Abstract
Chhurpe is a naturally fermented traditional dairy food of high altitude Western Himalayan region. They are generally prepared from cow or yak milk and are consumed during harsh winters. The present study was conducted to characterize the different Chhurpe samples traditionally prepared by the ethnic groups utilizing milk from different animal breeds such as cow, yak, Zomo (cow × yak), and Germo (Zomo × yak). Nutritional characterization revealed that 100 g of Chhurpe could completely meet the dietary protein requirements of children and adults with high concentrations of methionine and lysine. Tryptophan and valine were the limiting amino acids among all the Chhurpe samples. Palmitic, stearic, and oleic acids were the predominant fatty acids. The Chhurpe samples were a rich source of micronutrients such as calcium, iron, and zinc meeting above 70% of recommended dietary allowances (RDA) among children (3–10 years) and up to 20% RDA for adults. Culture-independent metagenomic analysis revealed that lactic acid bacteria were the predominant group, consisting of genera such as Lactobacillus, Leuconostoc, Lactococcus, and Streptococcus followed by acetic acid bacteria, mainly Acetobacter. At the species level, Lactobacillus delbrueckii was the abundant strain among all the Chhurpe samples. Species diversity was significantly higher in Chhurpe prepared from Zomo milk. Probiotic bacterial strains such as Lactobacillus helveticus, L. delbrueckii, L. brevis, and Leuconostoc mesenteroides were identified in the Zomo Chhurpe indicating their superior quality. The present study was an attempt to popularize Chhurpe and promote its wider consumption by highlighting its nutritional properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Agro-pastoralism is the main form of livelihood in the cold desert regions of the Western Himalayas such as Ladakh, Kinnaur, Lahaul, and Spiti. Meat and dairy products occupy a significant proportion in ensuring the nutritional security of these cold arid regions due to the limited availability of vegetation [1]. The commonly farmed livestock includes sheep, dairy cattle, buffalo, yaks, goats, and double-humped camels in this region [2, 3]. The livestock present in high-altitude regions has unique physiological mechanisms to adapt to hypobaric hypoxia, cold-stress conditions, and high irradiation (6–7 kWh/mm) [2]. Among the bovines, Yak (Bos grunniens) locally known as Demo, and the indigenous Ladakhi cow (Bos primigenius) locally known as Balang, are farmed primarily for milk and other dairy products. These breeds are known to produce milk with unique protein composition and have been demonstrated with health benefits such as anti-hypertensive and anti-inflammatory properties [4, 5]. However, their health management and dairy production practices are not standardized resulting in sub-optimal milk yield and dairy farm economics [5]. For upgradation, mainly enhanced milk yield, indigenous Ladakhi cows and Yaks are cross-bred with Holstein-Frieswal (Holstein Friesian x Bos indicus—Sahiwal) and exotic Jersey breeds (Bos taurus) [2]. The most common milk-yielding crossbreed in the Ladakh region is Zomo (Bos grunniens x Bos primigenius) [6]. The male progenies obtained from crossing indigenous cow Balang, with Yak, are infertile. Therefore, Zomo is generally backcrossed with Yak to obtain further progenies. The F2 progeny known as Germo (Zomo x Male Yak), is another major crossbreed used for milk production in the region [7].
As discussed earlier, naturally fermented dairy products are the major food components in the diet of the native population. They comprise curd (Jho), buttermilk (tara), butter (Maar) and cottage cheese (labo), dried cheese (Chhurpe), and khambir roti [1, 8]. The health benefits of naturally fermented dairy products from high-altitude regions are well known as a source of probiotic bacteria [9, 10]. Apart from meeting their nutritional requirements, fermented dairy products such as butter and cheese are important components of the rural economy contributing directly to the livelihood of the region [6]. Chhurpe is a fermented cottage cheese prepared from buttermilk after the separation of the cream. Chhurpe consists mainly of two varieties, soft (consumed immediately after processing) and hard Chhurpe (sun-dried and stored for winter, long-term consumption). Chhurpe being a naturally fermented food, is unique compared to other conventional cheeses like mozzarella, and cheddar which are mostly prepared using rennet [11]. Hard Chhurpe is traditionally used as a chewing gum or masticator for obtaining extra energy for the body and movement of jaws during winter [1]. Chhurpe is the main ingredient used in the preparation of a variety of dishes such as Thukpa (soups), Thuth and Femer/Dhuru (Sweet dishes), and Tsunalik (noodle-like preparation) [12,13,14]. Chhurpe is traditionally prepared from yak and crossbreeds such as Zomo owing to their higher milk yield [6]. Similar to the Western Himalayas, naturally fermented dairy products obtained from indigenous cows, buffalo, yak, sheep, and goat are extensively consumed in Eastern Himalayan regions comprising Nepal, Sikkim, Darjeeling, Bhutan, and Arunachal Pradesh. The traditional cottage cheese obtained through the natural fermentation process in the Eastern Himalayan region is called Churpi or Chhurapi [15]. The traditional use and method of preparation of Churpi in the Eastern Himalayas is similar to Western Himalayas. In recent years, the demand for Chhurpe has increased tremendously as a source of protein food and pet food with a tenfold increase in the annual growth and production and a five-fold increase in the total sales of Chhurpe [16]. Despite their popularity and wider consumption in both Eastern and Western Himalayan regions, Chhurpe is not nutritionally characterized, unlike other conventional naturally fermented dairy products such as kefir or commercially produced cheddar or mozzarella cheese. In this context, Chhurpe obtained from different animal breeds were evaluated for their proximate, amino acid, fatty acids, and mineral composition. Additionally, the microbiome composition of the Chhurpe was also determined through a metagenomics approach to understand the microbial diversity and abundance of various probiotic bacteria present in these naturally fermented foods. The present study is an attempt to popularize traditional fermented food, Chhurpe from the Himalayan region as a nutritious probiotic food.
2 Materials and methods
2.1 Collection of samples
Milk, from four different animal breeds viz., Jersey cow (Bos. taurus taurus), Demo (Bos grunniens), Zomo (Bos. grunniens x Bos. primigenius), and Germo (Bos. grunniens x Zomo) were collected between 08 and 16, September 2021, from different areas of cold desert regions of the Western Himalayas. The photographs of different animal breeds from which the milk samples were obtained are presented in Fig. 1A. The fresh milk samples were collected in sterile plastic containers (HiMedia, Mumbai), and were immediately stored at 4 °C in refrigerated boxes. The collected samples were transported to the nearest town and temporarily stored at − 20 °C. Samples (milk, curd, and sun-dried Chhurpe) of the Jersey breed were collected from the village Tholang, Lahaul & Spiti, Himachal Pradesh, located in the GPS 32.57373077099872°N, 76.9577265041242°E. Samples from the Demo breed were collected from Kargyak village, Ladakh located at 33.064192252895815°N, 77.22450928275538°E, whereas samples of the Germo breed were collected from Lungnak, Ladakh located in the GPS 33.52491550328321°N, 76.9274889853959°E, and samples of Zomo were collected form Zanskar, Ladakh (33.56113693436301°N, 76.99525483729121°E). The animals from which the samples were collected were grazing on natural pastures and did not have any supplementary feeding. The samples were brought to the laboratory under cold storage and preserved at − 20 °C for further analysis.
2.2 Traditional method of Chhurpe preparation
The method of Chhurpe preparation was recorded based on detailed conversations with locals from the different regions of sample collection. A typical Chhurpe processing method followed in the Western Himalayan region consisted following steps viz., (i) boiling of milk (ii) formation of curd, locally known as zho, following back slopping method, (iii) preparation of buttermilk through separation of cream (iv) heating of buttermilk at 45–50 °C, locally known as tara, to obtain coagulated material (v) thorough mixing of the coagulated material, (vi) shaping and mattering of coagulated material to give soft Chhurpe, (vii) sun drying of soft Chhurpe. The different processes involved in Chhurpe preparation are presented in Fig. 1B. Samples of milk from different animal breeds and curd obtained after the initial step were collected for the determination of physicochemical properties.
2.3 Physicochemical properties of milk and curd samples
The frozen milk samples were thawed to room temperature (28 ± 1 °C) and thoroughly mixed without causing frothing or churning. Physicochemical properties such as the total solids (%), pH, acidity was determined as per the methods described in the manual of methods of analysis of foods (Milk and milk products) by the Food Safety Standards Authority of India (FSSAI, 2015) [17]. The percentage acidity was expressed as % lactic acid.
