Abstract
The market for microbial gums such as xanthan, gellan, dextran, and curdlan is continuously expanding, driven by their increasing application in various industries including petroleum, pharmaceuticals, cosmetics, and food, with the latter accounting for approximately 50% of global usage. To meet the growing demand and improve sustainability, there is a need to enhance production efficiency and reduce costs. This article addresses these issues by examining recent advancements and emerging trends in microbial gum production and application. By highlighting patented technologies and innovative approaches, the article aims to provide a comprehensive understanding of how the industry can achieve higher yields and economic viability. Despite being produced by different microorganisms, these gums are synthesized under similar conditions, such as pH, temperature, and medium composition. The purification or downstream processes for these gums are also comparable, primarily involving solvent precipitation, centrifugation for separation, and drying. Significant advances in gum production include genetic improvement of microbial strains to improve biopolymer performance. Additionally, alternative media are being explored, either by optimizing nutrient availability or deprivation, or by using agroindustrial by-products to reduce production costs. Engineering improvements are another strategy: bioreactor characteristics, fermentation conditions and modes of operation, and advances in downstream process are highlighted. Furthermore, the text explores emerging trends in the application of microbial gums in the food sector. Microbial gums applications are not limited to their traditional action as emulsifiers and stabilizers, but expands to new uses in biodegradable packaging films and as antioxidant and prebiotic food ingredient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Microbial gums are biopolymers of natural origin that have been intensely used in different types of industries, mainly in the food sector, where they can be applied as stabilizers or gelling agents to improve rheological and sensorial properties of different products [1]. Among the main gums derived from microbial sources, specifically bacteria, are: xanthan, composed of three types of monosaccharide (mannose, glucose, and glucuronic acid), each block containing five sugar units composed by two glucose units, two mannose and one glucuronic acid [2]; gellan, a tetrasaccharide polymer composed of two molecules of glucose, one of glucuronic acid, and one of rhamnose [3]; dextran, which is a polysaccharide composed of glucose linked by α (1–6) bonds in the main chain and α (1–4), α (1–3), α (1–2) bonds in the branches [4]; and curdlan, a polymer composed of D-glucose molecules linked together by β (1,3) bonds [5].
Among the main bacterial species that have been used for the production of gums there are: Xanthomonas campestris for the synthesis of xanthan gum [6]; Leuconostoc mesenteroides, Weisella cibaria, Oenococcus kitaharae for the synthesis of dextran gum [7, 8]; Sphingomonas paucimobilis synthesizes gellan gum [9, 10].; and Agrobacterium sp., Rhizobium sp., Gluconacetobacter xylinus, Bacillus cereus, Cellulomonas fimi, for curdlan gum [11]. The synthesis of the xanthan, gellan, and curdlan gum by these microorganisms starts with the degradation of the carbon source and phosphorylation of glucose 6-phosphate to glucose 1-phosphate, which will initiate a cascade of reactions that will synthesize the different nucleotides from donor sugars to more complex molecules for the polymerization of the gums. Dextran gum, on its turn, is characterized by its extracellular synthesis in the presence of sucrose and the enzyme dextransucrase, responsible for carrying out the assembly of this polymer. Microbial gums differ from each other mainly in relation to their physicochemical proprieties, such as physical state, moisture, ashes content, rate of nitrogen, acetate and pyruvate concentrations, salt content, and viscosity grade. These characteristics can affect its viscosity and water. Due to these differences, some gums they can be used in different industrial processes and productions, with some gums being used in higher concentrations compared to others (13).
Despite this, the production and purification processes of different gums are similar. For example, all are produced by submerged fermentation and under operating conditions of similar pH, temperature and aeration. The downstream processes are also similar, for example, all purification processes begin with the separation of cellular biomass from the biopolymers, followed by the precipitation or insolubilization of the biopolymer using solvents such as methanol, isopropanol and ethanol. The latter is one of the most used due to its cost, high efficiency and low or no toxicity [12]. Innovations regarding the production of cellular gums are focused on the genetic modification of microorganisms to improve yields, the use of new culture media based on by-products or waste, the use of agents that affect the metabolic pathway, among others.
However, the fermentation processes used in the production of microbial gums may have some bottlenecks. For example, batch-scale fermentation achieves high production efficiencies but it is more time-costly (more than 2 days), which can lead to an increase in viscosity and changes in oxygen and nutrient levels, affecting gum quality. An alternative to industrial scale processes is the continuous fermentation, which in turn, also presents difficulties, such as the requirement for constant maintenance of the configuration and the risk of contamination, disadvantages that can lead to a large loss of product [13]. Furthermore, fermentation processes can be very expensive due to the high costs of synthetic media components. In order to reduce overall process costs, new research has been carried out on the use of industrial and agricultural waste as substrates in the production of gums of microbial origin, which would help to reduce process costs and make them more sustainable [14].
The production of microbial gums is constantly increasing, so by the year 2023 the gum market reached USD 1.5 billion and is expected to grow between 4 and 7% by the year 2030. Although the gums have applications in different sectors such as oil, gas, pharmaceuticals, cosmetics and food and beverages, around 50% of world’s production is applied in this last sector [15,16,17]. Considering the importance of microbial gums mainly in the food industry, the main objectives of this work are to present an overview of the metabolic processes of gum production, advances in production and purification processes and emerging trends regarding their applications. The present work also supplies a survey of patents that demonstrate the great potential for industrial and commercial application of gums of microbial origin, and how it already has a great impact on market of food, pharmaceutical, cosmetics and biomedical fields.
2 Structural conformation and biosynthetic pathways of microbial gum synthesis
Microbial gums have become very important in recent years due to their great structural and functional diversity, which allows multiple applications in almost all biotechnological sectors, especially in the food industry. These properties are directly related to their composition, which generally consists of polysaccharides composed of one (homopolysaccharides) or more types of sugars (heteropolysaccharides) [18]. Gums are produced by several microbial species, mainly bacteria (Table 1). These polysaccharides are synthesized extracellularly, which is why they are known as exopolysaccharides or extracellular polymeric substances (EPS) [19]. Likewise, they play an important role on microbial growth in environmental conditions since they allow microorganisms to form a complex structure called biofilm, in which EPS provide viscosity, elasticity, cohesion, protection and nutrition to the microbial community that composes it [20].
Currently, there are several products made from microbial gums in the market, among which xanthan and dextran gums are the most popular microbial polysaccharides worldwide due to their growing demand in recent years [21, 22]. Likewise, other polysaccharides such as gellan and curdlan, have attracted the attention of the food industry. Therefore, their development and research is of vital importance to expand the market of gums. For this reason, it is important to know the mechanism of biosynthesis and the metabolic pathways involved in this process, since it will allow identifying the critical steps and control points of the synthesis, facilitating the optimization processes both at physiological and molecular level, using metabolic engineering tools [23].
According to Kumart et al. [23] four general mechanisms for microbial EPS production are currently known. The Wzx/Wzy (flippase/polymerase) dependent pathway, which is used for heteropolysaccharide biosynthesis; the ATP transporter-dependent pathway, which is used for capsular polysaccharide biosynthesis; the single extracellular synthesis pathway using protein sucrose synthase, which is located in the outer cell membrane and polymerizes monomers to form homopolymers and oligosaccharides; and the synthase-dependent pathway, which synthesizes homopolymers with a polymeric transport pathway spanning the entire cell membrane.
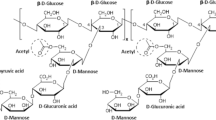
Xanthan gum is an EPS produced commercially by bacteria of the genus Xanthomonas, especially by the species Xanthomonas campestris, a gram-negative bacillus that under environmental conditions can be phytopathogenic to various crops. During one of the phases of its pathogenesis process, this bacteria produces EPS to fill the intercellular spaces of plant cells and thus facilitate their permeability through them [27]. Structurally, xanthan gum is a heteropolysaccharide made up of repeating pentasaccharide units consisting of two glucose units, two mannose units and one glucuronic acid unit, in a molar ratio of 2:2:1. Likewise, the main chain is linked by a β-D-glycosidic bond in positions 1 and 4, while the side chains are composed of a trisaccharide made up of a glucuronic acid unit between two mannose units in the O-3 position of every two glucose residues of the main chain. In addition, the terminal D-mannose contains a pyruvic acid linked by a keto group in positions 4 and 6, and the D-mannose unit of the main chain contains an acetate group in the O-6 position [12].
As a consequence of the presence of acetic acid and pyruvic acid in the molecular structure, xanthan gum presents an anionic characteristic [28]. Furthermore, the stability of this molecule will depend on the type of functional group in the external mannose unit, determined by the type of microorganism used and the culture conditions. Generally, a higher acetylation generates a higher stability, while a higher presence of pyruvic acid increases its viscosity [29].
Xanthan gum synthesis begins with the uptake of simple sugars, followed by their conversion into nucleotide derivatives, assimilation of monosaccharide subunits, assembly of pentasaccharide units, polymerization of repeating pentasaccharide units and finally their secretion to extracellular media [6]. First, glucose is used as the main substrate for the formation of the sugar nucleotides UDP-glucose, UDP-glucuronic acid, and UDP-mannose [30]. UDP-glucose results from the phosphorylation of glucose to 6-P-glucose by the enzyme glucokinase, followed by isomerization to 1-P-glucose by a phosphoglucomutase encoded by the XanA gene and subsequent activation to UDP-glucose by the addition of UTP by the enzyme UDP-glucose pyrophosphorylase. The formation of UDP-glucuronic acid occurs from a single oxidation reaction of UDP-glucose to UDP-glucuronic acid mediated by the enzyme UDP-glucose dehydrogenase and the transfer of two electrons donated by NAD + being reduced to NADH and the release of a proton. In the case of GDP-mannose formation, this results from a series of reactions which begin with the isomerization of 6-P-glucose to 6-P-fructose mediated by the enzyme phosphoglucose isomerase, followed by isomerization to 6-P mannose by the enzyme phosphomannose isomerase encoded by the XanB gene, then isomerization to 1-P mannose mediated by the enzyme phosphomannomutase encoded by the XanA gene and finally the reaction mediated by GDP-mannose pyrophosphorylase to obtain GDP-mannose [13, 30,31,32]. Subsequently, each of the synthesized sugar nucleotides will enter a series of reactions in sequence from the phosphate coupling of the polyisoprenol, starting with 2 mol of glucosyl 1-phosphate from UDP-glucose, 2 mol of D-mannose from UDP-glucose and 1 mol of D-glucoronic acid from UDP-glucoronic acid. Finally, acetyl-CoA transfers the O-acetyl and pyruvate groups to the internal and terminal mannose residue, respectively [13]. In this way, all the pentasaccharides are coupled to form the long chain of polysaccharides that will form the xanthan gum, that will be secreted through the transmembrane proteins B, encoded by the gumB gene. Likewise, this whole process is regulated by several genes known as gum genes: gumC, gumD, gumE, gumF, gumG, gumH, gumI, gumJ, gumK, gumL, and gumM [24].
In its turn, dextran is a homopolysaccharide composed of D-glucose linked mainly by consecutive al α-(1 → 6) bonds forming a backbone which presents branches of α-(1 → 3), α-(1 → 2) and α-(1 → 4) bonds [33], whose arrangement and distribution may vary according to the production method and the type of microorganism used in the fermentation [34]. Conventionally, the strains Leuconostoc mesenteroides NRRL B512F and NRRL B1299 are used to produce dextran at the industrial level, but dextran is also known to be produced by strains L. reuteri 180, Weisella cibaria CMGDEX3, W. cibaria JAG8, Oenococcus kitaharae DSM17330/DSR-OK and Streptococcus mutans [35,36,37,38,39,40]. Specifically, the structure of L. mesenteroides NRRL B512F consists of 95% α-(1 → 6) and 5% branched α-(1 → 3) linkages. Likewise, the length of the branched chain can contain up to 33 glucose residues [41, 42], however this is variable, reinforcing the hypothesis of its fermentation type-dependent variability.
