Abstract
Numerous under-researched edible plants are present in the desert regions of the world. These plants could be potential candidates to ensure food security and provide valuable bioactive compounds through diet. In general, the bioactives present in food manifest synergistic, additive, or antagonistic interactions. The current study investigates such interactions between food combinations traditionally consumed in (semi) arid regions. Five edible plants (representing three food categories) were selected: Prosopis cineraria and Acacia senegal (legume), Capparis decidua and Cordia dichotoma (non-legume), and Mangifera indica (fruit), in which the first four are largely underutilized. The antioxidant capacities of individual plant extracts and their binary mixtures were analyzed by DPPH free radical scavenging and FRAP assays. The total phenolic content (TPC) and total flavonoid content (TFC) were also determined. The highest antioxidant activity was obtained for Prosopis cineraria extract (EC50—1.24 ± 0.02 mg/ml, FRAP value—380.58 ± 11.17 μM/g), while Mangifera indica exhibited the lowest antioxidant activity (EC50—2.54 ± 0.05 mg/ml, FRAP value—48.91 ± 4.34 μM/g). Binary mixture of Prosopis cineraria (legume) and Mangifera indica (fruit) manifested maximum synergy (experimental EC50—0.89 ± 0.01 mg/ml, theoretical EC50—3.79 ± 0.05 mg/ml). Correlation studies [Pearson’s correlation coefficient (r) and Principal component analysis (PCA)] showed a high correlation of TFC with DPPH and TPC with FRAP values. LC–MS analysis of methanolic plant extracts detected 43 phenolic compounds (including phenolic acids, flavonoids, and isoflavonoids), possibly responsible for the observed food synergy. For edible plants of the (semi) arid zones, this study is a first-of-its-kind and provides scientific validation to the traditional wisdom of consuming these foods together. Such indigenous food combinations derived from desert flora could offer valuable insights into development of sustainable functional foods and nutraceuticals.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
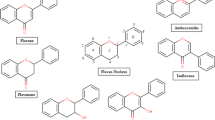
The plant-based foods that we eat not only provide macronutrients (proteins and carbohydrates) and micronutrients (vitamins and minerals) but also phytochemicals like phenolics and flavonoids. The latter are bioactive non-nutrient chemicals present in fruits, vegetables, legumes, and other plants, which they often accumulate under different biotic and abiotic stresses. A growing body of evidence suggests that the potential benefits of antioxidant capacity inherent in phytochemicals in fruits and vegetables may be even more significant than currently acknowledged. This is because oxidative stress caused by free radicals is implicated in the genesis of a wide range of non-communicable chronic disorders like cancer, obesity, diabetes, and cardiovascular disease, to name a few [1, 2]. Phytochemicals are classified as phenolics, nitrogen-containing compounds, carotenoids, terpenoids, and sulfur-containing compounds. Phenolic acids and flavonoids (flavone, isoflavone, flavanol, flavonol, flavonone, anthocyanidin) (Fig. 1), are the most studied phytochemicals owing to their vast structural and functional diversity. These phytochemicals and their synthetic derivatives have shown beneficial biological activities, such as anticancer, antifungal, antibacterial, and antioxidant activities [3]. Their antioxidant activity is linked to the structure, length of the side chain, and substitutions on the aromatic ring [4]. However, in the current era where consumption of isolated dietary supplements is more prevalent, it cannot be overemphasized that the same compounds can become toxic or antagonistic if consumed in excess or wrong combinations. In this context, wisdom lies in their intake via the food matrix, where the individual concentrations of the nutraceuticals are balanced.
In our body, cellular respiration, protein folding, and some metabolic activities constantly produce reactive oxygen species (ROS), such as hydroxyl radicals, superoxide anions, and singlet oxygen, which are essential for various signalling pathways and regulation of physiological activities. But, in the case of redox imbalance, excessive ROS can be harmful and cause oxidative stress, which can trigger inflammation or lead to chronic diseases [5]. According to epidemiological reports, regular intake of fruits and vegetables has been related to a lower risk of diseases, often heralded by this redox imbalance. Phytochemical extracts from vegetables and fruits have potent antioxidant and antiproliferative properties, and the majority of overall antioxidant activity comes from the combination of phytochemicals. Their strong antioxidant activity is often implicated to their additive and synergistic effects [1].
As a concept, food synergy highlights the positive interactions between nutrients and/or nutraceuticals, their absorption, and bioavailability in human body. It emphasizes the interaction of nutrients in various foods rather than a single component present therein. For example, green tea and black pepper were reported to have a synergistic effect, increasing the bioavailability of epigallocatechin gallate (EGCG), a compound present in green tea [6]. It is important to remember that phytochemicals may still cause cytotoxicity. The cytotoxic effects of phytochemicals may be worsened by excessive dosageor incompatible interactions. Because of these reasons, it is preferable to obtain bioactive compounds from whole plant-based foods that contain low-dose phytochemicals and have a generally favorable safety profile [7].
Plants growing in desert regions face harsh environmental conditions like excessive temperatures and low rainfall, and therefore accumulate unique anti-stress metabolites to combat the same [8]. Studies on synergistic interactions between traditional food combinations from the (semi) arid zones are missing. For this, five foods from three different categories, including Prosopis cineraria and Acacia senegal (legumes), Capparis decidua and Cordia dichotoma (non-legume), and Mangifera indica (fruit), were selected (Fig. 2). These plants are found in different regions worldwide and fruits/vegetables derived from them are dried/processed, and traditionally consumed in the arid and semi-arid parts of Indian subcontinent and Western Asia in different combinations, like ker-sangri (Capparis decidua-Prosopis cineraria), ‘Panchkuta’ (combination of all five above-mentioned plants), etc. Indigenous communities (such as the Bishnoi in Rajasthan, India) have been known to protect these plants/trees, particularly Prosopis cineraria, since ancient times. However, apart from corroboration through ethnic dietary practices, there is no scientific evidence till date on whether these foods have any synergistic effects. The objective of the current study is to investigate interactions between phenolic bioactives in the food matrix in order to scientifically validate the antioxidant synergy in traditional food combinations of the (semi) arid regions, by employing multi-mechanistic antioxidant assays.
