Abstract
Non-timber forest products are often disregarded in favor of primary crops in Burkina Faso, despite their nutritional significance and contribution to food self-sufficiency. However, the lack of scientific information regarding the amino acid content of Saba senegalensis pulp impedes its utilization in various foods production. Consequently, the primary objective of this investigation was to assess the free amino acid profile of S. senegalensis pulp in the three distinct climatic zones of Burkina Faso. Fruit samples were collected from these climatic regions, and the amino acid content was analyzed using HPLC methods. The analysis of S. senegalensis pulp revealed the presence of essential amino acids in mg/100 g, mainly isoleucine (300 ± 75–305 ± 15), leucine (370 ± 92.50–377 ± 95.50), lysine (200 ± 50–205 ± 70), methionine (90 ± 22.50–104 ± 50), phenylalanine (140 ± 35–196 ± 15), threonine (230 ± 57.50–241 ± 27.50), valine (260 ± 65–285 ± 40), and tryptophan (230 ± 57.50–237 ± 75.50), as well as non-essential amino acids. Statistical analysis indicated no significant difference (p > 0,5) in the essential and non-essential amino acids across the climatic zones. According to the revised model spectrum of ideal essential amino acids for humans by the WHO/FAO, the E/T values ranged from 43.12 to 43.40%, and the E/NE values ranged from 75.83 to 76.68%. The BC/E values ranged from 50.69 to 51.09%, while the BC/A values ranged from 1.24 to 1.37. Principal component analysis (PCA) revealed specific variations in the amino acid composition of the fruit pulp based on the climatic zones. This study demonstrates that S. senegalensis is a valuable source of amino acids and can potentially enhance food security.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
1 Introduction
In developing countries where multi-deficiency malnutrition and food insecurity are prevalent, it is crucial to reassess eating habits to fight these issues more effectively. As per FAO et al. [1], the number of people affected by hunger worldwide has reached around 828 million in 2021, and stell increaing for year.
The worsening of this food crisis may also be linked to the various environmental and security crises affecting the world. To combat malnutrition in its varied forms, diversifying dietary habits through the incorporation of non-timber forest products (NTFPs) with high nutritional value could offer a potential alternative solution [2]. NTFPs, despite their nutritional significance and contribution to food self-sufficiency, are often overlooked in favor of so-called primary crops [3]. This is exemplified by the case of Saba senegalensis, which is a food crop growing in the forests of Sub-Saharan Africa, specifically in Burkina Faso, Mali, and Côte d'Ivoire.
The pulp of S. senegalensis is not commonly used for domestic purposes or commercial exploitation [4]. However, various authors have reported that it contains significant levels of macronutrients, with averages of 144.2 g/kg total carbohydrates, 11.5 g/kg fat, 5.9 g/kg protein, and 5.9 g/kg ash, and mineral elements like iron, zinc, magnesium, calcium, and potassium), which could potentially fight multi-deficiency malnutrition with averages of 144.2 g/kg total carbohydrates, 11.5 g/kg fat, 5.9 g/kg protein, and 5.9 g/kg ash, [5, 6].
In same order, Parkouda et al. [7], demonstrated that S. senegalensis pulp S. senegalensis pulp contained a favorable nutritional quality with 8 g /100 g protein making this fruit a promising option for remedial strategies targeting chronic malnutrition.
According to local communities appreciation, the pulp of S. senegalensis is highly valued by certain communities in Sub-Saharan Africa, as it can be consumed as a whole or processed into various products such as puree, nectar, jams, preserves, and jellies [5, 8, 9]. Also the fruit has been reported to be highly nutritious and has economic potential [10].
However, despite existing quantitative data on its physicochemical and nutritional composition, there is limited knowledge about the qualitative composition of certain substances for a best nutritional and economic valorization of this NTFP. Indeed, the amino acid profile of S. senegalensis pulp lacks scientific data, limiting its use in other food products that could potentially reduce malnutrition. To address this gap, the present study aimed to determine the free amino acid profile of S. senegalensis fruit pulp in three different climatic zones of Burkina Faso.
2 Material and methods
2.1 Sampling and preparation of samples
2.1.1 Samples collection
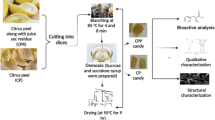
The ripe Saba senegalensis (A.DC.) Pichon fruits were collected from various localities in 3 different climate zones of Burkina Faso as indicated in Fig. 1. S. senegalensis (A.DC.), ID number: 102255 was identified by Pichon (1953) and verified by ARS Systematic Botanists in National Plant Germplasm System [11]. It is wild plant that grow in different areas of Burkina Faso. For the Sudanian zone, the fruits were gathered from Orodara, Wempea, and Gaoua, while Balongen, Passakongo, and Guirgho were selected for the Sudano-Sahelian zone, and Bourbon, RiguiRigui, and Bougouré for the Sahelian zone. The geographical coordinates were presented in the Table 1. The sampling was conducted between may and august 2022, and 25 samples of fruits each) per locality were collected in the same climatic zone, resulting in a total of 75 fruit samples per climate zone. Each sample weighed 1 kg, and a total of 225 samples were transported in plastic bag to the laboratory.