2.4 Proximate and nutrient analysis of milk, curd and dried Chhurpe
The proximate composition of thawed milk, curd and sun-dried Chhurpe samples was determined using standard procedures of the Association of Official Analytical Chemists (AOAC 2012) [18]. The crude protein was determined by the micro-Kjeldahl method, total fat was determined by n-hexane solvent extraction using a Soxhlet apparatus while ash was determined by igniting samples at 550 °C for 3 h in two cycles until constant weight. The total carbohydrate content was determined by the difference method. The total dietary fiber content was determined by serial enzymatic digestion method according to AOAC. Total starch content was determined by enzymatic digestion method where samples were digested with alpha-amylase and amyloglucosidase and the liberated glucose and total sugars were estimated by digesting samples in an acidic solution (2.5 N HCl) followed by determination using the phenol–sulphuric acid method as described in [19]. The mineral composition consisting of calcium, magnesium, sodium, potassium, iron, zinc, and copper was determined from the ash samples using atomic absorption spectrophotometry [18]. Atwater factors were applied to determine the total energy content of the samples, where 1 g of protein and carbohydrate contributes 4 kcal while 1 g of Fat contributes 9 kcal of energy. All analyses were performed in triplicates.
2.5 Amino acid and in-vitro protein digestibility analysis
The amino acid composition analysis was performed by digesting the Chhurpe samples (equivalent to 5.0 mg protein) in 6N HCl followed by derivatization with o-phthaldialdehyde. The amino acid derivatives were separated through reverse-phase high-performance liquid chromatography (RP-HPLC) and quantified. The amino acid content was expressed per 100 g of food product sample [19].
The in vitro protein digestibility (IVPD) of Chhurpe samples were measured by the sequential enzymatic digestion method as outlined by [20] with slight modifications. Briefly, 500 mg of samples were digested using pepsin derived from porcine mucosa (4520 U per mg protein) at pH 2.2, 37 °C for 30 min, followed by digestion with pancreatin at pH 6.8, incubated at 37 °C for 2 h in a shaking water bath. The reaction was terminated by the addition of 2% w/v trichloroacetic acid (TCA) solution. The samples were then centrifuged at 8000 g for 10 min and the protein content in the pellet was determined by the micro-Kjeldahl method following the AOAC protocol and the protein digestibility was estimated using the Eq. (1).
2.6 Fatty acid analysis
The fatty acid composition was determined by converting the crude fat obtained from Chhurpe samples to fatty acid methyl esters (FAME) as per the protocol described by [21]. The FAME conversion was achieved by reacting the extracted lipids with 5% (w/v) methanolic hydrogen chloride followed by extraction with n-hexane. The FAME-enriched hexane extracts were washed with 5% NaCl and 2% KHCO3 solutions and dried over anhydrous Na2SO4 followed by vacuum concentration. HPLC grade n-hexane was used for dissolving the FAME extracts and the fatty acid composition was determined using a GC–MS (Agilent 7890 series) equipped with a flame-ionization detector and a fused silica capillary HP-5 column (30 m length, 0.32 mm width, and 0.25 µm film thickness). The temperature program for the separation of fatty acids was followed as described by [19]. The injection volume was 0.5 µl of the FAME extract. The FAMEs were identified by comparing the retention times with the standard FAME mixture (C8–C24, FAME mix, Sigma-Aldrich, USA) and their fragmentation patterns. The data were expressed as the relative percentage composition of the fatty acids in each sample.
2.7 Indices for assessing the nutritional quality of fatty acids
Nutritional indices of dietary components, specifically fats, offer a method to evaluate the quality of fatty acid composition. Some of the most commonly used parameters are the index of atherogenicity (IA), index of thrombogenicity (IT), and health-promoting index (HPI). These indices were calculated using the Eqs. (2, 3 and 4) recommended by [22] but originally developed by [23].
IA was calculated using Eq. 1.
where, C12:0- Lauric acid; C14:0- Myristic acid; C16:0- Palmitic acid; Σ UFA- Sum of Unsaturated Fatty Acid.
IT was calculated using the Eq. 2.
where, C14:0- Myristic acid; C16:0- Palmitic acid; C-18:0 Stearic acid; Σ MUFA- Sum of Mono-unsaturated fatty acid; Σn-6 PUFA- linoleic acid; Σn-3 PUFA- alpha-linoleic acid.
HPI was calculated using Eq. 3.
where, C12:0- Lauric acid; C14:0- Myristic acid; C16:0- Palmitic acid; Σ UFA- Sum of Unsaturated Fatty Acid.
2.8 Total phenolics and flavonoids content analysis
For determination of total phenolic acids (TPC) and flavonoids content (TFC), the Chhurpe samples were extracted twice in 70% aqueous methanol and the extracts from each fraction were pooled and dried under vacuum. Aliquots of the extracts were prepared for TPC and TFC analysis. The TPC was determined using the Folin-Ciocalteu (FC) phenol method as described by [24]. Gallic acid was used as standard and the results were expressed as mg gallic acid equivalents (GAE) per gram dry extract (mg g−1). The total flavonoid content (TFC) was determined by the aluminium chloride (AlCl3) method suggested by [25]. Quercetin was used as reference standard and the results were expressed as mg quercetin equivalents (QUE) per gram dry extract (mg g−1).
2.9 Microbiological analysis
The dominant culturable bacteria were determined using the spread plate technique on the basis of colony-forming units (CFU) in selective media. The total bacteria count was determined as per the methods described in ISO 4833:1991 [26], reaffirmed in 2007 using the plate count agar. Yeast and mold count was determined on potato dextrose agar as per the method outlined by ISO 7954: 1987 [27]; reaffirmed in 2009. The lactic acid bacteria (LAB) were determined using de-Mann Rogosa Sharpe (MRS) agar medium at 37 °C for 24–48 h as described by [28]. The coliform content was determined as per ISO 4832:1991; reaffirmed in 2007 [29] while Escherichia coli was determined as per IS 5887, part 1, 1976; reaffirmed in 2009 [30] using MacConkey agar. The presence of pathogenic bacteria such as Salmonella, Shigella, and Staphylococcus aureus was determined using selective media by following ISO 6579: 1993; reaffirmed in 2009 [31], and IS 5887 (Part 2):1976 [32] respectively.
2.10 Microbiome composition and metagenomic analysis
The microbiota present in different Chhurpe samples was evaluated by 16S rRNA diversity analysis using the Illumina (NOVASEQ 6000) and performed at M/s. Neuberg diagnostics, India. The DNA was isolated from food samples and was checked for its concentration using NanoDrop 1000 (M/s. Thermofisher Scientific, USA). The DNA quality was confirmed by electrophoresis on a 1% agarose gel. The V4-V5 regions of 16S r RNA genes were amplified and the PCR products were utilized for the preparation of the libraries. The final libraries were quantified using Qubit 4.0 fluorometer with DNA HS assay kit (M/s. Thermofisher Scientific, USA) [19]. The insert size of the library was determined using Tapestation 4150 utilizing high-sensitive D1000 screencaps (M/s. Agilent Technologies, USA) as per the protocol outlined by the manufacturer. The raw data quality assessment was performed using Fast QC v.0.11.9 (default parameters) and summarized using Multi QC softwares. The primer fragments of matched sequences were removed with using Cutadapt plugin. The sequences were then denoised, merged and the chimera removed, using the DADA2 plugin using QIIME2 v2021.8 software with DADA2 quality settings of –p-trunc-len-f-p-trunc-len-r parameters of 251 and 251 respectively [19].
The Greengenes database was downloaded and sequences flanking the forward [CCTAYGGGRBGCASCAG] and reverse [GGACTACNNGGGTATCTAAT] primers were extracted followed by training the QIIME2 Naïve Bayes feature classifier. The representative sequences were classified by taxon using the fitted classifier. Amplicon Sequence Variants (ASVs) annotated as mitochondria or chloroplasts were removed by QIIME2 export options as they are considered unwanted. The features labelled as unassigned at different lineage levels viz., phylum, class, order, family, genus and species were removed. Sample-wise taxonomic abundances were plotted at different lineage levels using the ‘plot_bar’ function of the microeco R package software. Alpha rarefaction plot, PCA biplots and circular phylogenetic tree were plotted using Phyloseq tool. The Krona chart was plotted using the QIIME 2 Krona functions. The heatmaps and point plots based on the relative abundance for each lineage were plotted using the ampvis2 R package software [19]. A workflow describing the methodology used for metagenomic analysis is presented in the supplementary material Fig. S1.
2.11 Statistical analysis
All the experiments were carried out in triplicates, and the reported data are average ± standard deviation (SD). The statistical analyses were performed using one-way ANOVA followed by Tukey’s post hoc test and Chi-square test to find the significant differences among the samples at p ≤ 0.05 using the IBM® SPSS® statistics version 26.0 software, SPSS Inc., USA.
