Abstract
The potential of red sugarcane as a functional probiotic drink was investigated, with a focus on determining its physicochemical, ergogenic, and antioxidant activities. Three different variants of Malaysian red sugarcane, namely Ragnar, Kapur, and Serai, were selected for analysis. The concentrations of electrolytes (Na, K, Ca, Mg, Zn, and Fe) in sugarcane juice were determined using an inductively coupled plasma mass spectrophotometer (ICP-MS), while the antioxidant activity and Vitamin C were assessed through colorimetric assays. Additionally, the functionality of the optimal variant, Ragnar, was enhanced by inoculating it with lactic acid bacteria (LAB) Lactobacillus plantarum ATCC8014 and Lactobacillus casei Shirota. High antioxidative properties (TPC: 71.63 mg GAE/mL, FRAP: 2.76 mmol TE/mL, DPPH EC50: 55.66 µg/mL, and Vitamin C: 0.72 mg/100 g) were observed in the Ragnar, which tends to exhibit an attractive blue-yellow hue. K exhibited the highest concentration in all samples (126.31 – 229.95 mg/mL), followed by Na, Mg, Fe, and Zn, which exceeded the commercial isotonic drink. The viability of LAB (above 107 CFU/mL) and the production of gamma-aminobutyric acid (GABA) were satisfactory while reducing sugars were generally lower after the fermentation. The findings present red sugarcane as a potential natural source for the development of functional drinks.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sugarcane, belonging to the genus Saccharum and the Gramineae family, is an economically significant crop. In Malaysia, it has been cultivated since the nineteenth century primarily for sugar production. Certain sugarcane variants are known for their juice which possesses exceptional sensory characteristics, including taste, aroma, and flavor. The local identification of sugarcane is based on the color of the rind [1], with yellow being the most common type for juice production, followed by red or black sugarcane [2].
Consumption of sugarcane juice is often regarded as an instant energy booster due to its high content of simple sugars such as glucose, fructose, and sucrose, which are readily absorbed by the body [3]. Scientific studies have shown that sugarcane extract exhibits a wide range of biological effects, including immunostimulation, anti-thrombosis activity, anti-inflammatory activity, vaccine adjuvant properties, modulation of acetylcholine release, and anti-stress effects [4]. Moreover, sugarcane juice, being a natural plant-based source, contains significant antioxidant activities and compounds derived from phenolics and flavonoids [5, 6]. These antioxidants play a crucial role in neutralizing free radicals generated during physical activities, thereby reducing oxidative stress and protecting the cells [7, 8].
Furthermore, the presence of electrolytes in sugarcane juice contributes to its ergogenic properties, which aid in restoring and improving metabolic activity after strenuous catabolic processes [9, 10]. Well-known ergogenic aids such as calcium, sodium, and potassium are effective in replenishing lost metabolites in the body [10]. While artificial liquid energy drinks formulated with these metabolites are commonly used, they often lack the additional benefits derived from plant-derived biological compounds, such as phenolic compounds.
An interesting approach to enhance the nutritional quality of sugarcane juice is the addition of probiotics, which can improve substrate composition compositions [11, 12] and contribute to a positive impact on human health via live bacteria [13, 14]. Additionally, certain LAB and fermented foods can lead to the production of gamma-aminobutyric acid (GABA), as demonstrated by the use of Lb. plantarum in a whey protein drink [15]. GABA is present in specific plant-based foods, such as sugarcane, and has demonstrated various exercise-related benefits [6]. These include promoting exercise-induced muscle hypertrophy, regulating cardiac arrhythmias, controlling blood pressure and lipids, and enhancing liver function [16]. Together with the ergogenic- and health-inducing properties of sugarcane juice, inoculating red sugarcane juice (RSJ) with probiotics is sensible as a functional drink to replace conventional probiotic drinks for both regular and actively exercising individuals.
Therefore, this study aims to assess the qualities of different variants of RSJ, exploring their potential as a natural functional drink. The physicochemical screening was conducted to determine the sugars and antioxidant content, while mineral content was measured using inductively coupled plasma mass spectrometry (ICP-MS). Lastly, two strains of Generally Recognised as Safe (GRAS) Lactic Acid Bacteria (LAB), namely Lb. casei subs Shirota and Lb. plantarum ATCC8014 was introduced to evaluate their impact on the RSJ. The assessment included measurements of LAB viability, reduction in sugar content, and the production of the bioactive neurotransmitter, GABA.
2 Materials and methods
2.1 Sample collection
The samples of red sugarcane juice (RSJ) with varieties Ragnar, Kapur, and Serai were obtained from a sugarcane farm in Kedah, Malaysia. The collection was performed in accordance with local and national guidelines. They were freshly harvested using a three-roller power crusher and filtered using a 6-layered muslin cloth. In this study, the different varieties were designated based on the species names. The subsequent transformation into a probiotic beverage involved using an established probiotic drink (Yakult) as the positive control, while non-inoculated Ragnar RSJ served as the negative control. The introduction of probiotics into Ragnar RSJ, with Lb. plantarum is referred to as LP, and with Lb. casei Shirota as YSJ.
