Abstract
The combination of multiple strains and multiple species in lactic acid bacteria-based fermented milk offers the potential for nutritional and sensory attributes, making it an attractive option for developing high-quality and health-promoting probiotic yoghurt-like fermented milk products. In the present study, four combinations of yoghurt-like fermented milk samples: Sample 1 (Control), Sample 2 (Lactobacillus desidiosus), Sample 3 (Lactobacillus fermentum), and Sample 4 (Lactobacillus desidiosus and Lactobacillus fermentum) were developed. Proximate composition (moisture, ash, protein, fat, carbohydrate), physicochemical (pH, titratable acidity, syneresis, total soluble sloid), microbiological (Lactobacillus bacteria, yeast and mold, and coliform bacteria), and sensory properties (color, flavor, texture, taste, and overall acceptability) were assessed under refrigerator conditions (4 °C) from 0 to 28 days of storage period. Proximate composition differed significantly (p < 0.05) among samples for moisture (49.34–79.32%), ash (1.14–0.31%), protein (3.72–3.21%), fat (11.23–5.62%), and carbohydrate (33.11–12.98%). Yoghurt-like fermented milk containing Lactobacillus desidiosus as single or in combination with Lactobacillus fermentum resulted in reduced pH levels (6.02–3.49), total soluble solid (41.66–24.66%) and increased syneresis (30.04–65.52%), titratable acidity content (0.42–1.62%). Single or combination of Lactobacillus desidiosus and Lactobacillus fermentum significantly (p < 0.05) reduced Lactobacillus bacteria (8.17–6.01 Log CFU/g), yeast, and mold (1.45–1.19 Log CFU/g). Additionally, none of the samples of probiotic yoghurt-like fermented milk showed any detectable coliform count, indicating the possibility of prolonging the shelf life. The sample prepared by Lactobacillus desidiosus had the highest sensory ranking (8.10) at day 0 and during storage than other samples. In conclusion, the inclusion of Lactobacillus desidiosus and Lactobacillus fermentum strains in the composition of production starter cultures holds great promise. The utilization of these enriched starter cultures enables the production of healthy food products that maintain their quality throughout the entire storage period.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Probiotics are defined as live microorganisms that, when consumed in sufficient quantities, provide health benefits to the host. These beneficial effects can be attributed to a range of core benefits observed across different probiotic species. These core benefits include the regulation of intestinal transit, the restoration of disrupted microbiota balance, the turnover of intestinal cells, the prevention of pathogen colonization, the enhancement of colonization resistance, and the production of short-chain fatty acids. In addition to these core benefits, specific probiotic strains have been found to offer unique advantages such as neurological, immunological, and endocrinological effects and the production of bioactive compounds [1]. One popular form of delivering probiotics is through fermented milk that has been consumed for centuries [2,3,4].
In the pursuit of developing innovative and effective probiotic fermented milk, researchers have turned their attention to specific strains of lactic acid bacteria, such as Lactobacillus desidiosus and Lactobacillus fermentum [5, 6]. These strains have shown promising characteristics and have been extensively studied for their probiotic properties. Lactobacillus desidiosus, a species of lactic acid bacteria, has been isolated from various sources, including fermented foods and the gastrointestinal tract of animals. It possesses unique traits that make it an attractive candidate for probiotic applications. Studies have demonstrated that Lactobacillus desidiosus exhibits excellent survival rates in the harsh conditions of the digestive tract, allowing it to reach the intestines and exert its beneficial effects [7]. Additionally, it has been shown to have antimicrobial activity against harmful bacteria, which further contributes to its potential as a probiotic strain [8]. Similarly, Lactobacillus fermentum is another strain of lactic acid bacteria that has gained attention for its probiotic properties. It has been isolated from various sources, including fermented foods, human breast milk, and the gastrointestinal tract [9]. Lactobacillus fermentum has been extensively studied for its ability to survive gastric acid and bile salts, enabling its colonization in the gut. It also exhibits anti-inflammatory properties, supports the immune system, and aids in maintaining a healthy balance of gut microbiota [10].
The development of a probiotic food products based on Lactobacillus desidiosus and Lactobacillus fermentum offers several advantages [11]. Firstly, the inherent properties of these strains make them well-suited for survival and colonization in the gut. This ensures that a sufficient number of viable probiotic cells reach the intestines, where they can confer their health benefits. Secondly, the potential antimicrobial activity of these strains can help in inhibiting the growth of pathogenic bacteria in the gut, promoting a healthy gut microbiota. Lastly, the combination of Lactobacillus desidiosus and Lactobacillus fermentum in fermented milk can provide a diverse range of beneficial effects, as each strain has its own unique properties [12].
The main objectives of this study were to produce probiotic yoghurt-like fermented milk product with combination of Lactobacillus desidiosus and Lactobacillus fermentum and to investigate the effect of these combinations on proximate composition (moisture, ash, protein, fat, carbohydrate, titratable acidity content), physicochemical (total soluble solids, pH, syneresis), microbiological, and sensory properties during refrigerated storage.
2 Materials and methods
2.1 Isolation of Lactobacillus species
Lactic acid bacteria for probiotic characteristics were isolated from different commercially manufactured yoghurts and other milk samples. For the present study, twenty-three samples were collected from different regions of Bangladesh. Ten Lactobacillus spp. were isolated from twenty-three milk products. Two isolates (Lactobacillus desidiosus and Lactobacillus fermentum) were selected for the present study to prepare probiotic yoghurt-like fermented milk based on some probiotic confirmation tests (data not provided).
2.2 Development of probiotic yoghurt-like fermented milk
2.2.1 Preparation of lactobacillus spp. mother culture
The frozen stock cultures of Lactobacillus spp. (Lactobacillus desidiosus and Lactobacillus fermentum) were revived by spreading them on sterile De Man Rogosa and Sharpe (MRS) agar (69964, Sigma-Aldrich, Bangalore, India). Following that, the plates were placed in an incubator at 37 °C for a period of 48–72 h. Afterward, a single colony was selected and introduced into 10 mL of MRS broth, which was then incubated at 37 °C for 24 h. This process aimed to obtain samples with a concentration level of approximately 106 colony-forming units per mL (CFU/mL) for further processing [13]. 10 mL of the Lactobacillus spp. (Lactobacillus desidiosus and Lactobacillus fermentum) cultures cultivated in MRS broth were harvested individually subjected to centrifugation at 6000 × g (Avanti J-20 XPI, Beckman Coulter Inc., Fullerton, CA, USA) for 5 min at 4 ºC. The supernatant was then discarded and the pellets were reconstituted in 1 mL of phosphate buffered solution (PBS) (P3813, Sigma-Aldrich, Bangalore, India) and vortexed (58816-123, VWR, CA, USA) properly [13]. This process aimed to obtain bacterial pellets, which were subsequently washed twice with sterile PBS.