3 Results and discussion
3.1 Proximate composition of milk and curd
The nutritional composition of milk from different animal breeds is presented in Table 1. The protein content of the milk samples ranged between 3.21 g 100 g−1 and 4.13 g 100 g−1 with the highest protein content observed for the Germo (4.13 g 100 g−1). The protein content of the Jersey breed milk was in the range observed in earlier reports [33] while that of Demo was slightly lower compared to the generally observed range of 4.0–6.0 g 100 g−1 [6, 33]. The highest fat content was observed for Demo milk; however, the fat content was lower compared to the earlier reported range for Yak. In the case of the Jersey breed, the fat content was similar to the previously reported range [33]. The other parameters such as total solids content, pH, and acidity were in the general range observed earlier for these animal breeds. The variations in protein and fat content in the milk in comparison to the standard range could be attributed to several factors such as breed, age of the animal, parity, body condition, milking time, and milking methods [33]. Further, physiological factors such as the stage of lactation and udder health affect milk composition [34]. Another important factor that determines the milk quality and nutrient composition is the availability and quality of pasture. This variation in the milk quality, especially protein, fat, and micronutrient content could be attributed more to the pasture quality and availability than parity or other physiological conditions in animal breeds [35, 36]. This was very evident in yak and yak × cow breeds of alpine Himalayas of Nepal [37]. The milk production in Western Himalayas is primarily pasture-based and the distribution and availability of forage crops determine the milk composition in these animal breeds. The major forage crops distributed in the Tholang region during the sample collection period were Tanacetum sp., Taraxacum sp., Capsella bursa-pastoris, Parochetus sp., and Arnebia sp. (Supplementary Fig. S2). In the Kargyak, Lungnak, and Zanskar regions of Ladakh, red clover (Trifolium pratense), yellow sweet clover (Melilotus officinalis), alfalfa (Medicago sativa), Medicago falcata and Festuca kashmiriana were the predominant forage crops. The distribution of these forage crops has been earlier reported by [38,39,40]. Further, the ability to metabolize forage crops varies between animal breeds consequently affecting the milk quality.
The primary fermentation product derived from milk is curd. In the Western Himalayan region, the curd is generally prepared by the back-slopping technique and constitutes a significant component of their diet [41]. The nutritional composition of curd obtained from different animal breeds is presented in Table 1. The protein and fat content of the curd samples were more or less similar with very low statistical significance. Curd obtained from the milk of Zomo had slightly higher protein content compared to other samples while the fat content was highest in the Germo. Curd obtained from Jersey and Demo milk was slightly acidic compared to the other two breeds.
3.2 Nutritional quality analysis of Chhurpe
Chhurpe is a fermented cheese traditionally consumed in the high-altitude regions of both the Eastern and Western Himalayan regions [42]. Traditionally, Chhurpe is consumed as a masticator for obtaining sustained energy among the locals, cooked and consumed along with a variety of foods such as meat, soups, vegetables, and tea [13]. Despite being an important component of their diet, Chhurpe is poorly characterized in terms of nutritional and microbiome composition. In this context, the present study was taken up to characterize the Chhurpe obtained from different animal breeds commonly farmed in cold desert regions. The nutritional composition of the Chhurpe samples prepared from different milk sources is presented in Table 2. The moisture content of sun-dried Chhurpe was less than 1.5% w/w indicating their suitability for long-term storage as practiced traditionally. Generally, Chhurpe is prepared and stored for harsh winters when the availability of food is limited [1]. The total carbohydrate content of the Chhurpe ranged between 18.92 and 26.91 g 100 g−1. Jersey Chhurpe contained the highest starch content (19.14 g 100 g−1) while the least starch content was observed for Demo (4.93 g 100 g−1). The total sugar content ranged between 11.99 and 24.20 g 100 g−1 with the highest sugar content observed for the Jersey Chhurpe. Lactose content was highest in the Germo Chhurpe (1.86 g 100 g−1) whereas the lactose content was 3 folds lower in the Jersey Chhurpe (0.63 g 100 g−1) compared to Germo. Chhurpe is naturally a low dietary fiber food with values ranging between 1.3 and 2.1 g 100 g−1. Zomo Chhurpe contained the highest protein content (69.03 ± 1.23 g 100 g−1) followed by Demo (61.21 ± 1.47 g 100 g−1). The protein content between Jersey (57.38 g 100 g−1) and Germo (53.73 g 100 g−1) Chhurpe was statistically similar. The protein content observed in the present study for Jersey Chhurpe was lower compared to the earlier report of Panda et al. (2016), while that of Yak (Demo) Chhurpe was higher compared to the earlier report of Ahmed et al. (2018). The present study revealed that 100 g of Chhurpe could meet more than the complete recommended dietary allowances (RDA) of proteins for the Indian population (both children and adults) as per ICMR-NIN (2020) [43] (Supplementary Table S1). The total phenolic acids content of all the samples was statistically similar ranging between 1.42 and 1.61 mg g−1. The total flavonoid content was slightly higher in Zomo Chhurpe (7.54 mg g−1) followed by Demo Chhurpe (6.93 mg g−1) and least in Germo Chhurpe (5.61 mg g−1).
The proximate composition of the dried Chhurpe revealed that the cross-breed Germo was nutritionally superior in terms of total protein and fat contents when compared to other Chhurpe samples. The variations in the nutritional quality among the Chhurpe samples could be attributed to the fermentation conditions and the quality of the milk samples utilized for fermentation. For example, in a study conducted by [44], it was observed that the initial fat content of the milk samples affected the yield, texture and nutritional quality of Chhurpe obtained from cow and buffalo milks. The author reported that higher fat content in milk resulted in better Chhurpe yield, texture and sensory properties such as chewiness and gumminess. However, the trend was opposite with higher protein content and better shelf stability in Chhurpe obtained from low fat milk. The results obtained in the present study corroborates to this observation with Germo milk containing lower fat content yielding Chhurpe with highest protein content. Other parameters that affect Chhurpe quality are fermentation conditions and microflora of the inoculum [45]. Method of Chhurpe preparation significantly affects its chemical composition. It was observed that the free fat content of the Chhurpe increases due to simultaneous action of scraping and agitation during the heating of curd. Further improper heat treatment to the milk samples results in significant variations in coagulation of casein resulting in variations in the protein content of the Chhurpe samples [46]. The variations in total sugars, reducing sugars and lactose content in different Chhurpe samples could be attributed to the different degree of conjugation of sugars with proteins during heat induced coagulation [46, 47]. In the present study, the variations in heating of buttermilk (Tara) during Chhurpe preparation could be the main reason behind the varied total sugar and reducing sugar content in the samples.
3.3 Mineral composition of Chhurpe
The micronutrient mineral composition of Chuurpe samples is presented in Table 2. Among the different Chhurpe samples, Germo contained the high amounts of essential micronutrients viz., iron and zinc, 7.82 mg 100 g−1 and 3.46 mg 100 g−1 respectively. However, with respect to calcium, Zomo contained the highest (384.73 mg 100 g−1) while Germo had three folds lower calcium content (117.94 mg 100 g−1) compared to all the other Chhurpe samples. In the case of magnesium, Germo contained 6–7 folds higher content compared to the other samples. Phosphorus content was highest with Zomo followed by Jersey and least in Germo while potassium content was 2.5 to 3 folds higher in Germo compared to the others. The variation in mineral composition of different Chhurpe samples could be attributed to the milk quality which is in turn determined by the availability and distribution of forage crops in the geographical region.
The analysis revealed that the one hundred grams of Chhurpe samples could meet between 23 and 95% of RDA of iron among children aged between 3 and 10 years. In the case of adults, the different Chhurpe samples could meet an average of 20% RDA of iron. Similarly, Chhurpe could satisfy between 28 and 100% RDA for zinc in 3 to 10-year-old children while for adults only 10% to 20% of zinc requirements were met. For calcium, Chhurpe samples could averagely meet between 20 and 75% amongst 3–10-year-old children. However, with adults up to 38% of dietary calcium requirements were satisfied (Supplementary Table S1). From the present study, it is evident that Chhurpe is a rich source of essential micronutrients.
3.4 Amino acid composition of Chhurpe
The total amino acid content (TAA) in Chhurpe was highest in Zomo (67.99 g 100 g−1) followed by Demo and Jersey which were statistically similar (56–57 g 100 g−1) while Germo contained the least (Table 3). A similar trend was observed for essential amino acid (EAA) contents such as sulphur, branched chain, and aromatic amino acids. The lysine and histidine content were highest in Zomo Chhurpe followed by Demo and Jersey and least in Germo. However, in the case of threonine, Germo and Zomo contained similar amounts (1.84–1.85 g 100 g−1) and were higher compared to Jersey and Demo. The present study is the first attempt to characterize the amino acid composition of Chhurpe from the Western Himalayan region.