2.2 Quality assessment of sugarcane juice
Various measurements were conducted on the RSJ, including Total Soluble Solids (TSS) using a refractometer, pH using a pH meter, and color analysis using a colorimeter (HunterLab, USA). Triplicate values were recorded and expressed in standard units (°Brix for total soluble solids and CIELAB color space for color analysis).
The reducing sugar content of the RSJ samples was determined using the dinitrosalicylic colorimetric (DNS) method. The DNS reagent was prepared by dissolving 1.5 g of DNS in 30 mL of 2M NaOH. A solution of sodium potassium tartrate (Rochelle salt) was prepared by dissolving 45 g in 75 mL of water. A standard glucose solution of 1 mg/mL was prepared. Samples were prepared by pipetting a standard sugar solution into test tubes, which were then filled to 3 mL with distilled water. Each sample was mixed with 1 mL of DNS reagent and incubated in a boiling water bath for 15 min. After cooling, 1 mL of a 40% Rochelle salt solution was added to stabilize the color. The absorbance was measured at 540 nm with a spectrophotometer, with all tubes cooled to room temperature before reading due to the temperature sensitivity of the absorbance.
2.3 High-performance liquid chromatography (HPLC)
The determination of gamma-aminobutyric acid (GABA) content was conducted using HPLC (Shimadzu LC 20AT, Shimadzu Corporation, Kyoto, Japan). The analysis utilized a Chromolith® RP-18 column (100 mm length × 4.6 mm internal diameter) provided by Merck KGaA (Darmstadt, Germany). In a nutshell, 1 g of RSJ underwent centrifugation, and 10 µL of the resulting supernatant was collected for vacuum evaporation (derivatization). The dried supernatant was reconstituted in a solution of ethanol, water, trimethylamine, and phenylisothiocyanate, followed by immediate vacuum evaporation. Subsequently, another solution comprising ethanol, water, triethylamine, and phenylisothiocyanate was added, and the sample was left for 20 min to facilitate phenylisothiocyanate-GABA formation. After vacuuming to remove excess solvent, the sample was diluted and subjected to HPLC analysis. Mobile phase A (pH 5.8) contained sodium acetate, trimethylamine, and acetic acid in deionized water, while mobile phase B consisted of acetonitrile and deionized water. Both mobile phases underwent filtration. The sample, injected at 5 µL, was eluted at a flow rate of 0.6 mL/min using an isocratic elution of 80% mobile phase A + 20% mobile phase B. Compound identification was performed at λ = 254 nm using a diode array detector, and GABA content was calculated by comparing the sample peak area with the GABA standard [17,18,19].
2.4 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity assay
DPPH free radical scavenging activity was assessed by adding varying concentrations of RSJ to a DPPH solution. Fresh DPPH reagent (3.94 mg in 100 mL methanol) was shielded from light. RSJ samples (6.25–100 µg/mL) were added to a 96-well microplate with DPPH reagent, incubated, and absorbance measured at 517 nm after 30 min. Results were obtained using a BIO-RAD 170–6930 Microplate Spectrophotometer. Controls included a negative control with distilled water and a sample blank. Radical scavenging activity was calculated as the percentage of inhibition, and the EC50 value was determined. All analyses were conducted in triplicate for accuracy and reproducibility. The EC50 value, representing the concentration required for 50% scavenging activity, was determined based on Eq. 1 [20].
2.5 Ferric ion reducing antioxidant power (FRAP) assay
The FRAP assay was conducted by adding diluted samples to a working FRAP reagent and incubating the mixture. In short, a fresh FRAP reagent was prepared by combining 300 mM acetate buffer (pH 3.6), a 10 mM solution of 4,6-tripyridyl-s-triazine (TPTZ) in 40 mM HCl, and a 20 mM ferric chloride solution in a ratio of 10:1:1 (v:v:v). Each sample underwent a ten-fold dilution, with 20 µL added to a well in a 96-well microplate, followed by the addition of 180 µL of the prepared FRAP reagent. A reagent blank was established by mixing 20 µL of distilled water with 180 µL of the FRAP reagent. The absorbance of the reaction mixture was measured at 593 nm using the BIO-RAD 170–6930 Benchmark Plus Microplate Spectrophotometer after incubating the plate at 37 °C for 4 min. To create a standard calibration curve, the procedure was replicated using aqueous solutions of ferrous sulfate heptahydrate (FeSO4·7H2O) at various concentrations (0.1, 0.2, 0.4, 0.6, 0.8, and 1.0 mM). FRAP assay results were expressed as antioxidant activity in millimoles of Trolox equivalent per milliliter (mmol TE/mL) of the sample, utilizing a standard calibration curve of the aqueous solution of FeSO4·7H2O [21, 22]. Each sample analysis was conducted in triplicate.