2 Materials and methods
2.1 Chemicals and reagents
DPPH, ascorbic acid, quercetin, ferric chloride (FeCl3), sodium hydroxide (NaOH), sodium nitrite, and sodium carbonate were purchased from HiMedia (Mumbai, India), methanol, aluminium chloride, sodium acetate, and Folin–Ciocalteu reagent (FCR) were obtained from Sigma Company (United States), glacial acetic acid and 2,4,6-tripyridyl-s-triazine (TPTZ) were purchased from SRL (Mumbai, India).
2.2 Sample collection and extraction
Commercially available dried pods of Prosopis cineraria (PC; Local name in Rajasthan: Sangri), berries of Capparis decidua (CD; Local name in Rajasthan: Ker), seeds of Acacia senegal (AS; Local name in Rajasthan: Kumatiya), the fruit of Cordia dichotoma (CDI; Local name in Rajasthan: Lasora), and pulp of Mangifera indica (MI; Mango) were purchased from a local grocery store (Nagaur district, Rajasthan, India; N 27° 11′ 55.0644″, E 73° 44′ 4.9848″).
2.2.1 Sample preparation and extraction
The dried edible parts were powdered using a Waring blender. For each plant, 10 g of the dry powder was extracted with 80% methanol (1:5 w/v) at room temperature (1 h) in an orbital shaker incubator. The samples were further centrifuged at 3000×g for 10 min. The supernatant was filtered with a filter paper (Whatman). This step was performed twice, followed by concentrating the pooled supernatants to dryness using a rotary evaporator (Aditya Scientific, India). The resulting extracts were stored at 4 °C for further analysis.
2.3 Evaluation of antioxidant activity
Different concentrations of methanolic extracts were prepared to evaluate their antioxidant activities. For binary combinations, the food extracts were mixed in a 1:1 (v/v) ratio. Individual extracts were twofold diluted before testing.
2.3.1 Total phenolic content (TPC)
The TPC was determined by the Folin–Ciocalteu method described by Tsao et al. [9] and Wang et al. [10] with some modifications. Briefly, 1 ml of the FCR (Folin–Ciocalteu Reagent) and 0.8 ml of 7.5% sodium carbonate were mixed with 0.2 ml of the extract. After gently shaking, the mixture was incubated for 30 min at room temperature, and the absorbance was recorded at 765 nm. A standard curve of gallic acid was prepared (50–250 μg/ml), and the TPC values were expressed as micrograms of gallic acid equivalents (GAE) per gram of sample. All tests were performed in triplicates.
2.3.2 Total flavonoid content (TFC)
The TFC was calculated by the Aluminum chloride colorimetric assay described by John et al. [11]. Briefly, in a flask containing 4 ml distilled water, an aliquot (1 ml) of extracts or standard solutions of quercetin (200–1000 μg/ml) was added. To the flask was added 0.30 ml 5% NaNO2, followed by 0.30 ml 10% AlCl3 after 5 min. Further, 2 ml of 1 M NaOH was added after five minutes, and the volume was adjusted to 10 ml with distilled water. After shaking absorbance of the sample was read at 510 nm. The TFC values were expressed as mg of quercetin equivalents (QE) per g of sample. All tests were performed in triplicates.
2.3.3 DPPH free radical scavenging assay
The DPPH assay was performed as described by Hidalgo et al. [12]. A 100 μM DPPH solution was prepared in methanol, and to 290 μl of this solution, 10 μl of individual food extract or extract combination was added. The concentration of methanolic extracts ranged between 0.5 and 2.5 mg/ml (final concentration). The above reactions were carried out in a 96-well microplate, followed by incubation in the dark at room temperature for 1 h, and absorbance was measured at 517 nm using a microplate reader (Thermo Scientific Multiskan GO). The percentage DPPH radical scavenging activity was calculated using the following equation:
where Ac represents the absorbance of the control while As represents that of the sample. Solution devoid of the sample was taken as control. The results were expressed as EC50 (μM) obtained by plotting a graph between concentration and inhibition percentage.
2.3.4 FRAP assay
The FRAP assay was performed using the protocol described by earlier researchers [13, 14]. Briefly, the FRAP reagent was prepared by mixing 300 mM acetate buffer, pH 3.6, 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) in 40 ml of 40 mM HCl, and 20 mM FeCl3 at 10:1:1 (v/v/v). 10 μl of individual extract/binary combinations and 300 μl of freshly prepared FRAP reagent were mixed in a 96-well plate, incubated at 37 °C, and the absorbance was read at 593 after 12 min. The final FRAP value was expressed as μM ascorbic acid equivalent (AAE) per g of extract.
2.3.5 Synergy percentage and synergy effect (SE)
Synergy percentage for DPPH assay was calculated by the following equation [12]
Here Mix EC50 is the value of EC50 obtained by the mixture of two extracts. A EC50 is the EC50 of extract A, and B EC50 is the EC50 of extract B. Synergy percentage for FRAP assay was calculated by the following equation [12, 15].
Here the mixture FRAP value is obtained by the binary mixture of extracts. A FRAP value and B FRAP value are the values of individual extract A and individual extract B. In both assays, positive values signify synergistic interaction, whereas negative values signify antagonistic interaction.