2.1.2 Pulp preparation
Fruits of good quality and maturity collected were first washed thoroughly using potable water and soap. Afterward, they were disinfected in a water solution that contained a few drops of sodium hypochlorite. To extract the pulp, a knife and spoon method was used, and then the pulps were blended with a Nasco 500 W5 blender. For analysis purposes, samples of 500 g of mixed pulp without seeds per locality in the climatic zones were taken and dried in an oven at 40 °C, for 14 days. Part of the pulp obtained was stored in freezer bags at 4 °C and kept frozen for analysis. The dried pulp was then powdered using a MINA MIQABO electric grinder. A composite sample of pulp extracted from each area was monitored.
2.2 Methods of analysis
2.2.1 Determination of crude protein
The protein content of the pulp was determined using AOAC, 1990 methods. The crude protein quantity was estimated by applying the macro-Kjeldahl method and then calculated by multiplying the measured nitrogen by a factor of 6.25. To evaluate the crude fiber, the defatted sample (2 g) was digested in a solution containing 1.25% HCl and 1.25% NaOH, following AOAC [12] guidelines.
2.2.2 Free amino acid extraction
To analyze free amino acids, aliquots of approximately 200 mg of frozen powdered fruit samples were used as edible parts.
The samples were precipitated using 80% cold ethanol (1.0 mL) in the presence of nor-Leu (50 nmol) as an internal standard. The mixture was homogenized using a Teflon pestle and centrifuged at about 14000 × g, at 4 °C. The obtained supernatant was lyophilized and then treated with 6% sulfosalicylic acid (500 μL) to precipitate any protein fraction that was still present. The mixture was then centrifuged again following method of Iriti et al. [13]. Generally, 30 μL of this extract was directly analyzed. Each sample was individually prepared and analyzed in triplicate.
2.2.3 Determination of free amino acid profile using high-performance liquid chromatography (HPLC)
Method describe by Dadwa et al. [14]was used. The detection and quantification of amino acids were carried out on a Waters 996 HPLC system that was equipped with a photodiode array detector (PDA), Waters 717 plus autosampler, Waters 600 controller, Waters TM pump, and Waters inline degasser AF.
The Lichrospher RP-18 column (250 mm × 4.6 mm, 5 μm, Merck, Germany) that was used in the analysis was fitted with a suitable guard column. Mobile phase A was composed of 0.14 M sodium acetate with 500 µL triethylamine and pH was adjusted to 6.7 with glacial acetic acid and methanol (90:10 (v/v)). On the other hand, mobile phase B was composed of acetonitrile: water (60:40 (v/v)). The binary gradient varied from 0 to 75% B in 15 min followed by 75–100% in 15 min. The flow rate was 1 mL min−1 and the amino acids were monitored at 355 nm. Limit of Detection (LOD) was 0.74 ng/mL [14].
2.2.4 Methods for evaluating amino acids for nutritional value
According to the WHO/FAO (World Health Organization/Food and Agriculture Organization of the United Nations, 1991) revised pattern spectrum of ideal essential amino acids for the human body [15]. The different ratios were calculated using the method of Heger [16], Yuan et al. [17], and Odukoya et al. [18].
E/T (%) = EAA/TAA, E/N (%) = EAA/NEAA, SW/T (%) = SWAA/TAA, BI/T (%) = BIAA/TAA, BC/E (%) = BCAA/EAA and BC/A = BCAA/AAA.
E/T: Essential amino acids/Total amino acids.
EAA/TAA: Essential amino acids / Total amino acids.
E/N: essential/non-essential.
EAA/NEAA: essential amino acids / non-essential amino acids.
SW/T: Sweet /Total amino acids.
SWAA/TAA: Sweet amino acids /Total amino acids.
BI/T: bitter amino acids/Total amino acids.
BIAA/TAA: Bitter amino acids / Total amino acids.
BC/E: Branched-chain/ Essential.
BCAA/EAA: Branched-chain amino acids / Essential amino acids.
BC/A: Branched-chain/ Aromatic amino acid.
BCAA/AAA: Branched-chain amino acids / Aromatic amino acids.