2.6 Total phenolic content (TPC) assay
TPC was determined using a modified Folin-Ciocalteu assay. A 10% Folin-Ciocalteu reagent (FCR) solution was prepared by diluting 1 mL of FCR with 9 mL of distilled water, while a 7.5% sodium carbonate solution was made by dissolving 0.75 g of sodium carbonate in 10 mL of distilled water. Subsequently, 20 µL of ten-fold diluted RSJ samples were added to 330 µL wells in a 96-well microplate, followed by the addition of 100 µL of the prepared 10% FCR to each well and a five-minute incubation at room temperature. Afterward, 80 µL of the 7.5% sodium carbonate solution was added to each well, mixed, and incubated for 30 min under aluminum foil cover to shield from light. Absorbance was measured at 750 nm using a microplate spectrophotometer, and readings were recorded with software. Standard calibration curves were generated using gallic acid solutions, and results were expressed as milligrams of gallic acid equivalent per milliliter (µg GAE/mL) of the sample calculated using Eq. 2 [23]. All experiments were performed in triplicate, and absorbance readings were taken using a microplate spectrophotometer.
2.7 Vitamin C analysis
The Vitamin C analysis was conducted using the 2,6-dichlorophenolindophenol (DCPIP) dye method. Firstly, the DCPIP (C12H7Cl2NO2) dye solution is prepared by dissolving 26 mg of DCPIP dye and 21 mg of sodium bicarbonate in a 100 mL volumetric flask filled with distilled water. After filtering, the solution is stored in a dark-colored glass bottle. A Vitamin C (ascorbic acid) stock solution at a concentration of 0.1 mg/mL is prepared by dissolving 100 mg of Vitamin C in a 100 mL volumetric flask filled with 4% oxalic acid solution, followed by a serial dilution.
During titration, 5 mL of the Vitamin C working standard solution is titrated against the DCPIP solution using a burette. The DCPIP solution is added drop by drop until a pale pink color appears, indicating the endpoint of the titration. This color change signifies the reaction between Vitamin C in the solution and the DCPIP dye, allowing for the determination of Vitamin C content in the sample (V1).
To prepare the test sample, 10 g of sample (RSJ) is placed into a 100 mL beaker, transferred to a 100 mL volumetric flask, and diluted to 100 mL with 4% oxalic acid solution. 5 mL of test sample (RSJ) is titrated against DCPIP solution until a similar pale pink colour is achieved and the volume was recorded (V2).The Vitamin C content was calculated based on Eq. 3.
V1 = Volume of the DCPIP used for standard vitamin C (mL).V2 = Volume of DCPIP used for RSJ (mL).
2.8 Reducing sugar estimation
The dinitrosalicylic colorimetric (DNS) method was utilized to assess the reducing sugar content in RSJ samples based on the previous publication [24]. Preparation of the DNS reagent involved dissolving 5 g of dinitrosalicylic acid in 250 mL of distilled water at 80 °C ("DNS solution"). To this solution, 100 mL of sodium hydroxide (2 N) and 150 g of potassium sodium tartrate-4-hydrate were added at 27 °C, with the volume adjusted to 500 mL using distilled water (all reagents sourced from Sigma-Aldrich, Missouri, USA). Standard calibration curves were generated with glucose concentrations ranging from 0.0625% to 1%. Sample dilution was carried out at a ratio of 1:10 by adding 10 μL of the sample to 990 μL of distilled water. Subsequently, 1 mL of the DNS solution was added and thoroughly mixed. The mixture was incubated at boiling temperature (100 °C) for 5 min, followed by immediate cooling. Then, 10 mL of distilled water was added, and the absorbance was measured at 540 nm using a spectrophotometer (Thermo Spectro, USA).
2.9 Determination of electrolytes by inductively coupled plasma mass spectrophotometer (ICP-MS)
The electrolyte content of RSJ was determined using an ICP-MS. The RSJ samples and standards were subjected to ICP-MS (Perkin Elmer, USA) for the quantification of electrolytes in sugarcane juice with the following conditions: peak processing mode: average- signal profile processing mode: average- detector mode: dual- dead time (ns): 55- calibration type: external calibration- the number of replicates- 3. Prior to analysis, all glassware and centrifuge tubes were treated with 15% nitric acid and dried in an oven at 60 °C (Memmert, USA) for 24 h. Sodium (Na), potassium (K), calcium (Ca), and magnesium (Mg) in samples were determined after dilution with Milli-Q water at a ratio of 1:2000, while iron (Fe) and zinc (Zn) were assessed at a 1:200 dilution. After dilution, the samples underwent filtration through a 0.45 µm nylon filter. Calibration curves for each element were established within the range of 0.01–0.20 µg/mL [9].