The synergy effect (SE) was calculated for the DPPH assay. Experimental antioxidant capacities (EAC) of binary combinations were compared with theoretical antioxidant capacities (TAC) of mixtures to find the synergy effect. Calculation of theoretical values was done using the following equation [5, 16]:
EAC A and EAC B are the experimental antioxidant activities of individual extracts A and B, respectively. SE is calculated as EAC/TAC. Value of SE < 1 indicates synergistic effect, while SE equal to 1 and > 1 represent additive and antagonistic effects, respectively. This method is suitable when the antioxidant activity is expressed as EC50 values. Here, lower EC50 values imply higher antioxidant activity [17]. As a result, the synergy effect adopts reverse values, i.e., for synergism, it is higher than 1, and for antagonism, it is lower than 1.
2.4 LC–MS/MS analysis of the plant extracts
The crude methanolic extracts of the five plants were analyzed by LC–MS; HPLC-TQD Mass Spectrometer (Waters Corporation, Milford, USA). Samples were separated on an Accucore C-18 column (150 mm × 2.1 mm, 2.6 µm, ThermoScientific). The mobile phase consisted of acetonitrile (A) and 5 mM ammonium acetate (B). Gradient elution flow was: 0–10 min, 5% A; 10–16 min, 30% A; 16–24 min, 60% A; 24–35 min, 80% A; 35–40 min, 5% A. The flow rate was 0.25 ml/min; injection volume 5 µl; and column temperature 35 °C. A photodiode-array (PDA) detector was used to record the spectral data from 210 to 650 nm. Mass spectrometry data using electrospray ionization was collected in both positive and negative ionization modes, covering a mass range of m/z 150 to m/z 750. The detection parameters were: ion spray voltage 3.5 kV; capillary temperature 348 °C; gas flow 50 l/h; collision energy 20 V. The mass spectrum fragments were evaluated by mzCloud Mass Spectral Library (Thermo Fisher Scientific), and the reported relevant literature.
2.5 Statistical analysis
Experiments were performed in triplicate, and the values were estimated as mean ± standard deviation. One-way analysis of variance (ANOVA) was done to assess the statistically significant differences between the mean values. P-value ≤ 0.05 was considered statistically significant. Pearson’s correlation coefficient (r) was estimated using Microsoft Excel. Principal component analysis (PCA) was done using XLSTAT software (Addinsoft, Paris, France).
3 Results and discussion
In the current study, crude methanolic extracts prepared from edible portions of selected desert legumes (Prosopis cineraria and Acacia senegal), non-legumes (Cordia dichotoma and Capparis decidua), and fruit (Mangifera indica) were tested for the antioxidant activity, individually and in binary combinations by DPPH and FRAP assays. TPC and TFC values were calculated as the phenolic/flavonoid content and antioxidant activity were correlated [18]. Preliminary studies have shown that aqueous methanol is the best solvent for extracting phytochemicals, and methanolic extracts have the highest antioxidant activity [19, 20]. The experimental results obtained are presented and discussed below.
3.1 Antioxidant activity of individual plant extracts
The antioxidant activity of selected five foods was evaluated by four assays (TPC, TFC, DPPH, and FRAP) (Fig. 3). Prosopis cineraria (legume) showed the highest antioxidant activity among the five foods in all four assays. This result is in agreement with previous studies, which report that legumes (family Fabaceae) are the main source of isoflavones [21, 22], and antioxidant activity of isoflavones has been proven both in vitro and in vivo [22, 23]. Variations in antioxidant activity occur both within and between food categories. In legumes, the antioxidant activity of Prosopis cineraria (EC50—1.24 ± 0.02 mg/ml, FRAP value—380.58 ± 11.17 μM/g) was 1.7-folds and 2.4-folds higher than Acacia senegal (EC50—2.1 ± 0.02 mg/ml, FRAP value- 158.69 ± 7.34 μM/g), and in non-legumes, Cordia dichotoma (EC50—1.34 ± 0.04 mg/ml, FRAP value—280.24 ± 20.74 μM/g) showed 1.45-folds and 5.4-folds higher activity than Capparis decidua (EC50—1.94 ± 0.03 mg/ml, FRAP value—52.47 ± 5.7 μM/g) in DPPH and FRAP assays.
The order of highest to lowest antioxidant activities in all the five foods is as follows: (TPC estimation), Prosopis cineraria > Capparis decidua > Mangifera indica > Corida dichotoma > Acacia senegal; (TFC and DPPH assay), Prosopis cineraria > Corida dichotoma > Capparis decidua > Acacia senegal > Mangifera indica; (FRAP assay), Prosopis cineraria > Cordia dichotoma > Acacia senegal > Capparis decidua = Mangifera indica. The high antioxidant capacity of the legume Prosopis cineraria makes it an ideal candidate to be explored further as a source of not only protein but also natural antioxidants. These plants are also good sources of ascorbic acid, with 523 mg/100 g in Prosopis cineraria, 7.81 mg/100 g in Capparis decidua, 16 mg/100 g in Mangifera indica [24], 4 mg/100 g in Acacia senegal [25], and 0.93 mg/g in Cordia dichotoma [26]. The results showed that the antioxidant activity of foods is more due to the flavonoid content. For instance, the TPC of Cordia dichotoma was found to be 24% lower than Capparis decidua and 10% lower than Mangifera indica, but it has higher antioxidant activity because of 74% and 126% higher TFC than Capparis decidua and Mangifera indica. Similarly, the TPC of edible portions of Mangifera indica is more than Acacia senegal but it has lowest antioxidant activity because of lowest TFC among the five chosen plants.
The correlation of TPC as well as TFC with both DPPH and FRAP values of individual food extracts is shown in online resource Figure S4. The total flavonoid content (TFC) showed a strong significant correlation with DPPH and FRAP (r = 0.98 and r = 0.95 respectively, p < 0.05). However, the total phenolic content (TPC) showed a moderate non-significant correlation with DPPH and FRAP (r = 0.65 and r = 0.63, respectively). The correlation between DPPH and FRAP was significantly strong with r = 0.9319 (p < 0.05).
