbecker et al. [15] and Dopazo et al. [16] have developed a transient simulation model for predicting heat and mass transfer effects during the hot-gas defrosting cycle. They predicted the time required for complete frost melt. The latter model predicted the time required to defrost is around 14 min. They analysed the effect of refrigerant mass flow rate and inlet temperature, on the defrost time. It was found that the defrosting time increases, and energy supplied decreases as the refrigerant mass flow rate decreases. Hu et al. [17] and Wang et al. [18] carried out an experimental study of various hot-gas defrost methods for an air source transcritical CO2 heat pump for the water heater. They observed that for the compressor discharge temperature of + 60 °C, the time required to defrost was about 10 min. The heating required for melting the frost was 2659.5 kJ with a defrost efficiency of 41.6–50.84%. Soylemez et al. [19] have reported on a dedicated CO2 compressor for hot-gas defrosting which utilizes vapour from the cascade condenser to deliver heat to the evaporators in an CO2-NH3 cascade system for fishing vessels.
Due to climate concerns, the reintroduction of CO2 as a refrigerant for both transcritical and cascade refrigeration system has become significant. It can be noted from the literature that various studies on performance of cascade refrigeration systems have been conducted. Along with the freezing, defrosting is also an important process, especially in fish processing units where moisture content is high. Only a few studies are carried out on the defrosting techniques required in cascade deep freezers operating at − 40 °C. This paper proposes a mathematical model for CO2-NH3 cascade refrigeration system for blast freezers and different hot-gas defrosting configurations that can be adopted. In conventional hot-gas defrosting, hot gas from the discharge is redirected to the evaporators. However, in the case of a cascade system, the CO2 condensing temperature will be in the range of − 15 to − 5 °C, which is not adequate to remove the frost formed on the evaporators. A necessary temperature above 0 °C is required for efficient defrosting. Rising the temperature of the cascade condenser above the freezing point of ice is not a viable solution. Therefore, an analytical study of various defrosting methods such as the prospect of utilizing superheat, existing compressor and dedicated defrost compressor is presented, and the application of each configuration in a blast freezer is explored in this study.
2 System description and mathematical modelling
2.1 Compressor performance
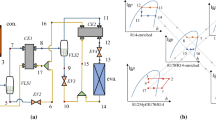
An CO2-NH3 cascade refrigeration system with basic components is shown in Fig. 1. The system consists of compressors, a condenser, an expansion device, a low-pressure liquid receiver, and an evaporator for both the high-temperature (HTC) and low-temperature circuits (LTC). The heat is transferred through a cascade plate heat exchanger (PHE) between LTC and HTC. The evaporation of NH3 and condensation of CO2 takes place on either side of the cascade plate heat exchanger (PHE). The cooling is achieved by two 90-kW evaporators.
For HTC circuit Bitzer OSKA7462-K model NH3 open screw compressor with a displacement volume of 220 m3/h and for LTC circuit Bitzer 4NSL-30 K model CO2 semi-hermetic reciprocating with a displacement volume of 46.9 m3/h were selected. The compressor performance data based on EN12900 standard is provided by the manufacturer catalogue [20]. This compressor polynomial correlation gives power consumption (W), cooling capacity (W) and mass flow rate (kg/h) considering 5 K and 10 K superheat for NH3 and CO2 compressors respectively. The polynomial coefficient and operating temperature range for both compressors are shown in Table 1.