2.10 Microbiological inoculation and determination
The De Man–Rogosa–Sharpe (MRS) agar, obtained from HiMedia Laboratories Pvt. Ltd. (Mumbai, India), was used in the study. The LAB strain Lb. plantarum ATCC8014 from the Lab Bioprocessing, Faculty of Food Science and Technology UPM Culture Collection, and the LAB isolated from commercial probiotic drink Yakult (Lb. casei strain Shirota) were used in this study. Initially, Yakult (150 mL) was inoculated into MRS broth and allowed to incubate for 48 h. Following two washes with peptone water and centrifugation, a stock solution was created. To achieve a bacterial inoculum of 107, a 3% LAB culture was initiated by transferring 300 µL from the stock solution into 10 mL of MRS broth. After a 48-h incubation, the culture underwent washing, followed by resuspension in phosphate buffer. Dilution was performed using the equation M1V1 = M2V2. Subsequently, LABs isolated from the stock solution (1%) were introduced into pasteurised RSJ (using double boiler at 72 °C for 15 s), with 1 mL of LAB from the stock sample used for every 100 mL of RSJ. The storage temperature is maintained at 4 °C. To determine the viability of LAB, a serial dilution was performed and inoculated on the MRS agar under anaerobic condition (Anaerocult™, Sigma-Aldrich), and the formation of the colonies were counted.
2.11 Statistical analysis
All the data were reported in the form of graphic images of triplicate measurements. One-way analysis of variance (ANOVA) and Tukey’s test were performed to detect differences among the juices for each parameter considered using Minitab 16 (Minitab Inc. State College, Pa. U.S.A).
3 Result and discussion
3.1 Quality assessment of red sugarcane juice – physicochemical, minerals, and antioxidants
In order to create healthy and tasty plant-based functional drinks, pH is an important factor to be considered. pH also plays a vital role in these drinks' stability and functional properties. Based on Table 1, the pH range of all the RSJ variants is 5.30 to 5.39, which is consistent with the findings of a previous study that reported a pH of around 5.29 for sugarcane juice [25]. This pH range indicates that consuming the juice could provide a healthier alternative to energy drinks, which typically have a pH below 4 [26]. Lower pH levels in energy drinks can lead to dental erosion and various health issues, including gastrointestinal discomforts like acid reflux or heartburn [26]. However, when converting fruit juice into a functional drink via fermentation, a pH below 4.5 is often targeted. This lower pH is because of organic acid production by LAB, which is essential for preservation purposes [27]. Overall, the pH range of 5.30 to 5.39 observed in the juice variants suggests a favorable balance between taste and potential health implications. It indicates that the juice can serve as a healthier option compared to highly acidic energy drinks, while still maintaining a pH level suitable for functional drink conversion.
The color of RSJ, such as observed in the variant Ragnar from Table 1, tends to have a higher blue-yellow color and a lower green–red color hue, aligning with the typical yellow hue of sugarcane juice. This color characteristic, influenced by compounds like xanthophyll and anthocyanin, can be an important factor in attracting consumers and enhancing their preference for the product [28]. In the context of sports isotonic drinks, which often come in various attractive colors [29], the natural color of sugarcane juice can serve as an indicator of its phenolic compound content. Normally, anthocyanins contributed to red-blue color, while xanthophyll and carotene are responsible for yellow and orange, respectively [28]. Understanding the relationship between color variations and specific phenolic compounds in sugarcane juice can provide valuable insights into its nutritional value and consumer perception [29].
To select an ideal candidate for the development of a functional drink, the Total Soluble Solids (TSS) value of RSJ was assessed. TSS serves as a rough measure of sugar content and is commonly used in the beverage industry to evaluate sweetness. RSJ typically contains higher sugar content compared to the yellow variant [25]. Opting for sugarcane juice with higher TSS levels may be advantageous for probiotic growth, as it provides a plentiful carbon source and increased energy value. As probiotics consume sugar during fermentation, it can lead to a reduction in sugar content while simultaneously producing beneficial bioactive compounds like GABA, thereby enhancing its nutritional significance [17]. In this study, Ragnar variety exhibited a significantly higher TSS value (Table 1), making it optimal for probiotic inoculation. It is important to consider that TSS measurement encompasses not only traditional sugars but also other dissolved components, including "functional" sugars such as D-tagatose, D-allose, D-talose, and D-psicose, organic acids, minerals, and various substances [30]. As a result, TSS can only provide an estimation of the sugar content rather than an absolute value.
The presence of electrolytes is considered crucial as an ergogenic aid on top of sugars, as they help replenish lost electrolytes and enhance energy production [9]. Since our bodies are unable to produce electrolytes, we rely on obtaining them from food sources. Table 2 shows that Serai exhibited the highest mineral content in five out of the six minerals tested, while Kapur demonstrated the highest potassium content. In comparison to commercial isotonic drinks, all variants of RSJ demonstrated significantly higher levels of electrolytes, except for sodium. This is advantageous since sodium is often consumed in excess in our daily diets, and dietary guidelines often recommend limiting sodium intake. Therefore, the higher electrolyte content found in RSJ variants can be highly beneficial for individuals engaged in physical activity.