The synthesis of dextran by Leuconostoc strains is regulated by the presence of sucrose in the fermentation medium [43], since it induces the expression of the enzyme dextransucrase (Dsr), a glycosyltransferase that catalyzes the synthesis of dextran extracellularly by hydrolysis of sucrose into the monomers fructose and glucose (D-glucopyranosyl conformation). Consequently, the enzyme forms a covalent glucopyranosyl-enzyme intermediate complex which is coupled to the final acceptor, another dextranyl-enzyme intermediate complex, where the glucopyranosyl group is added to the reducing end dextranyl group, to finally form the dextran molecule, which can be branched depending on the type of organelle, as mentioned above [4]. It is also important to mention that the fructose released in the dextran synthesis reaction enters the cell through subunit II of the phosphotransferase system and participates in the reactions of energy metabolism in which D-lactate is obtained as a secondary product [7, 8]. In this way, the microbial cell can replicate itself optimally and at the same time produce dextransucrase enzyme, satisfying its metabolic demand.
On the other hand, a microbial group belonging to the genus Sphingomonas is responsible for the production of one of the microbial gums with the greatest presence in a wide variety of food products. This is gellan gum, an EPS produced mainly by the commercial strain Sphingomonas paucimobilis ATCC 31461 [44, 45], a gram-negative, chemolithotrophic, strictly aerobic bacterium with yellow pigmented colonies [46], which has a yield of 40–50% of gellan gum when using glucose as the fermentation carbon source [47]. Likewise, at the structural level, gellan is an anionic linear heteropolysaccharide composed of α-L-rhamnose, β-D-glucose and β-D-glucuronic acid in a 1:2:1 molecular ratio [48,49,50]. The chain is made up of repeating units following the pattern β-D-glucose, β-D-glucuronic acid, β-D-glucose and α-L-rhamnose [51, 52]; it also has two acyl substituents L-glycerol and acetyl (L-glycerol every two repeating units and acetyl every repeating unit), whose presence or absence alters the physical and chemical properties of gellan gum [25, 53].
The biosynthetic pathway of gellan gum focuses on the synthesis of the sugar nucleotides, UDP-D-glucose, dTDP-L-Rhamnose and UDP-D-glucuronic acid, which will be the donors of the monomers that make up the tetrasaccharide to form gellan [9, 10]. Synthesis begins with an isomerization reaction of 6-P-glucose to 1-P-glucose, mediated by the enzyme PgmG phosphoglucomutase (PgmG). The pathway then branches into a dual pathway, in which the first pathway catalyzes the reversible conversion of 1-P-glucose and UTP to UDP-D-glucose, mediated by the enzyme UDP-D-glucose pyrophosphorylase, which is expressed by the ugpG gene. In the same pathway, a part of the synthesized sugar nucleotide leads to the formation of UDP-glucuronic acid, whose reaction is catalyzed by the enzyme UDP-D-glucose dehydrogenase. In this sense, both synthesized sugar nucleotides are active donors in the formation of the tetrasaccharide repeat that makes up the gellan [54]. On the other hand, in the other synthesis pathway, 1-P-glucose is converted to dTDP-L-rhamnose, through a series of reactions catalyzed first by the enzyme dTDP-D-glucose pyrophosphorylase, which converts 1-P-glucose to dTDP-D-glucose, this being the starting point for the formation of dTDP-4 keto-6-deoxy-D-glucose, dTDP-4 keto-L-rhamnose and dTDP-D-rhamnose; catalyzed by the enzymes dTDP-D-glucose 4,6-dehydratase, dTDP-4 dehydrorhamnose 3,5-epimerase and dTDP-4 dehydrorhamnose reductase, respectively. This enzyme system is also known as the dTDP -L-rhamnose enzyme system or TRS [55]. Finally, the sugar nucleotide formed in this pathway transfers a 1-P-glucose to a lipid transporter (C55-isoprenyl phosphate), whose reaction is regulated by the gelB gene [56]. Subsequently, glucuronic acid donated by UDP-glucuronic acid is added to the previously formed glucosyl-α-pyrophosphoryl polyprenol, considering that this reaction is regulated by the gelK gene.
The transfer of remaining sugars is regulated by the gelL and gelQ genes. In turn, the acetate and glycerate substituent groups are coupled from the precursors acetyl-CoA and glyceraldehyde-3-P, respectively, which come from metabolic pathways related to sugar metabolism for cell development and growth [57]. In this way, the tetrasaccharide repeating unit is formed to form gellan and secreted through the outer cell membrane by means of membrane transporter proteins [3].
Lastly, curdlan gum is a microbial EPS produced commercially by various strains including Agrobacterium sp., Rhizobium sp., Gluconacetobacter xylinus, Bacillus cereus, Streptococcus pneumonia, Cellulomonas fimi, among others [11]. Curdlan is a homopolymer of non-ionic linear D-glucose, insoluble in water and alcohol, but soluble in alkaline solutions [58]. This polysaccharide is composed of 300 to 500 glucose units which are linked by β-1,3-glycosidic bonds [59], forming a three-helix spatial molecular structure that makes it a thermally reversible gel with low viscosity. At high temperatures (> 80 °C), however, the triple helix chain unwinds, forming a thermally irreversible gel, which can give it an additional usable quality [60, 61].
At the biosynthetic level, it is known that curdlan-synthesizing bacteria present this capacity as a response to environmental stress factors, especially nitrogen source limitation, which makes this an important factor in the regulation mechanism at the molecular level [62, 63], and consequently, of utmost importance for process optimization [64, 65]. In this context, the metabolic pathway begins with the phosphorylation of D-glucose to 6-P-glucose by the action of a hexokinase. Then, the enzyme phosphoglucomutase converts 6-P-glucose to 1-P-glucose. In parallel, 6-P-glucose enters the tricarboxylic acid (TCA) cycle and the respiratory chain for ATP synthesis. Subsequently, the enzyme pyrophosphorylase UDP-Glucose condenses 1-P-glucose and UTP (from the phosphorylation of UDP + ATP) to form the sugar nucleotide UDP-glucose, which is transferred to the cell membrane via phospholipids. Once in the cell membrane, curdlan is synthesized by the action of a curdlan synthase enzyme transferring the glucose units from UDP-glucose to the growing polysaccharide chain and linking them with β-1,3-glycosidic bonds [26, 66]. In Fig. 1, we present a schematic diagram that integrates and summarizes the metabolic pathways in the process of synthesis of the four microbial gums discussed, helping on the visualization of the relationship between them and the central carbon metabolism.

Fig. 1 Schematic diagram of the integrated metabolic pathways for the synthesis of the microbial gums Dextran, Xanthan, Gellan and Curdlan. The sugar donor nucleotides that synthesize Gellan Xanthan and Curdlan are framed in green dotted lines. Further abbreviations: Glc Glucose, Fru Fructose, Rha Rhamnose, Man Mannose, GlcA Glucuronic acid, P Phosphate, BP Biphosphate; UDP Uridine diphosphate, UTP Uridine triphosphate; ATP Adenine triphosphate, ADP Adenine diphosphate, dTDP Thymidine diphosphate; GDP Guanosine diphosphate, Pi pyrophosphate, Pyr Pyruvic acid, Ac Acetate, Gly, Glycerate, TCA Tricarboxylic acid cycle
3 Microbial gums production
Microbial gums are usually produced in an aqueous media (submerged fermentation), being released in the broth along process time. This will lead to an increase in media viscosity since these polysaccharides present pseudoplastic behavior [67]. Each gum is produced by a specific type of microorganism, and therefore, conditions of optimal yield and productivity will vary depending on the final molecule [68]. Xanthan gum, for example, is produced by aerobic bacteria of the Xanthomonas genera, usually different strains of X. campestris, with glucose or sucrose being the main carbon source. Process conditions are generally mild: 30ºC and pH around 7 [51, 52]. In its turn, dextran is produced by lactic acid bacteria (LAB), mainly from the genera Leuconostoc or Streptococcus. Usually, different strains of L. mesenteroides are applied in industrial processes. Physicochemical conditions for dextran production are like the ones applied to xanthan gum (30 ºC and pH 7) and sucrose is generally used as carbon source [4].
Gellan gum can be produced by bacteria of the Pseudomonas genera, mainly strains of P. paucimobilis and P. elodea. Different sugars can be used for gellan gum obtainment, such as glucose, fructose, mannose, among others. Besides, both organic and inorganic nitrogen sources can be applied. Optimal temperature for fermentative process can vary from 20 to 30 ℃ and optimal pH is around 7 [69]. Finally, curdlan can be produced by Agrobacterium or Alcaligenes species. Usually, glucose or sucrose are applied as carbon source, and once more the optimal temperature is around 30 ℃ and optimal pH is 7 [26]. Generally, microbial gums are produced under carbon excess and nutrient limitation conditions, with a high carbon to nitrogen (C:N) ratio. Fed-batch strategies can be applied in order to achieve these conditions [67]. As these molecules can be produced from glucose and sucrose, an alternative to reduce production costs and increase added value to the final product is to use subproducts for fermentation (e.g., hydrolysates from lignocellulosic biomass, molasses, among others) [70]. Both agitation and aeration play important roles during fermentation, being therefore important parameters that need to be optimized [68].
Although different sources of carbon and nitrogen can be used to produce gum, according to [12] the best sources of carbon are glucose and fructose in concentrations between 40 and 50 g/L. Regarding the best sources of nitrogen, it was found that these vary accordingly to each type of microorganisms. For example, for X. campestris, the best nitrogen source is monosodium glutamate in concentrations not exceeding 15 mM, since concentrations above this value might inhibit the growth of the microorganism. For other gums, significative yields were found using organic nitrogen sources such as corn steep liquor, yeast stratum, peptone and soy concentrates, and the inorganic sources ammonium nitrate and potassium nitrate [69]. The media to produce gums is also supplemented with salts of potassium, sulfur, magnesium, zinc, iron and calcium. Furthermore, traces of some organic acids such as citric and succinic can help to improve the production of biopolymers [71,72,73]
4 Microbial gums market
As discussed throughout this review, microbial gums show a growing market interest with new applications being developed constantly. These gums produced by microorganisms pose advantages to the ones obtained from plants and/or crops as they are not affected by seasonal changes and other factors [67, 68]. Therefore, the market of microbial gums is in a constant crescent, with each molecule having a respective significant market value and growth rate (Table 2). The main driver for this expansion is the substitution of conventional ingredients (e.g. gelatin, carrageenan, among others) by microbial gums in different foods and beverages, along with the potential applications of these molecules in the packaging, pharmaceutical, cosmetics and biomedical fields [51, 52]. Therefore, there are several companies producing and distributing these microbial gums in a business to business (B2B) manner (Table 3). Xanthan gum is the one molecule that hold the biggest market share and functioning enterprises, as it was the first microbial gum that was industrially produced and commercialized in 1960 by CP Kelco [70].