RC: Ratio coefficient.
Essential amino acids: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine.
Nonessential amino acids: alanine, arginine, asparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine, and tyrosine.
Sweet amino acids: glycine, threonine, proline, and alanine.
Bitter amino acids: valine, isoleucine, leucine, phenylalanine.
Branched-chain amino acids (BCAAs): valine, leucine and isoleucine.
Aromatic amino acids: phenylalanine, tryptophan, and tyrosine.
2.3 Statistical analysis
The analysis of variance (ANOVA) was carried out to compare the mean values of each amino-acid concentration means using Fisher's tests at the probability threshold p = 5%. The statistical analysis of the data indicated significant differences in the values at the P < 0.05 confidence level. To identify groups related to free amino acid profiles, a multivariate cluster analysis was carried out using XLSTAT. In addition, Principal Component Analysis (PCA) was performed using R-commander 4.3.1 (https://r-forge.r-project.org/projects/rcmdr). The using Principal Component Analysis (PCA) analysis was carried out to explicitly determine amino acid variations between climatic zones.
3 Results and discussion
3.1 Amino acid composition and content in different areas
The amino acid profile analysis of S. senegalensis pulp is presented in Table 2. The statistical analysis of the data indicated that there were no significant differences in the values at the p = 0.05 confidence level across different climatic zones. Leucine had the highest essential amino acid levels at 377 ± 95.50 mg /100 g MS, while methionine had the lowest at 90 ± 22.50 mg/100 g MS. The levels of the other standard acids varied between 220. ± 55 mg/100 g MS for alanine, the lowest level, and 391 ± 10 mg/100 g MS for aspartic acid, the highest level. Notably, Taurine was not detected in any of the samples analyzed.
However, the essential amino acid amounts detected were comparatively lower than those reported by Owon et al. [19] who found higher levels of essential amino acids in Moringa Oleifera leaves in Egypt. Specifically, Owon et al. [19] reported threonine levels of 4100 ± 100 mg/100 g and leucine levels of 7700 ± 200 mg/100 g. Furthermore, Darsini et al. [20] and Escuredo et al. [21] reported higher amino acid levels in samples of Limonia acidissima L and quinoa (Chenopodium quinoa Willd), respectively, than those found in our study. In contrast, lowest amino acid levels (359 ± 71 mg/100 g leucine and 32 ± 13 mg/100 g methionine) than those found in our study were reported by in Goji berry (Lycium barbarum) fruit samples from China. On the other hand, our research found higher levels of essential amino acids when compared to Goji berry (L. barbarum) fruit samples from various regions of China [22]. A study conducted by Yu et al. [23] found that the Goji berry (L. barbarum) contained significant levels of essential amino acids. Specifically, the fruit contained 210 ± 44 mg/ 100 g of isoleucine, 359 ± 71 mg/ 100 g of leucine, 32 ± 13 mg/ 100 g of methionine, and 238 ± 56 mg/ 100 g of valine. Moreover, the fruit had higher levels of essential amino acids with 211 ± 48 mg/ 100 g lysine, 273 ± 107 mg/ 100 g phenylalanine, and 313 ± 49 mg/ 100 g threonine. Interestingly, our study revealed lower essential amino levels compared to that of Goji berry (Lycium barbarum) fruit samples from various regions across China had acids [22]. Kouadio et al. [24] observed that the amino acid content of Cocos nucifera L. kernel fruits increased as the fruits ripened while evaluating their proximal and amino acid composition.
Leucine value was found to have the highest level of amino acids in S. senegalensis pulp samples, regardless of the climatic zone (refer to Table 2). On the other hand, the amino acid content of S. senegalensis was found to be higher than the recommended levels by FAO/OMS [25] from supplement foods. S. senegalensis pulp is a potential food supplement that can address nutritional deficiencies due to its high content of essential amino acids. According to Mota et al. [26], the essential amino acid composition of proteins is crucial for nutrition. Since the human body cannot produce essential amino acids, they must be obtained through diet. As such, S. senegalensis pulp can offer a valuable source of these essential nutrients. Indeed, it was demonstrated that essential amino acids play a significant role in aging and serve as a nutritional source for bone health in older adult [27]. The high tryptophan content of S. senegalensis pulp indicates its potential use in combating metabolic disorders and associated symptoms such as depression and sleep disorders, as suggested by Kałużna-Czaplińska et al. [28]. Analysis of S. senegalensis pulps allowed the identification of nine non-essential amino acids from samples collected across the three climatic zones, as presented in Table 2. Non-essential amino acids play multiple roles in tumor metabolism as they serve as precursors for macromolecule biosynthesis, control the redox state, and act as substrates for post-translational and epigenetic modifications [29]. Table 2 reveals an absence of Taurine in the pulp samples of S. senegalensis. This indicates that the pulp could be used in the production of food for children, individuals with hypotension, or pregnant women. It is worth noting that food products containing taurine are not advised for children, pregnant women, or individuals with certain metabolic conditions [30].