Sugarcane juices are known to be rich in flavonoid and phenolic compounds, which possess natural antioxidant properties and can effectively scavenge free radicals [31]. In the context of energy isotonic drinks, antioxidants play a crucial role in neutralizing free radicals generated during intense energy production in the mitochondria's electron transport chain. Based on the results depicted in Fig. 1, all RSJ exhibited significant antioxidant activity, with Kapur displaying the lowest EC50 value. However, when considering other antioxidant values such as TPC and FRAP, there was no significant difference in antioxidant capacity between Kapur and Ragnar varieties, indicating comparable antioxidant prowess. Taking into account the superior physicochemical properties of Ragnar, this particular variant was chosen for further development as a functional drink. Most importantly, Ragnar RSJ is among the most popular red sugarcane cultivated in Malaysia, owing to its excellent sensory properties [5].
The antioxidant capacities of three different variants of RSJ (Serai, Kapur, and Ragnar) were assessed using three representative values: EC50 (DPPH), TPC (total phenolic content), and FRAP (ferric-reducing antioxidant power). Significant differences between RSJ varieties (column) were observed based on the results of Tukey's test (p < 0.05). The line graph is not drawn to scale; its purpose is to illustrate trends rather than provide accurate proportional representation
Prior to the inoculation of these probiotics into the Ragnar RSJ, an independent Vitamin C analysis was conducted in comparison with the commercial isotonic drinks A and B. Our finding demonstrated that Ragnar RSJ contains significantly higher Vitamin C compared to commercial Isotonic A and B. The Vitamin C content in RSJ was measured at 0.72 mg/100 g, whereas Isotonic A and B contained only 0.12 mg/100 g and 0.00 mg/100 g, respectively. Typically, active individuals possess greater number of mitochondria, hence experience rapid energy generation and leading to increased free radicals and inflammation. Therefore, Vitamin C and other neutralising antioxidants are crucial for post-recovery treatment in athletes [32].
3.2 Inoculation of probiotics into Ragnar red sugarcane juice (RSJ)
Lactic acid bacteria (LAB) play a crucial role in the food industry and are classified into homofermentative and heterofermentative types. Homofermentative LAB, such as Streptococcus thermophilus and Lactococcus lactis ssp. lactis, are commonly used, while heterofermenters like Lb. acidophilus, Lb. delbrueckii ssp. bulgaricus, and Leuconostoc mesenteroides are also important [33]. Probiotics offer numerous health benefits, including metabolic support for active individuals, gut and brain health through the gut-brain axis mechanism, reduction of sugar content, production of bioactive compounds, and more [12, 34].
Probiotic bacteria produce a diverse range of metabolites that have positive effects on human health. These include bacteriocins, metabolic enzymes, amino acids, peptides, short-chain fatty acids, vitamins, antioxidants, anti-inflammatory and immune-modulating agents, and exopolysaccharides. Considering the potential of Ragnar sugarcane juice as an isotonic drink, further development into a probiotic drink was carried out using the generally recognized as safe (GRAS) strain Lb. plantarum (abbreviation: LP) and Lb. casei Shirota strain (abbreviation: YSJ). Meanwhile, negative control experiment was RSJ without probiotics and commercial Yakult sample as the positive control.
From Table 3, it is shown that the samples inoculated with either Lb. plantarum (LP) and Lb. casei Shirota (YS) have reached the minimum limit of probiotic count that is above log107 (7.25–9.36 log CFU/mL), to confer its beneficial effects on human health [35]. In the control experiment, the LAB levels were relatively low, and in some instances, they were undetectable. This can be attributed to the pasteurization process and immediate refrigeration of the RSJ (4 °C) during the investigation. In contrast, all other findings indicated a stable population of LAB within the range of 8 to 10 log CFU/mL within 30 days-period, showcasing the substrates' suitability for LAB maintenance. Since simple sugars in RSJ are often the preferred carbon source for LAB, the culture should remain stable as long as the carbon does not become a limiting nutrient [36].
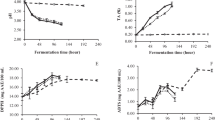
Moreover, throughout the fermentation process, there is a decrease in the quantity of reducing sugars (Fig. 2) attributed to their consumption by LAB. However, the reduction occurs at a slower rate in RSJ compared to the control probiotic drink, Yakult. Despite the slower reduction rate, the total reducing sugar content in RSJ samples still decreased over time, indicating the potential of L. plantarum as a probiotic strain to a healthier version of probiotic drink [37]. Yakult’s lower sugar content than other sugarcane drink samples suggests that Yakult's commercially prepared nature may involve sugar reduction during production [37].
The total reducing sugar in the sugarcane juice enriched with probiotics over a 30-days storage period at 4 °C. Each value from the table represents mean ± standard deviation (n = 3). Lowercase letters denote significance (p < 0.05) within the same time interval, whereas uppercase letters signify significance between the same treatments (as indicated by similar bar colors)
Control—Sugarcane juice without probiotics; YS – Lb. casei Shirota and sugarcane juice; LP – Lb. plantarum in sugarcane juice.