5 Advances in upstream microbial gum production
Industrial production of microbial gums occurs in bioreactors, which can be defined as systems or vessels in which biochemical reactions take place. Generally, microbial gums are produced in stirred tank reactors (STRs), which are the most common type of vessel used for different fermentation processes [67]. The STRs are composed by a tank with commonly a 1:1 ratio between their height (H) and reactor diameter (Dt). These systems can reach high agitation rates—and therefore, adequate aeration and oxygen diffusion—as different impellers’ configurations can be attached to a central rotating shaft [89, 90]. Studies have also reported the production of microbial gums in pneumatic bioreactors, mainly the airlift type (ALR). In this case, the main vessel counts with a H:D ratio that can range from 6:1 until 10:1 and aeration and agitation are provided by gas inlet at the bottom of the tank. The ALRs also count with two parallel panels that create a channel for air and media circulation, guaranteeing proper mixing on the system [89, 90]. This kind of bioreactor is useful for cultivating microorganisms that are sensitive to high shear rates (e.g. production of pullulan from filamentous fungi). Usually, STRs lead to higher overall productivities, while ALRs tend to be more energy-efficient since stirring is made pneumatically. Therefore, mass and energy balances need to be evaluated in order to determine the best type of bioreactor for microbial gum obtainment [91].
The microbial gums production process, however, counts with a central problematic: the high viscosity of the fermented broth. As fermentation occurs, the microbial gums—which are naturally viscous—are released in the extracellular media, therefore turning its behavior of a Newtonian fluid to pseudoplastic [3]. As discussed previously in other research, high viscosities in STRs can lead to system heterogeneity, since the regions near the impellers are highly agitated while remote zones of the bioreactor are poorly mixed [68, 90]. This heterogeneity also impacts in overall system aeration, as zones distant from the impellers will present a poor oxygen distribution. As many microorganisms that produces gums are aerobic, this can lead to poor microbial development and reduced gums’ yields.
Viscosity problematics also affects process controlling various parameters, such as optical density, pH, and dissolved oxygen. Since the media is heterogenous, a sample may not be able to represent the whole system [68]. High agitation rates can reduce overall broth viscosity, but this can also lead to high shear rates which may hinder cell growth and morphology. In order to counter these issues, process optimization is a crucial step for industrial microbial gum commercialization [51, 52]. As each molecule is produced by a different microorganism, screening of optimal parameters (e.g. temperature, pH, and agitation) should be performed when developing a fermentative process. Besides, media composition should also be evaluated for maximum gums yield and productivity. Usually, this optimization process is conducted in bench-scale flasks due to easy handling of many experiments developed.
However, regarding microbial gums production process, aeration and agitation play a major role on microbial development and product formation. Along with that, the polysaccharides may have their physicochemical and morphological characteristics altered depending on agitation and aeration rates [68]. Therefore, systems of small bioreactors that can better simulate and represent pilot or industrial sets should be applied in order to determine the optimal conditions of these parameters. Assis and collaborators [92] studied the effect of different aeration and agitation rated in the production of xanthan gum from crude glycerol in a bench scale STR (3 L operating volume). Researchers concluded that while low agitation contributed for biomass growth and gum production, high agitation altered the mannose content on xanthan gum. High aeration showed enhanced microbial development, while lower rates increased the product concentration and glucose and mannose content on the polymeric chain.
Microbial gum fermentation can also occur in different operational modes: batch, fed-batch and continuous. Production of microbial gums occurs, in its majority, with an imbalance of nutrients (usually with carbon excess) [67]. In this sense, Huang and collaborators [93] studied different carbon/nitrogen (C: N) relations for gellan gum production. While an increase in gum yield was observed with C:N increase, a higher viscosity of the media was observed which led to poor oxygen transferring and, therefore, a lower conversion of glucose to gellan. Fed-batch can help in increasing gums’ yield as this mode counts with intermittent or continuous feeding of fresh nutrients and no withdrawal of fermented broth [90]. Usually, fed-batch results in better gums productivities of microbial gums, however feeding strategy also needs optimization in small bioreactor systems since carbon excess will lead to nitrogen deficiency in the media, which can cause poor microbial development. Continuous operation can also be applied for microbial gums production. However, this mode has a higher risk of system contamination [91].
By determining the mode of operation and optimal conditions for both microbial growth and gum formation, the process can be scaled-up. This practice is based on maintaining similarities among scales [89, 90]. The first similarity is, obviously, the media composition and optimal conditions of pH and temperature. System geometry also needs to be taken into account—maintaining the same type of bioreactor among scales and similar H:D and other important ratios. Moreover, bioreactor scaling-up counts with other critical parameters and relations [e.g. volumetric oxygen transfer coefficient (kLa), potency per unit volume of medium (P/V), mixing time (tm), among others] that will be maintained constant and guarantee that the process developed in bench scale will be reproductible in higher ones [89]. Usually, prior to industrial implementation, there is a pilot scale in which process evaluation and validation will be conducted [90]. In this sense, Pan and colleagues [94] applied a 50-L pilot bioreactor for dextran production from Leuconostoc pseudomesenteroides. In a bench scale using flasks and an optimized media, maximum gum production was 26.02 g.L−1, while in the pilot scale, production rose to 40.07 g.L−1 by lowering system agitation and using a fed-batch mode of operation. The dextran was further applied in set yogurt and improved parameters of texture and viscosity during storage.
The industrial production of microbial gums still needs more research and development for as reports in literature even for pilot-scale production are still scarce [70]. Advances in new types and engineering of bioreactors will help to ensure a good balance between product yield and energy inputs, making therefore microbial gums more accessible. Besides, isolation of new microorganisms or modification of existing ones in order to increase yields and productivity is also a valid strategy [51, 52]. With the advancement of knowledge about the microbial routes used for gums production and molecular techniques, new strains with enhanced production and resistance to adverse conditions will be applicable to industrial sets. Also, optimization steps and microbial engineering opens the opportunity for tailoring molecules with unique properties [51, 52]. In Table 4 describes some of the most important parameters in the production of microbial gums. As well as the different systems of bioreactor operation and production at different scales.
6 Cell immobilization
Cell immobilization is a strategy utilized to enhance the productivity and stability of microbial metabolites, aiming for higher yields. Microbial cells can be immobilized in different kinds of supports to produce gums, as an alternative to using free cells [2, 108]. For example, xanthan gum was produced by X. campestris immobilized in various supports including smooth small pore size metal support, wavy large pore size metal support, plastic support, and calcium alginate beads. Compared with control (traditional submerged fermentation), xanthan productivity was improved [2, 108].
Immobilized systems often exhibit enhanced resistance to environmental fluctuations, reduced risk of contamination, and the ability to maintain high cell concentrations over prolonged periods. These factors contribute to higher productivity and consistent product quality, which are crucial for industrial applications [109, 110]. For example, immobilization of cells was also seen to enhance dextran production by L. mesenteroides KIBGE HA1. At 50 °C free cells stopped producing dextran, while calcium alginate beads immobilized cells continued to produce dextran even after 60 °C. Additionally, immobilized cells could produce dextran even after 12 days [111]. In the study by Martinez et al. Agrobacterium sp. was immobilized in a loofa sponge matrix, an abundant and inexpensive material. The immobilized cells produced curdlan gum for five consecutive cycles, and remained active after 300 days of storage at − 18 ℃. The gum produced was applied in a functional yogurt, resulting in a product with lower syneresis and thus more stable [110].
7 Downstream process for microbial gums
Downstream processes are the set of unit operations required to purify different bioproducts. The microbial gums addressed in the present work are extracellular, therefore no operation is required to extract the polymers from the microorganisms’ cells. There are different processes for purifying gums, even for the same type of gum. However, the most used processes on an industrial scale for the purification of microbial gums are mainly based on extraction or precipitation with solvents [1].
8 Xanthan gum purification
Xanthan gum can be purified via concentration by evaporation, however this process results in low quality gums with high levels of impurities such as microbial cells and unmetabolized substrates. Generally, the purification of xanthan gum begins with a process of thermal inactivation of microbial strains and enzymes (70–100 °C/10–20 min), followed by a centrifugation process to remove cell biomass. In the microbial and enzymatic inactivation process, chemical treatments can also be used, such as alkalis and hypochlorite, however these processes are more costly [112]. Furthermore, treatments at high pH can lead to depyruvylation of the biopolymer [113].
Subsequently, the gum is precipitated (decrease the solubility of the gum), process in which different compounds can be used such as calcium, aluminum and quaternary ammonium salts (CaCl2, MgCl2) [12, 113], as well as water-miscible solvents or organic solvents (isopropanol, acetone, ethanol) [2, 108]. The compounds mainly used to precipitate xanthan gum are alcohols such as ethanol and isopropanol, however the latter is no longer permitted by the Food and Drug Administration (FDA) when the gum is to be used as a food additive [12]. According to Palaniraj et al. [113] the amount of solvent used for precipitation is usually in a ratio of 4:1 solvent: fermented broth. Among the main advantages of using alcohols in precipitation are the high efficiency and the ease of operation [114]. The alcohol in the supernatant is recovered via distillation to be used again in the process (Fig. 2A).
When the gum is precipitated with alcohol, traces of impurities are left behind, meaning additional purification steps are required, for example washing with acid solutions (pH 2.0, chloric acid), or alkaline solutions (0.5 M NaOH), or applying a dialysis systems with 800 Da membranes. According to [108], this last method of purification after precipitation is the most recommended since efficiencies of up to 90% are obtained, while with the other processes efficiencies of 66% are obtained. Finally, the precipitated gum is separated from the supernatant by centrifugation, then it is washed to remove soluble impurities and finally the gum is dried (spray-dryer) to reach a humidity between 8.8% and 10%.
9 Gellan gum purification
Gellan gum purification is carried out in three macro stages: (i) pre-recovery of gellan gum (ii) recovery of gellan gum (iii) post-recovery of gellan gum (Fig. 2B). The objectives of the first macro step are the separation of the gum from the cells and the inactivation of these microbial cells and of the enzymes produced such as pectinases, lyase, and cellulases [3]. The inactivation of enzymes and microorganisms is carried out through thermal treatments (90–100 °C/20 min) [3], other authors have suggested treatments at higher temperatures in shorter times (115 °C/15 min) to inactivate microorganisms and enzymes. Gum cell separation occurs at high revolutions up to 7504 g /15 min [72]. In the first stage the deproteinization of the gum is also carried out. Industrially, this process is carried out via enzymatic means using neutral protease, papain and alkaline protease, being the latter the most efficient, reaching deproteinization efficiency up to 86% and gum recovery of approximately 90%. The enzyme concentrations used vary between 20–200 ppm and the process takes place at temperatures between 30 and 60 °C. With enzymatic treatment, intact cellular debris is decomposed, which enhances gum recovery.
In the second stage, the gum is separated from the fermentation broth. Ethanol is used as precipitating agent, however, the volume of solvent used is between two and four times greater than the broth volume [72] and the degrees of achieved gum purity are insufficient. According to [73], gellan gum is anionic in nature, therefore, in the alcohol precipitation process, the addition of salts such as CaCl2, MgCl2 or acids decreases the amount of solvent by up to three times [3]. This happens since the protonated form of gellan gum has low affinity for the solvent, therefore the polymer precipitates easily with a smaller amount of solvent (US8685698B2) [115]. After precipitation of the gum, the mixture is submitted to centrifugation to remove fermentation broth and ethanol, the latter is recovered via distillation.