Chemical scoring of EAA of different Chhurpes against the amino acid requirements for 3 to 10 years old children as per FAO [48] indicated that tryptophan, threonine, and valine were the deficient amino acids among the Chhurpe samples. In Jersey, the 1st, 2nd, and 3rd limiting amino acids were tryptophan, valine, and threonine respectively. In the case of Demo, the 1st, 2nd, and 3rd limiting amino acids were valine, tryptophan, and threonine. With respect to Germo, only valine was limited while all the other EAA scored > 1.00. In Zomo, tryptophan and valine were the 1st and 2nd limiting amino acids (Supplementary Table S2). Overall, the scores for tryptophan and valine were below 0.5 for all the Chhurpe samples while that of threonine was ~ 0.9 in Jersey and Demo. With respect to SAA, the score was multi-fold higher (6–7 folds), and in the case of histidine the scores were > 2.00, and with respect to lysine the scores were between 1.5 and 2.0 folds. An interesting observation is that, despite containing lower crude protein and total EAA, Germo scored best in terms of meeting the EAA requirements of 3–10 years old children as per FAO [48]. The present study revealed that the traditional Chhurpe is an excellent source of protein and EAA satisfying the nutritional requirements for different age groups. The in vitro protein digestibility scores of Chhurpe samples ranged between 75 and 85%. Chhurpe from Demo had relatively highest digestibility over other Chhurpe samples, however, the percent protein digestibility among the four different Chhurpe samples was statistically similar. The study indicates that Chhurpe is a highly digestible protein-rich food, meeting most of the EAA requirements.
3.5 Total fat and fatty acid composition
The total fat content of Chhurpe samples ranged between 11.5% and 14.3% and was statistically similar. Fatty acid analysis revealed that saturated fatty acids (SFA) were the predominant class of fatty acids constituting nearly 65% of the total fatty acid composition followed by monounsaturated fatty acids (MUFA) (32–33%) and polyunsaturated fatty acids (PUFA) (2.5–3%) (Table 4). Palmitic acid (C-16:0), oleic acid (C-18:1, c9), and stearic acid (C-18:0) were the major fatty acids. The present study is the first one to report the detailed fatty acid composition of Chhurpe. The nutritional quality of the fat composition of Chhurpe was determined using nutritional indices such as the index of atherogenicity (IA), index of thrombogenicity (IT), and, health-promoting index (HPI) [22]. The IA of Chhurpe samples ranged between 2.01 and 2.24 with Zomo and Germo showing the lowest and highest index respectively. However, the values were statistically not significant. The IA observed in the present study was in the range earlier observed for dairy products [49]. Similar to IA, thrombogenicity is an important parameter that determines food quality with respect to cardiovascular health [22]. The IT values of Chhurpe obtained from different animal breeds ranged from 3.22 to 3.75 with the highest and lowest scores observed for Germo and Jersey Chhurpe respectively. The values were in the range observed for dairy products such as cheese and milk obtained from Jersey and Holstein breeds [49, 50].
Ulbricht and Southgate [23] suggested that the presence of high amounts of SFA lauric acid (C12:0), myristic acid (C14:0), and palmitic acid (C16:0) may have pro-atherogenic and thrombogenic properties such as adhesion of lipids to cells of circulatory and immunological systems and promoting clot formation in blood vessels. Dairy products are known to have high amounts of SFA that may promote atherogenicity and thrombogenicity [49]. It is recommended to consume foods that are lower in IA and IT scores. In the case of dairy products, the incorporation of polyunsaturated fats (both MUFA and PUFA) rich oils such as fish and flax seed oils to the dairy products from bovine (such as cheese and yogurt) enhanced both the sensory scores and health indices such as IA, IT, and HPI of the dairy products [49, 51]. Further, the fatty acid composition of milk and dairy products is influenced by diet composition, management practices, and feed supplements that are being provided to the animals [52]. It is noteworthy that the inclusion of unsaturated-rich oil sources such as sunflower oil, linseed oil, and tocopherols in animal feed has improved the milk fatty acid composition, especially their nutritional indices [50, 53].
3.6 Microbiological analysis
The microbial populations in all the samples of Chhurpe were examined and represented in Table 5. The total bacterial count ranged between 11300 CFU g−1 and 17800 CFU g−1 with Demo and Germo showing higher total bacterial count. Lactic acid bacteria (LAB) were the predominant group constituting between 38 and 50% of total bacterial count with the highest percent LAB observed in Demo Chhurpe. No pathogenic bacteria such as Salmonella, S. aureus, Shigella, and E. coli were observed in any of the Chhurpe samples. Analysis of freshly fermented soft Jersey Chhurpe revealed that the total bacterial count was greater than 40000 CFU g−1 with nearly 50% constituted by LAB. Contaminants such as yeast and molds, coliforms, and E. coli were detected in soft Chhurpe derived from Jersey milk (Supplementary Table S3). This could be attributed to the quality of water and utensils used during the course of preparation of Chhurpe. A similar observation was made by [54], who reported a notable number of contaminants like coliforms, yeast and molds, pathogenic bacteria such as E. coli, S. aureus, Salmonella, and Shigella in the soft Chhurpe from Sikkim and Darjeeling regions. Microbiological analysis of hard and soft Chhurpe from Sikkim regions revealed that drying of Chhurpe reduced the total bacterial count, LAB count, and other contaminants and pathogenic bacteria [13]. Drying of Chhurpe reduces the water activity of the product thereby reducing the total bacterial count and consequent spoilage. Thus, the traditional practice of drying Chhurpe to very low moisture content is essential for long-term storage in high-altitude regions where the availability of food is limited during harsh winters.
3.7 Bacterial taxonomic diversity
The most abundant phylum present in all the Chhurpe samples was Firmicutes (Jersey: 96.76%, Demo: 92.14%, Germo: 86.69%, and Zomo: 52.68%) followed by proteobacteria (Jersey: 3.06%, Demo: 7.85%, Germo: 13.26%, Zomo 47.22%). The other groups identified in very low numbers were Actinobacteria among all the Chhurpe samples. However, the phyla Bacteroidetes and Plantomycetes were detected only in Zomo Chhurpe (Fig. 2A).
At the genus level, the predominant group was Lactobacillus in Jersey, Demo and Germo Chhurpes constituting greater than 50%, particularly constituting up to 85% of the total abundance in Jersey sample. In the case of Zomo Chhurpe, Acetobacter was the predominant group constituting 44% of the total abundance (Fig. 2B). The abundance of Lactococcus group was highest in Zomo (22.7%) followed by Germo (17.7%) and least in Demo (0.2%). In contrast, Streptococcus group was abundant in Demo Chhurpe (40.7%) followed by Germo (10.69%), Jersey (8.17%) and least in Zomo (5.84%). Gluconobacter was detected in maximum abundance in Demo Chhurpe (2.37%) while they constituted less than 0.5% in other three Chhurpe samples. Acinetobacter was present only in Germo and Zomo Chhurpe (up to 1.2%) and very negligible in Jersey and Demo samples while Pseudomonas was present only Zomo (1.54%) and was very negligible in other Chhurpe samples (less than 0.1%). Certain genera were identified only in one or two Chhurpe samples such as Stenotrophomonas was detected in Germo and Zomo samples while Kocuria and Gardeneralla were detected only in Germo Chhurpe. Following genera namely Gluconacetobacter, Weisella, and Chryseobacterium were detected only in Zomo Chhurpe. Genus Tanticharoenia was detected in Jersey and Demo Chhurpe. The study indicated that Zomo had relatively higher bacterial abundance and genera diversity compared to other Chhurpe samples.