Flavonoids show a structure–antioxidant activity relationship. In general, structural features such as the ortho-dihydroxy structure or catechol group in the B-ring, which stabilizes the radical formed; the 2-3-double bond in conjugation with a 4-oxo function, which helps in the delocalization of electron from the B-ring; and the presence of the 3- and 5-OH functions, responsible for scavenging the free radicals, are thought to be closely linked to a compound’s antioxidant potency [12]. The presence of these hydroxyl groups is essential for determining the antioxidant activity using various methods, including DPPH and FRAP, which work on hydrogen atom transfer (HAT) and single electron transfer (SET) mechanisms, discussed further in the next section. Among the chosen plants, the highest TFC was observed for P. cineraria. Many studies show that the antioxidant activity of plant extracts is directly proportional to their phenolic/flavonoid content [18, 27]. The phytochemical composition of different plant foods depends on various factors, like cultivar, growing and storage conditions (climate, soil, water), and time of harvest. Our research group has earlier reported the variation in total flavonoid content of P. cineraria pod extracts obtained from trees in different geographical regions, in which we found that pods from the semi-arid zone (300–500 mm average annual rainfall) had higher TFC in comparison to those from the arid zone (100–350 mm average annual rainfall) [28]. Therefore, cultivar selection and environmental growth conditions would be of immense significance if these traditional edible plants were to be propagated for commercial purposes or developed into future-smart functional foods for better food security.
3.2 Antioxidant activity of plant combinations
The interaction between the bioactive compounds present in different foods can be synergistic, additive, or antagonistic. When the effect of a binary food combination is equal to the sum of effects of individual foods, it refers to an additive effect; when the effect is greater than the sum of individual foods, it refers to a synergistic effect; and antagonism occurs when the effect is less than the sum of individual foods. Studies related to the interaction of different food extracts are scarce, especially in the combination of traditional foods of desert regions. As mentioned earlier, binary combinations selected from five indigenous food practices were prepared in a 1:1 (v/v) ratio and tested for their antioxidant activities by four chemical-based assays. A total of 10 combinations were tested. The experimental value of each combination was compared with the theoretical value, which is the sum of values obtained from individual extracts, evaluated at twofold dilution (Fig. 4). For TPC, TFC, and FRAP assays, if the experimental value is significantly higher than the theoretical one, it is considered a synergistic effect; if the experimental value is significantly lower than the theoretical one, it is an antagonistic effect. If there is no significant difference in the two values, it indicates an additive effect. Except for the DPPH assay, all assays were evaluated this way. For DPPH assay, however, the experimental values lower, higher, or equal to the theoretical values indicate synergistic, antagonistic, and additive effects, respectively [10, 15]. As seen from Fig. 4, almost all combinations manifested antioxidant synergy.
The correlation of TPC and TFC with DPPH and FRAP values of food combinations is shown in online resource Figure S5. The correlation of total flavonoid content (TFC) with DPPH (r = 0.81, p < 0.05) was higher than with FRAP values (r = 0.75, p < 0.05). But, the correlation of total phenolic content (TPC) with FRAP (r = 0.735, p < 0.05) was significantly higher than with DPPH (r = 0.579; non-significant). The DPPH activity showed a strong correlation with FRAP values (r = 0.937, p < 0.05). These results justify to an extent the selection of both DPPH and FRAP assays for calculation of antioxidant activities in the current study. The mechanistic aspects to differentiate both the antioxidant assays (DPPH and FRAP) have been discussed below.
Principal component analysis (PCA) was performed to determine the relationship between four variables (TPC, TFC, DPPH, and FRAP). The total variance of data matrix was 94.62%, out of which principal component 1 (PC1) accounted for 87.81%, whereas principal component 2 (PC2) accounted for 6.81% of the variance (Fig. 5). Positive factor loadings were shown by PC1 for all the variables, with DPPH showing the maximum factor loading (0.964), followed by FRAP (0.947). PC2 showed positive factor loading for TPC (0.456), indicating that it could be the best selection for individual factor loading. TFC, FRAP, and DPPH showed significant effects on PC1, while TPC had strong effect on PC2. Figure 5 also illustrates correlations between variables: positively correlated variables appear close to each other on the plot (angle < 90°), while negatively correlated variables appear diagonally opposite each other (angle > 90°) [29]. In this context, PCA demonstrates a positive correlation of TPC and TFC with antioxidant activity (measured by DPPH and FRAP assays). This result can be explained by the known antioxidant characteristics of phenolic compounds [30]. In this study, we observed that majority of extracts with binary combinations were present on the right side of the biplot indicating that synergistic interactions were responsible for their activity as represented by different variables. For instance, PCA showed that the combination of Prosopis cineraria (PC) and Cordia dichotoma (CDI) had better (synergistic) antioxidant activity (present in the lower right quadrant) in comparison to their individual extracts.