Using the coefficient from Table 1, the cascade heat exchanger cooling capacity (\({\dot{Q}}_{cas}\)), compressor power consumption (\({\dot{W}}_{H}\)) and mass flow rate (\({\dot{m}}_{H}\)) for NH3 cycle can be determined by substituting in polynomial Eq. (1):
Similarly, freezer evaporator cooling capacity (\({\dot{Q}}_{E}\)), compressor power consumption (\({\dot{W}}_{L}\)) and mass flow rate (\({\dot{m}}_{L}\)) for CO2 cycle can be determined by substituting in polynomial Eq. (2):
2.2 Thermodynamic analysis
Based on the compressor factory performance data depicted in Section 2.1, the thermodynamic analysis of the cascade system is conducted using Engineering Equation Solver EES® which has NH3 and CO2 thermophysical property functions build-in. To find the unknown values, the first argument is the name of the refrigerant followed by a minimum of two independent thermodynamic properties such as temperature, pressure, refrigerant quality, enthalpy or entropy. For example temperature = f(R744, h, P) and pressure = f(R744, T, X). The reference according to IIR is 200 kJ/kg and 1 kJ/kg for specific enthalpy and entropy respectively. Considering the state point at each component from Fig. 1, a simplified thermodynamic model has been developed based on the assumptions as follows:
-
i.
All process including defrosting is at steady state. Heat losses/gains from ambient and pressure drop across the component and pipes are neglected.
-
ii.
Superheat at the compressor inlet is maintained according to compressor factory data.
-
iii.
Saturated liquid refrigerant is obtained at the outlet of NH3 condenser and CO2 cascade condenser.
-
iv.
Expansion devices undergo isenthalpic expansion.
-
v.
Isentropic efficiency of 0.75 is assumed for defrost compressor.
The following series of equations are applied on each component; simultaneously, energy and mass balance were verified.
For low-temperature circuit as follows:
-
CO2 compressor:
$$\dot{{W}_{L}}=\dot{\frac{{m}_{L}}{3600}}({h}_{2}-{h}_{1})$$(3) -
Evaporator:
$$\dot{{Q}_{E}}=\dot{\frac{{m}_{L}}{3600}}({h}_{1}-{h}_{4})$$(4) -
Electronic expansion valve:
$${h}_{3}={h}_{4}$$(5) -
Cascade heat exchanger temperature difference:
$${T}_{3}-{T}_{8}=\Delta T$$(6)
where \(\dot{{W}_{L}}\), \(\dot{{Q}_{E}}\) and \(\dot{{m}_{L}}\) are compressor power input (kW), evaporator cooling capacity (kW) and mass flow rate of CO2 respectively.
Similarly, for high-temperature circuit as follows:
-
NH3 compressor:
$$\dot{{W}_{H}}=\dot{\frac{{m}_{H}}{3600}}({h}_{6}-{h}_{5})$$(7) -
Condenser:
$$\dot{{Q}_{C}}=\dot{\frac{{m}_{H}}{3600}}({h}_{6}-{h}_{7})$$(8) -
Electronic expansion valve:
$${h}_{7}={h}_{8}$$(9) -
Cascade heat exchanger:
$${\dot{Q}}_{E,cas}=\dot{\frac{{m}_{H}}{3600}}({h}_{5}-{h}_{8})$$(10)
where \(\dot{{W}_{H}}\), \(\dot{{Q}_{C}}\) and \(\dot{{Q}_{E, cas}}\)\(\dot{{m}_{H}}\) are compressor power input (kW), condenser capacity (kW), cascade heat exchanger capacity (kW) and mass flow rate of NH3, respectively.
Coefficient of performance (COP) is the scale used to determine the cooling performance, which is given by Eq. (11):
The performance of the system (COP) was studied for different condenser temperatures, evaporator temperatures and cascade temperature differences. The fixed values of the operating parameters considered for the thermodynamic analysis are given in Table 2. As per literature [3, 21], the maximum COP depends upon the evaporator, condenser and cascade temperature difference. They have emphasized that COP will be the maximum for an optimal cascade condensing temperature. However, for realistic analysis, compressor factory performance data was used for which the NH3 evaporating pressure of the cascade heat exchanger was set initially. Therefore, instead of optimum cascade condensing temperature, the cascade evaporating pressure was initialized for maximum COP.