Finally, post recovery processes take place. This stage is made up of filtration, drying and grinding operations. Filtration is carried out in batteries of filter presses since they have large surface areas, meaning large volumes can be treated. Then, the polymer is subjected to drying processes to remove water or any other solvent and the product becomes stable. In the case of gellan gum, drying is carried out at low temperatures, that is, using vacuum drying or freeze-drying to avoid damaging the polymeric structure [3]. The commercial gellan gum produced contains large amounts of divalent cation contaminants such as Ca2+ and Mg2+ from salts used in precipitation at levels sufficient to neutralize around a third of the carboxyl groups, as a result of which the biopolymer can only be dissolved at temperatures higher than 90 °C [116].
10 Dextran gum purification
The dextran gum purification process begins with the separation of cells from the fermentation broth. This process can be carried out by filtration or centrifugation at high speeds. However, this last operation is mostly used on an industrial scale. According to revolutions ≥ 8000 rpm must be used to separate cellular biomass from dextran gum. The purification of the gum after separation from the microbial biomass is carried out in two stages: (i) solvent extraction [117], and (ii) chemical treatment (Fig. 2C) [118].
Precipitation of the gum from the fermentation broth is done using ethanol in a 2:1 ethanol:broth ratio. After precipitation, the polymer is recovered using centrifugation operations. Next, chemical treatment is carried out with 10% (v/v) trichloroacetic acid to precipitate the protein fractions and remove them. Finally, the supernatant is treated with ethanol to precipitate the polymer, which is subsequently dried. New trends in gum purification are focused on the use of physical methods such as barriers. Díaz-Montes et al. [1] used microfiltration processes for the purification of dextran gum using 0.1 µ membranes and operating pressures between 0.4 to 1.7 bar. According to researchers, the use of micromembranes reduces the use of ethanol solvents by up to 75%.
11 Curdlan gum purification
The curdlan gum purification process begins with the separation of microbial cells from the fermentation broth. The main unit operation used to separate biomass from fermentation broth is centrifugation (Fig. 2D). However, to facilitate this process, some alkali agents can be used, such as NaOH. One of the disadvantages on curdlan gum recovery is the large amounts of alkali needed. According to Al-Rmedh et al. [71] the volumetric ratio used is 2:1 (volume of NaOH solution (1N)/volume of fermented broth). Cellular biomass is removed via centrifugation using speeds above 5000 rpm. The supernatant is treated with acids to recover the gum, generally the acid used is hydrochloric acid at high concentrations (3N), the acidic solution is mixed with the supernatant to precipitate the gum, which is subsequently separated by centrifugation. Finally, the gum is subjected to washing processes to eliminate water-soluble impurities such as salts, and the gum is concentrated and dried [119]. Currently, other technologies that do not affect the properties of the gums are being evaluated. For example, Mangolim et al. [5] evaluated a mixed process (pre-gelation and precipitation) for purifying curdlan gum. According to the researchers, gums purified by pre-gelation and precipitation showed greater water, gelation and thickening capacity than commercial gums, which improved the texture parameters of yogurts and reduced syneresis.
12 Development and improvement strategies
12.1 Development about strains
As discussed before, several species can be used to produce microbial gums, and the choice of strain directly impacts the process yield and cost, with some of them already being well studied and applied at industrial scale production. However, new wild strains with the capability to produce gum can also be explored. Recently, a marine bacteria isolated from red seaweed was selected among 78 screening candidates to produce curdlan gum with high efficiency. Aniline blue agar was used for primary screening, and an isolate from red seaweed (Gracilaria foliifera) presented high curdlan gum yield, which then had its production optimized [120]. Factors such as pH, inoculum percentage, production time, and different carbon source were tested, with the highest curdlan gum yield for starch as carbon source (11.5 g L−1). Patent CN106399145A refers to a species of Rhizobium radiobacter with high capacity of producing curdlan gum, which was isolated and identified from soil, and then preserved in China General Microbiological Culture Collection Center (number CGMCC 12099). The patent also establishes a method for producing curdlan gum through fermentation using glucose and a cheap inorganic nitrogen source, attaining a successful curdlan gum yield of 65.27 g L−1 [121].
Synthetic biology approaches are currently contributing to a better understanding of microbial gums production, allowing for enhanced production or for the improvement of the characteristics of the molecules. Through variations in microbial gene expression levels, enzyme activities, or metabolic pathways, it is possible to modify the structure of xanthan gum molecules to improve their thickening or stabilizing properties in food products, for example. Synthetic biology tools promise to revolutionize the future of gums by enabling tailored properties, sustainable production, and novel applications for diverse industries [122, 123].
Corn straw offers a plentiful and sustainable source for microbial biopolymer production. In the study by Wu et al. [124], Sphingomonas sanxanigenens was engineered to utilize both glucose and xylose from corn straw hydrolysate to produce xanthan gum. This strain was obtained by introducing the xanthan gum synthetic operon gum as a module into the genome of the constructed chassis strain NXdPE, and the transcriptional levels of gum genes were optimized by screening for a more appropriate promoter. The strain yielded 9.48 ± 0.34 g kg−1 of xanthan gum when using glucose as a carbon source, representing a 2.1-fold increase over the original strain. Moreover, batch fermentation with corn straw hydrolysate resulted in the production of 12.72 ± 0.75 g kg−1 xanthan gum with ultrahigh molecular weight (6.04 × 107 Da), marking a 15.8-fold increase [124]. Ultra-high molecular weight xanthan gum offers several advantages over conventional xanthan gum, including improved rheological properties, enhanced stability (better performance in a variety of formulations and resistance to factors such as temperature, pH, and salinity changes) and potential for reduced dosage [124].
13 Development about culture media
Culture medium in fermentation for producing gums can account for near 30% of the total gum cost [13]. It needs to present a composition that furnishes all nutrients necessary to microorganism growth and gum biosynthesis, which include carbon and nitrogen sources, salts, and micronutrients. The use of conventional carbon sources, such as glucose, can be very expensive at large-scale fermentations [106]. Utilizing alternative culture media for gum production can offer significant advantages, especially from an industrial point of view. By employing alternative culture media, manufacturers can potentially reduce production expenses, thus leading to more cost-effective gum products. Date juice is a thick liquid made from the juice extracted from dates, the fruit of the date palm tree, being considered an agricultural waste product. Production of curdlan gum was demonstrated in a low-cost culture media using date juice as a carbon source by a local isolate of Agrobacterium leguminum. The production of curdlan was increased to 30.7 g L−1 by using the modified medium that contained date juice at a concentration of 264 mL L−1 [125]. Brewers’ Spent Grain (BSG) was also demonstrated to act as a good carbon source for the growth of Xanthomonas campestris and xanthan gum production [106].
Additionally, alternative culture media may provide environmental benefits. Traditional culture media often relies on ingredients that have associated environmental concerns, such as high water usage or reliance on agricultural crops that compete with food production. By exploring alternative sources for culture media, such as waste products or sustainable alternatives, the environmental impact of gum production can be minimized. Researchers have investigated the feasibility of incorporating olive mill wastewater (OMW) into the culture medium for producing xanthan gum, reducing both environmental impact and production costs. Various concentrations of OMW were tested to determine the optimal ratio for maximizing gum yield, with 5% OMW giving the best result: xanthan gum yield increased by up to 39% compared to conventional media without OMW. This notable enhancement in gum production suggests the effectiveness of utilizing OMW as a supplementary component in the fermentation process [97].
Moreover, alternative culture media have the potential to enhance product quality and consistency. Variations in traditional culture media can sometimes lead to fluctuations in gum properties, affecting characteristics such as viscosity and texture. Alternative culture media formulations may offer more consistent results, leading to improved product quality. L. mesenteroides was cultivated in milk permeate media and MRS broth (control) for dextran production, and the gum rheological properties were studied. Evaluation of molecular weight by high-performance size exclusion chromatography showed that dextran produced in milk permeate had a lower molecular weight (< 10,000 Dalton) compared to the dextran produced in MRS broth (< 40,000 Dalton), and the viscosity of dextran solution (2%) produced in MRS was 0.005–0.01 Pa.s while in the synthetic medium it was < 0.005 Pa.s. It was observed that the permeate medium had a significantly positive effect on the reduction of the molecular weight of dextran and increased its solubility attributed to the presence of lactose. Such properties can facilitate the use of gum in applications where fine-tuning of texture or mouthfeel is required, such as in sauces, dressings, and dairy products [4].
Carbon-to-nitrogen (C/N) ratio in culture media also plays an important role in gum production and quality, as outlined in patent CN109251950A. In this work, the use of high carbon–nitrogen ratio fermentation medium directly impacted the growth rate and metabolic activity of the microorganism Sphingomonas elodea. The culture media was composed of glucose or maltose at 3–4%, soybean protein isolate at 0.1–0.3%, sodium nitrate at 0.1–0.2%, calcium carbonate at 0.1–0.15%, potassium dihydrogen phosphate at 0.1%—0.25%, and organic silicon defoamer at 0.05–0.15%. The high C/N ratio in the culture media impacted in gellan gum yield and composition. The resulting gum was characterized by high acyl content, high dissolving speed, and high gel strength [126].
14 Production enhancers
The addition of precursor molecules plays a significant role in driving polysaccharide synthesis metabolically. Specifically in polysaccharide production, it has been observed that elevated intracellular concentrations of nucleotide phosphate sugars, particularly under nitrogen-limited conditions, can boost the metabolic flux involved in exopolysaccharide synthesis [69].
Surfactants can also be applied to enhance gum production. They act by improving cell membrane permeability within highly viscous broth, aiding in the transport of nutrients and oxygen [3]. Surfactants such as Triton X-100 enhance mass transfer efficiency, thereby improving both the yield and molecular weight of gellan gum. In the study by Huang et al. the addition of Triton X-100 at 0.75 g L−1 and the use of corn steep liquor as nitrogen source significantly improved gellan gum production [127].
15 Development in downstream processes
Recent advancements in downstream processes have improved the recovery yield of microbial gums. New techniques involving heat treatment and enzymatic processes have shown promise. For instance, a patented process for xanthan gum effectively solubilizes water-insoluble residues without compromising the gum's viscosity. Innovations in the purification of welan gum have focused on overcoming issues related to high viscosity and the presence of residual bacterial cells and carotenoids. Techniques such as NaOH treatment, filtration through fine membranes, and genetic modifications of microbial strains have been explored. Despite these advancements, there is a continuous pursuit of more efficient, cost-effective, and environmentally friendly methods due to limitations in scalability and economic feasibility (COLOCAR REFERENCIAS QUE FALTAN).
16 Application in the food industry
Recognized as safe (GRAS) by the FDA, microbial gums have a wide range of physicochemical properties suitable for applications in the food industry, being able to be used as gelling agents, stabilizers, and thickeners. Furthermore, these biomaterials can potentially enhance the properties of biodegradable packaging films [128].
Microbial gums applied to foods alter product textures through changes in the rheological properties of water. There is also a relationship between the presence of gums in foods and flavors. Additionally, these bioproducts can stabilize food textures and prevent or reduce the formation of ice crystals in frozen foods, besides playing an important role in appearance, color, and flavor of prepared foods [51, 52].
In relation to emulsification activity, gums function by increasing the viscosity of the aqueous phase in foods, thereby contributing to the long-term stability of emulsions and preventing the coalescence of oil particles [129]. For example, in products such as creams and sauces, oil-in-water emulsions are fundamental components requiring the use of polysaccharides to stabilize the emulsion and allow further processing and commercialization [130]. However, most gums naturally do not meet the criteria to be classified as emulsifiers.