Based on the climate zones, there isn't a noticeable variation in the amino acid content (as shown in Fig. 2). However, the content of free amino acids differs depending on the climate zone, which can be attributed to the edaphic condition of that particular climate zone. Indeed, Kabre et al. [4] have discovered that the diversity of S. senegalensis trees varies across the three climate zones of Burkina Faso. The diversity of these trees across different climate zones can affect the proximate composition of their fruits.
The variability in the standard amino acid profile of S. senegalensis pulps may be due to factors such as soil composition and climatic conditions, and in line with report maked by De Araujo et al. [31] who also demonstred the variation in free amino acids of PH-16 dry cacao beans under different edaphic crop conditions.
Studies have revealed that the amino acid content of plants is not only influenced by the soil's element content but also by other factors like latitude and altitude [32]. S. Senegalensis is a highly adaptable plant that can grow well in low fertility and harsh environments where traditional crops, vegetables, and fruit trees are unable to survive [33]. The high amino acid content of S. Senegalensis can be attributed to its characteristics and ability to thrive in such adverse conditions.
3.2 Nutritional value of amino acids in S. senegalensis pulp
Table 3 presents the ratios of various amino acids found in S. senegalensis pulp. The sweet amino acids (SW), including proline and alanine, contribute to the fruit's flavor and range from 690 to 746 mg/100 g for Z1, Z3, and Z2. Our study aligns with the recommended sweet amino acid threshold of 650–750 mg/100 g for food taste. The mean values of sweet amino acid content in the pulp suggest its potential contribution to food flavor. On the other hand, bitter amino acids, with mean values ranging from 1070 to 1163 mg/100 g for Z1, Z3, and Z2, respectively, exceed the bitter amino acid threshold of 410 mg/100 g. However, they enhance the freshness and sweetness of other amino acids [34].
The amounts of Branched-chain amino acids (BCAAs) were recorded at 930, 942, and 967 mg/100 g for Z1, Z3, and Z2 respectively, which were found to be below the taste thresholds of 900 mg/100 g. These BCAAs serve as essential building blocks for the body's protein synthesis and also function as regulators for protein, glucose, and energy metabolism, as well as brain function. Shimomura and Kitaura [35] and Jin et al. [36] have suggested that BCAAs possess antioxidant potential and play various significant roles within the body. Furthermore, our investigation's findings align with Tobias et al. [37], who have reported that vegetable proteins also serve as sources of BCAAs.
The content of aromatic amino acids in the pulp of S. senegalensis varied across three zones, with values of 680, 693, and 781 mg/100 g for Z1, Z3, and Z2, respectively. These values align with the threshold values of 600 mg/100 g as stated by Li and Zhang [38]. Additionally, Ntzouvani et al. [39] have associated aromatic amino acids, specifically phenylalanine and tyrosine, with cardiometabolic risk. The analysis of the amino acid profile reveals that the S. senegalensis pulp meets the essential amino acid standard required by the human body and possesses significant nutritional value. The ratios of amino acids in the S. senegalensis pulp from the three zones (Z1, Z2, and Z3) indicate that the E/T (essential amino acids/total amino acids) values ranged from 43.12% to 43.40%. The E/NE (essential amino acids/non-essential amino acids) ratios were 75.83%, 75.96%, and 76.68%, while the SW/T (sweet amino acids/total amino acids) ratios were 16.35, 16.56, and 16.49 for Z1, Z2, and Z3, respectively. The BI/T (bitter amino acids/total amino acids) ratios of the S. senegalensis pulp ranged from 25.35 to 25.74%. Furthermore, the BC/E (branched-chain amino acids/essential amino acids) values gradually increase from 50.69 to 51.09% for Z3, Z2, and Z1. The BC/A (branched-chain amino acids/aromatic amino acids) ratios ranged from 1.24 to 1.37.
The recommended amino acid ratios for the daily requirements of the human body, as stated by FAO [40], are approximately 40% for E/T (essential amino acids/total amino acids) and a minimum of 60% for E/NE. The BI/T ratio should be around 20%, while the SW/T ratio should exceed 60%. Additionally, the BCAA/EAA ratio should range from 40 to 45%. In the case of the pulp of S. senegalensis, the value of E/T ratio was found to be higher than the recommended percentage. It is important to note that EAA cannot be synthesized by the human body and must be obtained from dietary sources [41]. Furthermore, the BC/A.
value was observed to be lower than the threshold mean. According to Holecek [42] and Vinayashree and Vasu [43] the normal human ratio of BC/A should range from 3.0 to 3.5.