3.3 Assessing the functionality of probiotic-enriched Ragnar red sugarcane juice (RSJ)
As a next step in evaluating the functionality of this probiotic-enriched drink, the analysis of gamma-aminobutyric acid (GABA) was conducted on the probiotic drinks to investigate its functionality and potential health benefits. Based on Fig. 3, all samples of RSJ possess at least an initial amount of GABA, whereby the control sample had the lowest GABA content (52.22 mg/100 mL), while the Yakult (107.80 mg/100 mL), YS (324.70 mg/100 mL), and LP (190.85 mg/100 mL) samples displayed higher GABA. The fermentation process appeared to influence GABA production in the RSJ samples. Notably, on day one, the Yakult and LP samples exhibited a significant increase in GABA content, with values of 457.15 mg/100 mL and 547.20 mg/100 mL, respectively. This suggests that the probiotic strains present in Yakult and YS played a role in enhancing GABA synthesis during fermentation. These findings are consistent with previous studies, which demonstrated the production of GABA through the use of Lb. plantarum in a whey protein drink [15]. However, GABA content fluctuates in all the samples over the 30 days of storage. GABA may be unstable, thus random degradation and reformation can occur during storage [38].
GABA content of different Ragnar RSJ treatments and its associated controls. Each value from the table represents mean ± standard deviation (n = 3). Lowercase letters denote significance (p < 0.05) within the same time interval, whereas uppercase letters signify significance between the same treatments (as indicated by similar bar colors). Control—Sugarcane juice without probiotics; YS – Lb. casei Shirota and sugarcane juice; LP – Lb. plantarum in sugarcane juice
4 Conclusion
Among the three varieties of red sugarcane juice (RSJ) investigated, Ragnar exhibited the highest potential for the development of a functional drink. This is attributed to its remarkable antioxidant, vitamin C, and essential mineral content, particularly beneficial for highly active individuals. The fortification of Ragnar RSJ with suitable probiotics, like Lactobacillus, not only enhances its functionality by promoting GABA production and reducing sugars but also ensures optimal growth of beneficial probiotics, imparting health benefits.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Hajar-Azhari S, Shahruddin R, Abd Rahim MH. The effect of heat treatment and sonication on physicochemical and colour attributes of yellow sugarcane juice. Malaysian Appl Biol. 2018;47:129–34.
Jasmi N, Mansor N, Lim EJ, Yusof NL, Hajar-Azhari S, AbdRahim MH. The effect of sonication and heat treatment on the physicochemical, nutritional and microbiological properties of different sugarcane variants. Food Sci Technol. 2020;40:551–6. https://doi.org/10.1590/fst.12619.
Hajar-Azhari S, Abd Rahim MH, RazidSarbini S, Muhialdin BJ, Olusegun L, Saari N. Enzymatically synthesised fructooligosaccharides from sugarcane syrup modulate the composition and short-chain fatty acid production of the human intestinal microbiota. Food Res Int. 2021;149:110677. https://doi.org/10.1016/j.foodres.2021.110677.
Singh A, Lal UR, Mukhtar HM, Singh PS, Shah G, Dhawan RK. Phytochemical profile of sugarcane and its potential health aspects. Pharmacognosy Rev. 2015;9:45–54. https://doi.org/10.4103/0973-7847.156340.
Mansor N, RamLi NS, Azhari SH, AbdRahim MH. Effects of different preservation treatments on nutritional profile on juices from different sugar cane varieties. Sains Malays. 2020;49:293–291.
Hajar-Azhari S, AbdRahim MH, Wan-Mohtar WAAQI, Sarbini SR, Saari N. Novel fructooligosaccharide conversion from sugarcane syrup using a specialised enzymatic pH-stat bioreactor. Process Biochem. 2020;95:55–63. https://doi.org/10.1016/j.procbio.2020.04.031.
Kadum H, Hamid AA, Abas F, RamLi NS, Mohammed AKS, Muhialdin BJ, Jaafar AH. Bioactive compounds responsible for antioxidant activity of different varieties of date (Phoenix dactylifera L.) Elucidated by 1H- NMR Based Metabolomics. Int J Food Prop. 2019;22:462–76. https://doi.org/10.1080/10942912.2019.1590396.
Mohd Zaini NS, Khudair AJD, Gengan G, Abd Rahim MH, Hussin ASM, Idris H, Mohsin AZ. Enhancing the Nutritional profile of vegan diet: a review of fermented plant-based milk as a nutritious supplement. J Food Compos Anal. 2023. https://doi.org/10.1016/j.jfca.2023.105567.
Halim HH, WilliamsDee E, PakDek MS, Hamid AA, Ngalim A, Saari N, Jaafar AH. Ergogenic attributes of young and mature coconut (Cocos nucifera L.) water based on physical properties, sugars and electrolytes contents. Int J Food Propert. 2018;21:2378–89. https://doi.org/10.1080/10942912.2018.1522329.