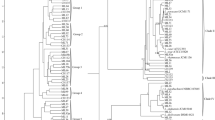
At the species level, the most abundant microbial strain in all the Chhurpe samples was Lactobacillus delbrueckii (Jersey: 99.45%, Demo: 99.94%, Germo: 95.2% and Zomo: 78.37%, (Fig. 2C). Among the various species identified, three probiotic strains viz., Lactobacillus delbrueckii, L. helveticus and L. brevis have been previously approved for human use as probiotics by Food Safety and Standards Authority of India, 2021 [55]. L. helveticus and L. brevis were detected in very low quantities among the different Chhurpe samples with Zomo showing highest relative abundance (Fig. 2C). In addition, other potential probiotic strain, Leuconostoc mesentroides was identified in significant quantities in Zomo (10.92%) followed by Germo (2.62%) and was negligible in the other two Chhurpe samples. Apart from these strains, Pseudomonas fragi and Acinetobacter johnsonii were present in significant abundance in Zomo Chhurpe samples which were very negligible in other samples. Apart from these few abundant strains, certain species were identified in only one or two Chhurpe samples. For example, Pseudomonas veronii was present only in the sample of Jersey and Zomo, while Weissella viridescens was present only in Demo and Zomo Chhurpe. Stenotrophomonas retroflexus was present only in Germo and Zomo, while Tenticharonia sakaeratensis was detected only in Jersey and Demo samples. Acinetobacter rhizosphaerae was present only in Demo and Germo while Acetobacter aceti was only present in Germo sample. Aggregatibacter segnis was detected in Demo while Pseudomonas viridiflava was present only in Chhurpe sample of Zomo. Similar to the pattern observed for genera distribution, highest species diversity was observed for Zomo sample (Supplementary Fig. S3). A combined circular phylogenetic tree was also constructed after 16S sequencing analysis of all the samples (Supplementary Figure S4). In the present study at the species level, unclassified sequences of bacteria were also detected in all the samples (Jersey: 17.2%, Demo: 51%, Germo: 42%, and Zomo: 76.3%) indicating the need for detailed characterization of microorganisms (Supplementary Figure. S5).
3.8 Multivariate analysis
3.8.1 PCA biplots and correlation matrix
Multivariate analysis revealed that Zomo Chhurpe was significantly different in terms of bacterial diversity and abundance which was evident in the heatmap (Fig. 2a, b, c). The PCA biplots revealed that Zomo Chhurpe was unique and distant in comparison to other samples at all the lineage (phylum, genus and species) levels (Fig. 3a, b, c). The Jersey, Demo and Germo Chhurpe were clustered together between the PC1 and PC2 at all the lineage levels indicating homogeneity in the bacterial population. However, between PC1 vs PC3, stark differences were observed among the samples. The Demo and Zomo Chhurpe lie on the opposite quadrants at all the lineage levels whereas Jersey and Germo were clustered together at phylum and genus level. However, at species level, all the four samples lie on the opposite quadrants indicating wide diversity (Fig. 3a, b, c). The detailed variations between PC1, PC2 and PC3 among the four samples are presented in supplementary material, Fig. S6.
Further the differences between the four Chhurpe samples at all the lineage levels were elucidated using correlation matrix (Supplementary Fig. S7a, b, c). At the phylum level, the Jersey, Demo and Germo samples showed very high correlation (between 0.99 and 1.00) whereas Zomo showed relatively lower correlation (between 0.69 and 0.77) when compared against the other samples (Supplementary Fig. S7a). Similarly, at the genus level, Jersey, Demo and Germo samples showed relatively higher correlation (0.82–1.00) among each other whereas Zomo was distinct and showed very low correlation when compared with Jersey (0.36) and Demo (0.32) (Supplementary Fig. S7b). Surprisingly, at species level, the correlation between all the samples were very high (0.99 -1.00). This could be attributed to the abundance of Lactobacillus delbrueckki as the predominant species among all the Chhurpe samples (Supplementary Fig. S7c).
The differences in the species diversity, and their distribution between the different Chhurpe samples were evaluated for statistical significance using a Chi-square test. The Zomo Chhurpe emerged significantly different from the rest of the Chhurpe samples based on the read data abundance distribution (χ2 -test: 4176, DF = 14, p < 0.001). Supporting these variations, the bacterial diversity and abundance were further elucidated at all the lineage levels using kernel density estimation (KDE) plots (Fig. 4a, b, c). The plots at phylum and genus level showed more or less similar pattern between the samples (Fig. 4a, b). However, a sharp difference was observed with respect to Zomo over others Chhurpe samples in terms of bacterial abundance showing a three-fold higher density (Fig. 4c).
The unique difference in Zomo Chhurpe over others could be attributed to the microflora of the raw milk of the Zomo breed. It was previously reported that the inherent microflora of milk, handling of product, and fermentation conditions significantly affect the bacterial composition of the naturally fermented foods [56]. Further the variations in bacterial composition could be corroborated to the fact that Chhurpe production is not controlled and purely dependent on the quality of starter culture and individual process variations. Thus, it is important that a standardized process may be developed for large-scale manufacturing of this unique Himalayan cheese.
3.9 Alpha diversity
A total number of 24, 31, 31 and 37 OTUs were detected in samples of Jersey, Demo, Germo and Zomo, respectively. Shannon–Wiener Diversity (H), Shannon–Wiener Evenness (EH), and Simpson Dominance (D), Indices were employed to determine and compare microbial diversity in the samples, based on the metagenomic data of all the Chhurpe samples (supplementary Fig. S8). The calculated value of the diversity indices to species evenness and richness were shown in supplementary Fig. S8 and Supplementary Table S4. The α-diversity metrics Demonstrated more species diversity in Zomo as compared to all other Chhurpe samples. This variation may be due to the abiotic and biotic factors of used for the preparation of Chhurpe which provide a habitable environment to flourish/support a rich microbial community.
There are very few reports describing the overall microbial diversity in traditional fermented foods. In case of curd generated from cow’s milk by back-slopping technique, the major phyla were Firmicutes and Proteobacteria [57]. Previous reports on naturally fermented dairy products like curd (dahi), churpi (cottage cheese), churkam (dried hard cheese) and marr (butter) from North Eastern Regions indicated that Firmicutes and Proteobacteria were predominant phyla [56]. In the case of fermented milk kefir samples, metagenomics analysis indicated that Acidobacteria, Actinobacteria, Proteobacteria and Firmicutes were predominant phyla [58] while the abundant phyla in Chinese traditional fermented milk product Koumiss were Firmicutes, Proteobacteria, and Actinobacteria [59].
Microbial diversity analysis of natural cottage cheese, churpi from North Eastern Region (NER) such as Sikkim and Darjeeling revealed that Lactobacillus sp., were predominant group constituted by Lactobacillus casei, L. plantarum, L. delbrueckii, L. paracasei, and L. brevis [54]. Similarly, metagenomics analysis of churpi, from North Eastern Himalayas indicated that Lactococcus lactis, L. helveticus and Leuconoctoc mesenteroides were predominant strains present in most of the fermented foods of India along with other species such as Acetobacter lovaniensis, Acetobacter pasteurianus, Acetobacter syzygii, and Gluconobacter oxydans [56]. Apart from these strains, Enterococcus durans, Enterococcus lactis and Enterococcus faecium were detected in hard churpi obtained from Yak milk from Sikkim Himalaya [60, 61]. It was observed that in vitro gastrointestinal digestibility of yak and cow milk-derived churpi from NER improved the bioactivity, specifically antioxidant and anti-hypertensive properties, owing to the presence of bioactive peptides derived from milk proteins such as α-S1-casein, α-S2-casein, β-casein, κ-casein, α-lactalbumin, and β-lactoglobulin [62]. The results obtained in the present study corroborate to that of earlier reports from NE Himalayas with a significant abundance of lactic acid bacteria such as Lactobacillus sp., and L. mesenteroides among the different Chhurpe samples. A similar observation of a relatively high abundance of lactic acid bacteria mainly of the genus Lactobacilli such as L. helveticus, L. plantarum, L. kefiranofaciens, and L. parabuchneri was observed in fermented milk kefir beverage [58]. However, apart from lactic acid bacteria, acetic acid bacteria such as Aacetobacter sp., Gordonia sp., were also equally abundant in the fermented kefir beverage. Metagenomic analysis of kefir inoculum, i.e., kefir grains revealed that the predominant genera were Bifidobacterium and Lactobacillus with a significant diversity of Lactobacillus species such as L. kefiranofaciens, L. helveticus L. amylolyticus, L. buchneri [63]. Metagenomics analysis of Chinese traditional fermented milk product Koumiss indicated that they were abundant in lactic acid bacteria specifically L. helveticus, L. kefiranofaciens, Lactoccoccus lactis, Streptococcus infantarius and Streptococcus thermophilus [59].
The microbial population during the process of fermentation determines the functionality of fermented food. In the Western Himalayan region, Chhurpe is used as a main ingredient for enhancing the nutritional and sensory properties of traditional dishes such as soups (Thukpa), sweet dishes (femer/dhuru), and noodle like preparations (Tsunalik) in the Western Himalayas region. Addition of Chhurpe gives a milky aroma to the products [12]. The characteristic aroma of the Chhurpe samples could be attributed to the natural microbial fermentation process involving Lactobacillus sp., and Pseudomonas sp. [15]. Short-chain fatty acids in milk fat are hydrolyzed by lipolytic enzymes in Pseudomonas fragi which then interact with ethanol to produce ethyl esters leading to a fruity aroma [64]. In addition to L. delbrueckii, presence of Leuconostoc p., also influences aroma generation during fermentation. In a recent report it was observed that fermentation of milk using L. delbrueckii sub sp., bulgaricus caused production of different aroma compounds such as 2,3-butanedione, δ-decalactone, acetaldehyde, butanoic acid, acetic acid and hexanoic acid attributing to the characteristic aroma profile of fermented dairy products [65]. Further, metabolite analysis of curds prepared by back-slopping technique indicated presence of volatile compounds such as 10-methyl dodecan-5-olide that nay have been produced by fermentation through Leuconostoc sp. under acidic conditions [57].