A widely used example in the industry is xanthan gum, which has various applications in dry mix food products, preventing the constituents from reverting to their separate phases, thereby increasing stability, which is essential for commercialization [51, 52]. Xanthan gum is applied as a food additive due to its rheological behavior, low-concentration viscosity, pseudoplasticity, and high stability over a wide temperature range (Fig. 3). It is a bioproduct with significant market growth over the years, with an estimated compound annual growth rate between 2023 and 2030 of 4.73%. Applications in ice creams, syrups, and dessert toppings for thickening, stabilizing, fat substitutes, gelling agents, among other ingredients used in the food industry, are the main factors responsible for this growth [104, 131]. In the rapidly expanding market of 3D food printing, xanthan gum can be an alternative as a thickening agent, potentially improving the texture, smoothness, and viscoelasticity of the product, as demonstrated for fish paste [132].
As thickening agents, microbial gums have a wide range of applications, as is the case with one of the main applications of curdlan, which is widely applied to increase creaminess, stability, and viscoelasticity. It holds significant importance for the food industry due to its thermo-gelling characteristics, making it suitable for application in ice creams and frozen foods, as well as minimizing water loss during frying processes [51, 52]. Gellan also presents these characteristics and can be a strategy as emulsifying, stabilizing, and thickening agents in the food industry [51, 52]. For example, gellan (0.1%) applied to soy yogurt significantly improved the product's stability and brought more desirable sensory characteristics, besides reducing the water precipitation rate in the yogurt, and enhancing the viscosity, hardness, and consistency of the product [133]. As a gelling agent, gellan can improve the overall density of the network, reinforcing the structure of the gel [134].
In addition to thickening, gelling, foaming, flocculating, stabilizing, and other aspects in which microbial gums can be applied, some can favor the improvement of quality aspects in food products, such as pullulan, which can be used to provide low-calorie dietary fiber value as part of functional foods. This is due to its antioxidant and prebiotic properties, as well as its ability to improve the adhesion and gloss of foods without altering odor, taste, and caloric value. The presence of pullulan in foods also promotes the formation of films in frozen foods, preventing damage due to low temperature, and the construction of matrices that can act as carriers of bioactive compounds in functional foods [135, 136]. This scenario of expanding industrial use of pullulan is reflected in market growth data, which indicate an increase of 4.59% in the compound annual growth rate up to 2030 [137]. The drive in the economic market for pullulan is also due to its wide application in the pharmaceutical and cosmetic industries.
Biopolymer-based film formation has been an area explored for the use of microbial gums, highlighting that there is an emerging field of studies in full development for the application of gums in the production of biodegradable packaging [138,139,140]. The production of biodegradable films for bio-based packaging is a scenario that has gained strength in research in the food area, and when based on microbial gums they can improve the oxygen barrier and reduce humidity, helping to extend the shelf life of a food product, without compromising quality [139].
In biopolymer-based film development, the addition of gum forms the polymeric structure, allowing for the incorporation of additives (such as plasticizers and surfactants) aimed at modifying intermolecular bonds between polymers or even bioactive compounds (e.g., fungicides and antioxidants) to inhibit pathogen propagation [4]. Davidović et al. [141] developed dextran-based edible films plasticized by sorbitol, which significantly improved mechanical properties and low water vapor permeability. Additionally, nanoparticles have been evaluated for the preparation of gum-based film, such as gellan and xanthan, intending to act as antimicrobials and to improve the rheological properties of the biomaterial. The addition of zinc oxide nanoparticles significantly reduced water vapor permeability rates increased tensile strength and exhibited excellent antimicrobial activity against Staphylococcus aureus and Escherichia coli [142]. Nevertheless, it should be noted that cytotoxicity studies in human cells are important for assessing the toxicity of ZnO concentrations that come into contact with food and health care products. Other alternatives have also been evaluated, such as gellan gums films with eggshell nanoparticles [143], antifungal biodegradable films made from nanocellulose and gellan [144], chitosan films with essential oil [145] or gellan gum film incorporated with red cabbage anthocyanins extract [146].
This prospecting can accelerate the production and development market of this sector, driving the production of new technologies and raw materials, as well as efficient downstream processes for these markets. Another market trend for the application of gums is the expansion of the marketing of products for celiacs. Microbial gums can be alternatives to the use of gluten-containing products, providing texture, elasticity, and structures like those obtained with gluten, as well as improving the texture and quality of food products [147,148,149].
17 Conclusion
Xanthan, gellan, dextran and curdlan gums are extracellular polymers produced by submerged fermentation in batch processes by different bacteria of the genera Xanthomonas, Leuconostoc, Oenococcus, Sphingomonas, Gluconacetobacter and Bacillus. In the production processes of microbial gums, it is essential to optimize variables such as pH, temperature, aeration and agitation, which significantly affect the production of biopolymers. Different strategies have been evaluated to improve the yield of biopolymers and reduce the production costs, including alteration of the microbial genome, design of new culture media based on agro-industrial waste or by-products and addition of precursor molecules that can improve the production of biopolymers, such as the use of surfactants. The purification of microbial gums is a complex process that involves the use of different unit operations, among which the most applied are precipitation, centrifugation and drying. The structural and functional diversity of microbial gums allows for versatile applications, making them valuable ingredients in different industrial sectors. However, the greatest relevance of these molecules is in the food sector, which consumes around 50% of world production. In the food industry, gums are mainly used to improve sensory aspects (product appearance, flavor, texture), prevent deterioration processes, such as syneresis in emulsions, and provide resistance to factors such as temperature, pH and changes in salinity.
Data availability
No datasets were generated or analysed during the current study.
References
Díaz-Montes E, Yáñez-Fernández J, Castro-Muñoz R. Microfiltration-mediated extraction of dextran produced by leuconostoc mesenteroides SF3. Food Bioprod Process. 2020;119:317–28. https://doi.org/10.1016/j.fbp.2019.11.017.
Nejadmansouri M, Shad E, Razmjooei M, Safdarianghomsheh R, Delvigne F, Khalesi M. Production of xanthan gum using immobilized xanthomonas campestris cells: effects of support type. Biochem Eng J. 2020;157: 107554. https://doi.org/10.1016/j.bej.2020.107554.
Dev MJ, Warke RG, Warke GM, Mahajan GB, Patil TA, Singhal RS. Advances in fermentative production, purification, characterization and applications of gellan gum. Bioresour Technol. 2022;359: 127498. https://doi.org/10.1016/j.biortech.2022.127498.
Díaz-Montes E. Dextran: sources, structures, and properties. Polysaccharides. 2021;2:554–65. https://doi.org/10.3390/polysaccharides2030033.
Mangolim CS, Silva TT, et al. Description of recovery method used for curdlan produced by Agrobacterium Sp. IFO 13140 and its relation to the morphology and physicochemical and technological properties of the polysaccharide. PLoS ONE. 2017;12:1–19. https://doi.org/10.1371/journal.pone.0171469.
Donot F, Fontana A, Baccou JC, Schorr-Galindo S. Microbial exopolysaccharides: main examples of synthesis, excretion, genetics and extraction. Carbohydr Polym. 2012;87:951–62. https://doi.org/10.1016/J.CARBPOL.2011.08.083.
Prechtl RM, Janßen D, Behr J, Ludwig C, Küster B, Vogel RF, Jakob F. Sucrose-induced proteomic response and carbohydrate utilization of Lactobacillus SakeiTMW 1.411 during dextran formation. Front Microbiol. 2018;9:422346. https://doi.org/10.3389/FMICB.2018.02796/BIBTEX.
Besrour-Aouam N, Fhoula I, Hernández-Alcántara AM, Mohedano ML, Najjari A, Prieto A, Ruas-Madiedo P, López P, Ouzari HI. The role of dextran production in the metabolic context of leuconostoc and Weissella tunisian strains. Carbohydr Polym. 2021;253: 117254. https://doi.org/10.1016/J.CARBPOL.2020.117254.
West TP. Synthesis of the microbial polysaccharide gellan from dairy and plant-based processing coproducts. Polysaccharides. 2021;2:234–44. https://doi.org/10.3390/POLYSACCHARIDES2020016.
Aragão D, Fialho AM, Marques AR, Mitchell EP, Sá-Correia I, Frazão C. The Complex of Sphingomonas elodea ATCC 31461 glucose-1-phosphate uridylyltransferase with glucose-1-phosphate reveals a novel quaternary structure, unique among nucleoside diphosphate-sugar pyrophosphorylase members. J Bacteriol. 2007;189:4520–8. https://doi.org/10.1128/JB.00277-07/SUPPL_FILE/SUPPLEMENTARY_MATERIAL_TABLE_1.DOC.
Verma DK, Niamah AK, Patel AR, Thakur M, Singh Sandhu K, Chávez-González ML, Shah N, Noe Aguilar C. Chemistry and microbial sources of curdlan with potential application and safety regulations as prebiotic in food and health. Food Res Int. 2020;133: 109136. https://doi.org/10.1016/J.FOODRES.2020.109136.
García-Ochoa F, Santos VE, Casas JA, Gómez E. Xanthan gum: production, recovery, and properties. Biotechnol Adv. 2000;18:549–79. https://doi.org/10.1016/S0734-9750(00)00050-1.
Bhat IM, Wani SM, Mir SA, Masoodi FA. Advances in xanthan gum production, modifications and its applications. Biocatal Agric Biotechnol. 2022;42: 102328. https://doi.org/10.1016/J.BCAB.2022.102328.
Rončević Z, Grahovac J, Dodić S, Vučurović D, Dodić J. Utilisation of winery wastewater for xanthan production in stirred tank bioreactor: bioprocess modelling and optimisation. Food Bioprod Process. 2019;117:113–25. https://doi.org/10.1016/J.FBP.2019.06.019.
Market-Research Xanthan Gum Market 2022–2031. 2022–2024. https://www.Transparencymarketresearch.Com/Xanthan-Gum-Market.Html. Accessed 20 Feb 2024.
Allied-Market-Research Gellan Gum Market Research, 2030. 2022–2024. https://www.Alliedmarketresearch.Com/Gellan-Gum-Market-A13722. Accessed 20 Feb 2024.
Business-Research-Hinsights Dextran Markket Report Overview. 2022–2025. www.Businessresearchinsights.com/Market-Reports/Dextran-Market-100530. Accessed 20 Feb 2024.
Sun X, Zhang J. Bacterial exopolysaccharides: chemical structures, gene clusters and genetic engineering. Int J Biol Macromol. 2021;173:481–90. https://doi.org/10.1016/J.IJBIOMAC.2021.01.139.
Flemming HC. EPS—then and now. Microorganisms. 2016. https://doi.org/10.3390/MICROORGANISMS4040041.
Decho AW, Gutierrez T. Microbial extracellular polymeric substances (EPSs) in ocean systems. Front Microbiol. 2017;8: 265214. https://doi.org/10.3389/FMICB.2017.00922/BIBTEX.
Moshaf S, Hamidi-Esfahani Z, Azizi MH. Statistical optimization of xanthan gum production and influence of airflow rates in lab-scale fermentor. Appl Food Biotechnol. 2014;1:17–24. https://doi.org/10.22037/AFB.V1I1.7132.
Kumar AS, Mody K, Jha B. Bacterial exopolysaccharides—a perception. J Basic Microbiol. 2007;47:103–17. https://doi.org/10.1002/JOBM.200610203.
Schmid J, Sieber V, Rehm B. Bacterial exopolysaccharides: biosynthesis pathways and engineering strategies. Front Microbiol. 2015;6: 147519. https://doi.org/10.3389/FMICB.2015.00496/BIBTEX.
Hahn J, Koch D, Niehaus K, Ortseifen V. Analysis of gum proteins involved in xanthan biosynthesis throughout multiple cell fractions in a “single-tube.” J Proteomics. 2022;257: 104513. https://doi.org/10.1016/J.JPROT.2022.104513.