BC/A is linked to hypertension and has the potential to forecast cardiac events in individuals with heart failure. Additionally, it may impact insulin resistance during pubertal development in girls. This association was observed in studies conducted by Zhang et al. [44] and Hiraiwa et al. [45]. BCAAs play a crucial role in normal growth and function at both the cellular and organ levels. They have significant effects on protein synthesis, glucose homeostasis, anti-obesity mechanisms, and nutrient-sensitive signaling pathways. These findings were reported by Ruiz-Canela et al. [46] and Nie et al. [47].
3.3 Principal component analysis of amino acids
The correlation between amino acids and their distribution in different climate zones was analyzed using Principal Component Analysis (PCA) and presented in Fig. 2. The first two axes Dim1/Dim2 showed a variability of 100%, indicating a good representation. All amino acid were positive correlation with the Dim1 axis. Zone 2 was near the Dim1 axis, while zones 1 and 3 had a more nuanced contribution, sharing subspaces formed by the intersection of the Dim1 and Dim2 dimensions. The variables studied showed a strong correlation with the Sahelo-Sudanian zone. The better amino acid profile was found in the Sudano-Sahelian zone. Based on this analysis, S. senegalenis fruit, grown abundantly in the Sahelo-Sudanian climate, has a high mobilization of essential amino acids. Kabré et al. [4] found high densities of adult S. senegalensis in the Sahelian and Sudanian zones, with young plants having a satisfactory amino acid profile in the Sudano-Sahelian zone.
3.4 Correlation analysis of the contents of amino acids
Table 4 provides Interrelationships among the free amino acids profile of S. senegalensis pulp. These correlations were analyzed to identify interrelationships that could indicate the development of nutritional quality variability based on the climatic zones. The majority of the amino acids showed significant correlations (p < 0.05). The correlation between most of the amino acids was high, with a value greater than 0.75. However, the correlation between Alanine, Asparaginic acid, and Cysteine was relatively low. Among all the amino acids, Leucine had the highest correlation with Hydroxyproline, Serine, Tryptophan, and Arginine. The correlation between Leucine and Arginine was the strongest, with a value of 0.999. On the other hand, the correlation between Valine and Asparagine acid was the lowest, with a value of 0.298. Interestingly, there was a negative correlation between Alanine and Asparagine acid, with a value of -0.701. Similarly, Aspartic acid also showed a negative correlation with Alanine, with a value of −0.596.
The multivariate analysis of the PCA biplot presented in Fig. 2 is in accord with the absence of nonsignificant differences between the average contents of amino acids determined in S. senegalensis pulp. In the biplot of the PCA (Fig. 2), isoleucine is in perfect correlation (r = 1.00) with phenylalanine, threonine, and proline as presented in Table 2. Also, proline is in perfect correlation (r = 1.00) with phenylalanine and threonine. However, a very weak correlation has been found between alanine and hydroxyproline (r = 0.397), lysine and aspartic acid (r = 0.189), and asparaginic acid (r = 0.052). But, lysine was strongly correlated with cystine (r = 0.998) and tryptophan (r = 0.996) (Table 2). Leucine and isoleucine are the most abundant free amino acids in S. senegalensis pulps, and leucine was highly correlated with isoleucine (r = 0.915). Negative correlations have been observed between some free amino acids. Indeed, cystine was negatively correlated with asparaginic acid (r = −0.006); alanine was also negatively correlated with tyrosine (r = -0.159), with asparaginic acid (r = −0.701) and with aspartic acid (r = −0.596).
It was found that the amino acids in samples of S. senegalensis pulp were significantly correlated. This could be attributed to the similar amino acid profile resulting from the prevailing climatic conditions. As per Zhao et al. [48], the synthesis and accumulation of metabolic products such as amino acids in plants are mainly influenced by climatic conditions and management models with local characteristics.
4 Conclusion
Through this study, we were able to determine the amino acid composition of Saba senegalensis fruit pulp in the three climatic zones of Burkina Faso. The creeper's pulp is rich in both essential and non-essential amino acids, providing a strong foundation for the argument that this fruit when transformed into other food products, could help maintain a balanced diet and address malnutrition. To gather data on the distribution of proximal compositions throughout the three climatic zones, multivariate analysis techniques are necessary. The parameters of composite samples analyzed revealed that Sudano-Sahelian zone had the better amino acid profile. Conservation measures must be taken to protect the ageing species in the Sahelian and Sudanian zones. This would improve the nutritional profile of S. senegalensis fruits in Burkina Faso. It would be interesting to study amino acid profile during fruit maturity.