2.2.2 Preparation of probiotic yoghurt like-fermented milk
The toned milk (PRO1005, Milk Vita Company Ltd., Dhaka, Bangladesh), following to the prescribed standards (PFA) for toned milk with 3% fat and 8.5% solids-not-fat, was purchased from a local market and milk was tested within 24 h of purchase to minimize any potential quality degradation or microbial growth. The collected liquid milk was then homogenized at 55–65 °C and 15–20 MPa, followed by pasteurization at 85–90 °C for 30 min, and immediate cooling to incubation temperature (40–45 °C) (Fig. 1). Once the pasteurized milk reached a temperature of 42 °C, a total of 20 previously sterilized food grade plastic jars were divided into four categories, each consisting of five jars. These categories were as follows: (i) Sample 1: control; (ii) Sample 2: Lactobacillus desidiosus; (iii) Sample 3: Lactobacillus fermentum; and (iv) Sample 4: mixed culture of Lactobacillus desidiosus and Lactobacillus fermentum (Fig. 2). At first, 100 mL of pasteurized milk was poured into each jar. Each category of jars was inoculated with a different bacterial culture. For the control group, a popular brand of fermented milk called Bonoful was used as the starter culture, with 2 g added to each control jar. Sample 2 and Sample 3 were inoculated with 2% (equivalent to 2 mL) of previously prepared Lactobacillus mother culture of Lactobacillus desidiosus and Lactobacillus fermentum, respectively. Sample 4 was inoculated with 1% (equivalent to 1 mL) of Lactobacillus desidiosus and 1% (equivalent to 1 mL) of Lactobacillus fermentum. Following inoculation, the combination of pasteurized milk and starter culture was vigorously stirred to ensure the even distribution of the fermented milk starter culture throughout the cooled milk. Subsequently, the jars were placed in an incubator and incubated for a period of 18 h at a temperature of 42 °C and approximately pH 4.5 [14]. Once the 18-h incubation period was completed, the yoghurt-like fermented milk were developed. They were then allowed to cool down to room temperature. After cooling, the yoghurt-like fermented milk samples were transferred and stored inside a refrigerator, maintaining a temperature of 4 ± 1 °C.
2.3 Determination of proximate composition
2.3.1 Moisture content
The moisture content was ascertained using the oven method specified by the Association of Official Analytical Chemists (AOAC) [15]. A quantity of 2 g from the sample was subjected to evaporation in a water bath to eliminate any surplus water and subsequently dried in a hot air oven at a temperature of 105 °C for a duration of 3 h. The reduction in weight was measured and documented as the moisture content, in accordance with Eq. 1:
2.3.2 Ash content
The ash content was evaluated using the direct heating technique outlined by AOAC [15]. A quantity of 2 g from the sample was measured and subjected to evaporation in a water bath to eliminate any surplus water, followed by burning to ash in a muffle furnace for a duration of 3 h at a temperature of 550 °C. Subsequently, the sample was cooled in a desiccator, and the weight of the resulting ash was determined using Eq. 2:
2.3.3 Protein content
The crude protein content was assessed using the macro Kjeldahl method outlined in AOAC [15]. A portion of 2 g from the samples was introduced into the digestion flask. In the flask, a mixture of 10 g of copper sulphate and sodium sulphate in a 5:1 ratio, along with 25 mL of concentrated sulphuric acid, was added. The flask was then placed in a digestion block located in a fume cupboard and heated until the frothing ceased, resulting in a clear and light blue coloration. After allowing the mixture to cool, it was diluted with distilled water until reaching a volume of 25 mL in a volumetric flask. Subsequently, 10 mL of the mixture was transferred to the distillation apparatus, and 10 mL of 40% sodium hydroxide was added. The released ammonia was allowed to continue until 10 mL of boric acid reacted with 0.02 M of hydrochloric acid, causing the green color to transition to purple. The nitrogen content in the sample was then determined from Eq. 3. Finally, the percentage of cruden protein in the sample was calculated using Eq. 4.
2.3.4 Fat content
The fat content was determined utilizing the Soxhlet solvent extraction technique outlined in AOAC [16]. In this method, a 2 g sample was carefully weighed and placed into a flat-bottom flask of known weight, with the extractor securely attached. The thimble was positioned at a midpoint within the extractor, and the measured sample was meticulously transferred into the thimble, which was subsequently sealed using cotton wool. The extraction process was carried out throughout 8 h, maintaining a temperature range of 40 to 60 °C. Following this, the solvent was evaporated to eliminate it entirely, and the remaining contents in the flask were subjected to drying in an oven at 80 °C for 30 min. After sufficient cooling within a desiccator, the flask underwent reweighing, facilitating the calculation of the percentage of fat (Eq. 5).
2.3.5 Carbohydrate content
The difference determined the total carbohydrate content. The sum of the percentage moisture, ash, and crude protein was subtracted from 100 as described by Ihokoronye and Ngoddy [17], summarized in Eq. 6:
2.4 Determination of physicochemical properties
2.4.1 Titratable acidity content
The titratable acidity content of different yoghurt-like fermented milk samples was determined by AOAC method 947.05 [18]. A total of 10 g of the sample was measured and combined with 20 mL of boiled and cooled distilled water. Additionally, 1 mL of phenolphthalein indicator, which was prepared at a concentration of 1% in 95% ethyl alcohol, was added to the mixture. The resulting mixture was subjected to titration using standardized 0.1N NaOH solution. The titration process involved adding the NaOH solution gradually to the mixture while observing for the first color change, which indicated the neutralization of the lactic acid. The color change observed was a transition to pink. Once the pink color appeared, it was allowed to persist for 30 s. To ensure complete neutralization of the lactic acid, an additional drop of 0.1N NaOH solution was added to the mixture. The final volume of 0.1N NaOH required to achieve the desired color change and neutralization was carefully noted. The titratable acidity was calculated according to the Eq. 7.
2.4.2 Total soluble solids
The method described by Mazumdar and Majumder [19] was used to determine the total soluble solids using a Digital-Bench Refractometer (model: RX-5000i, Thomas Scientific, New Jersey, USA). The instrument was cleaned and adjusted to zero at a temperature of 25 °C using distilled water before it was used. Using a glass rod, an appropriate quantity of each product sample was placed on the refractometer's prism-plate, with the cover folded back. The instrument was calibrated with distilled water for each sample. The reading shown on the screen was directly noted as the total soluble solids, expressed in terms of Brix.
2.4.3 pH
The measurement of pH was conducted by employing a digital pH meter (model: Jenway 3505, Cadmus, Essex, UK) that underwent calibration using pH 4 and 7 buffers. A volume of 25 mL of the sample was carefully moved into a 50 mL beaker. Subsequently, the pH probe was submerged into the sample, and the beaker was gently swirled until the pH reading stabilized, at which point the value was recorded [13].
2.4.4 Syneresis
Yoghurt-like fermented milk samples syneresis was determined using a bench-top centrifuge (model: Eppendorf 5810R, Eppendorf South Pacific Pty. Ltd, New South Wales, Australia) following the method outlined by Motoki and Seguro [20]. A 20 g sample was placed in a 50 mL glass tube and subjected to centrifugation at 3500 rpm for 15 min at a temperature of 20 °C. The syneresis was quantified by calculating the proportion of whey released relative to the initial weight of the gel.