2.3 Hot-gas defrosting criteria
Defrosting is a dynamic process, which is subdivided into different stages such as preheating, frost melting, water draining and dry heating. The dynamics of defrosting have been discussed profoundly in previous literatures [22, 23, 24]. Therefore, in this study, various defrost configurations (DeConfig) required for hot-gas defrosting were focused on considering the total amount of heat (kJ) and CO2 mass flow rate (kg/h) require to melt the frost. The time taken to melt the frost generally varies from 15 to 30 min in the food processing industry; beyond this, time limit would cause economic loss to the production line [12]. Here, a 90-kW evaporator coil with 1 mm of frost deposit is considered for defrosting. The evaporator is made ready for defrosting by shutting off the liquid CO2 supply first. The evaporator outlet valve is kept open for 180 to 400 s, with evaporator fans in a running state to boil off residual liquid CO2. Then, the evaporator fans are switched off, and the solenoid valve for the hot-gas bypass is opened. After defrosting is completed, the hot-gas bypass solenoid is closed. At last, liquid CO2 feed to the evaporator is opened, and evaporator fans are restarted [25]. The amount of heat required to melt the frost is depicted in Table 3.
DeConfig0 shown in Fig. 1 is a basic conventional method used for hot-gas defrosting. The schematic layout and p–h diagram of newly proposed defrost configurations are shown in Figs. 2, 3, 4 and 5. Throughout the analysis, one evaporator is on cooling, and the other will undergo defrosting so that a constant supply of hot gas is obtained. In DeConfig0, hot gas from CO2 compressor discharge is redirected to the evaporator by the opening of a solenoid valve. Superheated vapour from the compressor discharge is utilized for defrosting because the saturation temperature at the cascade condenser is lower than 0 °C. Management of condensed liquid CO2 from the evaporator after the defrosting could be avoided; on the other hand, the mass flow rate of hot gas will be high which is impractical for the NH3 refrigerant but feasible for CO2 refrigerant because of its comparatively low specific volume. In DeConfig1 (Fig. 2), one of the multi-compressor is used to elevate the pressure and temperature of hot gas above the cascade condenser saturation temperature so that latent heat can also be utilized for defrosting. A stop valve is used to block discharge from the main compressor, and the suction vapour is compressed above cascade condenser pressure so that temperature above the melting point of ice is obtained. The condensed CO2 after melting the frost is throttled through a pressure regulator valve and collected in the liquid receiver. In DeConfig2 (Fig. 3), an additional defrost compressor is installed where the discharge hot gas from the CO2 compressor is fed into the suction of defrost compressor. Here, the hot-gas pressure and temperature are elevated above the saturation pressure of the cascade condenser to a maximum temperature of + 10 °C. The hot gas will reject heat to melt the frost deposit, and condensed CO2 is collected in the liquid receiver after throttling through the pressure regulator valve. In DeConfig3a (Fig. 4), the existing compressor among the multi-compressor can be used as defrost compressor for batch process defrosting in the case of a blast freezer. To maintain the compressor in its operating temperature envelope and to have stable lubrication, an internal heat exchanger (IHX) or desuperheater (DSH) is required between the discharge of the main refrigeration compressor and the suction of defrost compressor. By using ambient temperature or return condensed CO2, the hot gas can be cooled before entering into defrost compressor, but not near to the saturation temperature. Alternatively, desuperheating of hot gas can also be achieved from the cascade condenser for controlled cooling of the hot gas as shown in DeConfig3b (Fig. 5). The choice of either configuration depends on the selection of the compressor and its operating parameters as the same compressor is used for refrigeration and defrosting. Therefore, drastic changes in the compressor capacity can be avoided.
The defrost compressor work input for DeConfig1, DeConfig2 and DeConfig3a and DeConfig3b is expressed in Eqs. (12), (13), (14) and (15) respectively.
The defrosting configurations are evaluated and compared by its defrosting efficiency. For numerical analysis, defrost efficiency can be defined as the ratio of amount energy required to melt the frost deposit, to the total energy consumed by the compressors.