Sallehuddin NA, Abdul-Hamid A, Salleh SZ, Abdul-Majid N, Halim HH, RamLi NS, Shukri R, Jaafar AH, Hussin M, Anwar F. Ergogenic, anti-diabetic and antioxidant attributes of selected Malaysian herbs: characterisation of flavonoids and correlation of functional activities. Int Food Res J. 2020;27:37571.
Faizal FA, Ahmad NH, Yaacob JS, Abdul-Halim Lim S, Yaacob JS, Abd Rahim MH. Food processing to reduce anti-nutrients in plant-based food. Int Food Res J. 2023;30:25–45. https://doi.org/10.47836/ifrj.30.1.02.
Abd Rahim MH, Hazrin-Chong NH, Harith HH, Wan-Mohtar W, Sukor R. Roles of fermented plant-, dairy- and meat-based foods in the modulation of allergic responses. Food Sci Hum Wellness. 2023;12:691–701. https://doi.org/10.1016/j.fshw.2022.09.002.
Abadl MMT, Mohsin AZ, Sulaiman R, Abas F, Muhialdin BJ, Meor Hussin AS. Biological activities and physiochemical properties of low-fat and high-fat coconut-based kefir. Int J Gastron Food Sci. 2022;30:100624. https://doi.org/10.1016/j.ijgfs.2022.100624.
Mohsin AZ, Marzlan AA, Muhialdin BJ, Wai LK, Mohammed NK, MeorHussin AS. Physicochemical characteristics, GABA content, antimicrobial and antioxidant capacities of yogurt from Murrah buffalo milk with different fat contents. Food Biosci. 2022;49:101949. https://doi.org/10.1016/j.fbio.2022.101949.
Zarei F, Leila N, Eshaghi MR, Abadi MET. Production of gamma-aminobutyric acid (GABA) in whey protein drink during fermentation by lactobacillus plantarum. J Microbiol. 2021;9:1087–92.
Wang H, Cheng L, Han Y. Effect of oral administration of GABA on thermoregulation in athletes during exercise in cold environments: a preliminary study. Front Nutr. 2022. https://doi.org/10.3389/fnut.2022.883571.
Shin Yee C, Sohedein MNA, Poh Suan O, Weng Loen AW, Abd Rahim MH, Soumaya S, Ilham Z, Wan-Mohtar W. The Production of Functional γ-Aminobutyric Acid Malaysian Soy Sauce Koji and Moromi Using the Trio of Aspergillus Oryzae NSK, Bacillus Cereus KBC, and the Newly Identified Tetragenococcus Halophilus KBC in Liquid-State Fermentation. Futur Foods. 2021;4:100055. https://doi.org/10.1016/j.fufo.2021.100055.
Sassi S, Ilham Z, Jamaludin NS, Halim-Lim SA, Shin Yee C, WengLoen AW, Poh Suan O, Ibrahim MF, Wan-Mohtar WA. Critical optimized conditions for gamma-aminobutyric acid (GABA)-producing Tetragenococcus Halophilus Strain KBC from a commercial soy sauce moromi in batch fermentation. Fermentation. 2022;8:409.
Wan-Mohtar WA, Sohedein MN, Ibrahim MF, Ab Kadir S, Suan OP, Weng Loen AW, Sassi S, Ilham Z. Isolation, identification, and optimization of γ-Aminobutyric Acid (GABA)-producing bacillus cereus strain KBC from a commercial soy sauce moromi in submerged-liquid fermentation. Process. 2020;8:652.
AhmadFauzi NS, AbdRahim MH, AbdulMajid N, Othman R, Yaacob JS. Evaluation of the effect of jasmonic acid elicitation on composition of pigments and biological activities in green callus of Neem (Azadirachta indica). Front Sustain Food Syst. 2022. https://doi.org/10.3389/fsufs.2022.1017398.
Amirah AS, Nor Syazwani S, Radhiah S, Anis Shobirin MH, Nor-Khaizura MAR, Wan Zunairah WI, Nurul Shazini R. Influence of raisins puree on the physicochemical properties, resistant starch, probiotic viability and sensory attributes of coconut milk yogurt. Food Res. 2020;4:70–84.
Halim HH, PakDek MS, Hamid AA, Saari N, MohdLazim MI, Abas F, Ngalim A, Ismail A, Jaafar AH. Novel sources of bioactive compounds in coconut (Cocos nucifera L.) water from different maturity levels and varieties as potent skin anti-aging strategies and anti-fatigue agents. Food Biosci. 2023;51:102326. https://doi.org/10.1016/j.fbio.2022.102326.
Mediani A, Abas F, Tan CP, Khatib A. Effects of different drying methods and storage time on free radical scavenging activity and total phenolic content of Cosmos Caudatus. Antioxidants. 2014;3:358–70. https://doi.org/10.3390/antiox3020358.