Morris ER, Nishinari K, Rinaudo M. Gelation of gellan—a review. Food Hydrocoll. 2012;28:373–411. https://doi.org/10.1016/J.FOODHYD.2012.01.004.
Yuan M, Fu G, Sun Y, Zhang D. Biosynthesis and applications of curdlan. Carbohydr Polym. 2021;273: 118597. https://doi.org/10.1016/J.CARBPOL.2021.118597.
Hayward AC. The hosts of xanthomonas. Xanthomonas. 1993. https://doi.org/10.1007/978-94-011-1526-1_1.
Aspinall GO. The Polysaccharides; Academic Press, 1982; ISBN 9780120656028.
Kool MM, Gruppen H, Sworn G, Schols HA. The influence of the six constituent xanthan repeating units on the order-disorder transition of xanthan. Carbohydr Polym. 2014;104:94–100. https://doi.org/10.1016/J.CARBPOL.2013.12.073.
Becker A, Katzen F, Puè A, Ielpi L. Xanthan gum biosynthesis and application: a biochemical /genetic perspective. Appl Microbiol Biotechnol. 1998;50:145–52.
Harding NE, Raffo S, Raimondi A, Cleary JM, Ielpi L. Identification, genetic and biochemical analysis of genes involved in synthesis of sugar nucleotide precursors of xanthan gum. J Gen Microbiol. 1993;139:447–57. https://doi.org/10.1099/00221287-139-3-447/CITE/REFWORKS.
Leigh JA, Coplin DL. Exopolysacharides in plant -bacterial interactions. Annu Rev Microbiol. 1992;46:307–53.
Li X, Wang X, Meng X, Dijkhuizen L, Liu W. Structures, physico-chemical properties, production and (potential) applications of sucrose-derived α-d-glucans synthesized by glucansucrases. Carbohydr Polym. 2020;249: 116818. https://doi.org/10.1016/J.CARBPOL.2020.116818.
Zhao Y, Jalili S. Dextran, as a biological macromolecule for the development of bioactive wound dressing materials: a review of recent progress and future perspectives. Int J Biol Macromol. 2022;207:666–82. https://doi.org/10.1016/J.IJBIOMAC.2022.02.114.
Irague R, Rolland-Sabaté A, Tarquis L, Doublier JL, Moulis C, Monsan P, Remaud-Siméon M, Potocki-Véronèse G, Buléon A. Structure and property engineering of α-d-glucans synthesized by dextransucrase mutants. Biomacromol. 2012;13:187–95. https://doi.org/10.1021/BM201453R/ASSET/IMAGES/MEDIUM/BM-2011-01453R_0003.GIF.
Van Geel-Schutten GH, Flesch F, Ten Brink B, Smith MR, Dijkhuizen L. Screening and characterization of lactobacillus strains producing large amounts of exopolysaccharides. Appl Microbiol Biotechnol. 1998;50:697–703. https://doi.org/10.1007/s002530051353.
Vuillemin M, Grimaud F, Claverie M, Rolland-Sabaté A, Garnier C, Lucas P, Monsan P, Dols-Lafargue M, Remaud-Siméon M, Moulis C. A Dextran with unique rheological properties produced by the dextransucrase from Oenococcus kitaharae DSM 17330. Carbohydr Polym. 2018;179:10–8. https://doi.org/10.1016/J.CARBPOL.2017.09.056.
Tingirikari JMR, Kothari D, Goyal A. Superior prebiotic and physicochemical properties of novel dextran from Weissella Cibaria JAG8 for potential food applications. Food Funct. 2014;5:2324–30. https://doi.org/10.1039/c4fo00319e.
Ahmed RZ, Siddiqui K, Arman M, Ahmed N. Characterization of high molecular weight dextran produced by Weissella cibaria CMGDEX3. Carbohydr Polym. 2012;90:441–6. https://doi.org/10.1016/J.CARBPOL.2012.05.063.
Kelstrup J, Funder Nielsen TD. Adhesion of dextran to streptococcus mutans. J Gen Microbiol. 1974;81:485–9. https://doi.org/10.1099/00221287-81-2-485/CITE/REFWORKS.
Monchois V, Remaud-Simeon M, Russell RRB, Monsan P, Willemot RM. Characterization of leuconostoc mesenteroides NRRL B-512F dextransucrase (DSRS) and identification of amino-acid residues playing a key role in enzyme activity. Appl Microbiol Biotechnol. 1997;48:465–72. https://doi.org/10.1007/S002530051081.
Walker GJ, Pulkownik A. Degradation of Dextrans by an α-1,6-glucan glucohydrolase from streptococcus mitis. Carbohydr Res. 1973;29:1–14. https://doi.org/10.1016/S0008-6215(00)82066-2.
Neely WB, Nott J. Dextransucrase, an induced enzyme from leuconostoc mesenteroides. Biochemistry. 1962;1:1136–40. https://doi.org/10.1021/BI00912A027/ASSET/BI00912A027.FP.PNG_V03.
Kang KS, Veeder GT. Polysaccharide S-60 and bacterial fermentation process for its preparation. Biotechnol Adv. 1983;1:157.
Kang KS, Colegrove GT, Veeder GT Deacetylated Polysaccharide S-60 1983.
Yabuuchi E, Yano I, Oyaizu H, Hashimoto Y, Ezaki T, Yamamoto H. Proposals of Sphingomonas paucimobilis Gen. Nov. and Comb. Nov., Sphingomonas parapaucimobilis Sp. Nov., Sphingomonas yanoikuyae Sp. Nov., Sphingomonas adhaesiva Sp. Nov., Sphingomonas capsulata Comb, Nov., and two genospecies of the genus sphingomonas. Microbiol Immunol 1990, 34, 99–119, https://doi.org/10.1111/J.1348-0421.1990.TB00996.X.
Vartak NB, Lin CC, Cleary JM, Fagan MJ, Saier MH. Glucose metabolism in “Sphingomonas elodea”: pathway engineering via construction of a glucose-6-phosphate dehydrogenase insertion mutant. Microbiology (N Y). 1995;141:2339–50. https://doi.org/10.1099/13500872-141-9-2339/CITE/REFWORKS.
Jansson PE, Lindberg B, Sandford PA. Structural studies of gellan gum, an extracellular polysaccharide elaborated by Pseudomonas elodea. Carbohydr Res. 1983;124:135–9. https://doi.org/10.1016/0008-6215(83)88361-X.
Milas M, Shi X, Rinaudo M. On the physicochemical properties of gellan gum. Biopolymers. 1990;30:451–64. https://doi.org/10.1002/BIP.360300322.
Osmałek T, Froelich A, Tasarek S. Application of gellan gum in pharmacy and medicine. Int J Pharm. 2014;466:328–40.
Jindal N, Singh Khattar J. Microbial polysaccharides in food industry. In Biopolymers for Food Design; Elsevier, 2018; pp. 95–123.
Yildiz H, Karatas N. Microbial exopolysaccharides: resources and bioactive properties. Process Biochem. 2018;72:41–6. https://doi.org/10.1016/j.procbio.2018.06.009.
Chandrasekaran R, Radha A, Thailambal VG. Roles of potassium ions, acetyl and l-glyceryl groups in native gellan double helix: an x-ray study. Carbohydr Res. 1992;224:1–17. https://doi.org/10.1016/0008-6215(92)84088-A.
Sá-Correia I, Fialho AM, Videira P, Moreira LM, Marques AR, Albano H. Gellan gum biosynthesis in Sphingomonas paucimobilis ATCC 31461: genes, enzymes and exopolysaccharide production engineering. J Ind Microbiol Biotechnol. 2002;29:170–6.
Silva E, Marques AR, Fialho AM, Granja AT, Sá-Correia I. Proteins encoded by Sphingomonas elodea ATCC 31461 RmlA and UgpG genes, involved in gellan gum biosynthesis, exhibit both DTDP- and UDP-glucose pyrophosphorylase activities. Appl Environ Microbiol. 2005;71:4703–12. https://doi.org/10.1128/AEM.71.8.4703-4712.2005/ASSET/AAB8057B-1B80-4E5A-88E9-B8C494F3D376/ASSETS/GRAPHIC/ZAM0080556850006.JPEG.
Pollock TJ, Van Workum WAT, Thorne L, Mikolajczak MJ, Yamazaki M, Kijne JW, Armentrout RW. Assignment of biochemical functions to glycosyl transferase genes which are essential for biosynthesis of exopolysaccharides in sphingomonas strain s88 and Rhizobium leguminosarum. J Bacteriol. 1998;180:586–93. https://doi.org/10.1128/JB.180.3.586-593.1998/ASSET/D25BE93C-33F7-48B8-9DAA-168662C37D8C/ASSETS/GRAPHIC/JB0381281002.JPEG.
Li A, Hu T, Luo H, Alam NU, Xin J, Li H, Lin Y, Huang J, Huang K, Meng Y, et al. A Carotenoid-and poly-hydroxybutyrate-free mutant strain of Sphingomonas elodea ATCC 31461 for the commercial production of gellan. 2019. https://doi.org/10.1128/mSphere.
Laroche C, Michaud P. New developments and prospective applications for & #946; (1,3) glucans. Recent Pat Biotechnol. 2008;1:59–73. https://doi.org/10.2174/187220807779813938.
Zhang R, Edgar KJ. Properties, chemistry, and applications of the bioactive polysaccharide curdlan. Biomacromol. 2014;15:1079–96. https://doi.org/10.1021/BM500038G/ASSET/IMAGES/MEDIUM/BM-2014-00038G_0026.GIF.
Wu C, Yuan C, Chen S, Liu D, Ye X, Hu Y. The effect of curdlan on the rheological properties of restructured ribbonfish (Trichiurus Spp.) meat gel. Food Chem. 2015;179:222–31. https://doi.org/10.1016/J.FOODCHEM.2015.01.125.
Chen T, Fang S, Zuo X, Liu Y. Effect of curdlan and xanthan polysaccharides on the pasting, rheological and thermal properties of rice starch. J Food Sci Technol. 2016;53:4076–83. https://doi.org/10.1007/S13197-016-2414-6/TABLES/3.
Lee IY, Kim MK, Lee JH, Seo WT, Jung JK, Lee HW, Park YH. Influence of agitation speed on production of curdlan by agrobacterium species. Bioprocess Eng. 1999;20:283–7. https://doi.org/10.1007/PL00009049.
Prakash S, Rajeswari K, Divya P, Ferlin M, Rajeshwari CT, Vanavil B. Optimization and production of curdlan gum using bacillus cereus PR3 isolated from rhizosphere of leguminous plant. Prep Biochem Biotechnol. 2018;48:408–18. https://doi.org/10.1080/10826068.2018.1451886.
Wang XYZ, Dong JJ, Xu GC, Han RZ, Ni Y. Enhanced curdlan production with nitrogen feeding during polysaccharide synthesis by rhizobium radiobacter. Carbohydr Polym. 2016;150:385–91. https://doi.org/10.1016/J.CARBPOL.2016.05.036.
West TP. Effect of Nitrogen source concentration on curdlan production by Agrobacterium Sp. ATCC 31749 grown on prairie cordgrass hydrolysates. Prep Biochem Biotechnol. 2016;46:85–90. https://doi.org/10.1080/10826068.2014.985835.
Liang Y, Zhu L, Ding H, Gao M, Zheng Z, Wu J, Zhan X. Enhanced production of curdlan by coupled fermentation system of Agrobacterium Sp. ATCC 31749 and Trichoderma harzianum GIM 3442. Carbohydr Polym. 2017;157:1687–94. https://doi.org/10.1016/J.CARBPOL.2016.11.055.