2.4 Exergy analysis
The exergy analysis for defrosting is carried out on the components involved in defrosting such as defrosting compressor, desuperheater and evaporator under defrosting. Total system exergy analysis has been conducted in previous literatures; therefore, it is not focused in this section [27]. The chemical, kinetic and potential exergies are neglected; thus, at a state point, the physical exergy of the system for the defrosting compressor, desuperheater and defrosting evaporator is shown in Eqs. (17), (18), (19) and (20), respectively, presented in Table 4.
where \({\dot{Ex}}_{des}\) is the exergy destruction/lost, \({T}_{0}\) is the ambient temperature in K and \({\dot{Q}}_{dsh}\) and \({\dot{Q}}_{de}\) are the desuperheater capacity and heat required for defrosting respectively in W. The outlet temperature of desuperheater and defrosting evaporator coil is denoted by \({T}_{dsh}\) and \({T}_{de}\) in K. The change in entropy is termed as \({s}_{in}\) and \({s}_{out}\) across the component under analysis.
3 Model validation
The mathematical model for the cascade system was validated with previous literatures [4, 7, 10]. For the analysis, one evaporator was in refrigeration mode, and the other evaporator was in defrosting mode so that a continuous supply of hot gas is obtained. The maximum COP of refrigeration that depends on optimum cascade condensing temperature was compared and validated as shown in Table 5. The maximum deviation in percentage when compared to the published data, Saini et al. [10], Lee et al. [4] and Dopazo and Fernández-Seara [7], is 5.1%, 7.9% and 13.6%, respectively. The present cascade refrigeration model shows a good agreement with previously developed thermodynamic models. Therefore, as the baseline mathematical model deviation is in the acceptable range, the defrosting analysis can be considered valid.
4 Results and discussion
In this study, the cascade heat exchanger evaporating pressure was analysed first. A constant value of evaporating pressure was fixed for a particular condenser outlet temperature for all defrost configurations. Secondly, the effect of operating parameters such as evaporating temperature TE, condensing temperature TC and cascade temperature difference ΔT on COP was depicted. Hereafter, the additional power consumed in different defrost configurations (DeConfig) was discussed.
4.1 Initialization of cascade heat exchanger evaporating pressure
The study was carried out using compressor factory performance data, for which the evaporating and condensing temperature for both circuits have to be decided initially. The parameters that need to be initialized were evaporator temperature, condenser outlet temperature and cascade temperature difference. For this, the cascade evaporating positive pressure had to be fixed. However, it was observed that by variation of cascade evaporating pressure, the COP of the system reaches a peak value and then declines as shown in Fig. 6. It can be seen that for a condensing temperature of + 40 °C and evaporating temperature of − 43 °C, the maximum COP obtained were 1.23, 1.18 and 1.13 for cascade temperature difference of 3 K, 5 K and 7 K respectively against nearby value of 2.9 bar cascade evaporating pressure.
4.2 Effect T C, T E and ΔT on system performance
Figure 7 shows the effect of condensing temperature TC on COP for various evaporating temperature TE of −40 °C, −45 °C and −50 °C and cascade temperature difference ΔT of 3 K, 5 K and 7 K, respectively. In Fig. 7, the COP decreases with an increase in condensing temperature and a drop in evaporating temperature. COP is linearly related to the operating parameters of TC, TE and ΔT. These are expected trends, because, when the NH3 condensing temperature increases, compressor power consumption will increase with rise in pressure ratio. When ΔT is large, the performance of the cascade system reduces for an invariant cooling capacity, TC and TE, because of the increase in entropy, resulting in more irreversibility of the refrigeration cycle. Consequently, the COP of the cascade system decreases. For a high condensing temperature of + 40 °C, COP values between 0.95 and 1.25 are obtained for different operating parameters as shown in Fig. 7.