Gonçalves C, Rodriguez-Jasso RM, Gomes N, Teixeira JA, Belo I. Adaptation of dinitrosalicylic acid method to microtiter plates. Anal Methods. 2010;2:2046–8.
Abd Rahim MH, Wan Mansor WN, Abd-Halim S, Wui LP. Physicochemical Changes of Malaysian Red Ragnar Sugarcane Juice Treated with Chemical Preservatives during Storage. Artic Songklanakarin J Sci Technol. 2022;44:1339–44. https://doi.org/10.14456/sjst-psu.2022.174.
Clapp O, Morgan MZ, Fairchild RM. The top five selling uk energy drinks: implications for dental and general health. Br Dental J. 2019;226:493–7. https://doi.org/10.1038/s41415-019-0114-0.
Mudgil D, Barak S. 3 Dairy-Based Functional Beverages. In: Milk-Based Beverages- Grumezescu AM, Holban AM, eds.: Woodhead Publishing, 2019: pp. 67–93 ISBN 978-0-12-815504-2.
Khoo HE, Azlan A, Tang ST, Lim SM. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res. 2017;61:1361779.
van Esch P, Gadsby CL. Marketing the healthiness of sports drinks: from physiological to cognitive based benefits. Austr Market J. 2019;27:179–86. https://doi.org/10.1016/j.ausmj.2019.04.001.
Ali S, El Gedaily R, Mocan A, Farag M, El-Seedi H. Profiling metabolites and biological activities of sugarcane (Saccharum officinarum Linn.) juice and its product molasses via a multiplex metabolomics approach profiling metabolites and biological activities of sugarcane (Saccharum officinarum Linn.) juice and its product molasses via a multiplex metabolomics approach. Molecules. 2019;24:934.
Duarte-Almeida JM, Salatino A, Genovese MI, Lajolo FM. Phenolic composition and antioxidant activity of culms and sugarcane (Saccharum officinarum L.) products. Food Chem. 2011;125:660–4. https://doi.org/10.1016/j.foodchem.2010.09.059.
Simioni C, Zauli G, Martelli AM, Vitale M, Sacchetti G, Gonelli A, Neri LM. Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget. 2018. https://doi.org/10.18632/oncotarget.24729.
Fazilah NF, Ariff AB, Khayat ME, Rios-Solis L, Halim M. Influence of probiotics, prebiotics, synbiotics and bioactive phytochemicals on the formulation of functional yogurt. J Funct Foods. 2018;48:387–99. https://doi.org/10.1016/j.jff.2018.07.039.
Kechagia M, Basoulis D, Konstantopoulou S, Dimitriadi D, Gyftopoulou K, Skarmoutsou N, Fakiri EM. Health benefits of probiotics: a review. ISRN Nutr. 2013. https://doi.org/10.5402/2013/481651.
Minelli EB, Benini A. Relationship between number of bacteria and their probiotic effects. Microb Ecol Health Dis. 2008;20:180–3. https://doi.org/10.1080/08910600802408095.
Abd Rahim MH, Harith HH, Montoya A, Abbas A. Growth and lovastatin production by aspergillus terreus under different carbohyrates as carbon sources. Biocatal Agric Biotechnol. 2017;10:379–85. https://doi.org/10.1016/j.bcab.2017.04.011.
Mantzourani I, Kazakos S, Terpou A, Alexopoulos A, Bezirtzoglou E, Bekatorou A, Plessas S. Potential of the probiotic lactobacillus plantarum atcc 14917 strain to produce functional fermented pomegranate juice. Foods. 2019;8:6352242.
Jewett B, Sharma S. Physiology, GABA. In StatPearls; StatPearls Publishing, 2022.
Acknowledgements
The authors thank Universiti Putra Malaysia Inisiatif Putra Siswazah Grant, with a reference of UPM.RMC.800-2/1/2022/GP-IPS/9740400, and Ministry of Higher Education, Malaysia (FRGS grant no. 01-01-20-2323FR, with reference code: FRGS/1/2020/STG01/UPM/02/2) for the financial support.
Author information
Authors and Affiliations
Contributions
Muhamad Hafiz Abd Rahim: Conceptualization, Methodology. Wan Nusrah Wan Mansor.: Data curation, Writing-Original draft preparation. Gengghatarani Gengan and Nurul Solehah Mohd Zaini: Visualization, Investigation. Muhamad Hafiz Abd Rahim: Supervision. Ariani Hoo Abdullah, Anis Zulaikha Roslan and Ainnur Adnin Mohd Sha'ari: Writing- Reviewing and Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wan Mansor, W.N., Mohd Zaini, N.S., Gengan, G. et al. Enhancing ergogenic performance and antioxidant benefits of red sugarcane juice through probiotic fermentation. Discov Food 4, 32 (2024). https://doi.org/10.1007/s44187-024-00092-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44187-024-00092-w