The present study validates the wide usage of Chhurpe as an important food during winter in the high-altitude region. Apart from its nutritional properties, the presence of health-promoting bacteria such as Lactobacillus sp., and Leuconostoc sp., indicate its potential as probiotic food. Strains belonging to Lactobacillus genus such as L. delbrueckii, L. helveticus, and L. brevis have been reported to show hypocholesterolemia properties and consequently anti-obesity and cardio-protective activities [66,67,68]. Further, Leuconostoc mesenteroides is a next-generation probiotic strain approved by the European food safety authority (EFSA) with excellent anti-microbial properties [69]. In addition, Acinetobacter johnsonii has been reported to enhance immunity and exhibit anti-inflammatory, and antioxidant properties [70]. The bioactive properties previously reported for a few bacterial strains detected in different Chhurpe samples from the present study are listed in supplementary Table S5.
The present work holds significant industrial implications in terms of validation of the nutritional quality of traditional food, Chhurpe from the Western Himalayas. Chhurpe obtained from the Zomo breed had the best nutritional quality and possessed highest bacterial diversity with the presence of many probiotic strains suggesting the unique quality of the breed adapted to high-altitude regions. The results obtained in this work hold societal relevance in terms of identifying a better milk source with better nutrition quality for the production of Chhurpe. Further, the presence of high amounts of protein and probiotic species such as Lactobacillus helveticus, L. delbrueckii, L. brevis, and Leuconostoc mesenteroides approved by Food Safety Standards Authority of India (FSSAI) offer tremendous opportunities in marketing Chhurpe as high protein probiotic foods [51]. The identification of antioxidant and ACE inhibitory bioactive peptides in Chhurpe obtained from Yak milk earlier by [71], indicates its potential application as a therapeutic food.
Apart from its use in local cuisines, Chhurpe has been recently exported as a food supplement and pet food, specifically as dog feed owing to its hard and chewy texture [16]. The sale of Chhurpe as a food ingredient is continuously increasing with an average growth rate of 10%-11% over the last five years with significant export opportunities. In Nepal, the annual exports of Chhurpe stood at 18.59 metric tonnes for the fiscal year 2020–2021 predominantly as dog food [16]. However, most of the Chhurpe production across the Indian Himalayan Region is unorganized and are mainly operated as cottage industry. Characterization of the predominant bacterial species in this naturally fermented food would help in standardization of fermentation process for commercial production of Chhurpe. In the present study, we identified Lactobacillus sp., to be the predominant bacterial group indicating that the product can be commercially manufactured with known Lactobacilli strains as inoculum. Thus, the present study is important from both academic as well as industrial point of view in popularizing the traditional fermented food unique to Indian Himalayan Region.
4 Conclusion
The present study for the first time compared the nutritional and microbiome composition of Chhurpe samples prepared using milk obtained from four different animal breeds that are commonly domesticated in the high-altitude Western Himalayan region. Among these, Chhurpe prepared from the milk of cross breed, Zomo had the highest protein, fat, calcium and total flavonoids content indicating its nutritional superiority over others. In addition, the metagenomic analysis revealed that Zomo Chhurpe possessed the highest bacterial species diversity with the presence of several probiotic bacterial strains such as Lactobacillus helveticus, L. delbrueckii, L. brevis, and Leuconostoc mesenteroides suggesting its potential application as natural probiotic food. Our work validates the potential application of Chhurpe as a low-cost protein supplement to combat malnutrition in the high-altitude regions where vegetation is scarce. Further, the results obtained in the present study suggest the need for the preservation and domestication of native breeds such as Zomo that are unique and well adapted to high-altitude regions. Currently, Chhurpe production is limited to cottage industries with wide variations in the product quality. The microbiome composition results obtained in the present work can be exploited for the optimization of large-scale fermentation for commercial production of Chhurpe by utilizing the predominant Lactobacilli species as starter cultures. Thus, the present work holds industrial importance along with its primary aim to characterize and popularize the traditional foods of the Western Himalayas, possessing unique resources and practices, towards their global outreach and wider consumption.
Data availability
The metagenomic sequences obtained in this study have been deposited in the NCBI database. The bio project number is PRJNA1036203 and the accession numbers are SRR26681221, SRR26681222, SRR26681223, and SRR26681224 for Zomo, Germo, Demo and Jersey Chhurpe samples respectively. The raw data obtained in the current study are available from the corresponding author upon reasonable request.
References
Raj A, Sharma P. Fermented milk products of Ladakh. IJTK. 2015;1:1.
Bharti VK, Giri A, Vivek P, Kalia S. Health and productivity of dairy cattle in high altitude cold desert environment of Leh-Ladakh: a review. Indian J Anim Sci. 2017;1(87):3–10. https://doi.org/10.56093/ijans.v87i1.66794.
Hamadani A, Ganai NA, Rather MA, Shanaz S, Ayaz AA, Mansoor S, Nazir S. Livestock and poultry breeds of Jammu and Kashmir and Ladakh. Indian J Anim Sci. 2022;92(4):409–16. https://doi.org/10.56093/ijans.v92i4.124009.
Gagoi DE, Sharma AN, Mann SA, Mukesh MA. Analysis of allelic pattern across milk trait genes in native cattle adapted to high altitude region of Leh-Ladakh. Indian J Animal Sci. 2020;90(11):1499–508. https://doi.org/10.56093/ijans.v90i11.111509.
Kumari P, Bharti VK, Kumar D, Mukesh M, Sharma I. Haematological and biochemical profiling of Ladakhi cow: a native cattle of high altitude Leh-Ladakh India. Indian J Animal Sci. 2020;90(4):599–602. https://doi.org/10.56093/ijans.v90i.104210.
Ahmed N, Abbas M, Bhat MI, Zargar KA, Dar NA, Ali L, Farooq I. Churpy-an important yak milk product and its traditional way of making for livelihood of tribal people of Zanaskar Ladakh. J Krishi Vigyan. 2018;7(1):12–7. https://doi.org/10.5958/2349-4433.2018.00149.6.
Jasra AW, Hashmi MM, Waqar K, Ali M. Traditional yak herding in high-altitude areas of Gilgit-Baltistan, Pakistan: transboundary and biodiversity conservation challenges. Yak on the Move. 2016;40:41–51.
Pohsnem JM, Ramakrisnan E, Parasar DP. Fermented food products in the Himalayan belt (North East India) and their health benefits. Int J Gastron Food Sci. 2023;9:100676. https://doi.org/10.1016/j.ijgfs.2023.100676.
Tamang JP, Cotter PD, Endo A, Han NS, Kort R, Liu SQ, Mayo B, Westerik N, Hutkins R. Fermented foods in a global age: east meets West. Compr Rev Food Sci Food Safety. 2020;19(1):184–217. https://doi.org/10.1111/1541-4337.12520.
Tamang JP, Lama S. Probiotic properties of yeasts in traditional fermented foods and beverages. J Appl Microbiol. 2022;132(5):3533–42. https://doi.org/10.1111/jam.15467.
Kondyli E, Pappa EC, Bosnea L, Vlachou AM, Malamou E. Chemical, textural and organoleptic characteristics of Greek semihard goat cheese made with different starter cultures during ripening and storage. Int Dairy J. 2023;1(145):105717.
Bhalla TC. Traditional foods and beverages of Himachal Pradesh.
Panda A, Ghosh K, Ray M, Nandi SK, Parua S, Bera D, Singh SN, Dwivedi SK, Mondal KC. Ethnic preparation and quality assessment of Chhurpi, a home-made cheese of Ladakh India. J Ethnic Foods. 2016;3(4):257–62. https://doi.org/10.1016/j.jef.2016.12.004.
Rai R, Tamang JP. In vitro and genetic screening of probiotic properties of lactic acid bacteria isolated from naturally fermented cow-milk and yak-milk products of Sikkim, India. World J Microbiol Biotechnol. 2022;38(2):25. https://doi.org/10.1007/s11274-021-03215-y.