Principal component analysis (PCA) biplot of antioxidant activity assays (DPPH and FRAP) versus phenolic (TPC) and flavonoid contents (TFC) with factor scores of 15 extracts (5 individual extracts and 10 binary extract combinations). For abbreviations, refer to the legend of Fig. 3
Tables 1 and 2 further represent the types of interactions possible in the binary combinations from the four assays. In TFC and DPPH assays, all 10 combinations showed a synergistic effect, whereas, in TPC and FRAP assays, differences in the type of interactions (synergistic, antagonistic, and additive) were observed in the food combinations. This observation is not uncommon. For instance, Hidalgo et al. [12] reported that anthocyanins (cyanidin-3-glucoside and malvidin-3-glucoside) manifested synergistic interactions in FRAP assay but not in DPPH assay. In our study, maximum synergism was shown by the binary combination of Prosopis cineraria (legume) and Mangifera indica (fruit) in both DPPH (experimental EC50—0.89 ± 0.01 mg/ml, theoretical EC50—3.79 ± 0.05 mg/ml) and FRAP assays (experimental value—542.02 ± 29.34 μM/g, theoretical value—429.49 ± 9.52 μM/g), which is in correlation with their total flavonoid content (experimental value—603.45 ± 4.12 mg QE/g, theoretical value—481.76 ± 3.79 mg QE/g). This result is in sync with the findings of Wang et al. [10], which indicated synergistic interaction between the combination of legumes and fruits while studying eleven different foods in Canada (that included a few berries, vegetables, and legumes. Bellik et al. [31] reported the synergistic antioxidant activity of honey-mint (Mentha spicata) combination, which can be used for the development of dietary supplements and functional foods. Our study brings out the health benefits of the traditional practice of preparing dishes containing edible parts of both Prosopis cineraria and Mangifera indica. The other synergistic combinations in all four assays include Capparis decidua–Acacia senegal and Acacia senegal–Mangifera indica. All the food combinations selected by us are distinctive as they are based on the indigenous food practices in arid regions of the world, which have been validated by their consumption through the ages.
The possible mechanisms for the antioxidant function (deactivation of free radicals) of different dietary phytochemicals include single electron transfer (SET) and hydrogen atom transfer (HAT) [10]. Such mechanistic differences might explain why no single antioxidant can provide the same health benefits as compared to a combination of natural phytochemicals found in our foods [2]. Therefore, while assessing synergistic effects is a worthy goal, the various available assays are based on multiple chemical mechanisms, making it more complicated. A single method's specificity and sensitivity do not ensure a reliable assessment of all types of dietary antioxidants; thus, a combination of various assays is considered a more accurate measure of antioxidant activity. HAT-based mechanisms assess an antioxidant’s capacity to scavenge free radicals through hydrogen donation. Methods based on SET detect an antioxidant’s capacity to transfer one electron to reduce any compound, including metals, carbonyls, and radicals [32].
The antioxidant activity of food mixtures may not always have an additive effect; it can either increase (synergism) or decrease (antagonism). The exact mechanism behind these interactions is still unclear. Some explanations proposed by researchers for synergistic interactions are (i) more robust antioxidants are regenerated by weaker antioxidants [33], (ii) stable intermolecular complexes with more potent antioxidant capacity are formed [17], (iii) depends on the type of antioxidant, and its concentration [34], (iv) unpredictable interactions. Hypotheses proposed for antagonistic interactions are (i) weaker antioxidants are regenerated by stronger antioxidants [17], (ii) antioxidants tend to polymerize, thus decreasing their antioxidant activity [35], (iii) free antioxidant radicals undergo irreversible reactions that lead to their disappearance (therefore they do not react with the neutralized radical). When studying antioxidant synergy between raspberry and adzuki bean extracts, Wang et al. [10] did not observe the appearance of new peaks on HPLC analysis of mixtures. In the current study, the formation of new compounds for synergy in the antioxidant activity of plant extracts could be ruled out, as similar phenolic compounds are also present in our extract, the metabolic aspects of which we have reported elsewhere [8].
Antioxidants may react differently to various radical or oxidant sources. Due to the involvement of different reaction mechanisms, characteristics, and various phase localizations, a single assay will be insufficient to precisely reflect all antioxidants or radical sources in a complex system (like food). Therefore, it is scientifically prudent to use both DPPH and FRAP assays to unambiguously ascertain and validate the antioxidant potential of phytochemicals in foods. FRAP assay utilizes SET reaction mechanism, hence cannot detect compounds that work by hydrogen transfer (radical quenching). However, the DPPH assay utilizes both SET and HAT reaction mechanisms. This radical can be quenched either by hydrogen transfer or neutralized by direct reduction via electron transfer [36]. Therefore, it is possible that a particular phytochemical combination might show synergy through DPPH assay but not FRAP assay, and vice versa.
In our case, we did not observe antagonism in DPPH assays, but antagonism was there in certain combinations when tested through the FRAP assay (e.g., Capparis decidua and Mangifera indica). Nonetheless, it is worthwhile to note that the FRAP assay is based on ferric-to-ferrous ion reduction, and antioxidants that do not have ferric-reducing ability will be ignored by the assay [37], and therefore the inherent antioxidant capacity of a food mixture will be underrepresented and might appear as antagonistic. Additionally, the composition of solvents used in the in vitro antioxidant assays has been reported to affect the phytochemical interactions resulting in chemical incompatibility. e.g., the reagent used in FRAP assay (TPTZ) is highly polar and might interfere with the polar reactive groups (–OH) present in the phenolic phytochemicals. Clarke et al. [38] have reported the DPPH method to be more reliable and reproducible. It is an enduring fact that certain phytochemical combinations show antagonistic interactions, at times due to intermolecular hydrogen bonding that reduces the availability of active hydroxyl groups (e.g., in flavonoids) for radical scavenging activities [12]. The available literature, including a recent report by our research group, also indicates that the traditional plants or medicines derived from them contain a number of hydroxyl-containing phenolics and flavonoids [28]. Therefore, antagonism among phytochemicals is definitely a possibility. This makes it all the more important to derive novel functional foods from time-tested combinations of traditional edible plants.