Data availability
Data relevant to this study can be provided upon request.
References
FAO, FIDA, OMS, PAM, UNICEF. L’État de la sécurité alimentaire et de la nutrition dans le monde 2022. Réorienter les politiques alimentaires et agricoles pour rendre l’alimentation saine plus abordable. FAO. L’État de la sécurité alimentaire et de la nutrition dans le monde 2022. Rome. 2022. 285 p. http://www.fao.org/documents/card/fr/c/cc0639fr. Accessed 1 Nov 2023.
FAO. Action contre la faim. Intégration de la nutrition dans le secteur de la foresterie de la théorie à la pratique - Une approche innovante appliquée à sept pays d’afrique francophone. Rome: FAO; 2021. p. 52. https://doi.org/10.4060/cb6788fr.
Loubelo E. Impact de la commercialisation des produits forestiers non ligneux ( PFNL) sur l’economie des menages et de la sécurité alimentaire: Cas de la république du Congo. Eur J Soc Law. 2015;27(2):232–52.
Belem KB, Ouédraogo M, Ouédraogo A. Variabilité démographique de Saba senegalensis (A. DC) Pichon suivant le gradient climatique au Burkina Faso. Bois Forêts des Trop. 2020;345(3):73–83.
Tiendrebeogo S, Ganou L, Compaore CS, Tapsoba FW, Dicko MH. Biochemical composition of Saba senegalensis fruits from Burkina Faso. African J Food Sci. 2020;14(10):322–9.
Gaye SM, Paul D, Cyrille AN, Mady C, Mama S, Mar DCG. Intake nutritional variabilities of Saba senegalensis fruits. Food Nutr Sci. 2022;13(10):826–34.
Parkouda C, Diawara B, Ganou L, Lamien N. Potentialités nutritionnelles des produits de 16 espèces fruitières locales du Burkina Faso. Sci Tech Sci Appl Technol. 2007;1(1):35–47.
Diabagate HMF, Traore S, Soro D, Cisse M, Brou K. Biochemical characterization and nutritional profile of jam and syrup from Saba senegalensis fruit in Côte d’Ivoire. J Food Res. 2020;9(6):67–78. https://doi.org/10.5539/jfr.v9n6p67.
Diabagaté HMF, Traoré S, Cissé M, Soro D, Brou K. Biochemical characterization and nutritional profile of the pulp of Saba senegalensis from Côte d’Ivoire Forest. Am J Food Nutr. 2019;7(1):19–25. https://doi.org/10.12691/ajfn-7-1-4.
Kouyaté AM, Diallo A, Diarra I, Antoine E, Traoré SD, Lykke AM, et al. Local knowledge of Saba senegalensis fruits against malnutrition in Mali Local knowledge of Saba senegalensis fruits against. For Trees Livelihoods. 2020. https://doi.org/10.1080/14728028.2020.1857310.
Pichon M. Monographie des Landolphiées. Mémoires de l'Institut Français d'Afrique Noire. 1953; 35: 316–324. https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomydetail?id=102255. Accessed 15 Dec 2023.
AOAC. Official methods of analysis. 15th ed. Washington D.C: Association of Official Analytical Chemists; 1990. p. 375–9.
Iriti M, Rossoni M, Borgo M, Ferrara L, Faoro F. Induction of resistance to gray mold with benzothiadiazole modifies amino acid profile and increases proanthocyanidins in grape: Primary versus secondary metabolism. J Agric Food Chem. 2005;53(23):9133–9.
Dadwa V, Agrawal H, Sonkhla K, Joshi R, Gupta M. Characterization of phenolics, amino acids, fatty acids and antioxidant activity in pulp and seeds of high altitude Himalayan crab apple fruits (Malus baccata). J Food Sci Technol. 2018;55:2160–9. https://doi.org/10.1007/s13197-018-3133-y.
Song J, Bi J, Chen Q, Wu X, Lyu Y, Meng X. Assessment of sugar content, fatty acids, free amino acids, and volatile profiles in jujube fruits at different ripening stages. Food Chem. 2018;270:344–52. https://doi.org/10.1016/j.foodchem.2018.07.102.
Heger J. Essential to non-essential amino acid ratios. Amin acids Anim Nutr. 2003;43(3):103–24.
Yuan Y, Xianyu Z, GuangGuang L, YanSong Z, Ding J, Hailong R, et al. Comprehensive evaluation and comparison of nutritional values of amino acids of Chinese flowering cabbage and related subspecies vegetables. Food Ferment Ind. 2019;45(14):102–7.