4.3 Analysis of different defrost configurations
The energy consumption required for defrosting by the individual compressor was investigated for various cascade temperature differences ΔT of 3 K, 5 K and 7 K and discharge pressure of defrosting compressor Pde of 39 bar and 45 bar with respect to different hot-gas mass flow rates \({\dot{m}}_{hotgas}\).
In DeConfig0 as shown in Fig. 8, the energy consumed by the system for different cascade temperature differences is 73.06, 76.18 and 79.53 kW. This shows the total power consumption of the baseline system. In this case, the superheat from the main compressor is utilized for defrosting. It is evident that a high mass flow rate of 982 kg/h is required for a standard duration of defrosting which is not feasible for a system with single or two evaporators. This configuration can be adopted only for multi-evaporators as shown in Fig. 9, which represents the number of 90-kW evaporators required in terms of cooling capacity for various defrosting time duration from 15 to 50 min. For a defrosting time up to 30 min and a hot-gas extraction ratio of 0.3, more than three evaporators are required to obtain sufficient hot gas for defrosting. In this figure, hot-gas extraction ratio is represented by the ratio of mass flow rate of hot gas by mass flow rate of mainline CO2 refrigerant. An increase in the extraction ratio will result in an inadequate supply of refrigerant to the cooling evaporators.
In DeConfig1 as shown in Fig. 10, the power consumption by defrost compressor increases linearly for various mass flow rates from 221 to 736 kg/h. For a mass flow rate of 316 kg/h, the power consumed is 8.40 and 9.24 kW for discharge pressure of 39 and 45 bar respectively. The effect of cascade temperature difference is negligible in this configuration as the suction vapours are supplied to the defrost compressor.
In DeConfig2 as shown in Fig. 11, similar to the previous case, the power consumption by defrost compressor increases linearly for various mass flow rates ranging from 212 to 707 kg/h. For a mass flow rate of 353 kg/h and defrost compressor discharge pressure of 45 bar, 2.80–3.62 kW of power is consumed for different cascade temperature differences as the hot gas from the main compressor discharge is directly supplied to defrost compressor suction. Highly superheated hot gas is available for defrosting; therefore, the defrosting compressor power consumption is low. However, the stability of lubricating oil and operating temperature envelop of defrost compressor should be supervised. Even though the total power consumption of the system is high for higher cascade temperature differences, the power consumed for defrosting is low due to the rise in cascade condensing temperature.
Figures 12 and 13 illustrates the power consumed for defrosting of DeConfig3a and DeConfig3b respectively. In DeConfig3a, the hot gas is desuperheated before entering the defrost compressor. One of the main compressors is modified to operate as defrost compressor, which should have a wide operating temperature envelope. Similarly, for DeConfig3b where the modified defrost compressor with narrow operative temperature envelope compared to DeConfig3a, a plate heat exchanger connected to the cascade evaporator can be used to reduce the hot-gas temperature to an acceptable level. According to Figs. 12 and 13, at defrost discharge pressure of 45 bar, for a mass flow rate of 425 kg/h and 470 kg/h, 2.62–3.59 kW and 6.68–6.88 kW of energy is consumed for DeConfig3a and DeConfig3b respectively. It can be deduced that to maintain lubricant oil stability, the hot gas can be desuperheated, where the defrost compressor power consumption is less. However, refrigeration/defrosting compressors should have a wide operating range.
The defrosting efficiency comparison of all four configurations for different cascade temperature differences and defrosting time of 30 min are depicted in Fig. 14. For all data set, average defrosting efficiency of 43.8%, 38.5%, 42.5%, 42%, and 39.7% was achieved for DeConfig0, DeConfig1, DeConfig2, DeConfig3a, and DeConfig3b respectively. DeConfig0 shows the highest defrosting efficiency because no additional energy is consumed for defrosting. Moreover, for all configurations, the efficiency remains in an average range of 38.5–43.8% which is in accordance with previous literatures where defrosting efficiency is reported in the range of 30–40% for hot-gas bypass defrosting for transcritical CO2 heat pumps [17, 18].