Freitas F, Torres CAV, Reis MAM. Engineering aspects of microbial exopolysaccharide production. Bioresour Technol. 2017;245:1674–83. https://doi.org/10.1016/j.biortech.2017.05.092.
Seviour RJ, McNeil B, Fazenda ML, Harvey LM. Operating bioreactors for microbial exopolysaccharide production. Crit Rev Biotechnol. 2011;31:170–85. https://doi.org/10.3109/07388551.2010.505909.
Bajaj IB, Survase SA, Saudagar PS, Singhal RS. Gellan gum: fermentative production, downstream processing and applications. Food Technol Biotechnol. 2007;45:341–54.
Barcelos MCS, Vespermann KAC, Pelissari FM, Molina G. Current status of biotechnological production and applications of microbial exopolysaccharides. Crit Rev Food Sci Nutr. 2020;60:1475–95. https://doi.org/10.1080/10408398.2019.1575791.
Al-Rmedh YSS, Ali HI, Al-Sahlany STG. Curdlan gum, properties, benefits and applications. IOP Conf Ser Earth Environ Sci. 2023. https://doi.org/10.1088/1755-1315/1158/11/112011.
Viswanathan HS, Rahman SSA, Venkatachalam P, Karuppiah S. Production of gellan gum using milk skin residue (MSR)—a tea shop waste: statistical optimization and downstream processing. Biomass Convers Biorefin. 2023;13:189–203. https://doi.org/10.1007/s13399-020-01026-z.
Zhang J, Dong YC, Fan LL, Jiao ZH, Chen QH. Optimization of culture medium compositions for gellan gum production by a halobacterium Sphingomonas paucimobilis. Carbohydr Polym. 2015;115:694–700. https://doi.org/10.1016/j.carbpol.2014.09.029.
Mordor Intelligence Xanthan Gum Market Size & Share Analysis - Growth Trends & Forecasts (2024–2029) https://www.mordorintelligence.com/industry-reports/xanthan-gum-market. Accessed 7 Feb 2024.
Future market insights gellan gum market. https://www.futuremarketinsights.com/reports/gellan-gum-market. Accessed 7 Feb 2024.
Business research insights dextran market size, share, growth and global industry analysis. https://www.businessresearchinsights.com/market-reports/dextran-market-100530. Accessed 7 Feb 2024.
Market Reports World Global Curdlan Gum Market 2023 by Manufacturers, Regions, Type and Application, Forecast to 2029 https://www.marketreportsworld.com/global-curdlan-gum-market-25171533.
CP Kelco. Xanthan Gum. https://www.cpkelco.com/products/xanthan-gum/. Accessed 7 Feb 2024.
Cargill Xanthan Gum. https://www.cargill.com/bioindustrial/xanthan-gum. Accessed 7 Feb 2024.
Meron Group Xanthan Gum. https://www.meron.com/products/xanthan-gum/. Accessed 7 Feb 2024.
Group, F. Hydrocolloids. http://en.fufeng-group.cn/product/list-8.html?sd=1. Accessed 7 Feb 2024.
CP Kelco Gellan Gum. https://www.cpkelco.com/products/gellan-gum/. Accessed 7 Feb 2024.
Meron group plant gel (Gellan Gum). https://www.meron.com/products/plant-gel-gellan-gum/. Accessed 7 Feb 2024.
Pharmacosmos the global leader in pharmaceutical dextran https://www.dextran.com/. Accessed 7 Feb 2024.
pK Chemicals Dextran. https://pkcas.dk/dextran/. Accessed 7 Feb 2024.
Meito sangyo co fine chemicals division. https://www.meito-sangyo.co.jp/kaseihin/index_e.html. Accessed 7 Feb 2024.
Organo food tech corporation curdlan compounds list. https://oft.organo.co.jp/english/product/curdlan_series/. Accessed 7 Feb 2024.
Meron group curdlan gum. https://www.meron.com/products/curdlan-gum/. Accessed 7 Feb 2024.
Vandenberghe LP de S, Herrmann LW, Penha R de O, de Mello AFM, Martínez-Burgos WJ, Junior AIM, Kirnev PC de S, de Carvalho JC, Soccol CR. Engineering Aspects for Scale-up of Bioreactors. Curr Dev Biotechnol Bioeng Adv Bioprocess En 2022, https://doi.org/10.1016/B978-0-323-91167-2.00002-2.
de Mello AFM, de Souza Vandenberghe LP, Herrmann LW, Letti LAJ, Burgos WJM, Scapini T, Manzoki MC, de Oliveira PZ, Soccol CR. Strategies and engineering aspects on the scale-up of bioreactors for different bioprocesses. Syst Microbiol Biomanufactur 2023, https://doi.org/10.1007/s43393-023-00205-z.
Schmid J, Sieber V. Fermentative production of microbial exopolysaccharides. Bioprocess Biomol Prod. 2019. https://doi.org/10.1002/9781119434436.ch7.
De Jesus Assis D, Brandão LV, De Sousa Costa LA, Figueiredo TVB, Sousa LS, Padilha FF, Druzian JI. A study of the effects of aeration and agitation on the properties and production of xanthan gum from crude glycerin derived from biodiesel using the response surface methodology. Appl Biochem Biotechnol. 2014;172:2769–85. https://doi.org/10.1007/s12010-014-0723-7.
Huang J, Jiang S, Xu X, Wu H, Zhu X, Ke Z, Cai J, Huang L, Xu Z. Effects of carbon/nitrogen ratio, dissolved oxygen and impeller type on gellan gum production in Sphingomonas paucimobilis. Ann Microbiol. 2012;62:299–305. https://doi.org/10.1007/s13213-011-0261-2.
Pan L, Wang Q, Qu L, Liang L, Han Y, Wang X, Zhou Z. Pilot-scale production of exopolysaccharide from leuconostoc pseudomesenteroides XG5 and its application in set yogurt. J Dairy Sci. 2022;105:1072–83. https://doi.org/10.3168/jds.2021-20997.
Liu Z, Xu Y, Wang Z, Zhu L, Li Z, Jiang Y, Zhan X, Gao M. Promoting substrates uptake and curdlan synthesis of Agrobacterium Sp. by attenuating the exopolysaccharide encapsulation. Carbohydr Polym. 2023;315:120941. https://doi.org/10.1016/J.CARBPOL.2023.120941.
Ben Salah R, Jaouadi B, Bouaziz A, Chaari K, Blecker C, Derrouane C, Attia H, Besbes S. Fermentation of date palm juice by curdlan gum production from rhizobium radiobacter ATCC 6466™: purification, rheological and physico-chemical characterization. LWT Food Sci Technol. 2011;44:1026–34. https://doi.org/10.1016/J.LWT.2010.11.023.
Rahman SSA, Pasupathi S, Karuppiah S. Conventional optimization and characterization of microbial dextran using treated sugarcane molasses. Int J Biol Macromol. 2022;220:775–87. https://doi.org/10.1016/J.IJBIOMAC.2022.08.094.
Yáñez-Fernández J, Herrera Ovando MG, Patlán Ramírez L, Ramírez-Sotelo G, Guarin CA, Castro-Rodríguez DC. Factorial design to optimize dextran production by the native strain leuconostoc mesenteroides SF3. ACS Omega. 2021;6:31203–10. https://doi.org/10.1021/ACSOMEGA.1C04856/ASSET/IMAGES/MEDIUM/AO1C04856_M005.GIF.
Nachtigall C, Hassler V, Wefers D, Rohm H, Jaros D. Dextrans of Weissella cibaria DSM14295: microbial production, structure and functionality. Int J Biol Macromol. 2023;246: 125631. https://doi.org/10.1016/J.IJBIOMAC.2023.125631.
Kamer DDA, Gumus T, Palabiyik I, Demirci AS, Oksuz O. The fermentation-based production of gellan from rice bran and the evaluation of various qualitative properties of gum. Int J Biol Macromol. 2022;207:841–9. https://doi.org/10.1016/J.IJBIOMAC.2022.03.168.
Giavasis I, Petrotos K, et al. Mary biovalorization of olive mill waste water for the production of gellan gum from Sphingomonas paucimobilis. Biotechnol J Int. 2016;11:1–15. https://doi.org/10.9734/BBJ/2016/22510.
Shakeri MS, Naji-Tabasi S. Characterization and optimization of gellan gum production by natural sphingomonas Sp. SM2. LWT. 2024;200:116164. https://doi.org/10.1016/J.LWT.2024.116164.
Huang J, Zhu S, Li C, Zhang C, Ji Y. Cost-effective optimization of gellan gum production by Sphingomonas paucimobilis using corn steep liquor. Prep Biochem Biotechnol. 2020;50:191–7. https://doi.org/10.1080/10826068.2019.1692215.
Demirci AS, Palabiyik I, Apaydın D, Mirik M, Gumus T. Xanthan gum biosynthesis using xanthomonas isolates from waste bread: process optimization and fermentation kinetics. LWT. 2019;101:40–7. https://doi.org/10.1016/J.LWT.2018.11.018.
Mohsin A, Zhang K, Hu J, Salim-ur-Rehman, Tariq M, Zaman WQ, Khan IM, Zhuang Y, Guo M. Optimized biosynthesis of xanthan via effective valorization of orange peels using response surface methodology: a kinetic model approach. Carbohydr Polym 2018;181:793–800. https://doi.org/10.1016/J.CARBPOL.2017.11.076.
Chetia R, Bharadwaj B, Dey R, Chatterji BP. The production of xanthan from brewer’s spent grain. Microbiol Biotechnol Lett. 2023;51:449–56. https://doi.org/10.48022/MBL.2309.09007.
Rajyaguru B, Varma A, Kharkwal A, Singh J. Biosynthesis of xanthan gum by xanthomonas campestris using cane molasses as a carbon source. Nat Environ Pollut Technol. 2017;2021:20. https://doi.org/10.46488/NEPT.2021.V20I05.018.
Li ZX, Chen JY, Wu Y, Huang ZY, Wu ST, Chen Y, Gao J, Hu Y, Huang C. Effect of downstream processing on the structure and rheological properties of xanthan gum generated by fermentation of melaleuca alternifolia residue hydrolysate. Food Hydrocoll. 2022;132: 107838. https://doi.org/10.1016/j.foodhyd.2022.107838.
West TP. Production of the polysaccharide pullulan by aureobasidium pullulans cell immobilization. Polysaccharides. 2022;3:544–55.
Ortiz Martinez C, Pereira Ruiz S, Carvalho Fenelon V, Rodrigues de Morais G, Luciano Baesso M, Matioli G. Characterization of curdlan produced by Agrobacterium Sp. IFO 13140 cells immobilized in a loofa sponge matrix, and application of this biopolymer in the development of functional yogurt. J Sci Food Agric 2016, 96, 2410–2417, https://doi.org/10.1002/jsfa.7357.
Qader SAU, Aman A. Low molecular weight dextran: immobilization of cells of leuconostoc mesenteroides KIBGE HA1 on calcium alginate beads. Carbohydr Polym. 2012;87:2589–92. https://doi.org/10.1016/j.carbpol.2011.11.046.
Dey R, Chatterji BP. Sources and methods of manufacturing xanthan by fermentation of various carbon sources. Biotechnol Prog. 2023. https://doi.org/10.1002/btpr.3379.