Odukoya JO, Odukoya JO, Mmutlane EM, Ndinteh DT. Phytochemicals and amino acids profiles of selected sub-saharan african medicinal plants’ parts used for cardiovascular diseases’ treatment. Pharmaceutics. 2021;13(9):1367.
Owon M, Osman M, Ibrahim A, Salama MA, Matthäus B. Characterisation of different parts from Moringa oleifera regarding protein, lipid composition and extractable phenolic compounds. OCL Oilseeds Fats Crop Lipids. 2021;28(45):1–12.
Darsini DTP, Maheshu V, Vishnupriya M, Nishaa S, Sasikumar JM. Antioxidant potential and amino acid analysis of underutilized tropical fruit Limonia acidissima L. Free Radic Antioxid. 2013;3:62–9.
Escuredo O, González Martín MI, Wells Moncada G, Fischer S, Hernández Hierro JM. Amino acid profile of the quinoa (Chenopodium quinoa Willd.) using near infrared spectroscopy and chemometric techniques. J Cereal Sci. 2014;60(1):67–74.
Guo M, Shi T, Duan Y, Zhu J, Li J, Cao Y. Investigation of amino acids in wolfberry fruit (Lycium barbarum) by solid-phase extraction and liquid chromatography with precolumn derivatization. J Food Compos Anal. 2015;42:84–90. https://doi.org/10.1016/j.jfca.2015.03.004.
Yu X, Gao Y, Zhao Z, Gao JM. Rapid determination of amino acids in Chinese Wolfberry (Lycium bararum L.) fruit by using fourier transform infrared spectroscopy and partial least square regression. Food Anal Methods. 2017;10(7):2436–43. https://doi.org/10.1007/s12161-017-0802-9.
Kouadio MK, Koné FMT, Soro PL, Doubi BTS, Konan KJL. Influence of maturity stage and cultivar on the proximate, mineral and amino-acid composition of Cocos nucifera L. Kernel from Côte d’ Ivoire coconut germplasm. J Food Nutr Sci. 2023;11(5):146–53. https://doi.org/10.11648/j.jfns.20231105.12.
FAO/OMS. Programme mixte FAO/OMS sur les normes alimentaires. Commission du Codex Alimentarius, 32ème session Rome (Italie), Rapport de la 30ème session du comité du codex sur la nutrition et les aliments diététiques ou de régime. Le Cap (Afrique du Sud). 2009; 1–223.
Mota C, Santos M, Mauro R, Samman N, Matos AS, Torres D, et al. Protein content and amino acids profile of pseudocereals. Food Chem. 2014;193:55–61. https://doi.org/10.1016/j.foodchem.2014.11.043.
Lv Z, Shi W, Zhang Q. Role of essential amino acids in age-induced bone loss. Int J Mol Sci. 2022;23(19):11281.
Kałużna-Czaplińska J, Gątarek P, Chirumbolo S, Chartrand MS, Bjørklund G. How important is tryptophan in human health? Crit Rev Food Sci Nutr. 2019;59(1):72–88.
Choi BH, Coloff JL. The diverse functions of non-essential amino acids in cancer. Cancers. 2019;11(5):675.
Abdikodirovna YN, Suratovich OF, Ogiloy B, Surayyo N. Influence of energy drinks components on different human organs and systems. Int Sci Res J. 2023;4(2):578–88.
De Araujo QR, de Loureiro GAHA, Póvoas CEM, Steinmacher D, De Almeida SS, Ahnert D, et al. Effect of different edaphic crop conditions on the free amino acid profile of ph-16 dry cacao beans. Agronomy. 2021;11(8):1–17.
Han WY, Huang JG, Li X, Li ZX, Ahammed GJ, Yan P, et al. Altitudinal effects on the quality of green tea in east China: a climate change perspective. Eur Food Res Technol. 2016;243(2):323–30.
Wang YK, Xue XF, Ren HY, Zhao AL, Wu H, Li DK. Advances of research and utilization of jujube (Ziziphus) germplasm in China. Acta Horticulturae. 2022. https://doi.org/10.17660/ActaHortic.2022.1350.7.
Lioe HN, Apriyantono A, Takara K, Wada K, Yasuda M. Umami taste enhancement of MSG/NaCl mixtures by subthreshold L-α-aromatic amino acids. J Food Sci. 2005;70(7):s401–5.
Shimomura Y, Kitaura Y. Physiological and pathological roles of branched-chain amino acids in the regulation of protein and energy metabolism and neurological functions. Pharmacol Res. 2018;133:215–7. https://doi.org/10.1016/j.phrs.2018.05.014.