2.5 Microbiological analysis
To determine the growth of microorganisms, the microbial count was carried out in triplicate using the standard spread plate method as per the IDF standard procedure [21]. The presence of Lactobacillus in the sample was specifically assessed by utilizing MRS agar as the culture medium. For the preparation of serial dilutions, each yoghurt-like fermented milk sample weighing 10 g was carefully measured and placed in sterile stomacher bags under sterile conditions. To obtain the initial dilution, 90 mL of 0.1% sterile peptone water was added to the stomacher bags for 90 s. Following that, 1 mL of each dilution (ranging from 10–3 to 10–8) was transferred onto MRS agar plates using a sterile pipette, and the spread was evenly distributed across the plates using a sterile glass spreader. All plates were incubated at 37 ± 1 °C for 48 h [22]. The yeast and mold counts were determined using the AACC 42–50 method [23], employing the same dilutions for the pour plate technique. The plates were then incubated at a temperature of 25 ± 1 °C for 3–5 days. Enumeration of coliform bacteria was performed on lauryl sulfate tryptone broth agar and incubated at 37 °C for 24–48 h [24]. After incubation, the colonies were counted on plates with between 3 to 300 colonies and the CFU/g was calculated by using following equation:
The results were expressed as a logarithm of colony forming units (Log CFU/g).
2.6 Sensory evaluation
A 9-point hedonic scale rating was utilized to evaluate the sensory characteristics of the different treatment samples. This scale spanned from 'like extremely' with a top score of '9' to 'dislike extremely' with a bottom score of '1' [25]. Intermediate scores were assigned according to the preferences of the panel members. A semi-trained panel consisting of 30 participants, comprising postgraduate students and faculty members from the Food Engineering and Tea Technology department at Shahjalal University of Science and Technology, was formed. They assessed various sensory parameters including color, flavor, taste, texture, and overall acceptability of the products.
2.7 Statistical analysis
The experiments were conducted in triplicate and repeated three times independently, and the collected data were analyzed utilizing GraphPad Prism version 9 (GraphPad Software Inc., San Diego, CA, USA). The outcomes are expressed as mean values accompanied by their corresponding standard deviation. To assess the statistical significance between the groups, a two-way analysis of variance (ANOVA) was performed, followed by Tukey's multiple comparison test. A p-value lower than 0.05 was considered statistically significant at a 95% confidence interval.
3 Results and discussion
3.1 Proximate composition
The proximate composition of change in moisture, protein, ash, carbohydrate, and fat content of yoghurt-like fermented milk samples are presented in Table 1. At 0-day moisture content of samples were 70.80 ± 0.47%, 54.96 ± 0.13%, 53.72 ± 0.60%, and 49.34 ± 0.33% for Sample 1 (Control), Sample 2, Sample 3, and Sample 4, respectively, indicating that Sample 1 (Control) and Sample 2 have the highest moisture contents. After 28 days of storage at refrigerator temperature (4 °C) there was a significant (p < 0.05) increase in moisture content across the samples (Sample 1 (Control): 79.32 ± 0.61%, Sample 2: 70.63 ± 0.44%, Sample 3: 66.22 ± 0.10%, and Sample 4: 68.62 ± 0.28%). These increases in moisture content of the samples are a result of decrease in carbohydrate, protein, fat, and titratable acidity content. This could be attributed to the fact that factors such as the concentration of milk proteins, and the activity of microbial enzymes can influence syneresis [26]. If Lactobacillus desidiosus and Lactobacillus fermentum do not produce sufficient amounts of exopolysaccharides or if other stabilizers are not present, syneresis can occur, leading to an increase in moisture content. In addition, Lactobacillus species, including Lactobacillus desidiosus and Lactobacillus fermentum, continue their metabolic activities even during refrigerated storage. They may consume lactic acid present in the yoghurt-like fermented milk, which can result in a decrease in acidity over time. This reduction in acidity can lead to a weakening of the gel structure and contribute to the release of moisture [27]. It is also possible that during storage, moisture can migrate within the yoghurt-like fermented milk matrix due to differences in water activity and concentration gradients. This movement of water can lead to an increase in moisture content in certain regions of yoghurt-like fermented milk. These results concurred with the finding of Nayla et al.[28], who reported an increase in moisture content of two different yoghurt samples during storage. The study conducted by Sanz et al. [29] demonstrated that the moisture content of yoghurt fell within the range of 80–85%. However, the moisture content of developed yoghurt also depends on composition of milk, addition of sugar, starter culture, etc.
Sample 1 (Control), Sample 2, Sample 3, and Sample 4 had protein contents of 3.59 ± 0.25, 3.71 ± 0.30, 3.80 ± 0.32, and 3.50 ± 0.36 at the start of the experiment (day 0) (Table 1). After 28 days of storage under refrigerated conditions, a significant (p < 0.05) decrease was observed (Sample 1: 3.33 ± 0.30, Sample 2: 3.41 ± 0.45, Sample 3: 3.49 ± 0.55, and Sample 4: 3.28 ± 0.15). Sample 3 exhibited the highest protein content, whereas sample 4 displayed the lowest protein content. Several factors contribute to the phenomenon of decrease in protein content. For instance, the lactic acid bacteria present in yoghurt-like fermented milk, including Lactobacillus desidiosus and Lactobacillus fermentum, continue their metabolic activities during storage. These bacteria produce enzymes that can degrade proteins over time. Enzymes such as proteases can break down proteins into smaller peptides or amino acids, resulting in a decrease in protein content [30]. In the yoghurt-like fermented milk environment, various microbial populations coexist, including lactic acid bacteria and potentially spoilage microorganisms. As the storage period progresses, competition among these microorganisms can occur. Some spoilage microorganisms may utilize proteins as a nutrient source, leading to their breakdown and a subsequent decrease in protein content [31]. Moreover, while lactic acid bacteria generally do not exhibit significant proteolytic activity, certain strains or conditions during storage can promote limited proteolysis. This proteolysis can contribute to a reduction in protein content over time. Sample 1(Control), Sample 2, Sample 3, and Sample 4 had protein content within the acceptable range of 3.2% of protein by weight, as defined by the CII [32] standard.