4.4 Effect of ΔT on defrosting exergy
Figure 15 depicts the influence of cascade temperature difference on the exergy lost for defrosting components such as defrosting compressor, desuperheater, cascade desuperheater, and defrosting evaporator. From the energy analysis in the previous Section 4.3, it is observed that cascade temperature differences have an influence on the defrosting energy consumption. For fixed operating conditions of heat required for defrosting, under which the elevated defrosting pressure of 45 bar and temperature of + 10 °C, the condensing temperature is + 40 °C for an ambient temperature of + 35 °C, and the exergy lost for different defrost configurations is shown in Fig. 15. It is obvious that exergy destruction is maximum for DeConfig0 because of high mass flow rate of superheated vapour. DeConfig3b shows the lowest exergy destruction.
5 Conclusions
Four hot-gas defrosting configurations for CO2-NH3 cascade refrigeration system for a blast freezer commonly used in seafood industry were investigated in this study. The main difference between these configurations was the arrangement of the defrosting compressor, and the additional energy consumptions required for the systems were numerically simulated. The DeConfig0 utilized high mass flow rate hot-gas superheat which is the conventional method adopted for defrosting. However, this configuration can be adopted for a centralized cascade refrigeration system with more than three evaporators so that an adequate supply of refrigerant for simultaneous cooling and hot-gas defrosting is obtained. For small-scale deep freezers, an additional defrosting compressor is required. When suction vapours are compressed above the saturation pressure and temperature of the cascade condenser, defrosting compressor (DeConfig1) consumed high energy in the range of 8.4–9.2 kW. An alternative to reduce the additional energy consumption, hot gas from the main compressor discharge was supplied to the defrosting compressor. The least energy demanding alternative was DeConfig2, which was in the range of 2.8–3.6 kW, where a dedicated compressor was used. Additionally, to reduce the cost of additional compressors, DeConfig3a and DeConfig3b can be selected where defrosting energy consumption was in the range of 2.6–3.6 kW and 6.7–6.9 kW, respectively. The lubricating oil stability was maintained by de-superheating the hot gas which depends on the operating temperature envelop of the compressors. For wide operating range compressor, DeConfig3a and for narrow operating range compressor, DeConfig3b configuration is recommended.
6 Nomenclature
COP coefficient of performance
DeConfig defrost configurations
DSH desuperheater
\({\dot{Ex}}\) Exergy (W)
h enthalpy (kJ/kg)
HTC high temperature circuit
IHX internal heat exchanger
LTC low temperature circuit
\({\dot{m}}\) mass flow rate (kg/h)
P pressure (bar)
\({\dot{Q}}\) amount of heat (W)
s entropy (J/K)
T temperature (°C)
\({\dot{W}}\) power input (W)
ΔT cascade temperature difference (K)
6.1 Greek symbol
η efficiency (%)
6.2 Subscripts
C condensing
cas cascade
de defrost compressor
des destruction
dsh desuperheater
E evaporating
H high temperature
hotgas CO2 hot-gas
L low temperature
Availability of data and materials
All the data is available in the manuscript.