Tamang JP. “Ethno-microbiology” of ethnic Indian fermented foods and alcoholic beverages. J Appl Microbiol. 2022;133(1):145–61. https://doi.org/10.1111/jam.15382.
Panta R, Paswan VK, Kanetkar P, Bunkar DS, Rose H, Bakshi S. Exploring trade prospects of Chhurpi and the present status of Chhurpi producers and exporters of Nepal. J Ethnic Foods. 2023;10(1):1–5. https://doi.org/10.1186/s42779-022-00165-0.
FSSAI. Manual of methods of analysis of foods–Milk and milk products. 2015. https://www.fssai.gov.in/upload/uploadfiles/files/MILK_AND_MILK_PRODUCTS.pdf. Accessed 12 June 2023.
AOAC, Official Methods of Analysis of AOAC International, AOAC INTERNATIONAL, Gaithersburg, MD, USA, 19th edn, 2012.
Kumar R, Sharma V, Das S, Patial V, Srivatsan V. Arthrospira platensis (Spirulina) fortified functional foods ameliorate iron and protein malnutrition by improving growth and modulating oxidative stress and gut microbiota in rats. Food Funct. 2023;14(2):1160–78. https://doi.org/10.1039/D2FO02226E.
Pasini G, Simonato B, Giannattasio M, Peruffo AD, Curioni A. Modifications of wheat flour proteins during in vitro digestion of bread dough, crumb, and crust: an electrophoretic and immunological study. J Agric food Chem. 2001;49(5):2254–61.
Christie WW. A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J Lipid Res. 1982;23(7):1072–5. https://doi.org/10.1016/S0022-2275(20)38081-0.
Chen J, Liu H. Nutritional indices for assessing fatty acids: a mini-review. Interna J Mol Sci. 2020;21(16):5695. https://doi.org/10.3390/ijms21165695.
Ulbricht TL, Southgate DA. Coronary heart disease: seven dietary factors. Lancet. 1991;338(8773):985–92.
Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc. 2007;2(4):875–7. https://doi.org/10.1038/nprot.2007.102.
Oh J, Jo H, Cho AR, Kim SJ, Han J. Antioxidant and antimicrobial activities of various leafy herbal teas. Food Control. 2013;31(2):403–9. https://doi.org/10.1016/j.foodcont.2012.10.021.
ISO 4833:1991—General guidance for the enumeration of microorganisms. Colony count technique at 30 °C.
ISO 7954:1987. General guidance for enumeration of yeasts and Molds. Colony count technique at 25 degrees C.
Baliyan N, Maurya AK, Kumar A, Agnihotri VK, Kumar R. Probiotics from the bovine raw milk of Lahaul valley showed cis-9, trans-11 conjugated linoleic acid isomer and antioxidant activity with food formulation ability. LWT. 2023;15(176):114553. https://doi.org/10.1016/j.lwt.2023.114553.
ISO 4832:1991. General guidance for the enumeration of coliforms. Colony count technique.
IS 5887 (Part 1): Methods for Detection of Bacteria Responsible for Food Poisoning, Part 1. Methods for Isolation, Identification and Enumeration of Escherichia Coli.
ISO 6579:1993. General guidance on methods for the detection of Salmonella.
IS 5887(Part-2): 1976. Methods for detection of bacteria responsible for food poisoning, Part 2: Isolation, identification and enumeration of Staphylococcus aureus and faecal streptococci.
Medhammar E, Wijesinha-Bettoni R, Stadlmayr B, Nilsson E, Charrondiere UR, Burlingame B. Composition of milk from minor dairy animals and buffalo breeds: a biodiversity perspective. J Sci Food Agric. 2012;92(3):445–74. https://doi.org/10.1002/jsfa.4690.
Lim DH, Mayakrishnan V, Lee HJ, Ki KS, Kim TI, Kim Y. A comparative study on milk composition of Jersey and Holstein dairy cows during the early lactation. J Animal Sci Technol. 2020;62(4):565.
Alothman M, Hogan SA, Hennessy D, Dillon P, Kilcawley KN, O’Donovan M, Tobin J, Fenelon MA, O’Callaghan TF. The “grass-fed” milk story: understanding the impact of pasture feeding on the composition and quality of bovine milk. Foods. 2019;8(8):350. https://doi.org/10.3390/foods8080350.
Timlin M, Tobin JT, Brodkorb A, Murphy EG, Dillon P, Hennessy D, O’Donovan M, Pierce KM, O’Callaghan TF. The impact of seasonality in pasture-based production systems on milk composition and functionality. Foods. 2021;10(3):607. https://doi.org/10.3390/foods10030607.
Barsila SR. Effect of parity in different grazing seasons on milk yield and composition of cattle× yak hybrids in the Himalayan alpines. J Appl Animal Res. 2019;47(1):591–6. https://doi.org/10.1080/09712119.2019.1697274.
Ahmad KS, Hameed M, Ahmad F, Sadia B. Edaphic factors as major determinants of plant distribution of temperate Himalayan grasses. Pak J Bot. 2016;48(2):567–73.
Ahmad S, Singh JP, Dev I, Radotra S, Bhat SS, Mir NH, Chaurasia RS. Evaluation of high yielding temperate perennial forages for augmenting forage resources in Ladakh (India). Appl Biol Res. 2021. https://doi.org/10.5958/0974-4517.2021.00022.7.
Kumar R, Joshi R, Kumar R, Srivatsan V, Satyakam CA, Patial V, Kumar S. Nutritional quality evaluation and proteome profile of forage species of Western Himalaya. Grassland Sci. 2022;68(3):214–25. https://doi.org/10.1111/grs.12357.
Mallappa RH, Balasubramaniam C, Nataraj BH, Ramesh C, Kadyan S, Pradhan D, Muniyappa SK, Grover S. Microbial diversity and functionality of traditional fermented milk products of India: current scenario and future perspectives. Int Dairy J. 2021;1(114):104941. https://doi.org/10.1016/j.idairyj.2020.104941.
Rai R, Shangpliang HN, Tamang JP. Naturally fermented milk products of the Eastern Himalayas. J Ethnic Foods. 2016;3(4):270–5. https://doi.org/10.1016/j.jef.2016.11.006.
ICMR-NIN, ICMR-NIN Expert group on Nutrient Requirement for Indians, recommended dietary allowances (RDA) and estimated average requirements (EAR), https://www.nin.res.in/RDA_short_Report_2020.html. Accessed 20 May 2023.
Sah DN, Bhandari RS, Singh YP, Vaidya P, Kansakar PB, Ghimire B, Kandel B, Sah JK, Lakhey PJ. Early outcome of Frey’s procedure for chronic pancreatitis: Nepalese tertiary center experience. BMC Surg. 2019;19:1–8.
Bhat NA, Gani A, Jhan F, Muzaffar K. Process standardization and characterization of chhurpi-a Himalayan homemade hard cheese. Appl Food Res. 2022;2(1):100116. https://doi.org/10.1016/j.afres.2022.100116.
Aktar Hossain S, Pal PK, Sarkar PK, Patil GR. Sensory characteristics of dudh churpi in relation to its chemical composition. Zeitschrift für Lebensmitteluntersuchung und-Forschung A. 1999;208:178–82.
Wu J, Li H, A’yun Q, Doost AS, De Meulenaer B, Van der Meeren P. Conjugation of milk proteins and reducing sugars and its potential application in the improvement of the heat stability of (recombined) evaporated milk. Trends Food Sci Technol. 2021;108:287–96. https://doi.org/10.1016/j.tifs.2021.01.019.
Food and Agriculture Organization, Dietary protein quality evaluation in Human Nutrition, Rome, Italy, 2013, 92:19–23, https://www.fao.org/ag/humannutrition/3597802317b979a686a57aa4593304ffc17f06.pdf. Accessed 23 May 2023.
Fehrmann-Cartes K, Toro-Mujica P, Garnsworthy PC. Influence of fish oil alone or in combination with hydrogenated palm oil on sensory characteristics and fatty acid composition of bovine cheese. Anim Feed Sci Technol. 2015;25:205. https://doi.org/10.1016/j.anifeedsci.2015.04.013.
Salles MS, D’Abreu LF, Júnior LC, César MC, Guimarães JG, Segura JG, Rodrigues C, Zanetti MA, Pfrimer K, Netto AS. Inclusion of sunflower oil in the bovine diet improves milk nutritional profile. Nutrients. 2019;11(2):481. https://doi.org/10.3390/nu11020481.
Ahmad N, Manzoor MF, Shabbir U, Ahmed S, Ismail T, Saeed F, Nisa M, Anjum FM, Hussain S. Health lipid indices and physicochemical properties of dual fortified yogurt with extruded flaxseed omega fatty acids and fibers for hypercholesterolemic subjects. Food Sci Nutr. 2020;8(1):273–80. https://doi.org/10.1002/fsn3.1302.