A significant (p < 0.05) decrease in ash content is observed (Sample 1(Control): 0 day—1.14 ± 0.05%, 28th day—0.31 ± 0.02%; Sample 2: 0 day—1.02 ± 0.08%, 28th day—0.76 ± 0.08%; Sample 3: 0 day—1.59 ± 0.57, 28th day—0.86 ± 0.08%; Sample 4: 0 day—1.06 ± 0.45%, 28th day—0.86 ± 0.07%) indicating significant variability in the percentage of ash contents among the samples stored under refrigerated conditions (Table 1). Ash content in yoghurt-like fermented milk primarily consists of minerals such as calcium, phosphorus, potassium, and magnesium. During storage, these minerals may leach out of the yoghurt-like fermented milk matrix into the surrounding liquid phase. Leaching can occur due to the concentration gradients and physical processes that take place during refrigerated storage, resulting in a decrease in ash content [33]. Also, Lactic acid bacteria, including Lactobacillus desidiosus and Lactobacillus fermentum, continue their metabolic activities during refrigerated storage. These bacteria may produce enzymes that can contribute to the breakdown of organic matter, including minerals [11]. Enzymatic processes may affect the availability or stability of minerals in yoghurt-like fermented milk, resulting in a decrease in ash content over time. In addition, various chemical reactions, such as precipitation or complexation, can occur between minerals and other components present in the yoghurt-like fermented milk during storage. These reactions may alter the solubility or form of minerals, leading to changes in ash content. This result is in agreement with the findings of Amove et al. [34], who also reported significant difference in the ash content of enriched whole soybean flour-based yoghurt (1.40 ± 0.82–0.39 ± 0.05%) stored under refrigerator storage conditions.
After 28 days of storage, there was a significant (p < 0.05) decrease in the fat content across all samples. The fat content of the yoghurt samples ranges from 8.75 ± 0.24–5.62 ± 0.04% in Sample 1 (Control), 11.23 ± 0.07–7.17 ± 0.09% in Sample 2, 8.91 ± 0.03–7.24 ± 0.05% in Sample 3, and 8.94 ± 0.03–6.39 ± 0.11% in Sample 4 after 28 days of storage period under refrigerator conditions (Table 1). Lactic acid bacteria can produce lipase enzymes that can hydrolyze or break down fats. During storage, these lipase enzymes may remain active, leading to the breakdown of fats present in the yoghurt-like fermented milk. This enzymatic activity can result in a gradual decrease in the fat content over time [30]. However, over extended periods of storage, fat globules may undergo creaming, which is the migration of fat to the surface of the yoghurt-like fermented milk. This creaming process can result in visible separation of fat, leading to a decrease in overall fat content when measured [35]. Furthermore, fats in yoghurt-like fermented milk are susceptible to oxidation, which can occur even under refrigerated conditions. Oxidation of fats can lead to the formation of off-flavors and degradation of the fat molecules. As a result, the fat content may decrease due to the breakdown and modification of fat molecules during storage [36]. As per the U.S. Department of Agriculture guidelines from 2001 [37], yoghurt with a fat content below 0.5% should be labeled as non-fat yoghurt, yoghurt with a fat content ranging from 0.5 to 2.0% should be labeled as low-fat yoghurt, and yoghurt with a fat content above 3.25% should be labeled as whole milk yoghurt. Consequently, all the yoghurt samples included in the present study would be classified as whole milk yoghurt.
The initial carbohydrate content of samples 1, 2, 3, and 4 were 11.63 ± 0.29, 29.74 ± 0.026, 33.11 ± 0.19, and 27.65 ± 0.22, respectively. Following a storage period of 28 days under refrigeration conditions, there was a significant (p < 0.05) reduction in the carbohydrate content of the samples (Sample 1(Control): 12.98 ± 0.08, Sample 2: 17.63 ± 0.08, Sample 3: 21.25 ± 0.16, and Sample 4: 20.50 ± 0.07) (Table 1). During fermentation, Lactobacillus desidiosus and Lactobacillus fermentum bacteria convert lactose into lactic acid, which lowers the pH of the yoghurt-like fermented milk and provides its characteristic tangy flavor. As lactose is consumed during fermentation, the overall carbohydrate content of the yoghurt-like fermented milk decreases [38]. In some cases, lactic acid bacteria possess enzymes capable of breaking down complex carbohydrates, such as starches or dietary fibers. During storage, these enzymes may hydrolyze complex carbohydrates into simpler sugars, which can then be utilized by the bacteria or further metabolized. This breakdown of complex carbohydrates can contribute to a decrease in the overall carbohydrate content of yoghurt-like fermented milk [39]. A reduction in carbohydrate content has also been observed in sweet orange (Citrus sinensis) marmalade yoghurt products, which utilize Streptococcus salivarius thermophilus and Lactobacillus delbrueckii bulgaricus as starter cultures [40].
3.2 Physicochemical properties
During the 28-day storage period, the pH value of the samples decreased significantly (p < 0.05). Sample 3 exhibited the highest pH value (5.31 ± 0.10) at the end of the storage period, while Sample 1 (Control) had the lowest pH value (3.49 ± 0.25) compared to Sample 2 (5.01 ± 0.20) and Sample 3 (5.23 ± 0.41) (Table 2). The decrease in pH value observed during the storage period can be attributed to the activity of the starter culture [41]. This activity includes post acidification resulting from the formation of lactic acid as well as bacterial growth during fermentation. The low pH levels can effectively limit microbial activity, leading to an extended shelf-life for the products. However, on the flip side, the low pH can also result in a sour taste, potentially leading to product rejection. Lactobacillus strains possess the capability to ferment lactose, converting it into lactic acid. This fermentation process leads to an increase in acidity and a subsequent decrease in the pH of yoghurt-like fermented milk, ultimately causing the coagulation of the mixture during fermentation. These findings align with the research conducted by Sokolinska et al. [42], which examined the influence of the proportion of yoghurt bacterial strains on milk souring and the development of curd qualitative characteristics. Their study revealed a decrease in pH from 4.34 to 4.11 over a 21-day storage period. The decrease in pH observed in our study aligns with the findings reported by Panesar and Shinde [43] in their investigation of the quality attributes of soy yoghurt during the storage period. Hossain et al. [44] also found a decrease in pH and an increase in acidity in probiotic apple and star fruit juices during chill storage.
The titratable acidity of the control and Samples were increased significantly (p < 0.05) during the storage period (28-day) at 4 ± 1 °C (Sample 1 (Control): 1.39 ± 0.025–1.62 ± 0.01%, Sample 2: 0.55 ± 0.01–0.62 ± 0.01%, Sample 3: 0.48 ± 0.01–0.58 ± 0.01%, and Sample 4: 0.42 ± 0.01–0.52 ± 0.01%) (Table 2). Amadarshanie et al.[45] conducted a study on control natural yoghurt, utilizing the Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus strains. They observed a decrease in pH from 4.92 to 3.58% and a two-fold increase in lactic acid content (to 1.24%) during a 21-day storage period. This change in pH is attributed to the fermentation of lactose by the lactic acid bacteria used in yoghurt production, resulting in the production of lactic acid. While the metabolic activity of the bacteria decreases after incubation due to cooling, their enzymatic activity continues during storage. As a result, changes in acidity are observed even after the initial incubation period [46]. In another study by Arslaner et al. [47], smaller differences were reported in the control yoghurts. Over a three-week storage period, the pH of the control yoghurts decreased by 0.15% (from 4.31 to 4.16%, p ≤ 0.01), while the lactic acid content increased by 0.08% points (from 1.00 to 1.08%, p ≤ 0.01). These findings highlight the ongoing fermentation process and the impact it has on the acidity and lactic acid content of yoghurt-like fermented milk during storage.