Palaniraj A, Jayaraman V. Production, recovery and applications of xanthan gum by Xanthomonas campestris. J Food Eng. 2011;106:1–12. https://doi.org/10.1016/j.jfoodeng.2011.03.035.
Li P, Li T, Zeng Y, Li X, Jiang X, Wang Y, Xie T, Zhang Y. Biosynthesis of xanthan gum by Xanthomonas campestris LRELP-1 using kitchen waste as the sole substrate. Carbohydr Polym. 2016;151:684–91. https://doi.org/10.1016/j.carbpol.2016.06.017.
Wu X, Wu R, Li O, Zhu L, Chen Y, Qian C, Chen M. Yellow pigments generation deficient sphingomonas strain and application thereof in gellan gum production. US Patent 8685698B2. 2014, 8685698.
Doner LW. Rapid purification of commercial gellan gum to highly soluble and gellable monovalent cation salts. Carbohydr Polym. 1997;32:245–7. https://doi.org/10.1016/S0144-8617(96)00168-3.
Naessens M, Cerdobbel A, Soetaert W, Vandamme EJ. Leuconostoc dextransucrase and dextran: production, properties and applications. J Chem Technol Biotechnol. 2005;80:845–60. https://doi.org/10.1002/jctb.1322.
Du R, Qiao X, Zhao F, Song Q, Zhou Q, Wang Y, Pan L, Han Y, Zhou Z. Purification, characterization and antioxidant activity of dextran produced by leuconostoc pseudomesenteroides from homemade wine. Carbohydr Polym. 2018;198:529–36. https://doi.org/10.1016/j.carbpol.2018.06.116.
Liu J, Tang J, Li X, Yan Q, Ma J, Jiang Z. Curdlan (Alcaligenes faecalis) (1→3)-β-d-glucan oligosaccharides drive m1 phenotype polarization in murine bone marrow-derived macrophages via activation of MAPKs and NF-ΚB Pathways. Molecules. 2019;24:1–16. https://doi.org/10.3390/molecules24234251.
Ezhilarasi P, Vanavil B. Curdlan gum production using marine bacteria isolated from red seaweeds: screening and optimization studies. Microbiology (Russian Federation). 2023;92:725–33. https://doi.org/10.1134/S0026261723600131.
Ye N, Jinjun D, Ruizhi H, Guochao X, Yuzhu WX. Rhizobium radiobacter and method for producing curdlan gum through fermentation of rhizobium radiobacter 2016.
Becker A. Challenges and perspectives in combinatorial assembly of novel exopolysaccharide biosynthesis pathways. Front Microbiol. 2015. https://doi.org/10.3389/fmicb.2015.00687.
Mahmoud YAG, El-Naggar ME, Abdel-Megeed A, El-Newehy MH. Recent advancements in microbial polysaccharides: synthesis and applications. Polymers (Basel). 2021;13:4136.
Wu M, Shi Z, Ming Y, Zhao Y, Gao G, Li G, Ma T. The production of ultrahigh molecular weight xanthan gum from a sphingomonas chassis capable of co-utilising glucose and xylose from corn straw. Microb Biotechnol. 2024. https://doi.org/10.1111/1751-7915.14394.
Al-Rumaidh YSS, Al-Sahlany STG, Ali HI. The impact of using date juice as a carbon source on curdlan produced by a local isolate of Agrobacterium leguminum. Basrah J Agric Sci. 2023;36:149–63. https://doi.org/10.37077/25200860.2023.36.1.13.
Zhang, Y.; Guopei, Z.; Shaohua, Z.; Xuezhen, L. Preparation method of high-gel-strength, high-viscosity and high-acyl gellan gum 2019.
Huang J, Zhu S, Li C, Zhang C, Ji Y. Cost-effective optimization of gellan gum production by Sphingomonas paucimobilis using corn steep liquor. Prep Biochem Biotechnol. 2020;50:191–7.
Alizadeh-Sani M, Ehsani A, Moghaddas Kia E, Khezerlou A. Microbial gums: introducing a novel functional component of edible coatings and packaging. Appl Microbiol Biotechnol. 2019;103:6853–66. https://doi.org/10.1007/s00253-019-09966-x.
Gao X, Pourramezan H, Ramezan Y, Roy S, Zhang W, Assadpour E, Zou J, Jafari SM. Application of gums as techno-functional hydrocolloids in meat processing and preservation: a review. Int J Biol Macromol. 2024;268: 131614. https://doi.org/10.1016/J.IJBIOMAC.2024.131614.
Chaturvedi S, Kulshrestha S, Bhardwaj K, Jangir R. A review on properties and applications of xanthan gum. Microb Polym. 2021. https://doi.org/10.1007/978-981-16-0045-6_4.
Market Research Future Xanthan Gum Market Research Report Information by Form, Function, Application and By Region. https://www.marketresearchfuture.com/reports/xanthan-gum-market-748?utm_term=&utm_campaign=&utm_source=adwords&utm_medium=ppc&hsa_acc=2893753364&hsa_cam=19912237177&hsa_grp=148712481999&hsa_ad=659589502539&hsa_src=g&hsa_tgt=dsa-2080758270360&hsa_kw=&hsa_mt=&hsa_net=adwords&hsa_ver=3&gad_source=1. Accessed 11 Feb 2024.
Yu X, Wang Y, Zhao W, Li S, Pan J, Prakash S, Dong X. Hydrophilic colloids (Konjac Gum/Xanthan Gum) in 3D printing of transitional food from fish paste. Food Hydrocoll. 2023;137: 108333. https://doi.org/10.1016/j.foodhyd.2022.108333.
Kong X, Xiao Z, Du M, Wang K, Yu W, Chen Y, Liu Z, Cheng Y, Gan J. Physicochemical, textural, and sensorial properties of soy yogurt as affected by addition of low acyl gellan gum. Gels. 2022;8:453. https://doi.org/10.3390/gels8070453.
Zhan L, Lan G, Wang Y, Xie S, Cai S, Liu Q, Chen P, Xie F. Mastering textural control in multi-polysaccharide gels: effect of κ-carrageenan, konjac glucomannan, locust bean gum, low-acyl gellan gum, and sodium alginate. Int J Biol Macromol. 2024;254: 127885. https://doi.org/10.1016/j.ijbiomac.2023.127885.
de Souza CK, Ghosh T, Lukhmana N, Tahiliani S, Priyadarshi R, Hoffmann TG, Purohit SD, Han SS. Pullulan as a sustainable biopolymer for versatile applications: a review. Mater Today Commun. 2023;36: 106477. https://doi.org/10.1016/j.mtcomm.2023.106477.
Ali K, Jiang B, Chen J, Ashraf W, Tahir A. Bin preparation and structural characterization of pullulan and whey protein isolate-based electrospun nanofiber. Food Biosci. 2023;56: 103218. https://doi.org/10.1016/j.fbio.2023.103218.
Medium Global Pullulan Market Insights and Forecast to 2030; 2023;
Rashid A, Qayum A, Liang Q, Kang L, Ekumah J-N, Han X, Ren X, Ma H. Exploring the potential of pullulan-based films and coatings for effective food preservation: a comprehensive analysis of properties, activation strategies and applications. Int J Biol Macromol. 2024;260: 129479. https://doi.org/10.1016/j.ijbiomac.2024.129479.
Agarwal N, Jyoti TM, Mishra BB, Singh SP. Preparation and characterization of biodegradable films based on levan polysaccharide blended with gellan gum. Environ Technol Innov. 2023;31:103231. https://doi.org/10.1016/j.eti.2023.103231.
Zheng M, Zhu Y, Zhuang Y, Tan KB, Chen J. Effects of grape seed extract on the properties of pullulan polysaccharide/xanthan gum active films for apple preservation. Int J Biol Macromol. 2023;241: 124617. https://doi.org/10.1016/j.ijbiomac.2023.124617.
Davidović S, Miljković M, Tomić M, Gordić M, Nešić A, Dimitrijević S. Response surface methodology for optimisation of edible coatings based on dextran from leuconostoc mesenteroides T3. Carbohydr Polym. 2018;184:207–13. https://doi.org/10.1016/j.carbpol.2017.12.061.
Rukmanikrishnan B, Ismail FRM, Manoharan RK, Kim SS, Lee J. Blends of gellan gum/xanthan gum/zinc oxide based nanocomposites for packaging application: rheological and antimicrobial properties. Int J Biol Macromol. 2020;148:1182–9. https://doi.org/10.1016/j.ijbiomac.2019.11.155.
Arredondo-Tamayo B, Méndez-Méndez JV, Chanona-Pérez JJ, Hernández-Varela JD, González-Victoriano L, Gallegos-Cerda SD, Martínez-Mercado E. Study of Gellan gum films reinforced with eggshell nanoparticles for the elaboration of eco-friendly packaging. Food Struct. 2022;34: 100297. https://doi.org/10.1016/J.FOOSTR.2022.100297.
Sripahco T, Khruengsai S, Pripdeevech P. Biodegradable antifungal films from nanocellulose-gellan gum incorporated with Anethum graveolens essential oil for bread packaging. Int J Biol Macromol. 2023;243: 125244. https://doi.org/10.1016/J.IJBIOMAC.2023.125244.
Khruengsai S, Phoopanasaeng P, Sripahco T, Soykeabkaew N, Pripdeevech P. Application of chitosan films incorporated with Zanthoxylum limonella essential oil for extending shelf life of pork. Int J Biol Macromol. 2024;262: 129703. https://doi.org/10.1016/J.IJBIOMAC.2024.129703.
Zhan S, Yi F, Hou F, Song L, Chen X, Jiang H, Han X, Sun X, Liu Z. Development of PH-freshness smart label based on gellan gum film incorporated with red cabbage anthocyanins extract and its application in postharvest mushroom. Colloids Surf B Biointerfaces. 2024;236: 113830. https://doi.org/10.1016/J.COLSURFB.2024.113830.
Rai S, Kaur A, Chopra CS. Gluten-free products for celiac susceptible people. Front Nutr. 2018. https://doi.org/10.3389/fnut.2018.00116.
Khorasani SM, Aalami M, Kashaninejad M, Tabarestani HS. Effect of adding millet flour and xanthan gum on the physicochemical and sensorial properties of gluten-free cake. Food Process Preservat J. 2021;13:57–70.
Widelska G, Wójtowicz A, Kasprzak K, Dib A, Oniszczuk T, Olech M, Wojtunik-Kulesza K, Nowak R, Sujak A, Dobrzański B, et al. Impact of xanthan gum addition on phenolic acids composition and selected properties of new gluten-free maize-field bean pasta. Open Chem. 2019;17:587–98. https://doi.org/10.1515/chem-2019-0075.
Author information
Authors and Affiliations
Contributions
Walter José Martínez Burgos: conceptualization, writing—original draft preparation; Diego Yamir Ocán-Torres: conceptualization, writing—original draft preparation; Ariane Fátima Murawski de Mello: conceptualization, writing—original draft preparation; Roberta Pozzan: conceptualization, writing—original draft preparation; Thamarys Scapini:conceptualization, writing—original draft preparation; Maria Clara Manzoki:conceptualization, writing—original draft preparation; , Adriane Bianchi Pedroni Medeiros: conceptualization, resources, supervision Luciana Porto de Souza Vandenberghe: conceptualization, resources, supervision Carlos Ricardo Soccol: conceptualization, resources, supervision
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martínez-Burgos, W.J., Ocán-Torres, D.Y., Manzoki, M.C. et al. New trends in microbial gums production, patented technologies and applications in food industry. Discov Food 4, 49 (2024). https://doi.org/10.1007/s44187-024-00130-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44187-024-00130-7