3.3 LC–MS/MS analysis of plant extracts
The LC–MS analysis was performed to screen phenolics/flavonoids in the five plant extracts. The LC–MS chromatograms, in both positive and negative ionization modes, and the m/z values of the identified metabolites are given in online resource file (Figure S2) and Table 3, respectively [individual mass spectra of identified compounds have been given in online resource file (Figure S3)]. Isoflavonoids are often exclusively present in Leguminosae and play an important role as antioxidant phytochemicals [39]. The LC–MS analysis of two legumes (P. cineraria and A. senegal) showed the presence of isoflavonoids (including a few glycosides and malonylated derivatives) like daidzein, daidzin, genistein, sissotrin, puerarin, and glycitin. The highest antioxidant activity of P. cineraria extract might be attributed to the presence of different isoflavonoids, which are categories of flavonoids known for their phytoestrogenic and anticancer properties. The phenolic compounds should be bioavailable (in terms of absorption, distribution, metabolism, and excretion) to exert their potential activities. Phenolic acids, isoflavonoids, and low molecular weight phenolics are reported to have the highest rate of bioavailability [40]. Joshi et al. [41] reported the synergistic antioxidant interaction of rutin (and quercetin) with kaempferol in a 1:1 ratio. The synergistic antioxidant activity between binary mixtures of the plants reported in this study could be due to quercetin-kaempferol or rutin-kaempferol interaction (as one of the many synergistic combinations). Ferulic acid and ellagic acid have been reported to show synergistic antioxidant activity [42], which can also be responsible for the synergism between Acacia senegal and Mangifera indica extracts. Similarly, Reber et al. [43] reported synergism between coumaric acid and quercetin, therefore, the synergistic activity between Prosopis cineraria and Acacia senegal, could be partly attributed to the presence of these two phenolics. We could also identify a few polyphenolic compounds that have not been previously reported in the literature (Table 3), therefore highlighting these plants as their potential sources for further characterization.
In the LC–MS chromatogram of the five plants, maximum number of compound peaks of high intensity were shown in P. cineraria and C. dichotoma, whereas least number of compounds were detected in M. indica extract. However, M. indica shows more number of phenolic compounds than Acacia senegal. These results further relate to the trend in their TPC, TFC and antioxidant activities by DPPH and FRAP assays (represented in Fig. 3). It has been reported that flavonols, flavones, and anthocyanins/anthocyanidins offer various health benefits such as anti-cancer, anti-diabetic, anti-obesity, antibacterial, anti-viral, cardio-protective, anti-inflammatory, and anti-allergic properties [19, 44]. In the present study, flavonols (quercetin, rutin, myricetin, kaempferol, isorhamnetin), flavones (diosmin, vitexin, homoorientin), and anthocyanins/anthocyanidins (delphinidin, malvidin, procyanidin, petunidin) were identified. Recently, our research group reported the antioxidant activity enhancer effect of cluster bean (Cyamopsis tetragonoloba or guar) seed extract on dietary phenolic phytochemicals (like epigallocatechin gallate), again emphasizing the antioxidant synergy between phytochemicals and/or plant extract(s) [45].
A representative graphical abstract for the entire study is given in Online Resource File (Figure S1).
4 Conclusions
Foods obtained from plants growing in the wild in desert regions are often local, seasonal, and sustainable, as input in terms of water and chemical fertilizers is minimal. However, these still remain neglected to a large extent and present an interesting opportunity for the food industries to harness their potential as alternative nutrients (e.g., protein) and nutraceutical sources. In the same context, designing strategic food combinations for enhanced human health becomes crucial. In a recently published study by our research group, we have shown synergy between flavonoid (rutin hydrate, quercetin dihydrate, kaempferol) and non-flavonoid (resveratrol, hydroquinone) phenolics, often present in many of the foods that we consume. The current study showed that binary combinations prepared from different indigenous food plants of (semi) arid zones result in mostly synergistic interactions, with P. cineraria (legume) and M. indica (fruit) showing maximum synergism in both DPPH and FRAP assays. In most cases, the antioxidant activity of plant extracts and their combinations were significantly correlated to TPC and TFC values. Potential phenolic compounds were identified by LC–MS analysis. To the best of our knowledge, the current study is first-of-its-kind concerning the traditional edible plants from the desert regions. In the future, these underutilized plants would need more attention by researchers to ensure health and food security for growing human populations.
Most studies focus on the interactions between pure/isolated compounds and nutrients. Studies on food-food interaction are relatively few in general, and negligible in foods obtained from traditional and underutilized plants thriving in desert ecosystems. In this context, we can gather insights from traditional food practices and further invest in developing synergy-guided food products in order to maximize the beneficial health effects. Functional foods or nutraceuticals enriched with synergistic antioxidant combinations may be developed by food industries so that consumers could incorporate antioxidants in their conventional diets. Dietary supplements, which have a huge market today, could be tailored to address specific health concerns, such as aging, diabetes, obesity, cardiovascular disease, and cancer. However, more research is needed for understanding the mechanism of interaction between various foods from different categories, along with their bioactives bioavailability assessments, to design functional foods with improved antioxidant activity. Further purification of these extracts may lead to identifying and validating potential specific compounds responsible for the biological activity by FT-IR and NMR studies. Additionally, these interactions can be validated in in vitro cell culture and other experimental model systems for disorders such as diabetes and obesity.
Data availability
The datasets generated in present study are available from the corresponding author on reasonable request.
Abbreviations
- TPC:
-
Total phenolic content
- TFC:
-
Total flavonoid content
- DPPH:
-
2,2-Diphenyl-1-picryl-hydrazyl-hydrate
- FRAP:
-
Ferric ion reducing antioxidant power
- EAC:
-
Experimental antioxidant capacity
- TAC:
-
Theoretical antioxidant capacity
- SE:
-
Synergy effect
References
Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003;78:517S-520S. https://doi.org/10.1093/ajcn/78.3.517S.
Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr. 2004;134:3479S-3485S. https://doi.org/10.1093/jn/134.12.3479S.