Syneresis values for all Samples of probiotic yoghurt-like fermented milk stored at 4 ± 1 °C for 28 days are shown in Table 2. The initial value of syneresis for probiotic yoghurt-like fermented milk was found to be 47.94 ± 0.78% (Sample 1(Control)), 53.67 ± 0.50% (Sample 2), 30.04 ± 0.17% (Sample 3), and 54.27 ± 1.9% (Sample 4), which after storage of 28 days increased significantly (p < 0.05) to 60.08 ± 0.24%, 64.89 ± 0.36, 35.44 ± 1.9%, and 65.52 ± 0.17% for Sample 1(Control), Sample 2, Sample 3, and Sample 4, respectively. This finding is in the agreement with the report of Obi and Akpoka [48] and Panesar and Shinde [43], wherein they stated that the rate of syneresis is directly related to the acidity and therefore inversely related to pH. Moreover, a significant correlation existed between syneresis and the duration of storage. The rise in syneresis values can be attributed to the likely absence of stabilizers, which would have otherwise contributed to the firmness of the yoghurt-like fermented milk samples and minimized whey separation. Additionally, the presence of stabilizers would have enhanced the yoghurt-like fermented milk samples' ability to retain water. The increase in syneresis may also be influenced by the denaturation of beta-lactoglobulin in the processed milk and the natural aging of the curd during storage [49].
The changes in the total soluble solids of the probiotic yoghurt-like fermented milk samples are depicted in Table 2. Over the storage period, a significant (p < 0.05) increase in total soluble solids was observed for all samples. Sample 1(Control), Sample 2, Sample 3, and Sample 4 exhibited initial total soluble solid values of 24.66 ± 0.57%, 32.00 ± 1.00%, 33.66 ± 0.57%, and 30.66 ± 0.57%, respectively. By the end of the storage period, the total soluble solid values were 31.00 ± 1.00%, 35.00 ± 1.00%, 41.66 ± 0.57%, and 33.66 ± 0.57% for Sample 1(Control), Sample 2, Sample 3, and Sample 4, respectively. This increase in total soluble solids can be attributed to the metabolic activities of lactic acid bacteria, which continue to carry out processes such as the breakdown of complex carbohydrates and the conversion of sugars into organic acids. As these carbohydrates are metabolized, the concentration of soluble solids in the yoghurt-like fermented milk increases, leading to an overall increase in total soluble solids content [50]. A similar trend of increasing total soluble solids over the storage period was observed by Adeola et al. [51]. In their study on baobab-tiger nut, baobab-bambara, and baobab-coconut yoghurts, the researchers found that the control samples exhibited total soluble solid values ranging from 23.34 to 25.89°Brix over the storage period of day 1 to day 15. The other experimental yoghurt-like fermented milk samples showed total soluble solid values ranging from 20.20 to 25.12, 21.27 to 24.34, and 21.26 to 24.88°Brix, respectively, for the same storage period.
3.3 Microbiological characteristics
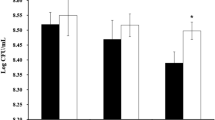
The initial Lactobacillus count in all samples at the start of storage ranged from 8.05 ± 0.02 (Sample 4) to 8.17 ± 0.02 Log CFU/g (Sample 2). However, by the end of storage, the count decreased to 6.01 ± 0.01 (Sample 4) to 6.14 ± 0.01 Log CFU/g (Sample 2) (Table 3). After the storage period, the prepared probiotic yoghurt-like fermented milk products continued to meet the criteria required to be classified as probiotic food. The combined count of Lactobacillus in all samples fulfilled the recommended minimum requirement of 6.0 Log CFU/g for probiotic products [52]. As mentioned by Salvucci et al. [53], an increase in storage time leads to a higher production of lactic acid. This increase in lactic acid production eventually results in a nutrient deficiency for lactic acid bacteria, leading to a phase of decline in their population known as the death phase. Based on Sah et al. [54], bacterial activity is hindered by the increasing acidity that develops. As a result, bacterial activity decreases due to the inhibitory effects of acidity. Furthermore, as the growth of bacteria accelerates, there is an increased utilization of sugars for both their growth and the production of lactic acid. Consequently, the sugar content progressively decreases. This decline in sugar content serves as a crucial substrate for bacterial growth, gradually depleting the available substrate. During the mortality phase, the rate of deceased probiotic bacteria continues to rise, while the rate of cell division becomes zero. The amount of Lactobacillus bacteria was nearly the same as in the study by Saccaro et al. [55] and Aini et al. [56], which stated that storage of yoghurt up to 28 days can maintain Lactobacillus bacteria of 4.72 to 7.1 Log CFU/g and 6.50–7.57 Log CFU/g, respectively.
In this study, yoghurt-like fermented milk samples appear to be the treatment that can resist spoilage with yeasts and molds (Table 3). According to Egyptian Organization for Standardization & Quality (EOSQ) [57], mold and yeasts count must not exceed 10 CFU/g in yoghurt. Sample 1(Control), Sample 2, Sample 3, and Sample 4 were within a permissible limit until 28 days of storage with a mean count of 1.44 ± 0.04, 1.47 ± 0.03, 1.29 ± 0.03, and 1.19 ± 0.01 Log CFU/g, respectively. The presence of yeast and mold on Day 0 in pasteurized probiotic yogurt-like fermented milk can be attributed to several factors. For instance, yeast and mold spores are particularly resistant to heat and can survive pasteurization temperatures that would kill most bacteria. If the thermal treatment is not uniformly applied or if the holding time is insufficient to reach the thermal death point of these organisms, they may survive. After pasteurization, the cooling and subsequent handling stages are critical points where contaminants can be introduced. Even with stringent sanitary practices, it's challenging to completely eliminate these microorganisms [2,3,4]. Interestingly, the increase in yeast and mold growth was observed in all probiotic yoghurt-like fermented milk samples during the 7th and 14th days of storage at refrigerator temperature (4 ± 1 °C), which can be attributed to several factors. For instance, firstly, during the initial stages of storage, the yoghurt-like fermented milk environment may still contain residual nutrients and moisture that can promote the growth of yeast and mold. This can be due to factors such as incomplete sterilization or the presence of naturally occurring microorganisms that are more tolerant to refrigeration conditions [58]. Secondly, the presence of lactic acid bacteria in the probiotic yoghurt-like fermented milk can contribute to the increase in yeast and mold growth. While lactic acid bacteria are beneficial probiotics, they can produce byproducts such as lactic acid and other organic acids during fermentation. These acids create an acidic environment that may inhibit the growth of undesirable microorganisms. However, as the storage period progresses, the acidity of the yoghurt-like fermented milk may decrease, providing a more favorable environment for yeast and mold growth [39]. Lastly, the decrease in yeast and mold growth observed after the 14th day and gradually up to 28 days of storage can be attributed to the depletion of available nutrients. As the yoghurt-like fermented milk ages, the nutrient content decreases as the lactic acid bacteria utilize the available sugars and other nutrients for their growth and acid production. This depletion of nutrients limits the resources available for yeast and mold growth, leading to a gradual decline in their population [50]. These findings are consistent with the results obtained by Abee et al. [59] and Hussien et al. [60].