References
NFBD (2023). National Fisheries Development Board, Department of Fisheries, Ministry of Fisheries, Animal husbandry & Dairying, Govt. of India. Available at: https://nfdb.gov.in/welcome/about_indian_fisheries. viewed on 15/07/2023
Pan, M., Zhao, H., Liang, D., Zhu, Y., Liang, Y., & Bao, G. (2020). A review of the cascade refrigeration system. Energies, 13(9). https://doi.org/10.3390/en13092254
Bingming, W., Huagen, W., Jianfeng, L., & Ziwen, X. (2009). Experimental investigation on the performance of NH3/CO2 cascade refrigeration system with twin-screw compressor. International Journal of Refrigeration, 32(6), 1358–1365. https://doi.org/10.1016/j.ijrefrig.2009.03.008
Lee, T. S., Liu, C. H., & Chen, T. W. (2006). Thermodynamic analysis of optimal condensing temperature of cascade-condenser in CO2/NH3 cascade refrigeration systems. International Journal of Refrigeration, 29(7), 1100–1108. https://doi.org/10.1016/j.ijrefrig.2006.03.003
Getu, H. M., & Bansal, P. K. (2008). Thermodynamic analysis of an R744–R717 cascade refrigeration system. International Journal of Refrigeration, 31(1), 45–54. https://doi.org/10.1016/j.ijrefrig.2007.06.014
Alberto Dopazo, J., Fernández-Seara, J., Sieres, J., & Uhía, F. J. (2009). Theoretical analysis of a CO2-NH3 cascade refrigeration system for cooling applications at low temperatures. Applied Thermal Engineering, 29(8–9), 1577–1583. https://doi.org/10.1016/j.applthermaleng.2008.07.006
Dopazo, J. A., & Fernández-Seara, J. (2011). Experimental evaluation of a cascade refrigeration system prototype with CO2 and NH3 for freezing process applications. International Journal of Refrigeration, 34(1), 257–267. https://doi.org/10.1016/j.ijrefrig.2010.07.010
Yilmaz, B., Mancuhan, E., & Erdonmez, N. (2018). A parametric study on a subcritical CO2/NH3 cascade refrigeration system for low temperature applications. Journal of Solar Energy Engineering, Transactions of the ASME, 140(9), 1–7. https://doi.org/10.1115/1.4039976
Bellos, E., & Tzivanidis, C. (2019). A theoretical comparative study of CO 2 cascade refrigeration systems. Applied Sciences (Switzerland), 9(4). https://doi.org/10.3390/app9040790
Saini, S. K., Dasgupta, M. S., Widell, K. N., & Bhattacharyya, S. (2021). Comparative analysis of a few novel multi-evaporator CO2-NH3 cascade refrigeration system for seafood processing & storage. International Journal of Refrigeration, 131(January), 817–825. https://doi.org/10.1016/j.ijrefrig.2021.07.017
Klingebiel, J., Hassan, M., Venzik, V., Vering, C., & Müller, D. (2023). Efficiency comparison between defrosting methods: A laboratory study on reverse-cycle defrosting, electric heating defrosting, and warm brine defrosting. Applied Thermal Engineering, 233(May), 121072. https://doi.org/10.1016/j.applthermaleng.2023.121072
Amer, M., & Wang, C. C. (2017). Review of defrosting methods. Renewable and Sustainable Energy Reviews, 73(October 2016), 53–74. https://doi.org/10.1016/j.rser.2017.01.120
Xi, Z., Yao, R., Li, J., Du, C., Yu, Z., & Li, B. (2021). Experimental studies on hot gas bypass defrosting control strategies for air source heat pumps. Journal of Building Engineering, 43(May), 103165. https://doi.org/10.1016/j.jobe.2021.103165
Bansal, P. K., & Jain, S. (2007). Cascade systems: Past, present, and future. ASHRAE Transactions, 113 PART 1, 245–252.