Jaradat N. Phytochemistry, traditional uses and biological effects of the desert plant Styrax officinalis L. J Arid Environ. 2020;182: 104253. https://doi.org/10.1016/j.jaridenv.2020.104253.
Mathew S, Abraham TE, Zakaria ZA. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J Food Sci Technol. 2015;52:5790–8. https://doi.org/10.1007/s13197-014-1704-0.
Chen X, Li H, Zhang B, Deng Z. The synergistic and antagonistic antioxidant interactions of dietary phytochemical combinations. Crit Rev Food Sci Nutr. 2021;62:1–20. https://doi.org/10.1080/10408398.2021.1888693.
Natarajan TD, Ramasamy JR, Palanisamy K. Nutraceutical potentials of synergic foods: a systematic review. J Ethn Foods. 2019;6:27. https://doi.org/10.1186/s42779-019-0033-3.
Kapinova A, Stefanicka P, Kubatka P, et al. Are plant-based functional foods better choice against cancer than single phytochemicals? A critical review of current breast cancer research. Biomed Pharmacother. 2017;96:1465–77. https://doi.org/10.1016/j.biopha.2017.11.134.
Asati V, Deepa PR, Sharma PK. Silent metabolism and not-so-silent biological activity: possible molecular mechanisms of stress response in edible desert legumes. J Plant Biochem Biotechnol. 2021;30:640–5. https://doi.org/10.1007/s13562-021-00728-3.
Tsao R, Yang R, Young JC, Zhu H. Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC). J Agric Food Chem. 2003;51:6347–53. https://doi.org/10.1021/jf0346298.
Wang S, Meckling KA, Marcone MF, et al. Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. J Agric Food Chem. 2011;59:960–8. https://doi.org/10.1021/jf1040977.
John B, Sulaiman CT, George S, Reddy VRK. Total phenolics and flavonoids in selected medicinal plants from Kerala. Int J Pharm Pharm Sci. 2014;6:406–8.
Hidalgo M, Sánchez-Moreno C, de Pascual-Teresa S. Flavonoid–flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010;121:691–6. https://doi.org/10.1016/j.foodchem.2009.12.097.
Tsao R, Yang R, Xie S, et al. Which polyphenolic compounds contribute to the total antioxidant activities of apple? J Agric Food Chem. 2005;53:4989–95. https://doi.org/10.1021/jf048289h.
Benzie IFF, Strain JJ. The Ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–6. https://doi.org/10.1006/abio.1996.0292.
Hajimehdipoor H, Shahrestani R, Shekarchi M. Investigating the synergistic antioxidant effects of some flavonoid and phenolic compounds. Res J Pharmacogn. 2014;1:35–40.
Zanfini A, Corbini G, La Rosa C, Dreassi E. Antioxidant activity of tomato lipophilic extracts and interactions between carotenoids and α-tocopherol in synthetic mixtures. LWT Food Sci Technol. 2010;43:67–72. https://doi.org/10.1016/j.lwt.2009.06.011.
Olszowy M. Synergistic, antagonistic and additive antioxidant effects in the binary mixtures. Phytochem Rev. 2020;19:63–103. https://doi.org/10.1007/s11101-019-09658-4.
Piluzza G, Bullitta S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm Biol. 2011;49:240–7. https://doi.org/10.3109/13880209.2010.501083.
Kumar A, Nirmal P, Kumar M, et al. Major phytochemicals: recent advances in health benefits and extraction method. Molecules. 2023;28:887. https://doi.org/10.3390/molecules28020887.
Phytochemical CS. In vitro free radical scavenging and antimicrobial activity of Cyamopsis tetragonoloba L. J Pharmacogn Phytochem. 2015;4:102–6.
Coward L, Barnes NC, Setchell KDR, Barnes S. Genistein, daidzein, and their.beta.-glycoside conjugates: antitumor isoflavones in soybean foods from American and Asian diets. J Agric Food Chem. 1993;41:1961–7. https://doi.org/10.1021/jf00035a027.
Křížová L, Dadáková K, Kašparovská J, Kašparovský T. Isoflavones. Molecules. 2019;24:1076. https://doi.org/10.3390/molecules24061076.
Ruiz-Larrea MB, Mohan AR, Paganga G, et al. Antioxidant activity of phytoestrogenic isoflavones. Free Radic Res. 1997;26:63–70. https://doi.org/10.3109/10715769709097785.
Rathore M. Nutrient content of important fruit trees from arid zone of Rajasthan. J Hortic For. 2009;1:103–8.
Mohammed N, Sati E, Abdallah F, et al. Carbohydrates and ascorbic acid contents in the acacia senegal and acacia seyal gum arabic. Dusunen Adam. 2019;10:613–20.
Kirankumar J, Gulhane PR. Evaluation of ascorbic acid (vitamin C) from some medicinal plants of Melghat region. J Glob Biosci. 2016;5:4638–42.
Jain D, Pancholi S, Patel R. Synergistic antioxidant activity of green tea with some herbs. J Adv Pharm Technol Res. 2011;2:177. https://doi.org/10.4103/2231-4040.85538.
Asati V, Deepa PR, Sharma PK. Desert legume Prosopis cineraria as a novel source of antioxidant flavonoids/isoflavonoids: biochemical characterization of edible pods for potential functional food development. Biochem Biophys Rep. 2022;29: 101210. https://doi.org/10.1016/j.bbrep.2022.101210.
Sapkota BK, Khadayat K, Sharma K, et al. Phytochemical analysis and antioxidant and antidiabetic activities of extracts from Bergenia ciliata, Mimosa pudica, and Phyllanthus emblica. Adv Pharmacol Pharm Sci. 2022. https://doi.org/10.1155/2022/4929824.
Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001;49:5165–70.
Bellik Y, Selles SMA. In vitro synergistic antioxidant activity of honey-Mentha spicata combination. J Food Meas Charact. 2017;11:111–8. https://doi.org/10.1007/s11694-016-9377-1.