In contrast, none of the samples of probiotic yoghurt-like fermented milk stored at refrigerated temperature conditions (4 ± 1 °C) for up to 28 days showed any detectable coliform count, which serves as an indicator of fecal contamination. Lactic acid bacteria possess antagonistic properties against other microorganisms, including coliform bacteria. They produce lactic acid and other metabolites that create an acidic environment, inhibiting the growth of coliform bacteria. This acidic condition, combined with the competition for nutrients and space, suppresses the proliferation of coliform bacteria in yoghurt-like fermented milk [61]. Furthermore, refrigeration at 4 ± 1 °C is an effective method for inhibiting the growth and survival of coliform bacteria. The low temperature slows down their metabolic activities, reproduction, and overall bacterial growth. Coliform bacteria are mesophilic organisms that thrive at higher temperatures, and refrigeration inhibits their growth and reduces their viability [62]. According to Aluko et al. [63], the presence of coliform bacteria, Escherichia coli, and Salmonella spp. was not detected in both the control and baobab pulp enriched probiotic yoghurts throughout the 28-day storage period under refrigerated temperature conditions (4 ± 1 °C).
3.4 Sensory properties
The results of sensory evaluation have been presented in Fig. 3. All the samples were evaluated for color, flavor, texture, taste, and overall acceptability during refrigerated storage from day 0 to day 28 at 7 days interval. Overall, sample 2 and 4 had the maximum sensory score at day 0 for almost all the parameters. The sensory characteristics were reduced slightly with the increasing storage days for sample 2, 3, and 4. However, the organoleptic rating scores were reduced drastically for control samples with increasing storage time. The sensory ratings for probiotic yoghurt-like fermented milk Samples (Sample 2, 3, and 4) were within acceptable limits until 21 days of refrigerated storage. However, the control sample becomes unacceptable after one week of storage at 4 ºC. The initial overall acceptability ratings of Sample 2, 3, and 4 were 8.1, 7.7, and 7.8, respectively, whereas for control sample it was 7.1. The overall acceptability of sample 1 reduced to 3.80 during days 14 although it was 7.1, 6.4, and 6.4 for sample 2, 3, and 4 after days 21. Hence, probiotic yoghurt made by Lactobacillus desidiosus exhibited the best sensory score than other samples. Lactobacillus fermentum and the co-culture of Lactobacillus fermentum and Lactobacillus desidiosus also gave satisfactory results, meaning that these bacteria strains could be used together to develop probiotic yoghurt-like fermented milk. These results signify that the yoghurt-like fermented milk developed by Lactobacillus desidiosus and Lactobacillus fermentum had better acceptability and shelf-life than the control. The poor sensory score of control sample after 7 days may be related to presence of yeast and mold in control sample which damage the yoghurt-like fermented milk quality. In probiotic samples, there was a lower presence of yeast and mold. In addition, the presence of Lactobacillus desidiosus and Lactobacillus fermentum may hinder the growth of yeast and molds, thereby the organoleptic quality remained good for a long time. These results were in line with the results of the previous research by Lestari et al. [64] who found that yoghurt prepared by Lactobacillus acidophilus and Bifidobacterium animalis gave better hedonic scores than control sample. Dias et al. [65] also found similar organoleptic test for probiotic yoghurts prepared by Streptococcus thermophilus, Bifidobacterium bifidum, and Lactobacillus delbrueckii.
4 Conclusion
After thorough evaluation of the findings, it is evident that varying combinations of lactic acid bacteria have a substantial impact on the nutritional, rheological, microbiological and sensory characteristics of yoghurt-like fermented milk during a refrigerated storage period of 28 days. During a 28-day storage period at 4 ± 1 °C, the presence of either Lactobacillus desidiosus or Lactobacillus fermentum, as well as their combined presence, resulted in yoghurt-like fermented milk with elevated pH levels, reduced moisture content, decreased titratable acidity, increased ash, protein, fat content, carbohydrates, syneresis, and total soluble solids. Additionally, it was noted that probiotic yoghurt-like fermented displays more pronounced alterations in its rheological, microbiological, and sensory attributes. The combination of strains has a greater impact on the nutritional, rheological, and sensory properties of the yoghurt-like fermented milk compared to control. The utilization of a combination of multiple strains and multiple species in probiotics led to enhanced texture and improved probiotic potential, with the combination of multiple species proving to be particularly effective. Consequently, when choosing between mono-strain or multi-strain probiotics, the decision should be based on the desired nutritional, rheological, microbiological, and sensory properties of the final product. The findings of this study underscore the significant potential of incorporating Lactobacillus desidiosus and Lactobacillus fermentum strains into yoghurt-like fermented milk production to enhance nutritional, sensory, and health-promoting attributes. Looking ahead, these results pave the way for further research into the optimization of fermentation processes and formulation strategies to maximize the probiotic and health benefits of yoghurt and other fermented dairy products. Future studies may also explore the interaction effects between different probiotic strains in mixed-culture fermentations to understand their synergistic or antagonistic impacts on the product's quality and shelf-life.
Data availability
Data relevant to this study can be provided upon request.
References
Sánchez B, Delgado S, Blanco-Miguez A, Lourenço A, Gueimonde M, Margolles A. Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res. 2017;61:1600240.
Zahran H, Mabrouk AMM, Salama HH. Evaluation of yoghurt fortified with encapsulated echium oil rich in stearidonic acid as a low-fat dairy food. Egypt J Chem. 2022;65:29–41.
El Sayed HS, Mabrouk AM. Encapsulation of probiotics using mixed sodium alginate and rice flour to enhance their survivability in simulated gastric conditions and in UF-Kariesh Cheese. Biocatal Agric Biotechnol. 2023;50: 102738.
Hamdy SM, Abdelmontaleb HS, Mabrouk AM, Abbas KA. Physicochemical, viability, microstructure, and sensory properties of whole and skimmed buffalo set-yogurts containing different levels of polydextrose during refrigerated storage. J Food Process Preserv. 2021;45: e15643.
Lim S-M, Lee N-K, Kim K-T, Paik H-D. Probiotic Lactobacillus Fermentum KU200060 isolated from watery kimchi and its application in probiotic yogurt for oral health. Microb Pathog. 2020;147: 104430.
Lutfiah NA. Isolasi Dan Identifikasi Bakteri Asam Laktat Pada Susu Kambing Saanen (Capra Aegagrus H.), Universitas Islam Negeri Maulana Malik Ibrahim, 2015; 2:1–59.
Davis CR. Ecology, properties and growth of lactic acid bacteria in Australian wines, UNSW Sydney, 1985.
Peynaud E, Sapis-Domercq S. A study of two hundred and fifty strains of lactic acid bacteria. Arch Mikrobiol. 1970;70:348–60.
Asan-Ozusaglam M, Gunyakti A. Lactobacillus Fermentum strains from human breast milk with probiotic properties and cholesterol-lowering effects. Food Sci Biotechnol. 2019;28:501–9.