Esposito G, Masucci F, Napolitano F, Braghieri A, Romano R, Manzo N, Di Francia A. Fatty acid and sensory profiles of Caciocavallo cheese as affected by management system. J Dairy Sci. 2014;97(4):1918–28. https://doi.org/10.3168/jds.2013-7292.
Bodas R, Manso T, Mantecon AR, Juarez M, De la Fuente MA, Gómez-Cortés P. Comparison of the fatty acid profiles in cheeses from ewes fed diets supplemented with different plant oils. J Agric Food Chem. 2010;58(19):10493–502. https://doi.org/10.1021/jf101760u.
Miglani R, Ahmed S, Reynolds JW, Parveen N, Kumar A, Bisht SS. A comprehensive checklist of the earthworms (Annelida: Oligochaeta) Of Uttarakhand, India. Megadrilogica. 2022;27(10).
FSSAI. Food safety and standards (health supplements, nutraceuticals, food for special dietary use, food for special medical purpose, functional food and novel food) regulations, 2016. 2021. https://www.fssai.gov.in/upload/uploadfiles/files/Compendium_Nutra_29_09_2021.pdf. accessed 12 Jun 2023.
Shangpliang HN, Rai R, Keisam S, Jeyaram K, Tamang JP. Bacterial community in naturally fermented milk products of Arunachal Pradesh and Sikkim of India analysed by high-throughput amplicon sequencing. Sci Reports. 2018;8(1):1532. https://doi.org/10.1038/s41598-018-19524-6.
Joishy TK, Dehingia M, Khan MR. Bacterial diversity and metabolite profiles of curd prepared by natural fermentation of raw milk and back sloping of boiled milk. World J Microbiol Biotechnol. 2019;35:1–2. https://doi.org/10.1007/s11274-019-2677-y.
Tenorio-Salgado S, Castelán-Sánchez HG, Dávila-Ramos S, Huerta-Saquero A, Rodríguez-Morales S, Merino-Pérez E, de la Fuente LFR, Solis-Pereira SE, Pérez-Rueda E, Lizama-uc G. Metagenomic analysis and antimicrobial activity of two fermented milk kefir samples. MicrobiologyOpen. 2021;10(2):e1183. https://doi.org/10.1002/mbo3.1183.
You L, Yang C, Jin H, Kwok LY, Sun Z, Zhang H. Metagenomic features of traditional fermented milk products. Lwt. 2022;1(155):112945. https://doi.org/10.1016/j.lwt.2021.112945.
Ghatani K, Tamang B. Assessment of probiotic characteristics of lactic acid bacteria isolated from fermented yak milk products of Sikkim, India: Chhurpi, Shyow, and Khachu. Food Biotechnol. 2017;31(3):210–32. https://doi.org/10.1080/08905436.2017.1335212.
Chourasia R, Phukon LC, Abedin MM, Sahoo D, Rai AK. Production and characterization of bioactive peptides in novel functional soybean chhurpi produced using Lactobacillus delbrueckii WS4. Food Chem. 2022;1(387):132889. https://doi.org/10.1016/j.foodchem.2022.132889.
Abedin MM, Chourasia R, Phukon LC, Singh SP, Rai AK. Characterization of ACE inhibitory and antioxidant peptides in yak and cow milk hard chhurpi cheese of the Sikkim Himalayan region. Food Chem X. 2022;30(13):100231. https://doi.org/10.1016/j.fochx.2022.100231.
Ilıkkan ÖK, Bağdat EŞ. Comparison of bacterial and fungal biodiversity of Turkish kefir grains with high-throughput metagenomic analysis. Lwt. 2021;1(152):112375. https://doi.org/10.1016/j.lwt.2021.112375.
Fuquay JW, McSweeney PL, Fox PF. Encyclopedia of dairy sciences. Cambridge: Academic Press; 2011. p. 25.
Liu A, Liu Q, Bu Y, Hao H, Liu T, Gong P, Zhang L, Chen C, Tian H, Yi H. Aroma classification and characterization of Lactobacillus delbrueckii subsp. bulgaricus fermented milk. Food Chem X. 2022;15:100385. https://doi.org/10.1016/j.fochx.2022.100385.
Damodharan K, Palaniyandi SA, Yang SH, Suh JW. Functional probiotic characterization and in Vivo cholesterol-lowering activity of Lactobacillus helveticus isolated from fermented cow milk S. J Microbiol Biotechnol. 2016;26(10):1675–86. https://doi.org/10.4014/jmb.1603.03005.
Karakula-Juchnowicz H, Rog J, Juchnowicz D, Łoniewski I, Skonieczna-Żydecka K, Krukow P, Futyma-Jedrzejewska M, Kaczmarczyk M. The study evaluating the effect of probiotic supplementation on the mental status, inflammation, and intestinal barrier in major depressive disorder patients using gluten-free or gluten-containing diet (SANGUT study): a 12-week, randomized, double-blind, and placebo-controlled clinical study protocol. Nutr J. 2019;18:1–3. https://doi.org/10.1186/s12937-019-0475-x.
Zhao X, Zhong X, Liu X, Wang X, Gao X. Therapeutic and improving function of lactobacilli in the prevention and treatment of cardiovascular-related diseases: a novel perspective from gut microbiota. Front Nutr. 2021;7(8):693412. https://doi.org/10.3389/fnut.2021.693412.
Wang Y, Li A, Jiang X, Zhang H, Mehmood K, Zhang L, Jiang J, Waqas M, Iqbal M, Li J. Probiotic potential of Leuconostoc pseudomesenteroides and Lactobacillus strains isolated from yaks. Front Microbiol. 2018;4(9):2987. https://doi.org/10.3389/fmicb.2018.02987.
Li X, Huang Z, Liu H, Wang X, Chen J, Dai L, Dong S, Xiao Y, Yang L, Liu W. Screening of antagonistic bacteria against Flavobacterium columnus and its effects on growth performance and immune function of Carassius auratus. Reproduct Breeding. 2022;2(4):138–48. https://doi.org/10.1016/j.lwt.2021.112801.
Chourasia R, Kumari R, Singh SP, Sahoo D, Rai AK. Characterization of native lactic acid bacteria from traditionally fermented chhurpi of Sikkim Himalayan region for the production of chhurpi cheese with enhanced antioxidant effect. LWT. 2022;15(154):112801. https://doi.org/10.1016/j.lwt.2021.112801.
Acknowledgements
Authors acknowledge the Director, CSIR-Institute of Himalayan Bioresource Technology, Palampur for supporting and providing all the necessary facilities. The CSIR-IHBT publication number for this manuscript is 5494.
Funding
The study was financially supported by the Department of Science and Technology (DST), Govt. of India under the Science and Heritage Research Initiative, vide grant no. DST/TDT/SHRI-11/2021(G)- GAP0287, Coordinated Programme for Arid, Semi-Arid and Cold Desert Regions (ASACODER), vide grant no. SEED/ASACODER-099-055-067/2018 (G)—GAP -0299 and Council of Scientific and Industrial Research (CSIR), grant no. MLP-0201. Sahdev and Shanu were supported by fellowships from ICMR and UGC respectively.
Author information
Authors and Affiliations
Contributions
Conceptualization—SD, VS; methodology, SC, ASH, VK, RK, SD; software, ASH, SC, VK; validation, SC, SK, ASH, SD, RS, VS; formal analysis, SC, SK, ASH, RK, RS, VS; investigation, SC, SK, ASH, SD & VS; resources, VS, SD; data curation, VS, SD, SC, SK, RS, VS; writing—original draft preparation, ASH, SC, SK, SD &VS.; writing—review & editing, ASH, SC, SK, SD, RS & VS.; visualization, ASH.; supervision, RS, SD & VS.; project administration, VS & SD; funding acquisition, VS & SD.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The present study did not involve any animal handling such as breeding or maintenance in an animal house facility. CSIR-Institute of Himalayan Bioresource Technology was informed before the milk samples were used for this experiment, confirmed approval was not required.
Informed consent
The authors have obtained written consent from the local body at the study site for using the photographs of respondents for the manuscript. No other personal details such as name, age, sex, or biometrical characteristics have been mentioned in the manuscript.
Competing interests
The authors declare no competing interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choudhary, S., Shanu, K., Hegde, A.S. et al. Nutritional quality and microbial diversity of Chhurpe from different milk sources: an ethnic fermented food of high-altitude regions of the Western Himalayas. Discov Food 4, 10 (2024). https://doi.org/10.1007/s44187-024-00073-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44187-024-00073-z