Jin HJ, Lee JH, Kim DH, Kim KT, Lee GW, Choi SJ, et al. Antioxidative and nitric oxide scavenging activity of branched-chain amino acids. Food Sci Biotechnol. 2015;24(4):1555–8.
Tobias DK, Clish C, Mora S, Li J, Liang L, Hu FB, et al. Dietary intakes and circulating concentrations of branched-chain amino acids in relation to incident type 2 diabetes risk among high-risk women with a history of gestational diabetes mellitus. Clin Chem. 2018;64(8):1203–10.
Li XX, Zhang X. Summary of free amino acids to improve crop flavor quality. J China Agric Univ. 2022;27:73–81.
Ntzouvani A, Nomikos T, Panagiotakos D, Fragopoulou E, Pitsavos C, McCann A, et al. Amino acid profile and metabolic syndrome in a male Mediterranean population: a cross-sectional study. Nutr Metab Cardiovasc Dis. 2017;27(11):1021–30. https://doi.org/10.1016/j.numecd.2017.07.006.
FAO. Dietary protein quality evaluation in human nutrition. Report of an FAO Expert Consultation. Vol. 92. Rome: FAO food and nutrition paper; 2013. p. 1–79.
Sá AGA, Moreno YMF, Carciofi BAM. Plant proteins as high-quality nutritional source for human diet. Trends Food Sci Technol. 2020;97:170–84. https://doi.org/10.1016/j.tifs.2020.01.011.
Holecek M. Ammonia and amino acid profiles in liver cirrhosis: effects of variables leading to hepatic encephalopathy. Nutrition. 2015;31(1):14–20. https://doi.org/10.1016/j.nut.2014.03.016.
Vinayashree S, Vasu P. Biochemical, nutritional and functional properties of protein isolate and fractions from pumpkin (Cucurbita moschata var. Kashi Harit) seeds. Food Chem. 2021;340:128177. https://doi.org/10.1016/j.foodchem.2020.128177.
Zhang X, Ojanen X, Zhuang H, Wu N, Cheng S, Wiklund P. Branched-chain and aromatic amino acids are associated with insulin resistance during pubertal development in girls. J Adolesc Heal. 2019;65(3):337–43.
Hiraiwa H, Okumura T, Kondo T, Kato T, Kazama S, Ishihara T, et al. Usefulness of the plasma branched-chain amino acid/Aromatic amino acid ratio for predicting future cardiac events in patients with heart failure. J Cardiol. 2020;75(6):689–96. https://doi.org/10.1016/j.jjcc.2019.12.016.
Ruiz-Canela M, Toledo E, Clish CB, Hruby A, Liang L, Salas-Salvado J, et al. Plasma branched-chain amino acids and incident cardiovascular disease in the PREDIMED Trial. Clin Chem. 2016;62(4):582–92.
Nie C, He T, Zhang W, Zhang G, Ma X. Branched chain amino acids: beyond nutrition metabolism. Int J Mol Sci. 2018;19(4):954.
Zhao X, Zhang B, Luo Z, Yuan Y, Zhao Z, Liu M. Composition analysis and nutritional value evaluation of amino acids in the fruit of 161 jujube cultivars. Plants. 2023;12(9):1–12.
Funding
For this research work, co-authors have benefited financial support of ISP-Sweden for laboratory analysis.
Author information
Authors and Affiliations
Contributions
Conceptualization, MKS, DK and MHD; methodology, KKTY, MKS, IM, MN; software, IM, MN, YD; validation, MKS, DK and MHD; formal analysis, KKTY, IM, YD and AO; investigation, KKTY, IM and AO; resources, YD, AO and DK; data curation, MKS, IM, DK and MHD; writing—original draft preparation, KKTY, IM and MN, writing—review and editing, MKS, DK and MHD; visualization, YD, AO, DK; supervision, MKS, DK and MHD; project administration, MKS and MHD; funding acquisition, MKS, DK and MHD. All authors have read and agreed to the published version of the manuscript.
Risk of plant extinction
S. senegalensis ((A.DC.) Pichon, 1953)) does not face any Risk of Extinction according to institutional, national, and international guidelines and legislation. Also this plant is not mentioned in the IUCN red list index of threatened species Convention on the Trade in Endangered Species of Wild Fauna and Flora.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors of this manuscript declare that no conflicts of interest exist for the present research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yao, K.K.T., Somda, M.K., Mogmenga, I. et al. Free amino acids profile of pulp of Saba senegalensis (A.DC.) Pichon fruit in the three climatic areas of Burkina Faso. Discov Food 4, 21 (2024). https://doi.org/10.1007/s44187-024-00080-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44187-024-00080-0