Naghmouchi K, Belguesmia Y, Bendali F, Spano G, Seal BS, Drider D. Lactobacillus fermentum: a bacterial species with potential for food preservation and biomedical applications. Crit Rev Food Sci Nutr. 2020;60:3387–99.
Hossain MA, Hoque MM, Kabir MH, Yasin M. Probiotification of mango juice by lactic acid bacteria and quality assessment at refrigerated storage condition. J Eng Res Innov Educ. 2019;1:1–10.
Hossain MA, Hoque MM, Hossain MM, Kabir MH, Yasin M, Islam MA. Biochemical, microbiological and organoleptic properties of probiotic pineapple juice developed by lactic acid bacteria. J Sci Res. 2020;12:743–50.
Soni R, Jain NK, Shah V, Soni J, Suthar D, Gohel P. Development of probiotic yogurt: effect of strain combination on nutritional, rheological, organoleptic and probiotic properties. J Food Sci Technol. 2020;57:2038–50.
Krisnaningsih AT, Radiati LE, Evanuarini H, Rosyidi D. The effect of incubation time to the physicochemical and microbial properties of yoghurt with Local Taro (Colocasia Esculenta (L.) Schott) starch as stabilizer. Curr Res Nutr Food Sci. 2019;7:547–54.
Official method of Analysis. 18th Edition, Association of officiating analytical chemists, Washington, DC. 2005, 4–5.
Official methods of analysis of association of official analytical chemists. 19th Edition, Washington, DC. 2012.
Ihekoronye AI, Ngoddy PO. Integrated food science and technology for the tropics, tropical fruits and vegetables. London and Oxford: Macmillan Education Ltd.; 1985.
Official Methods of Analysis. 17th Edition, The association of official analytical chemists, Gaithersburg, MD, USA. 2000.
Mazumdar BC, Majumder K. Methods on physico-chemical analysis of fruits, vol. 2003. Delhi: Daya Publishing House; 2003.
Motoki M, Seguro K. Transglutaminase and its use for food processing. Trends Food Sci Technol. 1998;9:204–10.
Sarif SNM, Tang JYH, Abd GA. Physicochemical properties and microbial count of bacterial survival in freeze dried goat milk yogurt powder. J Agrobiotechnol. 2022;13:74–84.
Shori AB, Albalawi A, Al Zahrani AJ, Al-sulbi OS, Baba AS. Microbial analysis, antioxidant activity, and sensory properties of yoghurt with different starter cultures during storage. Int Dairy J. 2022;126: 105267.
Seibel W. Approved methods of the American Association of cereal chemists, 8th Edition (Standardmethoden Der Amerikanischen Gesellschaft Für Getreidechemiker, 8. Ausgabe). Approved Methods Committee American Association of Cereal Chemists, Inc. St. Paul/Minnesota, 1989; Vol. 41.
Vanderzant C, Splittstoesser DF. Compendium of methods for the microbiological examination of foods, Washington D. DC Am. Public Heal Assoc. 1992;3:423–431.
Ahmed T, Sabuz AA, Mohaldar A, Fardows HMS, Inbaraj BS, Sharma M, Rana MR, Sridhar K. Development of novel whey-mango based mixed beverage: effect of storage on physicochemical, microbiological, and sensory analysis. Foods. 2023;12:237.
Gauche C, Tomazi T, Barreto PLM, Ogliari PJ, Bordignon-Luiz MT. Physical properties of yoghurt manufactured with milk whey and transglutaminase. LWT-Food Sci Technol. 2009;42:239–43.
Ale EC, Perezlindo MJ, Burns P, Tabacman E, Reinheimer JA, Binetti AG. Exopolysaccharide from Lactobacillus Fermentum Lf2 and its functional characterization as a yogurt additive. J Dairy Res. 2016;83:487–92.
Andleeb N, Gilani AH, Abbas N. Assessment of the quality of conventional yogurt as affected by storage. Pak J Agric Sci. 2008;45:218–22.
Sanz T, Salvador A, Jimenez A, Fiszman SM. Yogurt enrichment with functional asparagus fibre. Effect of fibre extraction method on rheological properties, colour, and sensory acceptance. Eur Food Res Technol. 2008;227:1515–21.
Garcia-Cano I, Rocha-Mendoza D, Ortega-Anaya J, Wang K, Kosmerl E, Jiménez-Flores R. Lactic acid bacteria isolated from dairy products as potential producers of lipolytic, proteolytic and antibacterial proteins. Appl Microbiol Biotechnol. 2019;103:5243–57.
Gao Z, Daliri EB-M, Wang JUN, Liu D, Chen S, Ye X, Ding T. Inhibitory effect of lactic acid bacteria on foodborne pathogens: a review. J Food Prot. 2019;82:441–53.
CII The prevention of food adulteration act and rules. 2004;6:1–216.
Kirdar SS, Toprak G, Güzel E. Determination of the mineral content in yogurt whey. Eur Int J Sci Technol. 2017;6:26–34.
Amove J, Af O, Pi A. Effect of yoghurt-milk enrichment with whole soy bean flour. J Nutr Heal Food Eng. 2019;9:97–103.
Wiking L, Gregersen SB, Hansen SF, Hammershøj M. Heat-induced changes in milk fat and milk fat globules and its derived effects on acid dairy gelation—a review. Int Dairy J. 2022;127: 105213.
Khalil OSF, Ismail HA, Elkot WF. Physicochemical, functional and sensory properties of probiotic yoghurt flavored with white sapote fruit (Casimiroa Edulis). J Food Sci Technol. 2022;59:3700–10.
Producers F. Recommendations of the summit. Am J Health Pharm. 2014;71:1390–1.
Hoxha R, Evstatieva Y, Nikolova D. Physicochemical, rheological, and sensory characteristics of yogurt fermented by lactic acid bacteria with probiotic potential and bioprotective properties. Foods. 2023;12:2552.
Wang Y, Wu J, Lv M, Shao Z, Hungwe M, Wang J, Bai X, Xie J, Wang Y, Geng W. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front Bioeng Biotechnol. 2021;9: 612285.
Al-Bedrani DI, ALKaisy QH, Mohammed ZM. Physicochemical, rheological and sensory properties of yogurt flavored with sweet orange (Citrus Sinensis) Marmalade. In Proceedings of the IOP Conference Series: Earth and Environmental Science; 2019; Vol. 388, p. 12052.
Donkor ON, Henriksson A, Vasiljevic T, Shah NP. Probiotic strains as starter cultures improve angiotensin-converting enzyme inhibitory activity in soy yogurt. J Food Sci. 2005;70:375–81.
Cais-Sokolinska D, Michalski MM, Pikul J. Role of the proportion of yoghurt bacterial strains in milk souring and the formation of curd qualitative characteristics. Bull Vet Inst Puławy. 2004; 48: 437–441.
Panesar PS, Shinde C. Effect of storage on syneresis, PH, Lactobacillus Acidophilus Count, Bifidobacterium Bifidum count of aloe vera fortified probiotic yoghurt. Curr Res Dairy Sci. 2012;4:17–23.