Waisundara AFDE-V. Recent advances in antioxidant capacity assays. IntechOpen: Rijeka; 2021. p. 29.
Marinova E, Toneva A, Yanishlieva N. Synergistic antioxidant effect of α-tocopherol and myricetin on the autoxidation of triacylglycerols of sunflower oil. Food Chem. 2008;106:628–33. https://doi.org/10.1016/j.foodchem.2007.06.022.
Shi J, Qu Q, Kakuda Y, et al. Investigation of the antioxidant and synergistic activity of lycopene and other natural antioxidants using LAME and AMVN model systems. J Food Compos Anal. 2007;20:603–8. https://doi.org/10.1016/j.jfca.2007.03.004.
Pinelo M, Manzocco L, Nuñez MJ, Nicoli MC. Interaction among phenols in food fortification: negative synergism on antioxidant capacity. J Agric Food Chem. 2004;52:1177–80. https://doi.org/10.1021/jf0350515.
Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53:4290–302. https://doi.org/10.1021/jf0502698.
Apak R, Güçlü K, Demirata B, et al. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules. 2007;12:1496–547. https://doi.org/10.3390/12071496.
Clarke G, Ting KN, Wiart C, Fry J. High correlation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, ferric reducing activity potential and total phenolics content indicates redundancy in use of all three assays to screen for antioxidant activity of extracts of plants from the Malaysian rainforest. Antioxidants. 2013;2:1–10. https://doi.org/10.3390/antiox2010001.
Trush K, Pal’ove-Balang P. Biosynthesis and role of isoflavonoids in legumes under different environmental conditions. Plant Stress. 2023;8: 100153.
García-Pérez P, Rocchetti G, Giuberti G, et al. Phenolic acids, lignans, and low-molecular-weight phenolics exhibit the highest in vitro cellular bioavailability in different digested and faecal-fermented phenolics-rich plant extracts. Food Chem. 2023;412: 135549. https://doi.org/10.1016/j.foodchem.2023.135549.
Joshi T, Deepa PR, Sharma PK. Effect of different proportions of phenolics on antioxidant potential: pointers for bioactive synergy/antagonism in foods and nutraceuticals. Proc Natl Acad Sci India Sect B Biol Sci. 2022;92:939–46. https://doi.org/10.1007/s40011-022-01396-6.
Mitra S, Tareq AM, Das R, et al. Polyphenols: a first evidence in the synergism and bioactivities. Food Rev Int. 2023;39:4419–41. https://doi.org/10.1080/87559129.2022.2026376.
Reber JD, Eggett DL, Parker TL. Antioxidant capacity interactions and a chemical/structural model of phenolic compounds found in strawberries. Int J Food Sci Nutr. 2011;62:445–52. https://doi.org/10.3109/09637486.2010.549115.
Barreca D, Trombetta D, Smeriglio A, et al. Food flavonols: nutraceuticals with complex health benefits and functionalities. Trends Food Sci Technol. 2021;117:194–204. https://doi.org/10.1016/j.tifs.2021.03.030.
Joshi T, Mandal SK, Puri S, et al. Investigating the antioxidant activity enhancer effect of Cyamopsis tetragonoloba seed extract on phenolic phytochemicals. Front Plant Sci. 2023;14:807. https://doi.org/10.3389/fpls.2023.1131173.
Bishnoi RS, Kumar M, Shukla AK, Jain CP. Development and validation of novel HPLC method for the estimation of Rutin in crude hydromethanolic leaf extract of Prosopis cineraria. J Drug Deliv Ther. 2018;8:68–73.
Neeraj NV, Jangir SSBL. Quality assessment and phytochemical profile of Capparis decidua (Forssk) Edgew. Pharma Innov. 2019;8:424–30.
Raghuvanshi D, Sharma K, Verma R, et al. Phytochemistry, and pharmacological efficacy of Cordia dichotoma G. Forst. (Lashuda): a therapeutic medicinal plant of Himachal Pradesh. Biomed Pharmacother. 2022;153: 113400.
Peng D, Zahid HF, Ajlouni S, et al. LC-ESI-QTOF/MS profiling of Australian mango peel by-product polyphenols and their potential antioxidant activities. Processes. 2019;7:764.
Ybañez-Julca RO, Asunción-Alvarez D, Quispe-Díaz IM, et al. Metabolomic profiling of mango (Mangifera indica Linn) leaf extract and its intestinal protective effect and antioxidant activity in different biological models. Molecules. 2020;25:5149.
Marcillo-Parra V, Anaguano M, Molina M, et al. Characterization and quantification of bioactive compounds and antioxidant activity in three different varieties of mango (Mangifera indica L.) peel from the Ecuadorian region using HPLC-UV/VIS and UPLC-PDA. NFS J. 2021;23:1–7.
Acknowledgements
Authors are grateful to the administration of Birla Institute of Technology and Science (BITS), Pilani–Pilani Campus for infrastructural and logistic support. TJ is grateful to University Grants Commission, New Delhi, India for providing Senior Research Fellowship. Authors are thankful to SAIF (Sophisticated Analytical Instrumentation Facility), Central Drug Research Institute, Lucknow (India) for LC–MS instrumentation facility.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
TJ performed the study and drafted the manuscript. KA and MM assisted in LC–MS data analysis. PRD and PKS supervised the study and interpreted the data. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Joshi, T., Agrawal, K., Mangal, M. et al. Measurement of antioxidant synergy between phenolic bioactives in traditional food combinations (legume/non-legume/fruit) of (semi) arid regions: insights into the development of sustainable functional foods. Discov Food 4, 11 (2024). https://doi.org/10.1007/s44187-024-00082-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44187-024-00082-y