Hoffenbecker, N., Klein, S. A., & Reindl, D. T. (2005). Hot gas defrost model development and validation. International Journal of Refrigeration, 28(4), 605–615. https://doi.org/10.1016/j.ijrefrig.2004.08.016
Dopazo, J. A., Fernandez-Seara, J., Uhía, F. J., & Diz, R. (2010). Modelling and experimental validation of the hot-gas defrost process of an air-cooled evaporator. International Journal of Refrigeration, 33(4), 829–839. https://doi.org/10.1016/j.ijrefrig.2009.12.027
Hu, B., Yang, D., Cao, F., Xing, Z., & Fei, J. (2015). Hot gas defrosting method for air-source transcritical CO2 heat pump systems. Energy and Buildings, 86, 864–872. https://doi.org/10.1016/j.enbuild.2014.10.059
Wang, Y., Ye, Z., Song, Y., Yin, X., & Cao, F. (2020). Experimental investigation on the hot gas bypass defrosting in air source transcritical CO2 heat pump water heater. Applied Thermal Engineering, 178(June), 115571. https://doi.org/10.1016/j.applthermaleng.2020.115571
Söylemez, E., Widell, K. N., Gabrielii, C. H., Ladam, Y., Lund, T., & Hafner, A. (2022). Overview of the development and status of carbon dioxide (R-744) refrigeration systems onboard fishing vessels. International Journal of Refrigeration, 140, 198–212. https://doi.org/10.1016/j.ijrefrig.2022.05.007
Bitzer software (2023). Version 6.18. https://www.bitzer.de/websoftware/. Accessed 15 July 2023
Aghazadeh Dokandari, D., Setayesh Hagh, A., & Mahmoudi, S. M. S. (2014). Thermodynamic investigation and optimization of novel ejector-expansion CO2/NH3 cascade refrigeration cycles (novel CO2/NH3 cycle). International Journal of Refrigeration, 46(94), 26–36. https://doi.org/10.1016/j.ijrefrig.2014.07.012
Badri, D., Toublanc, C., Rouaud, O., & Havet, M. (2021). Review on frosting, defrosting and frost management techniques in industrial food freezers. Renewable and Sustainable Energy Reviews, 151(January), 111545. https://doi.org/10.1016/j.rser.2021.111545
Huang, D., Li, Q., & Yuan, X. (2009). Comparison between hot-gas bypass defrosting and reverse-cycle defrosting methods on an air-to-water heat pump. Applied Energy, 86(9), 1697–1703. https://doi.org/10.1016/j.apenergy.2008.11.023
Song, M., Deng, S., Dang, C., Mao, N., & Wang, Z. (2018). Review on improvement for air source heat pump units during frosting and defrosting. Applied Energy, 211(December 2017), 1150–1170. https://doi.org/10.1016/j.apenergy.2017.12.022
Stoecker, W. F., Lux, J. J., & Kooy, R. J. (1983). Energy considerations in hot-gas defrosting of industrial refrigeration coils. ASHRAE Transactions, 89(pt 2A 2B), 549–573.
Thermofin (2023). Themofin heat exchangers Germany, Blast freezers, TOFL model for CO2. Available at: https://www.thermofin.de/en/blast-freezers-evaporators.php. viewed on 15/07/2023
Gholamian, E., Hanafizadeh, P., & Ahmadi, P. (2018). Advanced exergy analysis of a carbon dioxide ammonia cascade refrigeration system. Applied Thermal Engineering, 137, 689–699. https://doi.org/10.1016/j.applthermaleng.2018.03.055
Acknowledgements
The authors acknowledge the support received from the Indo-Norwegian project “Future Refrigeration India: INDEE+” (CIFT/FRI-INDEE 2021) funded by the Norwegians Ministry of Foreign Affairs, coordinated by Norwegian University of Science and Technology and SINTEF Ocean, Norway.
Funding
The author(s) acknowledge the funding received from the Indo-Norwegian project “Future Refrigeration India: INDEE+” (CIFT/FRI-INDEE 2021) funded by the Norwegians Ministry of Foreign Affairs, coordinated by Norwegian University of Science and Technology and SINTEF Ocean, Norway.
Author information
Authors and Affiliations
Contributions
BSA, conceptualization, methodology, and writing—original draft preparation. SK, software. MS, validation. GN, supervision. MS, supervision. VS, software and validation. MSD, writing—reviewing and editing. SKK, writing—reviewing and editing. AH, project administration. KNW, resources and analysis tools.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arun, B.S., Ninan, G., Murali, S. et al. Study on various hot-gas defrosting configurations for CO2-NH3 cascade deep freezer. Int. J. Air-Cond. Ref. 32, 6 (2024). https://doi.org/10.1007/s44189-024-00049-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44189-024-00049-9