Hossain MA, Das R, Yasin M, Kabir H, Ahmed T. Potentials of two Lactobacilli in probiotic fruit juice development and evaluation of their biochemical and organoleptic stability during refrigerated storage. Sci Study Res Chem Chem Eng Biotechnol Food Ind. 2022;23:131–40.
Amadarshanie DBT, Gunathilaka TL, Silva RM, Navaratne SB, Peiris LDC. Functional and antiglycation properties of cow milk set yogurt enriched with Nyctanthes Arbor-Tristis L. flower extract. LWT. 2022;154: 112910.
Shima AR, Salina HF, Masniza M, Atiqah AH. Viability of lactic acid bacteria in home made yogurt containing sago starch oligosaccharides. Int J Basic Appl Sci. 2012;12:58–62.
Arslaner A, Salik MA, Bakirci I. The effects of adding Hibiscus Sabdariffa L. flowers marmalade on some quality properties, mineral content and antioxidant activities of yogurt. J Food Sci Technol. 2021;58:223–33.
Akpoka OA, Obi TE. Viability of Lactobacillus Acidophilus and syneresis of probiotic yoghurt produced from reconstituted skim and whole milk powder during 35 days refrigerated storage at 4±2 OC. Bact Emp. 2019;2:54.
Gyawali R, Ibrahim SA. Effects of hydrocolloids and processing conditions on acid whey production with reference to Greek yogurt. Trends Food Sci Technol. 2016;56:61–76.
Shi C, Maktabdar M. Lactic acid bacteria as biopreservation against spoilage molds in dairy products—a review. Front Microbiol. 2022;12: 819684.
Adeola AO. Changes in the physicochemical, microbiological and sensory characteristics of plain yoghurt from Tigernut-Bambara-coconut milks supplemented with Baobab (Digitata Adansonia) fruit pulp emulsion during cold storage. J Food Technol Pres. 2023;7:172.
Rossi E, Restuhadi F, Efendi R, Dewi YK. Physicochemical and microbiological properties of yogurt made with microencapsulation probiotic starter during cold storage. Biodivers J Biol Divers. 2021. https://doi.org/10.13057/biodiv/d220450.
Salvucci E, LeBlanc JG, Pérez G. Technological properties of lactic acid bacteria isolated from raw cereal material. LWT. 2016;70:185–91.
Sah BNP, Vasiljevic T, McKechnie S, Donkor ON. Antibacterial and antiproliferative peptides in synbiotic yogurt—release and stability during refrigerated storage. J Dairy Sci. 2016;99:4233–42.
Saccaro DM, Tamime AY, Pilleggi ALOPS, Oliveira MN. The viability of three probiotic organisms grown with yoghurt starter cultures during storage for 21 days at 4 C. Int J Dairy Technol. 2009;62:397–404.
Aini N, Sustriawan B, Muthmainah MMR, Prihananto V, Wijonarko G. Estimation of the shelf-life of corn yoghurt packaged in polyethene terephthalate using the accelerated shelf-life method. Int J Adv Sci Eng Inf Technol. 2021;11:298.
Sultan A. Managing quality in Egypt A Directory of Services for SMEs In Collaboration With. 2017.
Viljoen BC, Lourens-Hattingh A, Ikalafeng B, Peter G. Temperature abuse initiating yeast growth in yoghurt. Food Res Int. 2003;36:193–7.
Abee T, Krockel L, Hill C. Bacteriocins: modes of action and potentials in food preservation and control of food poisoning. Int J Food Microbiol. 1995;28:169–85.
Hussien H, Abd-Rabou HS, Saad MA. The impact of incorporating Lactobacillus Acidophilus Bacteriocin with Inulin and FOS on yogurt quality. Sci Rep. 2022;12:13401.
Fidan H, Esatbeyoglu T, Simat V, Trif M, Tabanelli G, Kostka T, Montanari C, Ibrahim SA, Özogul F. Recent developments of lactic acid bacteria and their metabolites on foodborne pathogens and spoilage bacteria: facts and gaps. Food Biosci. 2022;47: 101741.
Jeantet R, Croguennec T, Schuck P, Brulé G. Handbook of food science and technology 1: food alteration and food quality. New Jersey: Wiley; 2016.
Aluko A, Kinyuru J, Chove L, Owino W, et al. Physico-chemical, microbiological and sensory qualities of probotic yoghurt enriched with baobab pulp. In Proceedings of the Fifth African Higher Education Week and RUFORUM Biennial Conference 2016," Linking agricultural universities with civil society, the private sector, governments and other stakeholders in support of agricultural development in Africa", Cape Town, South Africa; 2016; pp. 1027–1035.
Lestari LA, Nuriannisa F, Yuliani K, Ratnasari D, Farida IN, Azizah EF. Sensory and microbiological evaluation of probiotic yoghurt made with different types of probiotic cultures starter Lactobacillus Acidophilus LA-5 and Bifidobacterium Animalis Subsp. Lactis BB-12. Food Res. 2022;6:64–9.
Dias PGI, Sajiwani JWA, Rathnayaka R. Consumer perception and sensory profile of probiotic yogurt with added sugar and reduced milk fat. Heliyon. 2020. https://doi.org/10.1016/j.heliyon.2020.e04328.
Funding
This research was funded by Ministry of Science and Technology, Bangladesh. Project code: 39.012.002.02.01.018.2015/R&D-12/46.
Author information
Authors and Affiliations
Contributions
M. A. H.: Funding acquisition, Conceptualization, Methodology, Formal analysis, Writing—Original Draft; M. M. H.: Conceptualization, Methodology, Writing—Review and Editing, Supervision; M. M. A.: Conceptualization, Methodology, Writing—Review and Editing, Supervision, Funding acquisition; T.A.: Methodology, Writing—Original Draft, Review and Editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethical Committee of the Shahjalal University of Science and Technology (SUST), Sylhet 3114, Bangladesh, after the proposal was presented to the University Research Ethics Board. The present study did not involve any animal handling. The SUST Research Ethics Board (SREB) was informed before the milk samples were used for this study and confirmed approval was not required. Prior to participating in the sensory evaluation of yoghurt-like fermented milk, all panelists were provided with comprehensive information detailing the purpose, procedures, and potential implications of the study. Panelists were assured that their involvement was voluntary. Therefore, verbal informed consent was obtained from each sensory panelist before the sensory experiments were carried out as involving human voluntary participation. Moreover, our institute suggested that all ingredients used in the yoghurt-like fermented milk do not pose any health risk and have clean GRAS (Generally Recognized As Safe) status. Therefore, as per the guidelines of the institute, no approval of the ethical committee is required for sensory evaluation.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hossain, M.A., Hoque, M.M., Ahmed, M.M. et al. Probiotic yoghurt-like fermented milk product enriched with Lactobacillus desidiosus and Lactobacillus fermentum: proximate composition, physicochemical, microbiological, and sensory evaluation during refrigerated storage. Discov Food 4, 24 (2024). https://doi.org/10.1007/s44187-024-00093-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44187-024-00093-9